Abstract
We addressed the need for histological assessment of myocellular domains occupied by monocarboxylate transporters (MCT1, MCT2 and MCT4). From the perspective of lactate shuttle hypotheses we posited that MCT1 would be highly expressed in oxidative fibres, whereas MCT4 would be found in highly glycolytic fibres. Furthermore, we hypothesized that MCT1 would be detected at interfibrillar as well as at subsarcolemmal and sarcolemmal cell domains, whereas MCT2 and MCT4 abundances would be most prominent at the sarcolemma. To test these hypotheses, we examined cellular locations of MCT1, MCT2 and MCT4 transporter proteins in different fibre types (slow oxidative, SO; fast oxidative glycolytic, FOG; fast glycolytic, FG) in rat plantaris muscles by the avidin–biotin complex (ABC) as well as other methods. The plantaris was used as it is a mixed fibre skeletal muscle. MCTs, glucose transporter (GLUT4) protein, and mitochondrial constituent cytochrome oxidase (COX) abundances were assessed by immunohistochemistry and Western blotting using affinity-purified antibodies. The staining method was specific and stable, which allowed for semiquantitative assessment of MCT expression. As well, confocal laser scanning microscopy assessed MCT isoform localizations. Findings of the present study were: (1) MCT1 is located at the sarcolemma and throughout the cell interior in SO and FOG fibres where the mitochondrial reticulum was present; (2) in contrast, MCT4 was highly expressed in the sarcolemmal domain of FG and FOG fibres but poorly expressed in SO fibres; and (3) confocal laser-scanning microscopy demonstrated that MCT1 and COX are co-localised at both interfibrillar and subsarcolemmal cell domains, whereas MCT2 is only faintly detected at the sarcolemma of oxidative fibres. MCTs and associated proteins are positioned to facilitate the function of the lactate shuttles.
Lactate and pyruvate are exchanged across muscle cell (sarcolemmal) membranes by facilitated, proton-linked transport (Watt et al. 1988; Roth & Brooks, 1990a, b) involving a family of monocarboxylate transport (MCT) proteins (Garcia et al. 1994). MCT1 is widely expressed in different tissues (Halestrap & Price, 1999), and has been localised in muscle to sarcolemmal and mitochondrial membranes (Brooks et al. 1999b; McClelland & Brooks, 2002; McClelland et al. 2003; Butz et al. 2004). As part of the lactate shuttle mechanism, MCT1 facilitates uptake of lactate from interstitium and plasma (Bergman et al. 1999; Dubouchaud et al. 2000; Brooks, 2002). The putative role of MCT4 is cellular lactate extrusion (Dimmer et al. 2000). However, as sarcolemmal vesicle preparations are responsive to trans-stimulation (Brown & Brooks, 1994), and because working skeletal muscle can switch from net lactate release to consumption (Brooks et al. 1998), MCTs are recognized to be bidirectional in function, facilitating exchange down H+ and lactate anion concentration gradients (Roth & Brooks, 1990a, b). Additionally, the expression of MCT1 protein, but not the MCT4 isoform is responsive to endurance training (Pilegaard et al. 1999a; Dubouchaud et al. 2000). The role of MCT2, a high-affinity pyruvate transporter (Garcia et al. 1995; Lin et al. 1998; Bröer et al. 1999), is not clear in skeletal muscle.
The expression of MCT1 in different rat skeletal muscles is correlated with the percentage of slow oxidative (SO) and fast oxidative glycolytic (FOG) fibres in muscles (Wilson et al. 1998). The expression of MCT4 is reported to be similar in fast-twitch (fast glycolytic FG and FOG) skeletal muscles, but in soleus muscle, which is predominantly composed of slow oxidative fibres, MCT4 protein expression was seen to be relatively low (Wilson et al. 1998).
To better understand the physiological roles of MCTs and related proteins, such as cytochrome oxidase (COX), we sought to determine the distribution and relative abundances of MCTs in different muscle fibre types. Typically, the abundance of MCTs in muscle and other tissues has been assessed by homogenization and Western blotting. To date a few studies have used immunohistochemistry to show the distribution of MCTs in human and rat skeletal muscles (Garcia et al. 1994; Wilson et al. 1998; Pilegaard et al. 1999; Fishbein et al. 2002), but results are inconsistent. For instance, Pilegaard et al. (1999) showed little variation in the distribution of MCT1 between different human fibre types, whereas Fishbein et al. (2002) found that MCT1 was expressed predominantly in SO fibres. As well, Fishbein et al. (2002) demonstrated that MCT2 was expressed predominantly at sarcolemmal domains in SO fibres, but using tissue homogenization, differential centrifugation and Western blotting Benton et al. (2004) obtained results indicating the presence of MCT2 in both subsarcolemmal and interfibrillar mitochondria. Finally, localization of MCT1 solely to the sarcolemma by immunohistochemistry is inconsistent with the presence of MCT1 in the mitochondrial reticulum (Brooks et al. 1999b; Butz et al. 2004).
Up to now, no one has quantified MCT isoform expression by histochemical assessment. Consequently, it is unclear whether MCTs occupy the same or different cell domains in different muscle fibre types. We hypothesized that MCT1 is highly expressed in sarcolemmal, subsarcolemmal, and interfibrillar domains of oxidative (SO and FOG) fibres, whereas MCT4 is highly expressed in the sarcolemma of glycolytic (FG and FOG) fibres. To test these hypotheses, we histochemically examined a mixed fibre muscle, rat plantaris, to determine cellular locations and relative abundances of MCT1 and MCT4. Additionally, we hypothesized that compared to MCT1 and MCT4, MCT2 is not abundant in skeletal muscle and would be found predominantly in the sarcolemmal domain of oxidative fibres. Our suspicion about the low abundance and sarcolemmal localization of MCT2 was based on previous histological studies (Fishbein et al. 2002) as well as reported difficulties in detecting the mRNA of MCT2 in rat skeletal muscle (Jackson et al. 1997). To further test our hypotheses regarding the location and physiological roles of MCTs in mammalian skeletal muscle, we examined cellular locations of MCT1, MCT2, and COX by using confocal laser-scanning microscopy (CLSM).
