Abstract
Repetitive peripheral stimulation is associated with an enhancement of the intensity of corticomotor responses. We analysed the effects of hemicerebellectomy on the modulation of cortical motor output associated with repetitive electrical stimulation of the sciatic nerve in the rat. Hemicerebellectomy blocked the enhancement of the corticomotor response. The cerebellum is a key player in this form of short-term plasticity.
A sustained somatosensory stimulation increases corticomotoneuronal excitability (Luft et al. 2002). Repetitive electrical stimulation of the sciatic nerve in the rat is associated with enhanced motor responses evoked from the contralateral motor cortex by transcranial magnetic stimulation (TMS). The same phenomenon is found in humans (Luft et al. 2002; Kaelin-Lang et al. 2002; Knash et al. 2003). Since TMS excites predominantly corticocortical connections targeting pyramidal tract neurones, it has been suggested that the somatosensory input modulates motor pathways excitability by acting in the motor cortex. Decortication blocks these excitability changes (Luft et al. 2002; Kaelin-Lang et al. 2002).
The cerebellum plays critical roles in motor learning. One of the most widely used experimental models for the study of learning processes has been the classical conditioning of nictitating membrane/eyelid (Delgado-Garcia & Gruart, 2002). Cerebellar nuclei, especially the interpositus nuclei whose discharges are tuned in relation to sensory feedback, have been ascribed to critical roles in pairing of conditioned and unconditioned responses of the cutaneous eye blink reflexes. It is now established that the cerebellum is integral to classical associative learning. Relearning of motor functions following hemispherectomy requires cerebellar input. Animals undergoing both cortical hemispherectomy and contralateral cerebellar resection are unable to relearn motor tasks. Sensation is a critical component of such motor recovery and relearning (Mackel, 1987; Sasaki & Gemba, 1987). The notion that the cerebellum is involved in sensory processing is strongly supported by neuroimaging studies (Nixon, 2003). For instance, marked cerebellar-related activations have been reported during active discrimination of texture and shape, and during passive limb movements and sensory monitoring tasks (Roland et al. 1989; Gao et al. 1996; Jueptner et al. 1997; Tesche & Karhu, 2000). It has been suggested that the inferior olive, the origin of climbing fibres, acts to gate sensory inputs appropriate to motor learning, and that the cerebellum prepares motor responses to predictable sensory events with an unequalled degree of temporal precision (Devor, 2002; Nixon, 2003).
Little is known about the mechanisms through which the cerebellum exerts its influence on the cerebral cortex. Since the cerebellum participates in the processing of sensory information and is a major site for motor learning in the central nervous system, we wondered whether the cerebellum could play a role in the adaptation of the intensity of the corticomotor responses following peripheral repetitive stimulation. In a recent work, we showed that cerebellar nuclei contributed to the sensory modulation (Oulad Ben Taib et al. 2005). However, effects of lesions involving simultaneously cerebellar nuclei and ipsilateral cerebellar cortex were not investigated and control studies were partial. Moreover, we did not perform a time-course study of the sensory modulation. In the present study, we used the model of hemicerebellectomy because of the drastic effects on motor behaviour in rats and the major deficits in terms of spontaneous firing of neurones in the central neuronal loops projecting to spinal motoneurones (Tarnecki, 2003).
Methods
Description of the procedures
Studies were performed following approval of the institutional animal care committee of the Free University of Brussels. The facilities housing animals are inspected on a regular basis and meet the current national regulations of Belgium. The laboratory has received the agreement to perform surgery in animals (Number LA1230492, Ministère des Classes Moyennes et de l'Agriculture, Belgium). Adequate food, water, ventilation and space are provided. Procedures to minimize discomfort were used during the experiments. Surgical procedures were conducted by a neurosurgeon familiar with aseptic techniques (to minimize risk of infection), and animals were under close supervision on a daily basis. Postoperative monitoring and care was provided.
