Abstract
Regulation of GABAA receptors by extracellular pH exhibits a dependence on the receptor subunit composition. To date, the molecular mechanism responsible for the modulation of GABAA receptors at alkaline pH has remained elusive. We report here that the GABA-activated current can be potentiated at pH 8.4 for both αβ and αβγ subunit-containing receptors, but only at GABA concentrations below the EC40. Site-specific mutagenesis revealed that a single lysine residue, K279 in the β subunit TM2–TM3 linker, was critically important for alkaline pH to modulate the function of both α1β2 and α1β2γ2 receptors. The ability of low concentrations of GABA to reveal different pH titration profiles for GABAA receptors was also examined at acidic pH. At pH 6.4, GABA activation of αβγ receptors was enhanced at low GABA concentrations. This effect was ablated by the mutation H267A in the β subunit. Decreasing the pH further to 5.4 inhibited GABA responses via αβγ receptors, whereas those responses recorded from αβ receptors were potentiated. Inserting homologous β subunit residues into the γ2 subunit to recreate, in αβγ receptors, the proton modulatory profile of αβ receptors, established that in the presence of β2H267, the mutation γ2T294K was necessary to potentiate the GABA response at pH 5.4. This residue, T294, is homologous to K279 in the β subunit and suggests that a lysine at this position is an important residue for mediating the allosteric effects of both acidic and alkaline pH changes, rather than forming a direct site for protonation within the GABAA receptor.
The regulation of GABAA receptors by numerous endogenous factors, including phosphorylation, redox reagents and ions normally present in vivo (e.g. H+ and Zn2+) may be important for their physiological function (Kaila, 1994; Smart et al. 1994; Sieghart, 1995; Rabow et al. 1996; Moss & Smart, 2001). Generally, fast synaptic inhibition proceeds via GABAA receptors predominantly composed of αβγ subunits (McKernan & Whiting, 1996). In comparison, extrasynaptic GABAA receptors underlie continuous or tonic inhibition (Brickley et al. 1995; Brickley et al. 1996; Brickley et al. 1999; Mody, 2001) and their subunit composition is likely to vary depending on the type of neurone. Typical examples will include not only αβγ isoforms, but also receptors comprising α4, α5 or α6 and δ subunits, as well as the potential for αβ isoforms (Brickley et al. 2001; Mody, 2001).
Protons can exert various effects on GABAA receptor function depending upon their subunit composition (Krishek et al. 1996). Lowering external pH (to 6.4 or 5.4) potentiates GABA-activated currents recorded from αβ, αβδ and αβγδ GABAA receptors (Krishek et al. 1996). For the αβ isoform, this modulation has been largely attributed to the protonation of a single histidine residue, located in the ion channel lining contributed by the β subunit (H267) (Wilkins et al. 2002). However, the alkaline pH sensitivity profiles of recombinant GABAA receptors do appear to vary with their function either augmented, slightly inhibited, or remaining largely unaffected at pH 7.9–8.4 (Krishek et al. 1996; Huang & Dillon, 1999). The reasons for this variation are unclear. Although differences in experimental design may be relevant, such variability is also evident in the pH profiles of native neuronal GABAA receptors. Here, differences in subunit composition and/or varying proportions of receptor subpopulations offer more likely explanations. For example, alkaline pH inhibited responses to GABA in cerebellar granule cells (Robello et al. 1994) and sympathetic neurones (Smart, 1992; Krishek et al. 1996), whilst in hypothalamic neurones, responses were potentiated (Huang & Dillon, 1999). In acutely solated hippocampal pyramidal neurones, pH modulation was dependent upon the GABA concentration, with responses induced by low and high GABA concentrations being potentiated and inhibited, respectively, by alkaline pH (Pasternack et al. 1996). Furthermore, during neuronal development, the alkaline pH sensitivity of GABA-activated currents recorded from cultured cerebellar granule cells up to 11 days in vitro changed from initial potentiation to relative insensitivity thereafter (Krishek & Smart, 2001). The role of alkaline pH transients in GABA receptor function is still to be defined but it may be important in regulating neuronal excitability. Extracellular alkaline and acidic transients, of up to 0.2 pH unit, have been observed during synaptic transmission, as well as after the activation of GABAA or glutamate receptors (Chen & Chesler, 1991; Chen & Chesler, 1992; Kaila et al. 1992). Physiologically, during synaptic transmission, bicarbonate ions are thought to flow through GABAA receptors creating an extracellular alkaline environment for those receptors (Kaila, 1994), whereas extreme extracellular acidosis is more likely to occur in pathophysiological conditions such as ischaemia, brain trauma and hypothermia with deviations of 1 pH unit from physiological conditions possible (Kraig et al. 1987; Hoffman et al. 1996; Hoffman et al. 1999; Anderson & Meyer, 2002).