Methods
Animal care and use and tissue sampling
Male Wistar rats (n = 5) were housed in a temperature controlled room (22 ± 1°C) with a dark: light cycle of 12: 12 h and fed ad libitum. At 10 weeks of age, rats were anaesthetized with pentobarbital sodium, 50 mg kg−1, i.p. During ventilation with a rodent respirator, samples of plantaris muscle were removed from both hind limbs; one was for biochemical analysis, the other for histochemical analysis. Muscles were dissected free of connective tissues and immediately frozen in cooled isopentane or liquid nitrogen for histochemistry or biochemistry, respectively, and stored at −80°C until analysis. All experiments conformed to the Guiding Principle for the Care and Use of Animals approved by the Council of the Physiological Society of Japan and the American Physiological Society. As well, CLSM studies were conducted under Berkeley ACUP R017-1005.
Histochemical analysis
Muscle samples frozen for histochemical analyses were sectioned (10 μm thick) in a cryostat at −20°C. The sections were stained for myosin adenosine triphosphatase (ATPase), succinate dehydrogenase (SDH), and α-glycerophosphate dehydrogenase (α-GPD) according to previous studies (Wattenberg & Leong, 1960; Taguchi et al. 1985).
Immunohistochemical analysis
Procedures were performed on 10 μm thick unfixed cryosections of plantaris muscles in triplicate. Primary antibodies were against MCT1, MCT2, MCT4, GLUT4, and COX. MCT1 antibody (rabbit anti-MCT1), MCT2 antibody (chicken anti-MCT2), and glucose transporter (GLUT4) antibody (rabbit anti-GLUT4) were obtained from Chemicon (Temecula, CA, USA). MCT4 antibody was a donation from Dr H. Hatta (Department of Life Science, University of Tokyo, Tokyo, Japan). COX antibody was obtained from Molecular Probes (Eugene, OR, USA). Immunolabelling was performed using a commercial kit (Vector Vectastain Elite ABC Staining Kit, Vector Laboratories Inc., Burlingame, CA, USA). Briefly, unfixed cryosections were heated in citric acid buffer solution (pH 6.0) with 0.1% of Tween 20 at 95°C for 30 min. Sections were then blocked with horse serum for 20 min. After washing with phosphate-buffered saline (PBS), pH 7.4, individual sections were incubated overnight with either MCT1 antibody (1: 100), MCT4 antibody (1: 200), or GLUT4 antibodies (1: 2000) at 4°C, such that close serial sections obtained were stained for each protein. After a second washing with PBS, sections were incubated with a second antibody (Vector Vectastain Elite ABC Staining Kit, Vector Laboratories Inc.) for 1 h. Sections were then reacted with avidin–biotin complex (ABC) reagent (Vector Vectastain Elite ABC Staining Kit, Vector Laboratories Inc.) for 30 min. After again washing with PBS, sections were incubated with 3,3′-diaminobenzidine (DAB) kit (Vector Laboratories Inc.) for 10 min. In control sections the primary antibody was omitted and replaced by PBS.
Image analysis for histochemical enzyme activities and immunohistochemical protein expression
Images were captured by an optical microscope (Y2F-21, Nikon, Tokyo) with a CCD video camera (SSC-370, Sony, Tokyo) and image processing system (MacScope, Mitani Co., Fukui, Japan). To determine enzyme activities and protein expressions in individual muscle fibres of plantaris muscle, the following quantitative histochemical microphotometric procedure was used. First, ∼200 fibres were classified on the basis of ATPase staining. Individual fibres identified on the slides prepared for fibre type determination were then re-identified on the sections stained for SDH and α-GPD to assess oxidative and glycolytic enzyme activities, respectively (Peter et al. 1972). Individual fibres were re-identified on the sections stained for MCT1, MCT4, and GLUT4 as mentioned above. The image of each fibre was digitized with a resolution of 0.4 μm2 pixel size. Pixel intensity was quantified to 255 grey levels. The mean grey level value for each parameter in each fibre type was used as a measure of enzymatic activity or protein expression and recorded as optical density (OD). The OD determined for control sections was subtracted as background from data used to determine protein expression.
Confocal laser scanning microscopy (CLSM)
CLSM (Zeiss 510 META) was used for immunofluorescent detection of MCT1, MCT2, and COX. Briefly, unfixed cryosections were incubated overnight with MCT1, MCT2, and COX antibodies at 4°C. Anti-rabbit and anti-chicken Alexa fluor 488-conjugated secondary antibodies (Molecular Probes) were used for MCT1 and MCT2 detection, respectively. For COX, anti-mouse Cy3-conjugated secondary antibody (Chemicon, International) was used. Secondary antibodies were incubated for 1 h at room temperature. Antibody staining was detected at an emission of 500–530 nm and 550–600 nm after excitation at 488 nm and 543 nm, respectively.
Western blotting of MCT1, MCT4, and GLUT4
MCT1 antibody (rabbit anti-MCT1) and GLUT4 antibody (rabbit anti-GLUT4) were obtained from Chemicon (Temecula, CA, USA). Sample preparation, SDS-PAGE, and Western blotting for MCT1, MCT4, and GLUT4 were done as previously described (Hashimoto et al. 2004). Band imaging was scanned using a laser densitometer equipped with an integrator (Multi-Analyst, Bio-Rad, Hercules, CA, USA) (Brooks et al. 1999a, b).