Male Wistar rats (weight, 250–300 g; left hemicerebellar ablation, n = 7) were anaesthetized using an intraperitoneal administration of chloral hydrate (400 mg kg−1i.p.). In the seven rats, a caudal craniotomy was performed over one half of the cerebellum (Florenzano et al. 2002). The dura was exposed and incised, and the left hemicerebellum was removed. Subsequently, the overlying flaps of skin were opposed and sutured. Animals were allowed to recover from anaesthesia and surgical stress, and had free access to water and food. At the end of the experiments, brains were dissected to evaluate the extent of the lesion after administration of an overdose of chloral hydrate i.p. Histological verification of the lesion was performed. Complete ablation of the left cerebellar hemisphere and deep nuclei was carried out in all animals. There was no histological evidence of brainstem injury. We found no evidence of brain infection. Two of the seven rats died following surgery and were excluded from analysis (therefore, the results from five hemicerebellectomized rats were taken into account). Forty-eight hours after surgery, we investigated the responses evoked in the left gastrocnemius muscle following stimulation of the right motor cortex before (basal condition) and after repetitive electrical stimulation of the left sciatic nerve. We selected this timing because the severity of deficits observed both in human and in rodents is greatest early after ablation of the cerebellum. A second group of seven rats was used as the control group. Chloral hydrate was administered continuously using the i.p. route (CMA micropump; CMA, Sweden). Anaesthesia depth was adjusted for absence of abdominal contractions in response to tail pinch. The left sciatic nerve was surgically exposed for bipolar stimulation. Duration of stimulation was 1 h. Trains of stimulation were delivered at a rate of 10 Hz (a train being composed of five stimuli of a 1 ms duration; A310-A365 stimulator; World Precision Instruments, UK). Stimulus intensity was adjusted to produce constant somatosensory-evoked potentials (SEP) in EEG (Luft et al. 2002). Compound muscle action potentials (CMAPs) in the left gastrocnemius muscle were obtained using electrical stimuli (duration: 1 ms; square waves).
Stimulation of the right motor cortex
Stimuli were applied via screws which were fixed on the skull at the level of the right motor cortex (Paxinos & Watson, 1986). Peak-to-peak amplitudes in motor responses of the left gastrocnemius muscle were studied. Motor threshold was defined as the lowest intensity eliciting at least five out of ten evoked responses with an amplitude >20 μV. The sigmoid feature of the recruitment curve was checked in each rat. The intensity of stimulation was 130% of motor threshold. Filter settings were 30 Hz to 1.5 kHz (NeuroMax 4; Xltek, Canada).
H-reflex, F-waves, M responses
The H-reflex, the F-waves and the direct motor responses (M response) were studied in the left plantaris muscle using a method adapted from Gozariu et al. (1998). Electrical stimulation of the left tibial nerve was performed using needle electrodes inserted subcutaneously at the ankle, behind the medial malleolus. Electrical stimuli consisted of single square-wave shocks of 0.5 ms duration, delivered every 6 s. EMG recordings were made from the ispilateral plantaris muscle through a pair of needle electrodes inserted in the distal third of the sole (filters, 30 Hz to 1.5 kHz). Integrals of H and M responses were plotted against stimulus intensity to analyse the recruitment curves for the H/M ratios. We also assessed the F-wave persistence (percentage of F-waves present in a series of stimuli) and the ratio mean F/mean M wave amplitudes following 50 supramaximal stimuli. These studies were performed in the basal condition, and were repeated 1 h later after repetitive stimulation of the sciatic nerve.
Additional timing experiments
Because other factors associated with cerebellectomy in addition to operation itself, such as blood flow changes after surgery or responses to scar formation, could play a role, we studied the sensory modulation at other timings after operation. We applied the procedures described above 7–9 days (n = 5 rats), 2 weeks (n = 5 rats), 4 weeks (n = 5 rats; 3 died) and 5 weeks (n = 1 rat) after surgery. None of the rats developed a brain abscess.
Other control experiments performed 48 h after surgery
Assessment of the enhancement of the corticomotor response in left plantaris muscle and contralateral effects
In four of the control rats and in four of the hemicerebellectomized rats investigated 48 h after operation, we checked the enhancement of the corticomotor response in left plantaris muscle following repetitive stimulation of left sciatic nerve. In four of the hemicerebellectomized rats, we also analysed the effects of left hemicerebellectomy on the modulation of the cortical response on the right gastrocnemius muscle (stimulation of left motor cortex; repetitive stimulation of the right sciatic nerve).
Extracerebellar lesions
We also assessed the effects of a surgical lesion performed in left occipital region in four additional rats to exclude a ‘surgery’ effect (stimulation of the right motor cortex; recordings in left gastrocnemius muscle).
Statistical analysis
Acute phase (48 h after surgery)
We were particularly interested by the detailed study of the effects of hemicerebellar ablation at the acute phase. In each group (hemicerebellectomy group n = 5, control group n = 7), Student's t test was used to compare the intensities of the corticomotor responses before and after peripheral repetitive stimulation (Sigma Stat Software, Jandel Scientific, Germany). The same test was applied to compare the corticomotor responses in the basal condition and the enhancement of the motor potentials associated with repetitive stimulation in the two groups.
