Abstract
Diabetes induces oxidative stress and leads to attenuation of cardiac K+ currents. We investigated the role of superoxide ions and angiotensin II (ANG II) in generating and linking oxidative stress to the modulation of K+ currents under diabetic conditions. K+ currents were measured using patch-clamp methods in ventricular myocytes from streptozotocin (STZ)-induced diabetic rats. Superoxide ion levels, indicating oxidative stress, were measured by fluorescent labelling with dihydroethidium (DHE). ANG II content was measured using enzyme-linked immunosorbent asssay (ELISA). The results showed DHE fluorescence to be significantly higher in cells from diabetic males, compared to controls. Relief of stress by the NADPH oxidase inhibitor apocynin or by superoxide dismutase (SOD) but not by catalase reversed the attenuation of K+ currents and reduced DHE fluorescence. In cells from diabetic females, neither apocynin nor SOD augmented K+ currents, ANG II was not elevated and DHE fluorescence was significantly weaker than in cells from males. Reduced glutathione (GSH) also augmented K+ currents in cells from diabetic males but not females. In ovariectomized diabetic females K+ currents were augmented by GSH and apocynin. Current augmentation and the attenuation of DHE fluorescence by apocynin were significantly blunted by excess ANG II (300 nm). Diabetic male rats pretreated with the angiotensin-converting enzyme (ACE) inhibitor quinapril were hyperglycaemic, but their cellular ANG II levels and DHE fluorescence were significantly decreased. In cells from these rats, K+ currents were insensitive to apocynin. In conclusion, diabetes-related oxidative stress attenuates K+ currents through ANG II-generated increased superoxide ion levels. When ANG II levels are lower, as in diabetic females or following ACE inhibition in males, oxidative stress is reduced, with blunted alterations in K+ currents.
Diabetes mellitus is a growing epidemic (Grundy et al. 1999). Despite improved treatments complications develop over time, with cardiovascular disease established as the leading cause of morbidity and mortality (Mooradian, 2003). Diabetic cardiomyopathy, independent of coronary disease, results in mechanical and electric abnormalities in cardiac function (Belke et al. 2000; Bell, 2003). Diabetes-related glucose elevation produces cellular oxidative stress (Inoguchi et al. 2000; Penckofer et al. 2002; Ceriello, 2003), expressed as overproduction of reactive oxygen species such as superoxide and hydrogen peroxide, and/or depletion of antioxidant defence mechanisms (Guzik et al. 2002; Marra et al. 2002). It is important to note that hyperglycaemia can lead to oxidative stress by a wide variety of mechanisms, including glucose auto-oxidation, increased production of advanced glycosylation end products and activation of the polyol and hexosamine pathways (Baynes, 1991; Giugliano et al. 1996; Nishikawa et al. 2000; Evans et al. 2003; Ceriello, 2003). Activation of protein kinase C has also been suggested to lead to oxidative stress (Ceriello, 2003; Ungvari et al. 2003).
Diabetes also leads to activation of a localized cardiac renin–angiotensin system (RAS) in rats and humans (Sechi et al. 1994; Fiordaliso et al. 2000; Frustaci et al. 2000). Elevated levels of ANG II play a major role in adaptive and maladaptive cardiac function (Dostal, 2000). ANG II is one of the multiple sources of enhanced oxidative stress (Nakagami et al. 2003; Ungvari et al. 2004), as it directly activates NAD(P)H oxidase (Zafari et al. 1998; Harrison et al. 2003), a key source of reactive oxygen species. NAD(P)H oxidase activation produces superoxide ions, which are subsequently converted to hydrogen peroxide (H2O2) by superoxide dismutase (SOD). H2O2, in turn, is broken down by catalase. Both superoxide ions and H2O2 are thought to play a role in diabetic complications (Giugliano et al. 1996) and in the pathophysiological consequences of ANG II elevation (Zafari et al. 1998).
Inhibiting angiotensin formation or action reduces oxidative stress and improves function in cardiovascular disease and diabetes, in animal models and in clinical studies (De Cavanagh et al. 2001; Privratsky et al. 2003; Hamilton et al. 2004). We have demonstrated (Shimoni, 2001) that a well-established attenuation of transient and sustained K+ currents in ventricular cells isolated from type 1 diabetic rats and type 2 diabetic mice is partly due to activation of the RAS. Inhibition of the formation or action of ANG II reverses the attenuation of both K+ currents (Shimoni & Liu, 2003a). We also showed that the activation of autocrine/paracrine mechanisms is absent or less pronounced in cardiac cells from (type 1 and 2) diabetic females (Shimoni et al. 2003; Shimoni & Liu, 2003b), probably due to a protective action of oestrogen. ANG II content (measured by enzyme-linked immunosorbent asssay (ELISA)) increases in diabetic males but not in females (Shimoni & Liu, 2004). Thus, we hypothesized that ANG II-mediated oxidative stress and its effects on K+ currents may also be reduced in diabetic female rats.
Sex differences in oxidative stress and their electrophysiological implications have not been investigated in detail. The response to oxidative stress is reported to show some sex differences, based on oestrogen action (Hernandez et al. 2000) as well as on differences in the generation of hydrogen peroxide and superoxide anions, in rats and humans (Brandes & Mugge, 1997; Lacy et al. 2000). Some of the cytoprotective effects of oestrogen are related to its antioxidative properties (Si et al. 2001; Strehlow et al. 2003). This protective action may be due to an inhibition of the RAS (Gallagher et al. 1999; Gragasin et al. 2003).
In addition to insulin deficiency, diabetes-related oxidative stress is a major modulator of cardiac K+ currents (Rozanski & Xu, 2002; Ayaz et al. 2004). Recent reports demonstrate that under (STZ-induced) diabetic conditions the transient K+ current in cardiac cells is regulated by cellular redox status (Li et al. 2004) and is augmented following relief of oxidative stress by glutathione (GSH; Xu et al. 2002). However, the specific role of ANG II in mediating oxidative stress-related cardiac electrophysiological abnormalities has not been studied.
The aim of the present work was to study how oxidative stress contributes to cardiac dysfunction in diabetes, through induction of electrophysiological complications. Specifically, the aims were: (1) to investigate how different pathways of relieving diabetes-induced oxidative stress affect attenuated K+ currents; (2) to establish whether ANG II is the dominant mediator of hyperglycaemia-induced oxidative stress; (3) to try to establish the role of superoxide ions; and (4) to establish whether the generation of oxidative stress in diabetes and its electrophysiological consequences are sex-dependent.
Methods
This study conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health.
Animals
Control and diabetic male and female Sprague-Dawley rats (250–300 g) were used. Diabetes (type 1) was induced with a single i.v. injection of streptozotocin (STZ, 100 mg kg−1), given 8–13 days before cell isolation. STZ destroys pancreatic β-cells and produces severe insulin deficiency and hyperglycaemia, as characterized previously (Shimoni et al. 1998). Ovariectomized females were also made diabetic 2–3 weeks after ovariectomy, and cells were isolated 8–13 days after STZ injection.
Cell isolation
Ventricular myocytes were obtained by enzymatic dispersion. Rats were anaesthetized by CO2 inhalation and killed by cervical dislocation. Hearts were removed and the aortas cannulated. Hearts were perfused for 3–5 min with a solution (at 37°C, bubbled with 95% O2–5% CO2) consisting of (mm): NaCl 121, KCl 5.4, sodium acetate 2.8, MgSO4 1, Na2HPO4 5, NaHCO3 24, glucose 5 and CaCl2 1. This was switched to a calcium-free solution (other constituents unchanged) for 10 min, followed by the same solution containing collagenase (Yakult, 0.015 mg ml−1), protease (Sigma type XIV, 0.0075 mg ml−1), 20 mm taurine and 40 μm CaCl2. After 8 min, the free wall of the right ventricle was cut into pieces for further incubation in a shaker bath (at 37°C), in a solution containing 0.3 mg ml−1 collagenase, 0.15 mg ml−1 protease, 20 mm taurine and 10 mg ml−1 albumin. Aliquots of cells were removed over 10–20 min and stored at room temperature in a solution containing no enzymes, 20 mm taurine, 5 mg ml−1 albumin and 0.1 mm CaCl2.