Calculations and statistical analysis
Whole-muscle protein expression was estimated histochemically (HISTO) as the sum of each of the fibre type fractions multiplied by their corresponding mean OD, according to following equation
In this equation, PD (SO, FOG, or FG) represents the percentage of total area occupied by SO, FOG, or FG fibres, respectively. OD (SO, FOG, or FG) represents optical density of SO, FOG, or FG fibres, respectively.
Data are expressed as means ±s.e.m. To determine the significance of difference between mean values in SO, FOG, and FG fibres, one-way ANOVA and Fisher's protected least significant difference post hoc test were used. Regression analyses were performed using the least squares method. Pearson correlation coefficients were computed to determine the strength of association between histochemically and biochemically estimated protein expressions. Statistical significance was considered to be P < 0.05.
Results
Fibre-type assessment from myosin ATPase, SDH, and α-GPD staining in rat plantaris muscle
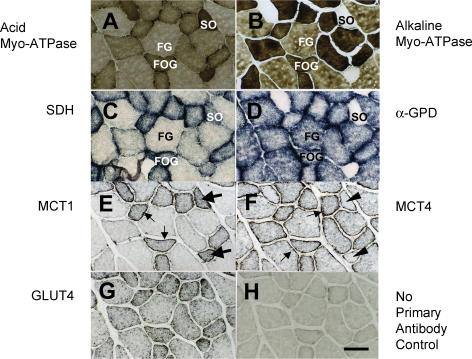
Fibre type identification was by comparison of histochemical staining for myosin ATPase after acid (Fig. 1A) and alkaline (Fig. 1B) preincubations. SO fibres appeared dark after acid preincubations, and light after alkaline preincubations (compare Fig. 1A and B). FOG fibres showed intermediate staining after acid preincubations, whereas they appeared dark after alkaline preincubations (Fig. 1A and B). FG fibres showed light and intermediate staining after acid and alkaline preincubations, respectively (Fig. 1A and B). Dark SDH staining revealed a high mitochondrial mass in SO and FOG fibres (Fig. 1C). SDH staining was especially prominent near the sarcolemma, but was also sizeable throughout oxidative fibres (compare SO and FOG with FG fibres in Fig. 1C). On the other hand, α-GPD staining was prominent in glycolytic fibres (FOG and FG) fibres (Fig. 1D). Little α-GPD activity was observed in SO fibres (compare Fig. 1B and D).
Figure 1. Serial cryostat sections stained for myosin ATPase.
A, acid preincubation; B, alkaline preincubation, C, SDH; D, α-GPD; E, MCT1; F, MCT4; and G, GLUT 4. Fibre-type identifications were made on the bases of myosin ATPase, SDH, and α-GPD staining (A–D). No primary antibody was reacted in control (H). Thin arrows in E indicate MCT1 abundance near the sarcolemma of oxidative (SO and FOG) fibres. Very little MCT1 is visible in FG fibres, either at sarcolemmal or interfibrillar cell domains. MCT4 was concentrated at the cell surface in fast (FOG and FG) fibres (thin arrows in F). Note that in SO fibres MCT1 is strongly stained (thick arrows in E), while little MCT4 is detected (arrowheads in F). Sections 10 μm; scale bar = 50 μm. Data obtained on four rats (Table 2), all panels shown from the same animal.
Transporter protein localization by immunostaining of rat plantaris muscle serial sections
Micrographs demonstrated characteristic distributions of MCT1, MCT4, and GLUT4 (Fig. 1E–G). MCT1 was especially abundant near the sarcolemma of oxidative (SO and FOG) fibres (Fig. 1E, thin arrows), but was also evident throughout the fibres as was SDH (compare Fig. 1C and E). In Fig. 1E, SO fibres with highest staining for MCT1 are indicated by thick arrows. Very little MCT1 was obtained in FG fibres, either in sarcolemmal or interfibrillar cell domains. In contrast, MCT4 was prominent in fast (FOG and FG) fibres (Fig. 1F). In fast fibres, MCT4 was concentrated at the cell surface (indicated by thin arrows), but visible also within the cell. Staining for MCT4 was light in SO fibres (indicated by arrowheads) as was α-GPD (compare Fig. 1F and D). Staining for GLUT4 was enriched in oxidative fibres, and localization was prominent not only at sarcolemmal but also at interfibrillar levels (Fig. 1G). Background staining is shown in the negative control not exposed to a primary antibody (Fig. 1H).
Quantitative analysis of protein expression
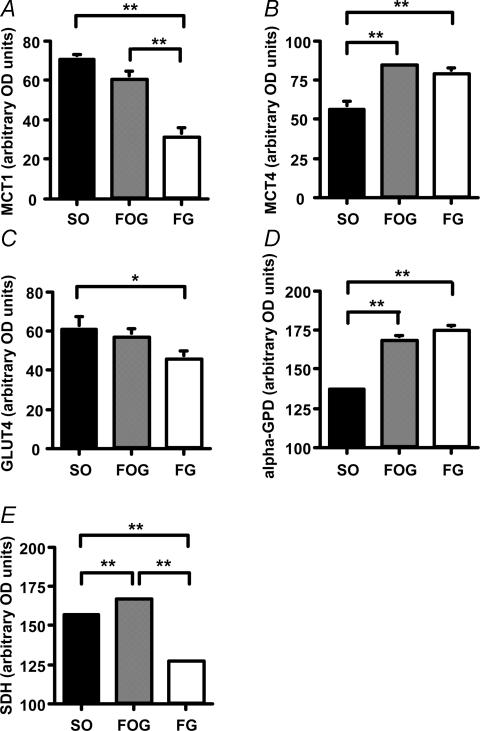
The relative abundances of each protein above background expressed in terms of optical density (OD) are shown in Fig. 2. MCT1 expression in both SO and FOG fibres was significantly higher than that in FG fibres (P < 0.01). In contrast, the amount of MCT4 expression in SO fibres was significantly lower than that in either FOG or FG fibres (P < 0.01). The pattern of GLUT4 expression was similar to that of MCT1, and there was a significant difference between SO fibres and FG fibres in the amount of GLUT4 (P < 0.05).