In each of the two groups of rats (hemicerebellectomy group n = 5, control group n = 7), the analysis of variance was used to compare the H-reflex and M response obtained as a function of increasing intensities of stimuli at time 0 and +1 h (repetitive stimulation effect). We also applied the analysis of variance to compare the amplitudes of M responses and H-reflex in the basal condition (time 0) in the two groups of rats (group effect, group by intensity interaction).
In each group (hemicerebellectomy group n = 5, control group n = 7), Student's test was used to compare the F-wave persistence and the ratio mean F/mean M at time 0 and 1 h later. The same procedure was applied to compare the F-waves both in the basal condition and following repetitive stimulation in the two groups. P values lower than 0.05 were considered as statistically significant.
Additional timing experiments
The question was: were the differences observed at 48 h were really due to the lack of the cerebellum? Therefore, in order to assess the consequences of hemicerebellar ablation in the two conditions (basal condition/following repetitive stimulation) as a function of time (time-course study, D'Agata et al. 1993), we applied the analysis of variance. The following parameters were analysed in four groups of rats (controls n = 7; hemicerebellar ablation 48 h, n = 5; hemicerebellectomy 7–9 days, n = 5; hemicerebellectomy 2 weeks, n = 5): corticomuscular responses in left gastrocnemius muscle, as well as H/M ratios (we selected the H response at 2.5 times M threshold, on the basis of recruitment curves of H reflex) and persistence of F-wave in left plantaris muscle.
Results
Results in control group and in rats 48 h after operation
Corticomuscular responses
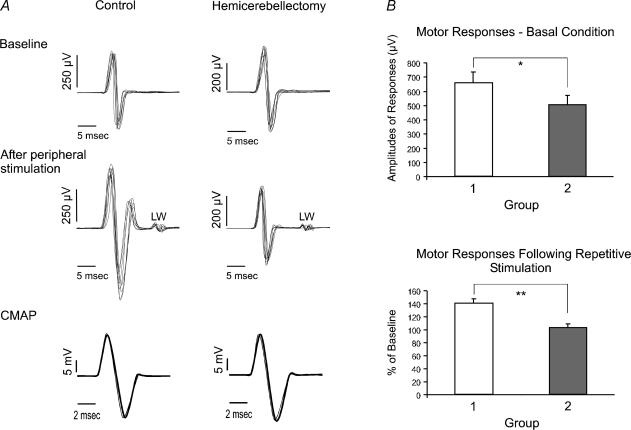
Typical findings are illustrated in Fig. 1A for a control rat and a rat with left hemicerebellar ablation. In the control rat, the corticomuscular response was enhanced following repetitive stimulation of the sciatic nerve. In addition, small amplitude late waves were observed, with latencies consistent with a transcortical reflex. In the basal condition, the amplitudes of responses were smaller in the hemicerebellectomized rat. Hemicerebellectomy blocked the increase of the intensity of the corticomotor response normally associated with repetitive peripheral stimulation. In both rats, CMAPs of the gastrocnemius muscle were unaffected by repetitive stimulation of the sciatic nerve.
Figure 1. Hemicerebellectomy impairs the adaptation of motor cortex.
A, corticomuscular responses evoked in left gastrocnemius muscle by electrical stimulation of the right motor cortex, before (top panels) and after (middle panels) repetitive stimulation of the left sciatic nerve. Superimposition of eight traces obtained in a control rat (left) and in a rat following left hemicerebellectomy (right, 48 h after operation). Lower panels, superimposition of compound muscle action potentials (CMAPs) of left gastrocnemius muscle before (thin trace) and after (thick trace) 1 h of repetitive stimulation. CMAPs are obtained by stimulating electrically the sciatic nerve following dissection. LW, late waves appearing after repetitive stimulation. B, upper panel, amplitudes of basal motor responses (recordings in left gastrocnemius muscle) in the two groups of rats before repetitive stimulation of the left sciatic nerve. Values are means ±s.d. and are expressed in microvolts. Group 1, no cerebellar intervention (7 rats). Group 2, left hemicerebellectomy (5 rats, 48 h after surgery). *P = 0.01, intergroup difference. Lower panel, corticomuscular responses evoked in left gastrocnemius muscle following stimulation of the right motor cortex (as compared to the basal condition; values are expressed in percentage of baseline values) in the two groups of rats. Means ±s.d. of motor responses are shown. Group 1, effects of 1 h of stimulation of the sciatic nerve in 7 control rats; group 2, effects in rats with hemicerebellectomy (n = 5). **P < 0.005, intergroup difference.