Current recording
Cells were placed on a stage of an inverted microscope and perfused (at 21–22°C) with a solution (pH 7.4) containing (mm): NaCl 150, KCl 5.4, CaCl2 1, MgCl2 1, HEPES 5 and glucose 5. CdCl2 (0.3 mm) was added to block L-type calcium currents. The whole-cell voltage-clamp method was used to record currents, elicited by 500-ms pulses to membrane potentials ranging from −110 to +50 mV. Currents were digitized (at 2 kHz) and normalized to cell size by dividing current amplitude by capacitance. This was measured by integrating current traces (digitized at 10 kHz) obtained in response to 5-mV steps from −80 mV. Recording pipettes (2–3-MΩ resistance) contained solutions consisting of (mm): potassium aspartate 120, KCl 30, Na2ATP 4, HEPES 10, EGTA 10, CaCl2 1 and MgCl2 1 (pH adjusted to 7.2, with KOH). Two currents were studied. The peak outward current (Ipeak), reflecting mainly the transient outward current, determines the sum of currents contributing to early repolarization and setting of the action potential plateau level. The sustained outward current (Isus), measured at the end of 500-ms voltage steps (with the transient current completely inactivated) reflects a mixture of delayed rectifier currents (Nerbonne, 2000) that determine late repolarization of the action potential. For each protocol, cells were divided into untreated and treated groups, and current densities compared in the absence or presence of different drugs.
Intrinsic variability of current density
It should be noted that STZ injection leads to slightly variable diabetic status in different rats. This results in some variability in oxidative stress as well. The effects on potassium current magnitude will therefore also be somewhat variable, leading to different baselines in mean Ipeak densities in the different experimental groups (e.g. see Figs 2, 4 and 6). There is also some variability due to transmural gradients in Ipeak (but not Isus) density, although this is reduced in the diabetic ventricle (Shimoni et al. 1995). However, the key point to consider is the effect (or lack thereof) of given treatments in each group of treated and untreated cells, rather than the actual baseline current magnitude. Thus, in earlier work (Shimoni & Liu, 2003b), currents from diabetic female cells were not much altered when compared to control females, but the additional key finding here was that there were no effects of inhibition of the angiotensin-converting enzyme (ACE), in marked contrast to the effects in males.
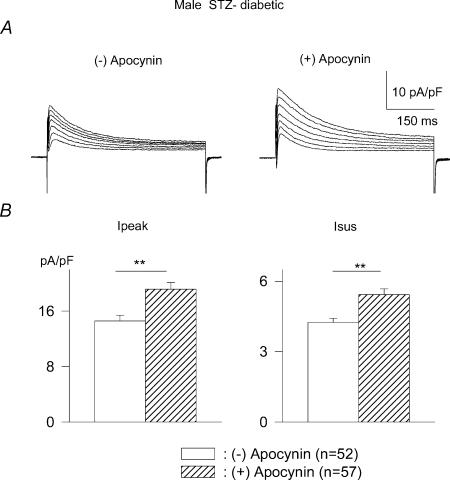
Figure 2. Effects of the NADPH inhibitor apocynin on outward currents in myocytes from male diabetic rats.
A, current traces (obtained in response to pulses from −80 mV to potentials ranging from −10 to +50 mV) from two cells in the absence (left) or following 8 h in the presence of 300 μm apocynin (right). B, mean current densities at +50 mV for Ipeak (left) and Isus (right) in the absence (open bars) or presence (hatched bars) of apocynin, which significantly enhances (P < 0.005) both currents.
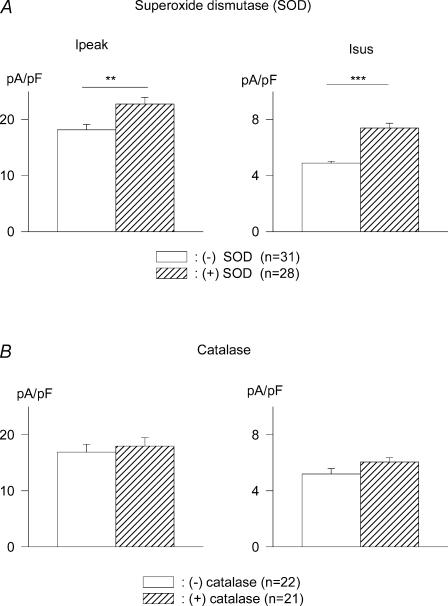
Figure 4. Effects of SOD and catalase on K+ currents.
A, superoxide dismutase (SOD, 300 U ml−1, 5–9 h) significantly augments K+ currents in myocytes from male diabetic rats. Mean Ipeak (left) and Isus (right) current densities (at +50 mV) are shown in the absence (open bars) and presence (hatched bars) of PEG-SOD. **P < 0.005; ***P < 0.0005. B, in contrast, catalase (500 U ml−1, 5–9 h exposure) has no effect on Ipeak (left) or Isus (right).
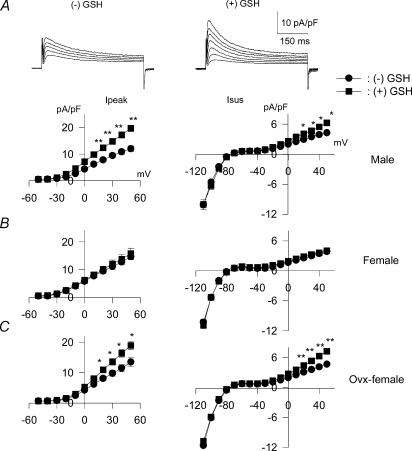
Figure 6. Sex-dependent effects of reduced glutathione (GSH) on outward currents in ventricular cells from STZ-induced diabetic rats.
A, current traces obtained from two cells obtained from males, in response to 500-ms voltage steps from −80 mV to potentials ranging from −10 to +50 mV, in the absence (left) or following 5.5 h in the presence of 1 mm GSH (right). Below are the current–voltage relationships, with mean (±s.e.m.) current densities plotted against membrane potentials in the absence (•) or following 5–8 h the presence of in GSH (▪). GSH significantly augments both Ipeak (left) and Isus (right). B, Current–voltage relationships obtained in cells from diabetic females (current traces omitted for clarity). No effects of GSH on Ipeak or Isus were observed in these cells. C, in contrast, the effects of GSH are restored in diabetic females that had been ovariectomized. *P < 0.05; **P < 0.005.
An added complexity is that current magnitudes are regulated by an interaction of many factors. These include insulin deficiency, elevated angiotensin and endothelin, as well as increased levels of reactive oxygen species. Thus, in cells from diabetic females there is still some reduction in current magnitudes due to insulin deficiency, despite the lack of activation of the RAS. In a large number of cells (to reduce the effects of variability discussed above) mean Ipeak density in males (at +50 mV) was 22.9 ± 0.8 pA pF−1 (n = 114) and 16.4 ± 0.6 pA pF−1 (n = 108) in control and diabetic conditions, respectively (P < 0.00005). In females, the corresponding values were 19.4 ± 0.6 (n = 127) and 17.5 ± 0.7 pA pF−1 (n = 101), respectively (P < 0.05). Control Ipeak was smaller in females (P < 0.005), and the reduction caused by diabetic conditions was significantly (P < 0.005) smaller in females (10%) than in males (26%). This may imply that in females reduction in Ipeak is dominated by insulin deficiency, whereas in males elevation of ANG II is an additional contributor, causing larger attenuation of Ipeak. Control Isus and its attenuation by diabetes were also significantly (P < 0.05) smaller in females (compared to control and diabetic males, respectively.
For each individual experiment, similar numbers of cells from the same heart were used for comparison between drug treatments. Results from different days were pooled, compensating for slight variations in diabetic status. Results are given as mean ±s.e.m.
Other biophysical characteristics of these currents were not investigated, because we had shown earlier that current attenuation associated with activation of the RAS is related to changes in channel protein expression (Shimoni & Liu, 2003a), with no effects on the recovery time course of the transient current or inactivation kinetics of the transient or the total outward currents (Shimoni, 2001; Shimoni & Liu, 2003a).