Figure 2. The quantitative analysis of MCT1, MCT4, and GLUT4 protein expression and SDH and α-GPD contents in terms of optical density (OD) in different fibre types are shown.
Values are expressed means ±s.e.m.*P < 0.05, **P < 0.01.
The α-GPD content in SO fibres was significantly lower than that of either FOG or FG fibres (P < 0.01). These results were in concordance with the pattern of MCT4 expression. The SDH contents in SO and FOG fibres were significantly higher than those in FG fibres (P < 0.01). Additionally, the SDH content in FOG fibres was significantly higher than that in SO fibres (P < 0.01).
Low variability in immunostaining
To assess variability in staining among similar cryosections, three serial cross sections of rat plantaris were made and stained for each protein of interest. Table 1 gives mean ±s.e.m. of ODs for MCT1 in SO, FOG, and FG fibres in three serial cross-sections of randomly selected muscle regions. There was little variability in any fibre type. Similar results were obtained in the staining for MCT4 and GLUT4 proteins, as well as control sections.
Table 1.
MCT1 protein expressions in different fibre types calculated in one part of three serial cross sections
| SO | FOG | FG | |
|---|---|---|---|
| Cross section 1 | 185.5 ± 2.0 | 171.2 ± 1.7 | 135.0 ± 2.8 |
| Cross section 2 | 185.5 ± 1.4 | 170.7 ± 1.9 | 135.3 ± 2.1 |
| Cross section 3 | 184.3 ± 1.1 | 172.6 ± 1.8 | 134.3 ± 2.3 |
Values are expressed as OD means ±s.e.m. The value of each fibre type is representative of 5, 8, and 7 fibres of SO, FOG, and FG fibres, respectively.
Comparison of protein expressions between histochemistry and biochemistry
Table 2 summarizes the fibre type distributions (FTD) and whole muscle transport protein expressions estimated by histochemistry (HISTO). In each case, we found positive correlations between the two methods of detection for (HISTO and Western blotting) MCT1 (r = 0.82), MCT4 (r = 0.73), and GLUT4 (r = 0.76), but low sample size precluded statistical assessment.
Table 2.
Rat plantaris fibre type distributions (FTD) and whole muscle transporter protein expressions obtained by histo chemistry (HISTO, from fibre area and OD)
| FTD | HISTO | |||||
|---|---|---|---|---|---|---|
| SO | FOG | FG | MCT1 | MCT4 | GLUT4 | |
| Rat 1 | 5.5 | 47.8 | 46.7 | 4925 | 7403 | 4104 |
| Rat 2 | 14.6 | 60.4 | 25.1 | 6298 | 8328 | 6089 |
| Rat 3 | 9.1 | 47.6 | 43.3 | 4737 | 8146 | 5758 |
| Rat 4 | 8.8 | 44.5 | 46.7 | 3746 | 7780 | 5025 |
Values are calculated as described in methods.
Micrographs obtained using CLSM
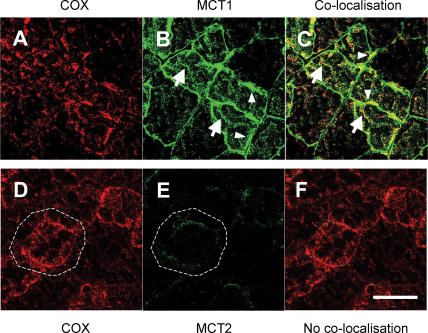
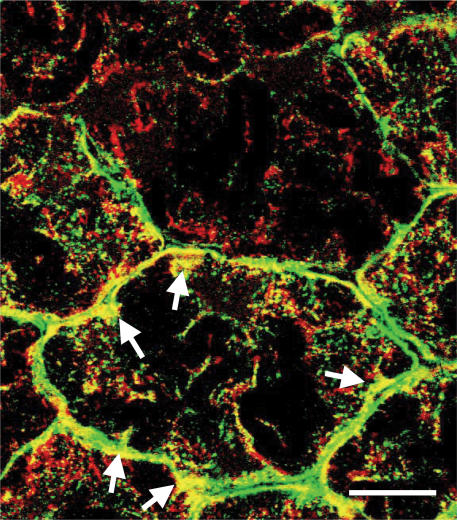
In Fig. 3, results for MCT1 localization are given in the first row (Fig. 3A–C), whereas results for MCT2 are given in the second row (Fig. 3D–F). Rat plantaris muscle fibres were stained with antibody against the mitochondrial inner membrane protein COX (red, Fig. 3A) and MCT1 (green, Fig. 3B). MCT1 was clearly detected at both sarcolemmal and intracellular domains (Fig. 3B), as was COX (Fig. 3A). Superposition of the two images (Fig. 3C) shows substantial overlap of the two probes (yellow). As well, there were some areas where the images did not coincide exactly, but that may reflect the relatively greater immunoreactivity of the MCT1 antibody. Co-localization of MCT1 and COX was clear at subsarcolemmal and interfibrillar levels under higher magnification (Fig. 4).