Latencies of corticomotor responses were between 7.9 and 9.1 ms in both the control rats and the rats with hemicerebellar ablation. Analysis of baseline motor responses (before concurrent somatosensory stimulation) showed that peak-to-peak values were lower in the hemicerebellectomized group (Fig. 1B; P = 0.01). In control rats, peripheral electrical stimulation was associated with an increase of motor response of 146.5 ± 9.6% (P < 0.001). In contrast, hemicerebellectomy prevented the enhancement of the response: mean ±s.d. of motor responses was 107.9 ± 7.4% of baseline measurements following stimulation of the sciatic nerve (P = 0.50). The enhancement of the motor potentials was higher in controls than in rats with hemicerebellectomy (P < 0.005).
H reflex and F-waves
Control rats
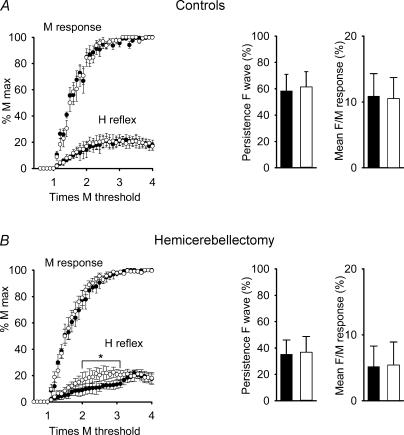
The M wave was of short latency (2.6–3.5 ms) (Gozariu et al. 1998). The latency of the H-reflex was longer (10.5–12.5 ms). The Hmax/Mmax ratios were found to be around 20%, as reported previously (Gozariu et al. 1998). The H-reflex had a lower thershold than the F-wave. The F-wave was observed at intensities of stimulation which elicited near-maximal M responses. The H-reflex amplitude increased as a function of stimulus intensity (Gozariu et al. 1998). Unlike H-reflex, F-waves did not decrease when the stimulus intensity was further increased and it disappeared at rates of stimulation >1 Hz. Repetitive peripheral stimulation of the sciatic nerve did not interfere with H-reflex (Fig. 2). The threshold, the slope and the Hmax/Mmax ratio were similar before and after stimulation (effect of repetitive stimulation on H-reflex amplitude, P = 0.24). Persistence of F-wave and ratios mean F/mean M wave were also unaffected (P = 0.26 and P = 0.30, respectively). M responses were unchanged (P = 0.40).
Figure 2. Effects of hemicerebellectomy on spinal cord.
M responses, H-reflex and F responses in control rats (A, top panels) and in hemicerebellectomized rats 48 h after surgery (B, bottom panels), before and after repetitive stimulation of the left sciatic nerve. Recordings in left plantaris muscle resulting from stimulation of the left tibial nerve. Left panels, M responses and H-reflexes at time 0 (basal state, filled circles) and time +60 min (open circles). Mean values ±s.d. are shown. Persistence and mean F/mean M wave ratios are illustrated in right panels (means ±s.d.; filled bar, time 0; open bar, +60 min). Values are means ±s.d.*P < 0.05.
Effects of hemicerebellectomy
M responses remained unchanged as compared with controls (P = 0.50). Hemicerebellectomy impaired the H-reflex recruitment curve. The comparison of the amplitudes of H-reflex in the basal condition (time 0) in the two groups of rats revealed a group effect (P = 0.02) and a group by intensity interaction (P = 0.015). Hemicerebellar ablation decreased the F-wave excitability ipsilaterally (intergroup comparison in the basal condition: P < 0.01 for both the persistence of F-wave and the ratios mean F/mean M response). Following repetitive stimulation, the shape of the recruitment curve of the H-reflex returned to normal. However, the depression of the F-wave was unchanged, with decreased persistence and reduced mean F/mean M wave ratios (intergroup comparison: P < 0.01 for both the persistence of F-wave and the ratios mean F/mean M response). In the hemicerebellectomized rats, these two parameters were unaffected by repetitive stimulation of the sciatic nerve (P > 0.30).
Additional timing experiments (time-course study)
Results are summarized in Table 1. A timing effect (P = 0.01) and a timing by condition effect (P = 0.002) were found for corticomuscular responses. Amplitudes of corticomotor responses in the basal condition returned to normal values 7–9 days after hemicerebellar ablation (intergroup difference, P = 0.40). However, hemicerebellectomy prevented the enhancement of the responses normally associated with repetitive stimulation until 5 weeks. In the basal condition, H/M ratios and persistence of F-wave returned to normal values 7–9 days following operation (intergroup difference, P = 0.32 and P = 0.25, respectively). These parameters remained unaffected by repetitive stimulation.