Superoxide production
Superoxide ions were detected using dihydroethidium (DHE, Molecular Probes). DHE is a cell-permeable fluorescent dye that is oxidized by superoxide to fluorescent ethidium bromide (EB). EB is a nucleic acid stain, trapped by intercalation with DNA. The fluorescence intensity indicates the relative level of superoxide production (Guzik et al. 2002; Bagi et al. 2003; Ungvari et al. 2004). Myocytes (from control and STZ-treated rats) were suspended in 800 μl buffer containing (mm): NaCl 120, KCl 5.4, MgSO4 1.2, Na H2PO4 1.2, glucose 5.6, NaHCO3 20, 2,3 butane-dione-monoxime (BDM) 10 and taurine 5, supplemented with 100 μm calcium and 0.2% fatty acid-free bovine serum albumin (BSA). DHE (prepared in DMSO as a 2 mm stock solution) was added to the cell suspension (final concentration, 5 μm) and the cells were incubated in a light-protected incubator for 30 min at 37°C. After 30 min, cell suspensions were centrifuged for 2 min at < 1000 g. Cell pellets were re-suspended in 20–50 μl phosphate-buffered saline (PBS). A small aliquot (10–15 μl) was fixed with 90% glycerol onto a slide.
In a previous study examining the production of superoxide in cardiac tissue sections and in cultured cells (Sayen et al. 2003), global DHE fluorescence was measured from thin sections or cell populations grown under different conditions. This method facilitates the analysis of fluorescence from uniform cell populations or within tissue; however, it could not be readily adapted to the analysis of the freshly isolated cardiac myocytes used in this study. Inherent in any isolated cardiac myocyte preparation is the presence of some cells that are hypercontracted and\or damaged. These cells, as well as cell fragments remaining from the isolation procedure, tend to pick up fluorescent labels. This, along with background fluorescence, would add uncertainty to any measurements of global fluorescence. For this study we therefore collected images of fields of cells with an Olympus IX70 inverted epifluorescence microscope and a SPOT™ RT cooled CCD camera. For each experiment, the groups of cells included in the analysis were prepared on the same day using the same protocols and reagents. All images for a given experiment were collected with the same camera settings and the fluorescence of individual nuclei in the images was analysed. To minimize the possibility that fluorescence from damaged cells was included in the analysis, only nuclei that were in focus and from rod-shaped robust-looking myocytes were analysed. To further reduce error, at least three different isolated cell preparations were analysed for each experimental situation. For each group of cells, fluorescence was measured from 10 to 50 nuclei, and the mean intensities compared. This was sufficient to indicate significant differences.
DHE fluorescence was initially analysed using two methods. In the first, a lower pixel intensity threshold was determined (using SPOT software) for images of individual nuclei so that only light from the nucleus remained in the image. The mean fluorescence intensity of pixels above threshold in the nuclear region was then determined using software written for this purpose. In the second method, an outline was drawn around the nucleus and the mean fluorescence intensity of the pixels within the nuclear boundary was determined from an intensity histogram using Photoshop software. Both methods gave similar results when images were compared, so that for most experiments the threshold method was used. It should be noted that in the following figures, the degree of difference between currents and DHE staining cannot be directly compared, as measurements had to be made using different cell populations.
Angiotensin II content
Angiotensin II content was measured in isolated cells by ELISA, using a commercial kit (Peninsula Laboratories). Standard curves were constructed, and optical densities of samples (in triplicate) were read from these curves (all values within the calibration curve range). Angiotensin II levels were normalized for protein content, measured in the same samples (using a bicinchoninic acid (BCA) protein assay kit from Pierce). The values obtained were similar to those reported by Fiordaliso et al. (2000).
Statistics
Mean values were compared using an unpaired t test or ANOVA, with the Student–Newman–Keuls multiple comparisons test. Differences with P < 0.05 were considered significant.
Results
Oxidative stress
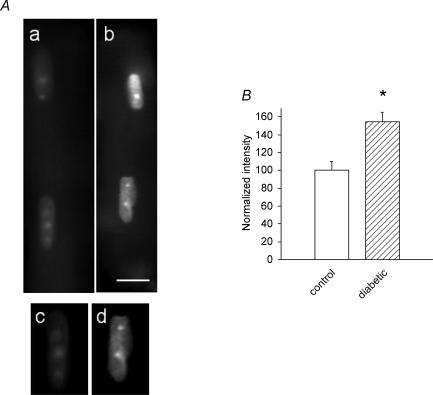
The first experiments established the validity of the fluorescent DHE measurements, confirming that ventricular myocytes from male diabetic rats demonstrate oxidative stress. Superoxide anions were detected (Guzik et al. 2002; Bagi et al. 2003) by DHE and their levels compared in myocytes from control and diabetic rats. In concordance with earlier reports, we found significant increases in superoxide levels in diabetic myocytes. Figure 1A shows typical labelled ventricular cells from a control (a) and a diabetic (b) male rat. Figure 1B shows the mean (±s.e.m.) levels of fluorescence intensity, measured by the threshold method (see above). The higher intensity of fluorescence in diabetic cells (155% of the control intensity, normalized to 100%) confirms the presence of oxidative stress under diabetic conditions. Two additional diabetic and non-diabetic cell preparations were analysed and gave similar results (for one the mean fluorescence intensity of 23 nuclei in the diabetic cells was 121 ± 6.2%, significantly different from non-diabetic (P < 0.01); for the second the mean intensity of 26 nuclei from the diabetic cells was 127 ± 7.7%, significantly different from non-diabetic at P < 0.005).
Figure 1. Comparison of DHE fluorescence in control and diabetic myocytes.
A, Images of myocytes from control (a) and diabetic (b) male rats. Panels c (control) and d (diabetic) show the uppermost nuclei in (a) and (b) after a lower threshold was chosen and pixels below threshold were set to an intensity of 0. All compared images were collected at the same camera settings. Scale bar in b, 10 μm. B, mean results obtained by analysis of DHE fluorescence images of nuclei from control and male diabetic rats. For this analysis, the mean fluorescence intensity of the pixels in seven nuclei in myocytes from non-diabetic animals (normalized as 100%) and 10 nuclei from male diabetic animals were compared using the threshold method of analysis. Mean DHE fluorescence intensity in nuclei from the diabetic myocytes was 155 ± 10%, compared to nuclei from the control rats (P < 0.003). Similar results were obtained when the nuclear images were analysed using a second method (see Methods), in which the fluorescence intensity in nuclei from the male diabetic rats was 151 ± 6% of that in nuclei from control rats.
We subsequently investigated several issues related to the effects of oxidative stress on attenuated K+ currents in diabetic conditions. Xu et al. (2002) had shown that replenishing antioxidative capacity by addition of reduced glutathione (GSH) significantly increased Ipeak in cells from diabetic rats. In our experiments, we examined whether inhibiting the production of superoxide ions, a major component of oxidative stress, would also augment the attenuated K+ currents. Superoxide ions are produced by the activation of NADPH oxidase (Inoguchi et al. 2000; McFarlane et al. 2003; Cotter & Cameron, 2003). Cells from diabetic males were exposed to the NADPH oxidase inhibitor apocynin (300 μm; Ungvari et al. 2004) for 5–9 h. Apocynin markedly increased the densities of both Ipeak and Isus. In this group, Ipeak (at +50 mV) was increased by apocynin from 14.6 ± 0.8 (n = 52) to 19.2 ± 1.0 pA pF−1 (n = 57, P < 0.005). Isus was increased from 4.3 ± 0.2 to 5.4 ± 0.2 pA pF−1 (P < 0.005). Figure 2A shows sample current traces from cells in the absence (left) or following 8 h (right) in the presence of apocynin, with summary data for current densities at +50 mV shown in Fig. 2B.
Apocynin had no effect when added to ventricular cells from control rats. The mean densities of Ipeak (at +50 mV) in the absence and presence of apocynin, respectively, were 22.4 ± 2.5 and 26.0 ± 2.3 pA pF−1 (n = 18 for both; P > 0.05). The corresponding values for Isus were 6.8 ± 0.4 and 7.0 ± 0.4 pA pF−1 (P > 0.05, results not shown).
In other experiments, DHE fluorescence was compared in cells from six diabetic rats, in the absence or presence (6 h) of apocynin (300 μm). In all cases, DHE fluorescence (corresponding to superoxide levels) was significantly reduced by apocynin (P-values for these comparisons ranging from P < 0.05 to P < 0.0001). One example is shown in Fig. 3.
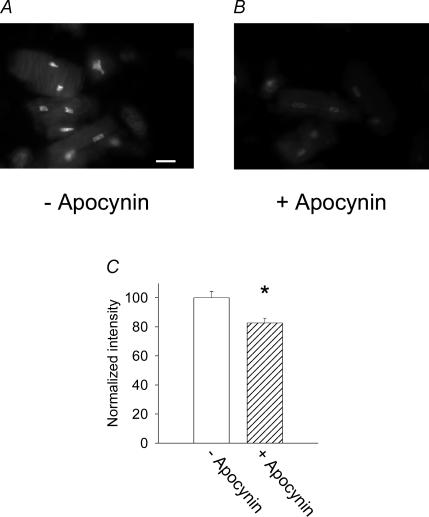
Figure 3. Effects of the NADPH inhibitor apocynin on DHE fluorescence (indicating superoxide anion levels).