Figure 3. Cellular locations of MCT1 and MCT2 lactate transporter isoforms and the mitochondrial reticulum (cytochrome oxidase, COX) determined using confocal laser scanning microscopy (CLSM) and fluorescent probes for the respective proteins.
A–C, comparisons for MCT1, and D–F, for MCT2. The localization of COX was detected in rat plantaris muscle (A and D). MCT1 was detected throughout the cells including subsarcolemmal (arrowheads) and interfibrillar (arrows) domains (B). MCT1 abundance was greatest in oxidative fibres where COX is abundant and the signal strong. When these MCT1 (green) and COX (red) were merged, superposition of the two probes was clear (yellow), a finding prominent at interfibrillar (arrows) as well as sarcolemmal (arrowheads) cell domains (C). In contrast, the signal for MCT2 (E) was weak, relatively more noticeable in fibres denoted by strong staining for COX (D and F, broken line is delineated around oxidative fibre to distinguish the faint signal for MCT2). Overlap of MCT2 and COX is insignificant, denoted by absence of yellow in F. Sections 10 μm scale bar = 50 μm. Sections from the same animal.
Figure 4. The co-localization of MCT1 (green) and COX (red) was observed as a yellow colour at interfibrillar as well as sarcolemmal cell domains.
Note that approximately half of the subsarcolemmal regions show yellow colour where MCT1 and COX are co-localized (arrows). Sections 10 μm; scale bar = 20 μm. Section from the same muscle as shown in Fig. 3.
In contrast to MCT1, we detected only faint signals for MCT2 in the sarcolemmal domain of oxidative fibres in which COX was abundant throughout the cell (Fig. 3D and E).
Discussion
We report the first histochemical determinations of MCT1 and MCT4 protein expression in SO, FOG, and FG fibres in rat plantaris muscles using the high sensitivity avidin–biotin complex (ABC) method. Our staining method used in this study was specific and reliable, which allowed our quantitative analysis of MCT expression. As well, we report first efforts to detect muscle MCT isoform expression using CLSM. Findings are: (1) MCT1 is located at the sarcolemma and throughout the cell interior in SO and FOG fibres where the mitochondrial reticulum is present; (2) in contrast, MCT4 is highly expressed in the sarcolemmal domain of FG and FOG fibres, but is present also within these fibre types; (3) MCT4 is little expressed in SO fibres; and (4) confocal laser-scanning microscopy demonstrates that MCT1 and COX are co-localized at both interfibrillar and subsarcolemmal cell domains, whereas MCT2 is weakly detected only at sarcolemmal cell domains. As well, we report novel findings based on our quantitative immunohistochemical, immunobiochemical and CLSM determinations and their concordance. Results are relevant to understanding the physiology of lactate shuttles in vivo.
Our results were that MCT1 protein is present throughout oxidative fibres, particularly in sarcolemmal, subsarcolemmal, and interfibrillar compartments of SO and FOG fibres of rat plantaris. MCT1 is much less abundant in FG fibres. Consistent with a mitochondrial location of MCT1, staining for MCT1 yields a similar pattern to that for both SDH activity and COX (Brooks et al. 1999b; Butz et al. 2004). Hence, our results are not in agreement with observations in previous reports (Wilson et al. 1998; Pilegaard et al. 1999b; Fishbein et al. 2002). One possible explanation of the discrepancy involves the difference in fixation methodology; we used unfixed sections followed by ABC staining for histochemical protein detection, while others used formalin fixation prior to staining (Wilson et al. 1998; Pilegaard et al. 1999b). This methodological distinction is significant because it is known that antibody binding can be blocked in formalin-fixed sections, as shown previously (Merezhinskaya et al. 2004). With this knowledge we also tested the formalin-fixed sections and found that the immunoreactivities of MCT1, MCT4, and GLUT4 were blocked (data not shown). Additionally, it is likely that because of the presence of MCT1 in both the sarcolemma and subsarcolemmal mitochondria, investigators using non-quantitative histochemical staining to detect MCT1 would find far more intense staining at the cell surface than at interfibrillar cell domains. These methodological differences probably explain our ability to obtain histochemical results consistent with those seen by CLSM as seen in Figs 3 and 4, where the presence of MCT1 at subsarcolemmal and interfibrillar domains within oxidative muscle fibres is readily apparent.
MCT4 protein was most concentrated near the sarcolemma in FOG and FG fibres, but little MCT4 was detected in SO fibres. This localization of MCT4 is consistent with results of previous investigations (Wilson et al. 1998; Pilegaard et al. 1999b; Fishbein et al. 2002). Additionally, while we detected MCT4 in interfibrillar as well as sarcolemmal domains in FOG and FG fibres, it did not appear that MCT4 co-localizes with mitochondria in the interfibrillar domain. Indeed, we observed little staining of MCT4 in the interfibrillar space of SO fibres where the volume density of mitochondria is known to be high. It has been observed (Bonen, 2001) that MCT4 could be detected in transverse (T)-tubular fractions obtained by muscle homogenization and differential centrifugation. Although results obtained by immunohistochemistry and light microscopy are not definitive, we may have detected MCT4 distributed along the T-tubular system extending deep into the cytoskeleton of FG and FOG fibres.
In the present study, we obtained low variation in replicate determinations on serial sections in each fibre type (Table 1). As a result, we had the sensitivity to demonstrate that MCT1 expression was significantly higher in SO and FOG fibres than in FG fibres. Similarly, we detected that the expression of MCT4 is significantly lower in SO than in FOG and FG fibres of rat plantaris. Because several studies have reported a correspondence between MCT1 expression and oxidative capacity in whole-muscle homogenates (Wilson et al. 1998; Pilegaard et al. 1999 Fishbein et al. 2002; Dubouchaud et al. 2000), it is notable that we could also replicate this correspondence in single fibres.