Table 1.
Electrophysiological parameters before and after repetitive stimulation of the left sciatic nerve
| Time after left hemicerebellar ablation | ||||||
|---|---|---|---|---|---|---|
| Condition | Control (n = 5)c | 48 h (n = 5)c | 7–9 days (n = 5)c | 2 weeks (n = 5)c | 4 weeks (n = 2)d | 5 weeks (n = 1)d |
| aCorticomuscular responses (μV)*, ** | ||||||
| Basal* | 659 ± 75 | 505 ± 68 | 666 ± 54 | 680 ± 41 | 638, 672 | 682 |
| Following repetitive stimulation* | 926 ± 45 | 522 ± 29 | 675 ± 69 | 691 ± 35 | 644, 693 | 701 |
| bH/M (%)*, ** | ||||||
| Basal* | 20.4 ± 4.3 | 10.9 ± 2.8 | 21.2 ± 3,7 | 20.2 ± 5.6 | 23.0, 18.5 | 17.6 |
| Following repetitive stimulation | 20.6 ± 4.9 | 20.3 ± 5.0 | 22.1 ± 5.0 | 21.4 ± 4.8 | 19.9, 22.7 | 19.1 |
| bPersistence of F-wave (%)* | ||||||
| Basal* | 58.4 ± 12.5 | 35.3 ± 10.8 | 51.5 ± 13.0 | 54.8 ± 12.1 | 59.2, 60.3 | 62.5 |
| Following repetitive stimulation* | 61.6 ± 11.4 | 36.9 ± 11.9 | 53.4 ± 10.7 | 56.7 ± 9.6 | 58.8, 59.1 | 61.0 |
Responses are recorded in left gastrocnemius muscle,
responses are recorded in left plantaris muscle.
Values are means ±s.d.,
individual values.
Timing effect, P < 0.05.
Time by condition effect, P < 0.05. Individual values obtained at 4 and 5 weeks are within mean ± 2.5 s.d. of control values.
Other control experiments
The amplitudes of the responses in left plantaris muscle following repetitive stimulation of the sciatic nerve increased to 142.3 ± 3.6% in the control group and to 104.7 ± 5.2% in the group with hemicerebellar ablation (intergroup difference, P = 0.006). In the four rats investigated, left hemicerebellectomy did not interfere with the modulation of the corticomotor response for the right gastrocnemius muscle following repetitive stimulation of the right sciatic nerve. Responses increased to 144.5, 153.6, 141.4 and 159.3% of baseline values. For extracerebellar lesions, responses in left gastrocnemius muscle increased to 151.9, 141.8, 156.1 and 147.2% of baseline values following repetitive stimulation of the left sciatic nerve.
Discussion
Our experiments confirm that baseline motor responses are depressed following acute cerebellar lesions (Hore & Flament, 1988). The main finding of this study is that hemicerebellectomy impairs the enhancement of the corticomotor response which is associated with repetitive stimulation of the sciatic nerve. So far, this form of plasticity was supposed to be under the sole control of the sensorimotor cortex (Luft et al. 2002; Kaelin-Lang et al. 2002).
Both the control experiments reported here and the time-course study of the effects of hemicerebellectomy on sensory modulation have not been described to date. Timing experiments have revealed that the impairment of adaptation of motor cortex is still present several weeks after hemicerebellectomy. In other studies addressing the time course of learning capacity as assessed by the shuttle-box test, a marked impairment was also found 1 month after cerebellectomy (D'Agata et al. 1993). A significant impairment of memory capacity was also detected in rats tested for avoidance behaviour 2 months after surgery, with a partial recovery 6 months after operation (D'Agata et al. 1993). The authors suggested a possible influence of cerebellum that was independent of its role in motor coordination.
Our main findings indirectly argue for cerebellar effects on supraspinal structures, in particular the motor cortical areas. The organization of the motor cortex is greatly dependent on the balance between excitatory and inhibitory influences over the network of cortical connections. The cerebellar ouput exerts an excitatory effect on the contralateral motor cortex via the cerebello–thalamo–cortical pathway. This tract is the most probable candidate for providing the input for gating the information flow (Molinari et al. 2002). Cerebellar informations are guided to the primary motor cortex via the ventro-lateral thalamic group which projects mainly to layers IV and V (Sanes & Donoghue, 2000). Through this channel, inputs can modulate the efficacy of the interconnections among cortical neurones, adjusting the circuitry of the motor cortex (Sanes & Donoghue, 2000).