Several cells and nuclei from diabetic male rats are shown either in the absence (A) or in the presence (B) of apocynin (300 μm, 6 h). Scale bar, 10 μm. C, mean (±s.e.m.) DHE fluorescence intensity in the absence (open bar, normalized as 100%) and presence of the drug (hatched bar), for one set of cells. *P < 0.05. A similar significant reduction of DHE intensity by apocynin was obtained in five other experiments.
Apocynin inhibits the formation of superoxide ions, which are removed in the cell by SOD. To further confirm the key role of superoxide ions, we incubated cells from diabetic rats for 5–9 h in 300 U ml−1 polyethylene glycol (PEG)-SOD (Liu et al. 2001; Pannirselvam et al. 2005). As with apocynin, this protocol also led to significant augmentation of Ipeak and Isus. At +50 mV, Ipeak density increased from 18.2 ± 0.9 pA pF−1 (n = 31) in the absence of PEG-SOD to 22.8 ± 1.1 pA pF−1 (n = 28) in the presence of PEG-SOD (P < 0.003). The corresponding values for Isus were 4.9 ± 0.1 and 7.4 ± 0.4 pA pF−1 (P < 0.0001). This result is shown in Fig. 4A. PEG-SOD also reduced DHE fluorescence (n = 3, data not shown), confirming that its action is related to reduction of oxidative stress.
H2O2 is another reactive species that is elevated under oxidative stress, such as in diabetes (Giugliano et al. 1996). ANG II is one of the triggers for H2O2 production (Zafari et al. 1998). Catalase has been reported to protect some (but not all) myocyte function in diabetes (Ye et al. 2004). It is interesting that SOD, but not catalase, was shown to antagonize hypertrophic effects of ANG II (Nakagami et al. 2003), suggesting differential roles for superoxide and H2O2. It was therefore of great interest to compare effects of SOD and catalase on K+ currents in diabetic myocytes. In further experiments, we exposed ventricular cells from diabetic rats (5–9 h) to 500 μ ml−1 catalase (Liu et al. 2001). This had no effect on Ipeak or Isus, as shown in Fig. 4B, concordant with the results of Nakagami et al. (2003).
Sex effects
We have previously shown substantial sex differences in the activation of cardiac autocrine mechanisms. Thus, in contrast to males, there is no increase in ANG II levels in cells from diabetic females. K+ currents are less attenuated and are insensitive to ACE inhibition (Shimoni et al. 2003; Shimoni & Liu, 2003b, 2004).
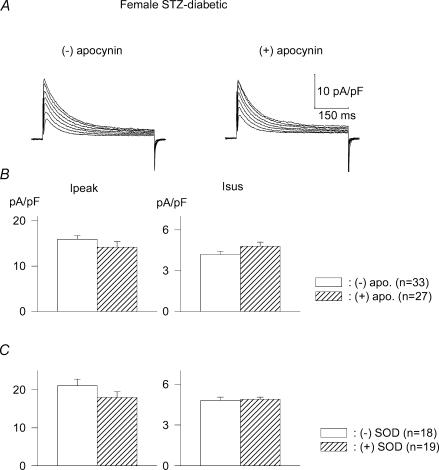
In the present study we investigated whether there are also sex-selective differences in the oxidative stress generated by ANG II. Cells from diabetic female rats were exposed to apocynin (300 μm, 5–9 h). In contrast to the effects in males, no alterations were seen in females. The mean densities (at +50 mV) of Ipeak were 15.9 ± 0.8 (n = 33) and 14.2 ± 124 pA pF−1 (n = 27; P > 0.05) in the absence and presence of apocynin, respectively. The corresponding values of Isus were 4.2 ± 0.2 and 4.8 ± 0.3 pA pF−1, respectively (P > 0.05).
Similar results were obtained with SOD (300 U ml−1). In this group, mean Ipeak values were 21.1 ± 1.7 and 18.0 ± 1.5 pA pF−1 in the absence and presence of SOD, respectively. The values for Isus were 4.8 ± 0.3 and 4.9 ± 0.2 pA pF−1, respectively. These results are shown in Fig. 5.
Figure 5. Absence of effects of oxidative stress inhibitors in cells from diabetic females.
A, current traces (same protocol as Fig. 2) from two cells, in the absence (left) or following (right) 9 h exposure to apocynin (300 μm). B, mean current densities (at +50 mV) in the absence (open bars) or presence (hatched bars) of apocynin (5–9 h). C, superoxide dismutase (300 U ml−1, 5–9 h) has no significant effect on Ipeak (left) or Isus (right), in marked contrast to the augmentation observed in cells from diabetic males.
We subsequently also investigated the sex-dependence of the effects of reduced glutathione (GSH). With cells obtained from diabetic males, we confirmed the results of Xu et al. (2002). Mean Ipeak densities (at +50 mV) increased from 12.0 ± 1.0 (n = 24) to 19.7 ± 1.2 pA pF−1 (n = 10, P < 0.0001). Isus was augmented from 4.3 ± 0.2 to 6.3 ± 0.6 pA pF−1 (P < 0.0005). Figure 6A shows sample current traces in cells from diabetic male rats in the absence (left) or following 5.5 h in the presence of 1 mm GSH (right). Below are the current–voltage relationships for Ipeak (left) and Isus (right). It is important to note that currents obtained in response to voltage steps to negative membrane potentials (−80 to −110 mV), reflecting the background inward rectifier current IK1, did not change in response to GSH, indicating selective effects of oxidative stress on K+ channels, rather than overall alterations in membrane conductance. In addition to effects on K+ currents, exposure to GSH reduced DHE fluorescence (n = 3, results not shown). This confirmed that the effects of GSH are directly related to the reduction of oxidative stress.
In myocytes from STZ-induced diabetic female rats, GSH (5–9 h) had no effect on either Ipeak or Isus, in marked contrast to the augmentation of currents in cells from diabetic males. This is shown in Fig. 6B. Current–voltage relationships are shown, with current traces omitted for clarity. In these cells, Ipeak densities (at +50 mV) were 14.7 ± 1.1 (n = 21) and 15.8 ± 1.9 pA pF−1 (n = 14; P > 0.05) in the absence or presence of GSH, respectively. The corresponding values for Isus were 3.9 ± 0.3 and 4.3 ± 0.2 pA pF−1 (P > 0.05), respectively. The lack of effect of GSH and apocynin in cells from diabetic females serve as additional controls, indicating a lack of direct effects on these K+ currents, unrelated to oxidative stress.
Our earlier work suggested that oestrogen is largely responsible for these sex differences. Oestrogen has been shown to suppress elements of the RAS (Brosnihan et al. 1997; Gallagher et al. 1999; Nickenig et al. 2000), and to exert antioxidative effects (Gallagher et al. 1999; Hernandez et al. 2000; Si et al. 2001; Gragasin et al. 2003; Strehlow et al. 2003). We previously showed that autocrine mechanisms are activated and K+ currents attenuated in diabetic ovariectomized female rats, as in males (Shimoni & Liu, 2003b). In the present work we found that K+ currents in cells from ovariectomized diabetic female rats are also sensitive to GSH and apocynin, as in males. Incubation of cells from diabetic ovariectomized rats with 1 mm GSH for 5–9 h significantly augmented Ipeak and Isus. This is shown in Fig. 6C. Ipeak was augmented (P < 0.01 to P < 0.05) between +10 and +50 mV. Isus was significantly increased (P < 0.0005 to P < 0.05) between −10 and +50 mV.
The preceding electrophysiological results suggest significant differences in the levels of oxidative stress in ventricular cells from diabetic males and females. We confirmed this directly by measuring DHE fluorescence. Cells were isolated from diabetic male and female rats on the same day, and the same reagents and procedures were performed in parallel. DHE fluorescence measurements were performed using identical conditions. In three paired experiments, the intensity of labelling was significantly (P < 0.005) smaller in cells from females, confirming that oxidative stress is less pronounced in diabetic females. An example and mean values from one paired comparison are shown in Fig. 7. In this experiment, DHE fluorescence in cells from females was 64% that of males. In two other comparisons (using the threshold method), DHE fluorescence in cells from females was 17 and 51% of the intensity in cells from males.