In terms of muscle physiology and metabolism, the differential cellular localizations and relative abundances of MCT1 and MCT4 proteins would contribute to the cell-to-cell lactate shuttle. Lactate formed in some muscle cells with high rates of glycolysis (e.g. FG fibres) could be readily released via MCT4 (and MCT1) and transported into SO and FOG fibres via sarcolemmal MCT1 lactate transporters. Further, imported lactate could be readily taken up and oxidized by subsarcolemmal mitochondria (Brooks, 1998; Brooks et al. 1999a, b). In the FOG fibres, both MCT1 and MCT4 protein expressions were high. Thus, FOG fibres could function in either efflux of glycolytically produced lactate or influx of lactate derived from other cells, depending on lactate concentration and pH gradients (Brooks, 2002).
GLUT4 was visible strongly near the sarcolemma, as well as among the fibrils, mostly in SO fibres as shown previously. As such, our results are in agreement with those of others who have seen greater expression GLUT4 in oxidative compared to glycolytic mammalian skeletal muscle fibres (Kern et al. 1990; Gaster et al. 2000).
Recently, using western blotting and mitochondrial preparations obtained using homogenization and protease treatment, Benton et al. (2004) reported that subsarcolemmal mitochondria obtained from rat skeletal muscle contained MCT1, MCT2 and MCT4, whereas interfibrillar mitochondria contained only MCT2. That report was of interest because it suggested a unique location and function of MCT2. However, our micrographs obtained with CLSM produced only faint signals for MCT2 protein in contrast to abundance of MCT1. Hence, our results regarding the relative abundances of MCT1 and MCT2 in rat muscle fibres are similar to those obtained previously on human muscle (Fishbein et al. 2002), but different from those of Benton et al. (2004) with regard to location of MCT2 in skeletal muscle fibres of any type. Additionally, our results showing an association between MCT1 and interfibrillar mitochondria are unlike those of Benton et al. (2004) who did not obtain such a result. In these and other respects, we are confident of our findings because of ongoing work in studies of MCT isoform expression in cultured L6 myocytes derived from rat (T. Hashimoto, R. Hussien, and G.A. Brooks, unpublished observations). With cultured myocytes we have confirmed in essence the results presented here including the low level of MCT2 protein expression and lack of concordance between localizations of either MCT2 or MCT4 with mitochondrial markers (e.g. by co-localization with COX, MitoTracker, or MCT1) in skeletal muscle.
In their studies, Benton and colleagues overlooked extensive evidence of the presence of a mitochondrial reticulum, and consequently the inevitability of mitochondrial disruption during cell fragmentation. In their attempts to isolate two mitochondrial populations (subsarcolemmal (SSM) and interfibrillar (IFM)), Benton and colleagues pooled skeletal muscle. This procedure not only made it impossible to detect fibre-type differences, but in terms of understanding how they and we could obtain divergent results, it is to be appreciated that the purported IFM population of Benton et al. (2004) was obtained using harsh homogenization followed by protease treatment. Such treatments are known to result in loss of mitochondrial constituents and function (Butz et al. 2004). Hence, the inability of Benton et al. (2004) to detect MCT1 by Western blotting in the supposed IFM population can be ascribed to loss during isolation. More difficult to explain is the result of Benton et al. (2004) concerning the presence of MCT4 in SSM, and MCT2 in both SSM and IFM of rat skeletal muscle, findings which we are unable to substantiate. However, possibilities include non-specific antibody binding or coalescence of mitochondrial and other organelle membranes during isolation, probably peroxisomes known to contain MCT2 (McClelland et al. 2003). In this respect it is important to note that our antibody to MCT2 reacts strongly to rat liver homogenates and isolated peroxisomes.
Using immunohistochemical and biochemical techniques we obtained results consistent with lactate transporter (MCT) distributions predicted by Cell–cell and intracellular lactate shuttle hypotheses. MCT1 is prominent in cell membranes of SO and FOG rat plantaris fibres. As well, MCT1 is also present within domains occupied by the mitochondrial reticulum of oxidative fibres. In contrast, MCT4 is highly expressed in FG and FOG fibres, but abundance is low in SO fibres. The latter finding causes us to question whether MCT4 is localized to the mitochondrial reticulum which is extensive in SO fibres. Rather, we conclude that the appearance of MCT4 inside fast fibres may reflect co-localization with another cytoskeletal structure, such as the T-system. Additionally, contrary to abundant evidence of co-localization of MCT1 and the mitochondrial reticulum, MCT2 abundance is low throughout mammalian muscle fibres. Thus, MCT1 should be the primary transporter operant in Cell–cell and intracellular lactate shuttles. Bidirectional in function, sarcolemmal MCT1 would facilitate myocyte lactate uptake or release depending on metabolic conditions causing lactate concentration or pH gradients. As well, mitochondrial MCT1 would facilitate lactate uptake, as oxidation is the major means of intramuscular lactate disposal and therefore an important process in establishing the concentration and pH gradients which drive cellular lactate uptake and release (Brooks, 2002; Miller et al. 2005).
Acknowledgments
The authors are very grateful to H. Hatta, Department of Life Science, University of Tokyo, Tokyo, Japan for helpful comments and donation of the MCT4 antibody. We are indebted to S. Lehman, T. Mau and G. Henderson, Integrative Biology, UC, Berkeley, and B. Miller, Sport and Exercise Science, University of Auckland for critical commentary. This research was supported in part by the Grants-in-Aid for Scientific Research (10680514) from the Ministry of Education, Science, Sports and Culture of Japan and by the Fund for Experimental Space Utilization U-37, and by NIH grant AR050459 to G.A.B. T.H. was a recipient of a fellowship of the Nakatomi Foundation.