Early after hemicerebellar ablation, we observed a decreased excitability of the spinal cord in hemicerebellectomized rats, with depressed H-reflex recruitment and decreased excitability of the anterior horn, as previously described in rodents and in human (Fox & Hitchcock, 1982; Drozdowski, 1995). The efferences of cerebellar nuclei modify the excitability of segmental motoneurones via ascending and descending pathways (Bantli & Bloedel, 1975). The specific effects of cerebellar nuclei stimuli on the excitability of lumbar alpha motoneurones are dependent upon the specific location within the nuclei at which the stimuli are applied. Experimental data support the existence of an excitatory cerebello-thalamo-corticospinal pathway which affects the excitability of motoneurones, since cooling of the motor cortex removes an excitatory component from the intracellularly recorded response evoked in lumbar motoneurones by dentate stimulation (Bantli & Bloedel, 1975). In addition, descending pathways from the brainstem, such as the rubrospinal tract, provide a part of the substrate by which the cerebellum participates in the control of muscle tension associated with limb movements. Our results suggest distinct anatomo–functional interactions between (1) the cerebellum and the motor cortex, and (2) the cerebellum and spinal motoneurones. Indeed, the time course of the deficit of the adaptation of the corticomotor responses to repetitive stimulation differs from the time course of the defect in spinal cord excitability. Whereas the first deficit persists at least 4 weeks after operation, spinal cord excitability returns to normal about 1 week after hemicerebellectomy. What could be the explanation for a short time course of spinal excitability changes? One of the important loops subserving the two-way communication between the sensory and motor systems is the spino–cerebello–rubro–spinal projection system (Tarnecki, 2003). This loop is linked to the musculature by the rubral projection to spinal motoneurones. Rubrospinal neurones receive major input from cerebellar nuclei (Courville, 1966) and show intense responses to sensory stimuli. Moreover, behavioural data indicate that the cerebello–rubral system is involved in learning and memory (Rosenfield & Moore, 1983; Tarnecki, 2003). Interestingly, the major decrease in the spontaneous firing of rubrospinal neurones, which appears after hemicerebellectomy, is reversible (Toyama et al. 1967; Tarnecki, 2003), and this could participate in the transient spinal excitability changes observed here. This is in contrast with the persistent cortical excitability deficits. Using TMS in human, it has been found that hemicerebellectomy is associated with permanent higher motor thresholds in motor cortex contralateral to the impaired hemicerebellum (Di Lazzaro et al. 1995). A reduction in the intrinsic excitability properties of the motor cortex functionally related to the impaired hemicerebellum has been observed. Our results argue in favour of a similar deficit in the intrinsic excitability properties in the rat.
Which parts of the cerebellum cause our findings and which are the mechanisms? We have found recently that cerebellar nuclei, the major output of cerebellar circuitry, could play an important role in the modulation of rodent cortical motor output after repetitive somatosensory stimulation (Oulad Ben Taib et al. 2005). Indeed, tetrodotoxin, a sodium channel blocker, administered locally in cerebellar nuclei blocks the adaptation of the corticomotor responses. Amongst cerebellar nuclei, the interpositus nucleus, which receives inputs from both the spinal cord and the sensorimotor cortex and which issues outputs to both the premotor neurones and the motor cortex, seems to have a major contribution. We aim to perform additional experiments such as cutting the superior or middle cerebellar peduncles or generating lesions in cerebellar cortex to increase our understanding of the results. Indeed, cerebellar cortex ablation removes the inhibitory effect of Purkinje cells on cerebellar nuclei (Tarnecki, 2003). This causes a considerable increase in the background firing and eliminates the pauses in discharges occurring in responses generated by somatosensory stimuli. Therefore, the areas of the cerebellar cortex which project upon interpositus nuclei are also good candidates.
Somatosensory input is implicated in motor learning, in the recovery of function following a cerebral lesion, and gives therapeutic benefits in functional recovery following vascular lesions (Johansson et al. 1993). Repetitive stimulation is currently used in humans to enhance reorganization of sensorimotor representations (Johansson et al. 1993). The motor pathways sensitized by peripheral input could increase their learning capacities (Ziemann et al. 2001). It is assumed that the increased excitability represents an early electrophysiological adjustment of the sensorimotor pathways. In a second stage, this functional change is transformed into a structural plasticity.