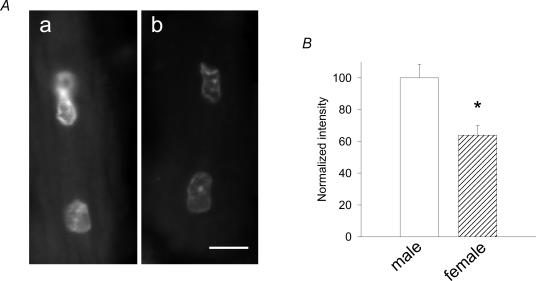
Figure 7. Comparison of superoxide ion levels in diabetic males and females.
A, DHE fluorescence images of myocytes from male (a) and female (b) diabetic rats. Images were collected at the same camera settings. Scale bar in b, 10 μm. B, results of analysis of images of nuclei from male and female diabetic rats. For this analysis, the mean fluorescence intensity of the pixels in 18 nuclei in myocytes from male diabetic animals and 19 nuclei from female diabetic animals were compared using the threshold method of analysis. The mean DHE fluorescence intensity in the nuclei from the female animals was 64 ± 6% of that in the nuclei from the male animals (normalized as 100%), which was significantly (P < 0.001) different. Similar results were obtained when the nuclear images were analysed using the second method (see Methods). This method of analysis indicated that the fluorescence intensity in the nuclei from the female animals was 63 ± 6% of that in the nuclei from male animals.
Involvement of angiotensin II
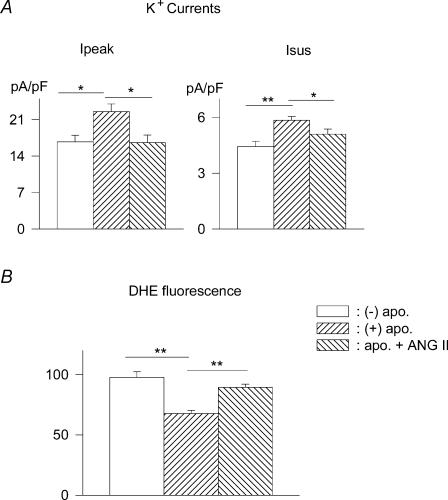
Earlier studies (Nakagami et al. 2003; Napoli et al. 2004) suggest that oxidative stress is mediated at least partly by elevated levels of ANG II. Diabetes elevates ANG II levels in animal models and in humans (Sechi et al. 1994; Frustaci et al. 2000; Shimoni & Liu, 2004). As ANG II activates and apocynin inhibits NADPH oxidase, we tested whether excess ANG II in the incubation medium would counter the effect of apocynin, presumably by maintaining oxidative stress. Cells were exposed to 300 nm ANG II for 1 h prior to addition of apocynin (300 μm). Currents were measured 5–8 h after application of apocynin (ANG II maintained throughout). The presence of ANG II significantly reduced the augmentation of both Ipeak and Isus by apocynin. The mean densities (at +50 mV) of Ipeak in the absence of apocynin, with apocynin alone, and with apocynin and ANG II were 16.8 ± 1.2 (n = 22), 22.5 ± 1.5 (n = 18) and 16.6 ± 1.5 pA pF−1 (n = 14), respectively (P < 0.05). The corresponding values for Isus were 4.5 ± 0.3, 5.9 ± 0.2, and 5.1 ± 0.3 pA pF−1 (P < 0.05). These results further indicate that apocynin does not increase the currents in a non-specific manner (unrelated to oxidative stress). In parallel experiments, the attenuating effects of apocynin on DHE fluorescence were also significantly blunted by ANG II. These results are shown in Fig. 8. ANG II also blunted the effects of GSH (results not shown).
Figure 8. The effects of apocynin are blunted by angiotensin in cells from male diabetic rats.
A, mean current densities (at +50 mV) are shown in the absence of drugs (−) apo., after 5–8 h incubation with 300 μm apocynin (+) apo., and in the presence of apocynin and 300 nm ANG II, added 1 h before apocynin (apo. + ANGII). ANG II significantly (P < 0.05) reversed current augmentation elicited by apocynin. Ipeak is shown on the left and Isus on the right. B, mean DHE fluorescence intensity mirrored these changes. Apocynin reduces DHE fluorescence in cells from diabetic rats (100%), but this effect is blunted by 300 nm ANG II.
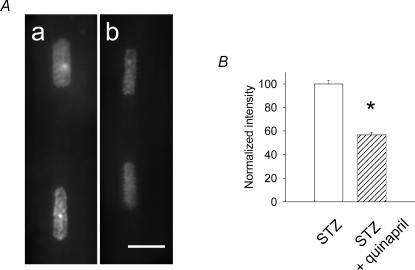
In the final experiments we investigated more directly the link between ANG II, oxidative stress and K+ current attenuation. The purpose was to determine whether oxidative stress is reduced by lowering ANG II, and whether the effects of the NAD(P)H inhibitor apocynin on K+ currents would then be reduced as well. Male rats were given the ACE inhibitor quinapril in their drinking water (6 mg l−1, 3 weeks), prior to induction of diabetes. Cells were isolated 7–10 days after STZ injection (quinapril continued). As expected, the ACE inhibitor greatly reduced the content of ANG II in cells from these rats (measured by ELISA). The mean value of ANG II in cells from quinapril-treated diabetic rats was 3.6 ± 0.5 pg (mg protein)−1 (n = 4), which was very significantly (P < 0.005) lower than in untreated diabetic rats, where the value was 47.1 ± 9.2 pg (mg protein)−1 (n = 4). In parallel, the ACE inhibitor was also found to greatly reduce oxidative stress in these cells, despite the persistence of hyperglycaemia, as also found by de Cavanagh et al. (2001). In three experiments, DHE fluorescence in cells from quinapril-treated rats was significantly reduced, as compared to untreated diabetic rats. The values in the quinapril-treated group were 82, 65 and 67% (P < 0.005 for each comparison) of the values in untreated cells. One example is shown in Fig. 9.
Figure 9. Effects of quinapril treatment on superoxide levels.
A, DHE fluorescence images from myocytes obtained from a diabetic rat (a) and from a diabetic rat after in vivo quinapril treatment (b). Scale bar, 10 μm. B, summary data obtained from analysis of the fluorescence of 78 nuclei (42 from untreated rats, normalized as 100%, and 36 from quinapril-treated rats). The intensity of fluorescence following quinapril-treatment was reduced by 43.2% (P < 0.0001).
However, the magnitude of K+ currents in these cells was not normalized to control levels (see Discussion). Nevertheless, in cells from quinapril-treated diabetic male rats, Ipeak and Isus were not affected by exposure to apocynin (300 μm, 5–9 h), confirming the reduced oxidative stress. This was in marked contrast to the augmentation of currents in cells from untreated diabetic rats (see Fig. 2). In the quinapril-treated diabetic group, mean current densities for Ipeak (at +50 mV) were 15.4 ± 1.3 (n = 21) and 14.3 ± 1.3 pA pF−1 (n = 22, P > 0.05) in the absence and presence of apocynin, respectively. The corresponding values for Isus were 5.6 ± 0.3 and 5.4 ± 0.3 pA pF−1 (P > 0.05), respectively.
Discussion
The present work addresses some of the mechanisms linking diabetes-induced oxidative stress and cardiac electrophysiological abnormalities, which play a major role in initiation of cardiac arrhythmias.
The novel and important aspects of the present work are the following: First, in addition to confirming (Fig. 1) the presence of oxidative stress in diabetic conditions, as previously suggested (Giugliano et al. 1996; Marra et al. 2002; Penckofer et al. 2002), we find that elevated superoxide ion levels play a major role in inducing oxidative stress and K+ current attenuation (Figs 2, 3, 4). Changes in H2O2 levels are of reduced significance in this context. Secondly, significant sex differences were found both in the presence of oxidative stress and in its modulation of K+ currents (Figs 5, 6, 7). Finally, angiotensin II plays a major role in inducing oxidative stress and the related electrophysiological changes under diabetic conditions (Figs 8 and 9).