References
- Benton CR, Campbell SE, Tonouchi M, Hatta H, Bonen A. Monocarboxylate transporters in subsarcolemmal and intermyofibrillar mitochondria. Biochem Biophys Res Commun. 2004;323:249–253. doi: 10.1016/j.bbrc.2004.08.084. [DOI] [PubMed] [Google Scholar]
- Bergman BC, Wolfel EE, Butterfield GE, Lopaschuk GD, Casazza GA, Horning MA, Brooks GA. Active muscle and whole body lactate kinetics after endurance training in men. J Appl Physiol. 1999;87:1684–1696. doi: 10.1152/jappl.1999.87.5.1684. [DOI] [PubMed] [Google Scholar]
- Bonen A. The expression of lactate transporters (MCT1 and MCT4) in heart and muscle. Eur J Appl Physiol. 2001;86:6–11. doi: 10.1007/s004210100516. [DOI] [PubMed] [Google Scholar]
- Bröer S, Bröer A, Schneider HP, Stegen C, Halestrap AP, Deitmer JW. Characterization of the high-affinity monocarboxylate transporter MCT2 in Xenopus laevis oocytes. Biochem J. 1999;341(3):529–535. doi: 10.1042/0264-6021:3410529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks GA. Mammalian fuel utilization during sustained exercise. Comp Biochem Physiol B Biochem Mol Biol. 1998;120:89–107. doi: 10.1016/s0305-0491(98)00025-x. [DOI] [PubMed] [Google Scholar]
- Brooks GA. Lactate shuttles in nature. Biochem Soc Trans. 2002;30:259–264. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- Brooks GA, Brown MA, Butz CE, Sicurello JP, Dubouchaud H. Cardiac and skeletal muscle mitochondria have a monocarboxylate transporter MCT1. J Appl Physiol. 1999b;87:1713–1718. doi: 10.1152/jappl.1999.87.5.1713. [DOI] [PubMed] [Google Scholar]
- Brooks GA, Dubouchaud H, Brown MA, Sicurello JP, Butz CE. Role of mitochondrial lactate dehydrogenase and lactate oxidation in the ‘intra-cellular lactate shuttle’. Proc Natl Acad Sci U S A. 1999a;96:1129–1134. doi: 10.1073/pnas.96.3.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks GA, Wolfel EE, Butterfield GE, Cymerman A, Roberts AC, Mazzeo RS, Reeves JT. Poor relationship between arterial [lactate] and leg net release during exercise at 4300 m altitude. Am J Physiol. 1998;275:R1192–R1201. doi: 10.1152/ajpregu.1998.275.4.R1192. [DOI] [PubMed] [Google Scholar]
- Brown MA, Brooks GA. Trans-stimulation of lactate transport from rat sarcolemmal membrane vesicles. Arch Biochem Biophys. 1994;313:22–28. doi: 10.1006/abbi.1994.1353. [DOI] [PubMed] [Google Scholar]
- Butz CE, McClelland GB, Brooks GA. MCT1 confirmed in rat striated muscle mitochondria. J Appl Physiol. 2004;97:1059–1066. doi: 10.1152/japplphysiol.00009.2004. [DOI] [PubMed] [Google Scholar]
- Dimmer KS, Friedrich B, Lang F, Deitmer JW, Broer S. The low-affinity monocarboxylate transporter MCT4 is adapted to the export of lactate in highly glycolytic cells. Biochem J. 2000;350:219–227. [PMC free article] [PubMed] [Google Scholar]
- Dubouchaud H, Butterfield GE, Wolfel EE, Bergman BC, Brooks GA. Endurance training, expression, and physiology of LDH, MCT1, and MCT4 in human skeletal muscle. Am J Physiol Endocrinol Metab. 2000;278:E571–E579. doi: 10.1152/ajpendo.2000.278.4.E571. [DOI] [PubMed] [Google Scholar]
- Fishbein WN, Merezhinskaya N, Foellmer JW. Relative distribution of three major lactate transporters in frozen human tissues and their localization in unfixed skeletal muscle. Muscle Nerve. 2002;26:101–112. doi: 10.1002/mus.10168. [DOI] [PubMed] [Google Scholar]
- Garcia CK, Brown MS, Pathak RK, Goldstein JL. cDNA cloning of MCT2, a second monocarboxylate transporter expressed in different cells than MCT1. J Biol Chem. 1995;270:1843–1849. doi: 10.1074/jbc.270.4.1843. [DOI] [PubMed] [Google Scholar]
- Garcia CK, Goldstein JL, Pathak RK, Anderson RG, Brown MS. Molecular characterization of a membrane transporter for lactate, pyruvate, and other monocarboxylates: implications for the Cori cycle. Cell. 1994;76:865–873. doi: 10.1016/0092-8674(94)90361-1. [DOI] [PubMed] [Google Scholar]
- Gaster M, Poulsen P, Handberg A, Schroder HD, Beck-Nielsen H. Direct evidence of fiber type-dependent GLUT-4 expression in human skeletal muscle. Am J Physiol Endocrinol Metab. 2000;278:E910–E916. doi: 10.1152/ajpendo.2000.278.5.E910. [DOI] [PubMed] [Google Scholar]
- Halestrap AP, Price NT. The proton-linked monocarboxylate transporter (MCT) family: structure, function and regulation. Biochem J. 1999;343:281–299. [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Kambara N, Nohara R, Yazawa M, Taguchi S. Expression of MHC-beta and MCT1 in cardiac muscle after exercise training in myocardial-infarcted rats. J Appl Physiol. 2004;97:843–851. doi: 10.1152/japplphysiol.01193.2003. [DOI] [PubMed] [Google Scholar]