In a previous experiment, a marked effect of somatosensory stimulation on motor potentials (especially the N1 component of motor potential) was identified (Luft et al. 2002). Moreover, a significantly smaller amplitude enlargement recorded from the contralateral nonstimulated hindlimb was observed, indicating a somatotopic effect. Bilateral ablation of the sensorimotor cortex resulted in the disappearance of N1 component. It was suggested that the increase of the motor response was mediated by supraspinal structures, probably the motor cortex, by a mechanism of short-term potentiation. It has been shown that synaptic potentiation is enhanced by rhythmic patterns of activation that generate short-term synaptic facilitation effects (Werk & Chapman, 2003). Enhanced polysynaptic activation of layer V, one of the targets of cerebellar circuitry, is induced by repetitive trains (Werk & Chapman, 2003). We suggest that the cerebellum is a key player in this short-term adaptation to somatosensory stimulation. The pathophysiological mechanism reported here could contribute to learning deficits in acute cerebellar disorders.
Acknowledgments
This work was supported by the FNRS-Belgium.
References
- Bantli H, Bloedel J. The action of the dentate nucleus on the excitability of spinal motoneurons via pathways which do not involve the primary sensorimotor cortex. Brain Res. 1975;88:86–90. doi: 10.1016/0006-8993(75)90952-x. [DOI] [PubMed] [Google Scholar]
- Courville J. Somatotopic organization of the projection from the nucleus interpositus anterior of the cerebellum to the red nucleus. An experimental study in the cat with silver impregnation methods. Brain Res. 1966;2:191–215. doi: 10.1007/BF00236713. [DOI] [PubMed] [Google Scholar]
- D'Agata V, Drago F, Serapide F, Cicirata F. Effects of cerebellectomy on motivation-related behavior: a time course study. Physiol Behav. 1993;53:173–176. doi: 10.1016/0031-9384(93)90027-d. [DOI] [PubMed] [Google Scholar]
- Delgado-Garcia JM, Gruart A. The role of interpositus nucleus in eyelid conditioned responses. Cerebellum. 2002;1:289–308. doi: 10.1080/147342202320883597. [DOI] [PubMed] [Google Scholar]
- Devor A. The great gate: control of sensory information flow to the cerebellum. Cerebellum. 2002;1:27–34. doi: 10.1080/147342202753203069. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Restuccia D, Nardone R, Leggio MG, Oliviero A, Profice P, Tonali P, Molinari M. Motor cortex changes in a patient with hemicerebellectomy. Electroencephalogr Clin Neurophysiol. 1995;97:259–263. doi: 10.1016/0013-4694(95)00110-k. [DOI] [PubMed] [Google Scholar]
- Drozdowski W. F wave in acute cerebellar damage. Acta Neurol Scand. 1995;91:141–144. doi: 10.1111/j.1600-0404.1995.tb00421.x. [DOI] [PubMed] [Google Scholar]
- Florenzano F, Viscomi MT, Cavaliere F, Volonte C, Molinari M. Cerebellar lesion up-regulates P2X1 and P2X2 purinergic receptors in precerebellar nuclei. Neuroscience. 2002;115:425–434. doi: 10.1016/s0306-4522(02)00397-4. [DOI] [PubMed] [Google Scholar]
- Fox JE, Hitchcock ER. Changes in F wave size during dentatotomy. J Neurol Neurosurg Psychiatry. 1982;45:1165–1167. doi: 10.1136/jnnp.45.12.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao JH, Parsons LM, Bower JM, Xiong J, Li J, Fox PT. Cerebellum implicated in sensory acquisition and discrimination rather than motor control. Science. 1996;272:545–547. doi: 10.1126/science.272.5261.545. [DOI] [PubMed] [Google Scholar]
- Gozariu M, Roth V, Keime F, Le Bars D, Willer J-C. An electrophysiological investigation into the monosynaptic H-reflex in the rat. Brain Res. 1998;782:343–347. doi: 10.1016/s0006-8993(97)01402-9. [DOI] [PubMed] [Google Scholar]
- Hore J, Flament D. Changes in motor cortex neural discharge associated with the development of cerebellar limb ataxia. J Neurophysiol. 1988;60:1285–1302. doi: 10.1152/jn.1988.60.4.1285. [DOI] [PubMed] [Google Scholar]