Earlier work (Xu et al. 2002; Li et al. 2004; Ayaz et al. 2004) has described some of the mechanisms by which oxidative stress and hyperglycemia attenuate K+ currents. The present work focused more on the pathways leading to generation of oxidative stress in diabetes. The key role of superoxide ions was elucidated by results showing that both inhibition of its formation by apocynin (Figs 2 and 3) and acceleration of its removal by SOD (Fig. 4) relieved oxidative stress and augmented Ipeak and Isus. In contrast, catalase did not augment these currents (Fig. 4). This suggests a minor role of hydrogen peroxide in modulating these currents under (type 1) diabetic conditions. This result is similar to the differential effects of SOD and catalase in preventing ANG II- induced hypertrophy (Nakagami et al. 2003). Interestingly, over-expression of catalase was also found to have selective effects in diabetic hearts, preventing some contractile derangements but not correcting some aspects of defective calcium handling (Ye et al. 2004).
Earlier work suggested possible sex differences in either generation of reactive oxidative species (Brandes & Mugge, 1997; Lacy et al. 2000) or in the response to oxidative stress (Hernandez et al. 2000). The novel aspect of the present work lies in the finding that the induction of a specific cardiac pathology such as diabetes leads in females to a lower level of oxidative stress, expressed as lower superoxide ion generation, in comparison to diabetic males (Fig. 7). Concomitantly, there are functional sex differences, observed as differential effects of apocynin and GSH (Figs 5 and 6). Our results link these sex differences to ANG II as the key mediator (Figs 8 and 9). Lower levels of ANG II in STZ-diabetic females were shown in earlier work (Shimoni & Liu, 2004).
The central role of ANG II in oxidative stress-mediated K+ current attenuation is indicated by several findings. (1) Excess in vitro ANG II prevents the restoration of K+ currents and the relief of oxidative stress by apocynin (Fig. 8). (2) In cells in which the RAS is not activated, following inhibition of ANG II formation by in vivo quinapril treatment, oxidative stress is lower (Fig. 9). In these cells there is also no augmentation of K+ currents by apocynin. This is also true in cells from diabetic females, which also lack RAS activation (Figs 5 and 6).
The linkage between diabetes, oxidative stress and K+ current attenuation can derive from multiple pathways (Giugliano et al. 1996; Nishikawa et al. 2000). Our work emphasizes the role of ANG II and superoxide ions. Interestingly, in other pathologies such as following acute or chronic elevations in blood pressure, superoxide ion production is elevated either independently of (Ungvari et al. 2003), or is partially mediated by activation of the renin-angiotensin system (Ungvari et al. 20047).
Our results establish a clear connection between ANG II-induced oxidative stress and K+ current attenuation in (type 1) diabetic conditions, since in the absence of elevated ANG II levels in ventricular cells from female diabetic rats (Shimoni & Liu, 2004) or in cells from quinapril-treated male diabetic rats, oxidative stress is significantly reduced. This underlies the smaller effects of diabetes on K+ currents (Shimoni & Liu, 2003b) and the lack of effect of GSH and apocynin on K+ currents in cells from diabetic females (Figs 5 and 6).
The modulation of K+ currents is complex. Oxidative stress is a major contributor to K+ current attenuation. However, relief from or absence of oxidative stress does not completely normalize current magnitudes (cf. Shimoni et al. 1998). Thus, current magnitudes are smaller than normal in cells from diabetic females and from quinapril-treated diabetic males, in which ANG II levels are not elevated. This implies that factors other than oxidative stress (such as insulin deficiency or additional autocrine factors) also contribute to current attenuation. These effects can persist even when oxidative stress is removed. Thus, the main difference between diabetic males and diabetic females, or diabetic males after quinapril treatment lies in the differences in the modulation of potassium currents, rather than in their magnitude per se. The effects of GSH and apocynin are sex-dependent, as are the effects of in vitro quinapril (Shimoni & Liu, 2003a).
The significance of the present work lies in the following: hyperglycemia is maintained in both diabetic females and in quinapril-treated males (also observed by de Cavanagh et al. 2001), in which both oxidative stress and ANG II levels are lower. This indicates that ANG II, rather than hyperglycemia per se, is a major contributor to oxidative stress-related electro-physiological abnormalities, compared to other derangements induced by hyperglycemia (Giugliano et al. 1996). The previously suggested protection of females from oxidative stress can now be extended to include a smaller oxidative stress-mediated attenuation of cardiac potassium currents. The persistence of GSH effects in diabetic ovariectomized females (Fig. 6) supports suggestions of antioxidative functions of oestrogen (Gallagher et al. 1999; Gragasin et al. 2003; Strehlow et al. 2003). The present results suggest that oestrogen also prevents oxidative-stress-dependent attenuation of K+ current magnitude, corresponding to earlier work suggesting that oxidative challenges are less cytotoxic in females (Lacy et al. 2000). The lower levels of oxidative stress in cells from females, as determined by measurements of K+ currents and of superoxide ion levels, results in a smaller attenuation in K+ currents (Shimoni & Liu, 2003b). This may provide a partial mechanism to explain the greater resistance of females to some types of cardiac arrhythmias (Larsen & Kadish, 1998), since reduction or elimination of the transient current was shown to be arrhythmogenic (Guo et al. 2000). Future work is required to determine other implications of reduced oxidative stress in cells from diabetic females as well as in other cardiac pathologies. Our results support the quest for treatment of diabetic complication by use of antioxidant therapy. However, the use of antioxidant agents as tools for prevention or treatment of diabetes is still controversial (Ceriello & Motz, 2004; Da Ros et al. 2004), suggesting that other cellular mechanisms also contribute to cellular damage in diabetes.
It should be noted that the attenuation of the transient outward current also has consequences for the mechanical function of the heart, by altering calcium influx and handling (Oudit et al. 2001), and possibly directly contributing to hypertrophy (Kassiri et al. 2002). Thus, the present results also have implications for understanding the contribution of oxidative stress for the mechanical abnormalities in the diabetic heart (Belke et al. 2000).
A major strength of the present study is the combined used of independent methodologies. Detection of superoxide ions with DHE provides independent confirmation that oxidative stress is indeed increased in cardiac myocytes from STZ-diabetic male rats (Fig. 1), and that oxidative stress is significantly lower in cells from diabetic females, compared to males (Fig. 7).
The validity of the DHE method is supported by comparison of cells from the same heart, in the absence or presence of apocynin. Apocynin blocks the generation of superoxide ions, decreases the DHE signal, and augments potassium currents. Furthermore, in vivo quinapril treatment of diabetic rats, which reduces ANG II levels, also reduces the DHE signal in ventricular cells from diabetic males (Fig. 9). Finally, the fact that DHE signals are weaker in diabetic females than in males indicates that the diabetic state does not significantly alter the sensitivity of the method.
In conclusion, in the STZ-induced model of diabetes, cardiac dysfunction associated with oxidative stress is largely triggered by angiotensin II, mediated by superoxide ions, and restricted to males.
Acknowledgments
This study was supported the Alberta Heart and Stroke Foundation and the Canadian Institutes of Health Research. We thank Gail McMartin for her generous help with imaging of DHE-labelled cells.