- Jackson VN, Price NT, Carpenter L, Halestrap AP. Cloning of the monocarboxylate transporter isoform MCT2 from rat testis provides evidence that expression in tissues is species-specific and may involve post-transcriptional regulation. Biochem J. 1997;324:447–453. doi: 10.1042/bj3240447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern M, Wells JA, Stephens JM, Elton CW, Friedman JE, Tapscott EB, Pekala PH, Dohm GL. Insulin responsiveness in skeletal muscle is determined by glucose transporter (Glut4) protein level. Biochem J. 1990;270:397–400. doi: 10.1042/bj2700397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin RY, Vera JC, Chaganti RS, Golde DW. Human monocarboxylate transporter 2 (MCT2) is a high affinity pyruvate transporter. J Biol Chem. 1998;273:28959–28965. doi: 10.1074/jbc.273.44.28959. [DOI] [PubMed] [Google Scholar]
- McClelland GB, Brooks GA. Changes in MCT 1, MCT 4, and LDH expression are tissue specific in rats after long-term hypobaric hypoxia. J Appl Physiol. 2002;92:1573–1584. doi: 10.1152/japplphysiol.01069.2001. [DOI] [PubMed] [Google Scholar]
- McClelland GB, Khanna S, Gonzalez GF, Butz CE, Brooks GA. Peroxisomal membrane monocarboxylate transporters: evidence for a redox shuttle system? Biochem Biophys Res Commun. 2003;304:130–135. doi: 10.1016/s0006-291x(03)00550-3. [DOI] [PubMed] [Google Scholar]
- Merezhinskaya N, Ogunwuyi SA, Mullick FG, Fishbein WN. Presence and localization of three lactic acid transporters (MCT1-2, and -4) in separated human granulocytes, lymphocytes, and monocytes. J Histochem Cytochem. 2004;52:1483–1493. doi: 10.1369/jhc.4A6306.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BF, Lindinger MI, Fattor JA, Jacobs KA, Leblanc PJ, Duong M, Heigenhauser GJF, Brooks GA. Hematological and acid-base changes in men during prolonged exercise with and without sodium-lactate infusion. J Appl Physiol. 2005;98:856–865. doi: 10.1152/japplphysiol.00753.2004. [DOI] [PubMed] [Google Scholar]
- Peter JB, Barnard RJ, Edgerton VR, Gillespie CA, Stempel KE. Metabolic profiles of three fiber types of skeletal muscle in guinea pigs and rabbits. Biochemistry. 1972;11:2627–2633. doi: 10.1021/bi00764a013. [DOI] [PubMed] [Google Scholar]
- Pilegaard H, Domino K, Noland T, Juel C, Hellsten Y, Halestrap AP, Bangsbo J. Effect of high-intensity exercise training on lactate/H+ transport capacity in human skeletal muscle. Am J Physiol. 1999a;276:E255–E261. doi: 10.1152/ajpendo.1999.276.2.E255. [DOI] [PubMed] [Google Scholar]
- Pilegaard H, Terzis G, Halestrap A, Juel C. Distribution of the lactate/H+ transporter isoforms MCT1 and MCT4 in human skeletal muscle. Am J Physiol. 1999b;276:E843–E848. doi: 10.1152/ajpendo.1999.276.5.E843. [DOI] [PubMed] [Google Scholar]
- Roth DA, Brooks GA. Lactate and pyruvate transport is dominated by a pH gradient-sensitive carrier in rat skeletal muscle sarcolemmal vesicles. Arch Biochem Biophys. 1990a;279:386–394. doi: 10.1016/0003-9861(90)90506-t. [DOI] [PubMed] [Google Scholar]
- Roth DA, Brooks GA. Lactate transport is mediated by a membrane-bound carrier in rat skeletal muscle sarcolemmal vesicles. Arch Biochem Biophys. 1990b;279:377–385. doi: 10.1016/0003-9861(90)90505-s. [DOI] [PubMed] [Google Scholar]
- Taguchi S, Hata Y, Itoh K. Enzymatic responses and adaptations to swimming training and hypobaric hypoxia in postnatal rats. Jpn J Physiol. 1985;35:1023–1032. doi: 10.2170/jjphysiol.35.1023. [DOI] [PubMed] [Google Scholar]
- Watt PW, Maclennan PA, Hundal HS, Kuret CM, Rennie MJ. L(+)-lactate transport in perfused rat skeletal muscle: kinetic characteristics and sensitivity to pH and transport inhibitors. Biochim Biophys Acta. 1988;944:213–222. doi: 10.1016/0005-2736(88)90434-8. [DOI] [PubMed] [Google Scholar]
- Wattenberg LW, Leong JL. Effects of coenzyme Q10 and menadione on succinic dehydrogenase activity as measured by tetrazolium salt reduction. J Histochem Cytochem. 1960;8:296–303. doi: 10.1177/8.4.296. [DOI] [PubMed] [Google Scholar]
- Wilson MC, Jackson VN, Heddle C, Price NT, Pilegaard H, Juel C, Bonen A, Montgomery I, Hutter OF, Halestrap AP. Lactic acid efflux from white skeletal muscle is catalyzed by the monocarboxylate transporter isoform MCT3. J Biol Chem. 1998;273:15920–15926. doi: 10.1074/jbc.273.26.15920. [DOI] [PubMed] [Google Scholar]