- Johansson K, Lindgren I, Widner H, Wiklund I, Johansson BB. Can sensory stimulation improve the functional outcome in stroke patients? Neurology. 1993;43:2189–2192. doi: 10.1212/wnl.43.11.2189. [DOI] [PubMed] [Google Scholar]
- Jueptner M, Ottinger S, Fellows SJ, Adamschewski J, Flerich L, Muller SP, Diener HC, Thilmann AF, Weiller C. The relevance of sensory input for the cerebellar control of movements. Neuroimage. 1997;5:41–48. doi: 10.1006/nimg.1996.0249. [DOI] [PubMed] [Google Scholar]
- Kaelin-Lang A, Luft AR, Sawaki L, Burstein AH, Sohn YH, Cohen LG. Modulation of human corticomotor excitability by somatosensory input. J Physiol. 2002;540:623–633. doi: 10.1113/jphysiol.2001.012801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knash ME, Kido A, Gorassini M, Chan KM, Stein RB. Electrical stimulation of the human common peroneal nerve elicits lasting facilitation of cortical motor-evoked potentials. Exp Brain Res. 2003;153:366–377. doi: 10.1007/s00221-003-1628-9. [DOI] [PubMed] [Google Scholar]
- Luft AR, Kaelin-Lang A, Hauser TK, Buitrago MM, Thakor NV, Hanley DF, Cohen LG. Modulation of rodent cortical motor excitability by somatosensory input. Exp Brain Res. 2002;142:562–569. doi: 10.1007/s00221-001-0952-1. [DOI] [PubMed] [Google Scholar]
- Mackel R. The role of the monkey sensory cortex in the recovery from cerebellar injury. Exp Brain Res. 1987;66:638–652. doi: 10.1007/BF00270696. [DOI] [PubMed] [Google Scholar]
- Molinari M, Filippini V, Leggio MG. Neuronal plasticity of interrelated cerebellar and cortical networks. Neuroscience. 2002;111:863–870. doi: 10.1016/s0306-4522(02)00024-6. [DOI] [PubMed] [Google Scholar]
- Nixon PD. The role of the cerebellum in preparing responses to predictable sensory events. Cerebellum. 2003;2:114–122. doi: 10.1080/14734220309410. [DOI] [PubMed] [Google Scholar]
- Oulad Ben Taib N, Manto M, Laute MA, Brotchi J. The cerebellum modulates rodent cortical motor output after repetitive somatosensory stimulation. Neurosurgery. 2005;56:811–820. doi: 10.1227/01.neu.0000156616.94446.00. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego, California: Academic Press; 1986. [Google Scholar]
- Roland PE, Eriksson L, Widen L, Stone-Elander S. Changes in regional cerebral oxidative metabolism induced by tactile learning and recognition in man. Eur J Neurosci. 1989;1:3–18. doi: 10.1111/j.1460-9568.1989.tb00769.x. [DOI] [PubMed] [Google Scholar]
- Rosenfield ME, Moore JW. Red nucleus lesions disrupt the classically conditioned nictitating membrane in rabbit. Behav Brain Res. 1983;10:393–398. doi: 10.1016/0166-4328(83)90043-8. [DOI] [PubMed] [Google Scholar]
- Sanes JN, Donoghue JP. Plasticity and primary motor cortex. Annu Rev Neurosci. 2000;23:393–415. doi: 10.1146/annurev.neuro.23.1.393. [DOI] [PubMed] [Google Scholar]
- Sasaki K, Gemba H. Plasticity of cortical function related to voluntary movement motor learning and compensation following brain dysfunction. Acta Neurochir Suppl (Wien) 1987;41:18–28. doi: 10.1007/978-3-7091-8945-0_4. [DOI] [PubMed] [Google Scholar]
- Tarnecki R. Responses of the red nucleus neurons to limb stimulation after cerebellar lesions. Cerebellum. 2003;2:1–5. doi: 10.1080/14734220309405. [DOI] [PubMed] [Google Scholar]
- Tesche CD, Karhu JJ. Anticipatory cerebellar responses during somatosensory omission in man. Hum Brain Mapp. 2000;9:119–142. doi: 10.1002/(SICI)1097-0193(200003)9:3<119::AID-HBM2>3.0.CO;2-R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyama K, Tsukahara N, Udo M. Nature of cerebellar influence upon the red nucleus neurons. Exp Brain Res. 1967;4:292–309. doi: 10.1007/BF00235697. [DOI] [PubMed] [Google Scholar]
- Werk CM, Chapman CA. Long-term potentiation of polysynaptic responses in layer V of the sensorimotor cortex induced by theta-patterned tetanization in the awake rat. Cereb Cortex. 2003;13:500–507. doi: 10.1093/cercor/13.5.500. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Muellbacher W, Hallett M, Cohen LG. Modulation of practice-dependent plasticity in human motor cortex. Brain. 2001;124:1171–1181. doi: 10.1093/brain/124.6.1171. [DOI] [PubMed] [Google Scholar]