References
- Ayaz M, Ozdemir S, Ugur M, Vassort G, Turan Effects of selenium on altered mechanical and electrical cardiac activities of the diabetic rat. Arch Biochem Biophys. 2004;426:83–90. doi: 10.1016/j.abb.2004.03.030. [DOI] [PubMed] [Google Scholar]
- Bagi Z, Koller A, Kaley G. Superoxide–NO interaction decreases flow- and agonist-induced dilations of coronary arterioles in Type 2 diabetes mellitus. Am J Physiol Heart Circ Physiol. 2003;285:H1404–H1410. doi: 10.1152/ajpheart.00235.2003. [DOI] [PubMed] [Google Scholar]
- Baynes JW. Role of oxidative stress in development of complications of diabetes. Diabetes. 1991;40:405–412. doi: 10.2337/diab.40.4.405. [DOI] [PubMed] [Google Scholar]
- Belke DD, Larsen TS, Gibbs EM, Severson DL. Altered metabolism causes cardiac dysfunction in perfused hearts from diabetic (db/db) mice. Am J Physiol Endocrinol Metab. 2000;279:E1104–E1113. doi: 10.1152/ajpendo.2000.279.5.E1104. [DOI] [PubMed] [Google Scholar]
- Bell DSH. Diabetic cardiomyopathy. Diabetes Care. 2003;26:2949–2951. doi: 10.2337/diacare.26.10.2949. [DOI] [PubMed] [Google Scholar]
- Brandes RP, Mugge A. Gender differences in the generation of superoxide anions in the rat aorta. Life Sci. 1997;60:391–396. doi: 10.1016/s0024-3205(96)00663-7. [DOI] [PubMed] [Google Scholar]
- Brosnihan KB, Li P, Ganten D, Ferrario CM. Estrogen protects transgenic hypertensive rats by shifting the vasoconstrictor-vasodilator balance of RAS. Am J Physiol. 1997;273:R1908–R1915. doi: 10.1152/ajpregu.1997.273.6.R1908. [DOI] [PubMed] [Google Scholar]
- Ceriello A. New insights on oxidative stress and diabetic complications may lead to a ‘causal’ antioxidant therapy. Diabetes Care. 2003;26:1589–1596. doi: 10.2337/diacare.26.5.1589. [DOI] [PubMed] [Google Scholar]
- Ceriello A, Motz E. Is oxidative stress the pathogenic mechanism underlying insulin resistance, diabetes, and cardiovascular disease? The common soil hypothesis revisited. Arterioscler Thromb Vasc Biol. 2004;24:816–823. doi: 10.1161/01.ATV.0000122852.22604.78. [DOI] [PubMed] [Google Scholar]
- Cotter MA, Cameron NE. Effect of the NADPH oxidase inhibitor, apocynin, on peripheral nerve perfusion and function in diabetic rats. Life Sci. 2003;73:1813–1824. doi: 10.1016/s0024-3205(03)00508-3. [DOI] [PubMed] [Google Scholar]
- Da Ros R, Assaloni R, Ceriello A. Antioxidant therapy in diabetic complications: what is new? Curr Vasc Pharmacol. 2004;2:335–341. doi: 10.2174/1570161043385538. [DOI] [PubMed] [Google Scholar]
- De Cavanagh EM, Inserra F, Tobili J, Stella I, Fraga CG, Ferder L. Enalapril attenuates oxidative stress in diabetic rats. Hypertension. 2001;38:1130–1136. doi: 10.1161/hy1101.092845. [DOI] [PubMed] [Google Scholar]
- Dostal DE. The cardiac renin-angiotensin system: novel signaling mechanisms related to cardiac growth and function. Regul Pept. 2000;91:1–11. doi: 10.1016/s0167-0115(99)00123-8. [DOI] [PubMed] [Google Scholar]
- Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Are oxidative stress-activated signaling pathways mediators of insulin resistance and β-cell dysfunction? Diabetes. 2003;52:1–8. doi: 10.2337/diabetes.52.1.1. [DOI] [PubMed] [Google Scholar]
- Fiordaliso F, Li B, Latini R, Sonnenblick EH, Anversa P, Leri A, Kajstura J. Myocyte death in streptozotocin-induced diabetes in rats is angiotensin II-dependent. Lab Invest. 2000;80:513–527. doi: 10.1038/labinvest.3780057. [DOI] [PubMed] [Google Scholar]
- Frustaci A, Kajstura J, Chimenti C, Jakoniuk I, Leri A, Maseri A, Nadal-Ginard B, Anversa P. Myocardial cell death in human diabetes. Circ Res. 2000;87:1123–1132. doi: 10.1161/01.res.87.12.1123. [DOI] [PubMed] [Google Scholar]
- Gallagher PE, Li P, Lenhart JR, Chappell MC, Brosnihan KB. Estrogen regulation of angiotensin-converting enzyme mRNA. Hypertension. 1999;33:323–328. doi: 10.1161/01.hyp.33.1.323. [DOI] [PubMed] [Google Scholar]
- Giugliano D, Ceriello A, Paolisso G. Oxidative stress and diabetic vascular complications. Diabetes Care. 1996;19:257–267. doi: 10.2337/diacare.19.3.257. [DOI] [PubMed] [Google Scholar]
- Gragasin FS, Xu Y, Arenas IA, Kainth N, Davidge ST. Estrogen reduces angiotensin II-induced nitric oxide synthase and NADPH oxidase expression in endothelial cells. Arterioscler Thromb Vasc Biol. 2003;23:38–44. doi: 10.1161/01.atv.0000047868.93732.b7. [DOI] [PubMed] [Google Scholar]
- Grundy SM, Benjamin IJ, Burke GL, Chait A, Eckel RH, Howard BV, Mitch W, Smith SC, Sowers JR. Diabetes and cardiovascular disease: a statement for healthcare professionals from the American Heart Association. Circulation. 1999;100:1134–1146. doi: 10.1161/01.cir.100.10.1134. [DOI] [PubMed] [Google Scholar]
- Guo W, Li H, London B, Nerbonne JM. Functional consequences of elimination of Ito,f and Ito,s: early afterdepolarizations, atrioventricular block, and ventricular arrhythmias in mice lacking Kv1.4 and expressing a dominant-negative Kv4 alpha subunit. Circ Res. 2000;87:73–79. doi: 10.1161/01.res.87.1.73. [DOI] [PubMed] [Google Scholar]
- Guzik TJ, Mussa S, Gastaldi D, Sadowski J, Ratnatunga C, Pillai R, Channon KM. Mechanisms of increased vascular superoxide production in human diabetes mellitus. Circulaion. 2002;105:1656–1662. doi: 10.1161/01.cir.0000012748.58444.08. [DOI] [PubMed] [Google Scholar]
- Hamilton CA, Miller WH, Al-Benna S, Brosnan MJ, Drummond RD, McBride MW, Dominiczak AF. Strategies to reduce oxidative stress in cardiovascular disease. Clin Sci. 2004;106:219–234. doi: 10.1042/CS20030379. [DOI] [PubMed] [Google Scholar]
- Harrison DG, Cai H, Landmesser U, Griendling KK. Interactions of angiotensin II with NADPH oxidase, oxidant stress and cardiovascular disease. J Renin Angiotensin Aldosterone Syst. 2003;4:51–61. doi: 10.3317/jraas.2003.014. [DOI] [PubMed] [Google Scholar]
- Hernandez I, Delgado JL, Diaz J, Quesada T, Teruel MJ, Llanos MC, Carbonell LF. 17 beta-estradiol prevents oxidative stress and decreases blood pressure in ovariectomized rats. AmJPhysiol Endocrinol Metab. 2000;279:R1599–R1605. doi: 10.1152/ajpregu.2000.279.5.R1599. [DOI] [PubMed] [Google Scholar]
- Inoguchi T, Li P, Umeda F, Yu HY, Kakimoto M, Imamura M, et al. High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C-dependent activation of NADPH oxidase in cultured vascular cells. Diabetes. 2000;49:1939–1945. doi: 10.2337/diabetes.49.11.1939. [DOI] [PubMed] [Google Scholar]
- Kassiri Z, Zobel C, Nguyen TTT, Molkentin JD, Backx PH. Reduction of Ito causes hypertrophy in neonatal rat ventricular myocytes. Circ Res. 2002;90:578–585. doi: 10.1161/01.res.0000012223.86441.a1. [DOI] [PubMed] [Google Scholar]
- Lacy F, Kailasam MT, O'Connor DT, Schmid-Schonbein GW, Parmer RJ. Plasma hydrogen peroxide production in human essential hypertension: role of heredity, gender, and ethnicity. Hypertension. 2000;36:878–884. doi: 10.1161/01.hyp.36.5.878. [DOI] [PubMed] [Google Scholar]
- Larsen JA, Kadish AH. Effects of gender on cardiac arrhythmias. J Cardiovasc Electrophysiol. 1998;6:665–667. doi: 10.1111/j.1540-8167.1998.tb00950.x. [DOI] [PubMed] [Google Scholar]
- Li X, Xu Z, Li S, Rozanski GJ. Redox regulation of Ito remodeling in diabetic rat heart. Am J Physiol Heart Circ Physiol. 2004;288:H1417–H1424. doi: 10.1152/ajpheart.00559.2004. [DOI] [PubMed] [Google Scholar]
- Liu Y, Terata K, Rusch NJ, Gutterman DD. High glucose impairs voltage-gated K channel current in rat small coronary arteries. Circ Res. 2001;89:146–152. doi: 10.1161/hh1401.093294. [DOI] [PubMed] [Google Scholar]
- McFarlane SI, Kumar A, Sowers JR. Mechanisms by which angiotensin-converting enzyme inhibitors prevent diabetes and cardiovascular disease. Am J Cardiol. 2003;91:30H–37H. doi: 10.1016/s0002-9149(03)00432-6. [DOI] [PubMed] [Google Scholar]
- Marra G, Cotroneo P, Pitocco D, Manto A, Di Leo MAS, Ruotolo V, Caputo S, Giardina B, Ghirlanda G, Santini SA. Early increase of oxidative stress and reduced antioxidant defenses in patients with uncomplicated type 1 diabetes. Diabetes Care. 2002;25:370–375. doi: 10.2337/diacare.25.2.370. [DOI] [PubMed] [Google Scholar]
- Mooradian AD. Cardiovascular disease in type 2 diabetes mellitus. Arch Inter Medical. 2003;163:33–40. doi: 10.1001/archinte.163.1.33. [DOI] [PubMed] [Google Scholar]
- Nakagami H, Takemoto M, Liao JK. NADPH oxidase-derived superoxide anion mediates angiotensin II-induced cardiac hypertrophy. J Mol Cell Cardiol. 2003;35:851–859. doi: 10.1016/s0022-2828(03)00145-7. [DOI] [PubMed] [Google Scholar]
- Napoli C, Sica V, de Nigris F, Pignalosa O, Condorelli M, Ignarro LJ, Liguori A. Sulfhydryl angiotensin-converting enzyme inhibition induces sustained reduction of systemic oxidative stress and improves the nitric oxide pathway in patients with essential hypertension. Am Heart J. 2004;148:172. doi: 10.1016/j.ahj.2004.03.025. [DOI] [PubMed] [Google Scholar]
- Nerbonne JM. Molecular basis of functional voltage-gated K+ channel diversity in the mammalian myocardium. J Physiol. 2000;525:285–298. doi: 10.1111/j.1469-7793.2000.t01-1-00285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickenig G, Strehlow K, Wassermann S, Baumer AT, Albory K, Sauer H, Bohm M. Differential effects of estrogen and progesterone on AT1 receptor gene expression in vascular smooth muscle cells. Circulation. 2000;102:1828–1833. doi: 10.1161/01.cir.102.15.1828. [DOI] [PubMed] [Google Scholar]
- Nishikawa T, Edelstein D, Du XL, Yamagishi SI, Matsumura T, Kaneda Y, et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycemic damage. Nature. 2000;404:787–790. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- Oudit GY, Kassiri Z, Sah R, Ramirez RJ, Zobel C, Backx PH. The molecular physiology of the cardiac transient outward current (Ito) in normal and diseased myocardium. J Mol Cell Cardiol. 2001;33:851–872. doi: 10.1006/jmcc.2001.1376. [DOI] [PubMed] [Google Scholar]
- Pannirselvam M, Wiehler WB, Anderson T, Triggle CR. Enhanced vascular reactivity of small mesenteric arteries from diabetic mice is associated with enhanced oxidative stress and cyclooxygenase products. Br J Pharmacol. 2005;144:953–960. doi: 10.1038/sj.bjp.0706121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penckofer S, Schwertz D, Florczak K. Oxidative stress and cardiovascular disease in type 2 diabetes: the role of antioxidants and pro-oxidants. J Cardiovasc Nurs. 2002;16:68–85. doi: 10.1097/00005082-200201000-00007. [DOI] [PubMed] [Google Scholar]
- Privratsky JR, Wold LE, Sowers JR, Quinn MT, Ren J. AT1 blockade prevents glucose-induced cardiac dysfunction in ventricular myocytes: role of the AT1 receptor and NADPH oxidase. Hypertension. 2003;42:206–212. doi: 10.1161/01.HYP.0000082814.62655.85. [DOI] [PubMed] [Google Scholar]
- Rozanski GJ, Xu Z. Glutathione and K+ channel remodeling in postinfarction rat heart. Am J Physiol Heart Circ Physiol. 2002;282:H2346–H2355. doi: 10.1152/ajpheart.00894.2001. [DOI] [PubMed] [Google Scholar]
- Sayen MR, Gustafsson AB, Sussman MA, Molkentien JD, Gottlieb RA. Calcineurin transgenic mice have mitochondrial dysfunction and elevated superoxide production. Am J Physiol Cell Physiol. 2003;284:C562–C570. doi: 10.1152/ajpcell.00336.2002. [DOI] [PubMed] [Google Scholar]
- Sechi LA, Griffin CA, Schambelan M. The cardiac renin-angiotensin system in STZ-induced diabetes. Diabetes. 1994;43:1180–1184. doi: 10.2337/diab.43.10.1180. [DOI] [PubMed] [Google Scholar]
- Shimoni Y. Inhibition of the formation or action of angiotensin II reverses attenuated K+ currents in type 1 and type 2 diabetes. J Physiol. 2001;537:83–92. doi: 10.1111/j.1469-7793.2001.0083k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoni Y, Chuang M, Abel ED, Severson DL. Gender-dependent attenuation of cardiac potassium currents in type 2 diabetic db/db mice. J Physiol. 2003;555:345–354. doi: 10.1113/jphysiol.2003.055590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoni Y, Ewart S, Severson DL. Type I and II models of diabetes produce different modifications of K+ currents in rat heart: role of insulin. J Physiol. 1998;507:485–496. doi: 10.1111/j.1469-7793.1998.485bt.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoni Y, Liu XF. Role of PKC in autocrine regulation of rat ventricular K+ currents by angiotensin and endothelin. Am J Physiol Heart Circ Physiol. 2003a;284:H1168–H1181. doi: 10.1152/ajpheart.00748.2002. [DOI] [PubMed] [Google Scholar]
- Shimoni Y, Liu XF. Sex differences in the modulation of K+ currents in diabetic rat cardiac myocytes. J Physiol. 2003b;550:401–412. doi: 10.1113/jphysiol.2003.041269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoni Y, Liu XF. Gender differences in the levels of angiotensin II and in its action on multiple pathways of K+ current modulation in diabetic rats. Am J Physiol Heart Circ Physiol. 2004;287:H311–H319. doi: 10.1152/ajpheart.01212.2003. [DOI] [PubMed] [Google Scholar]
- Shimoni Y, Severson D, Giles W. Thyroid status and diabetes modulate regional differences in potassium currents in rat ventricle. J Physiol. 1995;488:673–688. doi: 10.1113/jphysiol.1995.sp020999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si ML, Al-Sharafi B, Lai CC, Khardori R, Chang C, Su CY. Gender difference in cytoprotection induced by estrogen on female and male bovine aortic endothelial cells. Endocrine. 2001;15:255–262. doi: 10.1385/ENDO:15:3:255. [DOI] [PubMed] [Google Scholar]
- Strehlow K, Rotter S, Wassmann S, Adam O, Grohe C, Laufs K, Bohm M, Nickenig G. Modulation of antioxidant enzyme expression and function by estrogen. Circ Res. 2003;93:170–177. doi: 10.1161/01.RES.0000082334.17947.11. [DOI] [PubMed] [Google Scholar]
- Ungvari Z, Csiszar A, Huang A, Kaminski PM, Wolin MS, Koller A. High pressure induces superoxide production in isolated arteries via protein kinase C-dependent activation of NADPH oxides. Circulation. 2003;108:1253–1258. doi: 10.1161/01.CIR.0000079165.84309.4D. [DOI] [PubMed] [Google Scholar]
- Ungvari Z, Csiszar A, Kaminski PM, Wolin MS, Koller A. Chronic high pressure-induced arterial oxidative stress: involvement of protein kinase C-dependent NADPH oxidase and local renin-angiotensin system. Am J Pathol. 2004;165:219–226. doi: 10.1016/S0002-9440(10)63290-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Patel KP, Lou MF, Rozanski GJ. Up-regulation of K+ channels in diabetic rat ventricular myocytes by insulin and glutathione. Cardiovasc Res. 2002;53:80–88. doi: 10.1016/s0008-6363(01)00446-1. [DOI] [PubMed] [Google Scholar]
- Ye G, Metreveli NS, Donthi RV, Xia S, Xu M, Carlson EC, Epstein PN. Catalase protects cardiomyocytes function in models of type 1 and type 2 diabetes. Diabetes. 2004;53:1336–1343. doi: 10.2337/diabetes.53.5.1336. [DOI] [PubMed] [Google Scholar]
- Zafari AM, Ushio-Fukai M, Akers M, Yin Q, Shah A, Harrison DG, Taylor RW, Griendling KK. Role of NADH/NADPH oxidase-derived H2O2 in angiotensin II-induced vascular hypertrophy. Hypertension. 1998;32:488–495. doi: 10.1161/01.hyp.32.3.488. [DOI] [PubMed] [Google Scholar]