The present study examined the molecular mechanism by which alkaline pH affected GABA-activated currents and its apparent dependence on GABA concentration. Using site-directed mutagenesis, a single residue was identified which appeared to have a prime role in the modulation of the GABAA receptor in alkaline pH. In addition, by examining the differential modulation of αβ and αβγ GABAA receptors in acidic pH we have identified key residues that are involved in the modulation of GABAA receptors at both alkaline and acidic pH.
Methods
cDNA constructs and site-specific mutagenesis
Murine GABAA receptor α1, β2 and γ2s subunit cDNAs were cloned into the vector pRK5. Site-specific mutagenesis was performed using the QUICKCHANGE (Stratagene) primer-directed polymerase chain reaction method and cDNAs were prepared using the Plasmid Maxi Kit (Qiagen, Crawley, UK). The precision of the point mutations and integrity of the entire coding sequence was assessed using the BigDye ready reaction mix (PerkinElmer/Applied Biosystems) with an ABI 310 automated DNA sequencer (Applied Biosystems, Foster City, CA, USA).
Cell culture and transfection
Human embryonic kidney (HEK) cells were cultured in 10 cm dishes at 37°C in 95% air−5% CO2 in a growth medium consisting of Dulbecco's modified Eagle's medium (DMEM), 10% fetal calf serum (FCS), 2 mm glutamine, 100 units ml−1 penicillin G and 100 mg ml−1 streptomycin. Exponentially growing HEK cells attaining 70% confluence were washed with 5 ml Hanks' balanced salt solution (HBSS) and harvested using 2 ml of 0.5 mg ml−1 trypsin and 0.2 mg ml−1 EDTA. Trypsin activity was quenched by adding 10 ml of the growth medium. Cells were centrifuged at 84 g for 2 min and resuspended in 10 ml of DMEM with FCS. Cells were plated onto 22 mm glass poly-l-lysine-coated coverslips in 35 mm culture dishes and allowed to adhere at 37°C in a 95% air−5% CO2 incubator for at least 1 h.
Prior to DNA transfection, the cells were washed with 2 ml of HBSS and maintained in 1.5 ml of growth medium. For each 35 mm dish of cells, 4 μl of DNA solution for the receptor subunits and the reporter, GFP (total, approximately 4 μg), was mixed gently with 20 μl of 340 mm CaCl2 solution and 24 μl of double-strength HBSS (containing, 280 mm NaCl, 2.8 mm Na2HPO4, 50 mm Hepes, pH 7.2 with 1 n NaOH) and left to stand for at least 20 min for the DNA precipitate to form. The DNA suspension was then applied to the dish (48 μl per dish), which was incubated at 37°C in a 95% air−5% CO2 overnight. The medium was replaced with growth medium after ∼18 h and the cells used for electrophysiology for a further 24–48 h.
Patch clamp electrophysiology
Membrane currents were recorded from single HEK cells using the whole-cell patch clamp configuration in conjunction with an Axopatch 1C amplifier. Patch pipettes (resistances 3–5 MΩ) were pulled from thin-walled borosilicate glass and filled with a solution containing (mm): 120 KCl, 1 MgCl2, 11 EGTA, 30 KOH, 10 Hepes, 1 CaCl2, 2 adenosine triphosphate and 12 creatine phosphate; pH 7.11. The cells were continuously perfused with Krebs solution containing (mm): 140 NaCl, 4.7 KCl, 1.2 MgCl2, 2.52 CaCl2, 11 glucose and either 5 Hepes or 5 Mes (Hepes was used for Krebs solutions in the pH range 7.4–8.4; Mes was used for the pH range, 5.4–6.9). The Krebs solution's pH was adjusted to 5.4–8.4 with 1 or 5 n NaOH. Membrane currents were filtered at 5 kHz (−3 dB, 6th pole Bessel, 36 dB per octave) and stored on a Viglen pentium III computer for analysis with Clampex 8. Changes of more than 10% in the membrane input conductance or series resistance resulted in the recording being discarded. Drugs and solutions were rapidly applied to the cells using a modified Y-tube positioned approximately 300 μm from the recorded cell.
Analysis of whole-cell current data
Peak amplitude GABA-activated currents were determined at −50 mV holding potential. To construct concentration–response relationships for GABA, the current (I) was measured in the presence of each concentration of GABA applied at 2 min intervals to allow recovery from desensitization. The currents were normalized to the maximum GABA response at pH 7.4 (Imax) and the concentration response relationship fitted with the Hill equation:
| (1) |
where the EC50 represents the concentration of GABA ([A]) inducing 50% of the maximal current evoked by a saturating concentration of GABA and nH is the Hill coefficient. The GABA concentration–response curve data were subjected to an analysis of variance with Bonferroni's post hoc test. Significance for all data was determined at the P < 0.05 level.
The pH titration data were curve fitted as previously described providing estimates of pKa values assuming the receptor protein can behave as a weak diprotic acid possessing two sites for proton binding that will influence the GABA-activated conductance (Krishek et al. 1996). The proportions of charged and uncharged species of amino acids that coexisted in solution at particular pH values, were calculated using:
The level of spontaneous receptor activation was assessed by using picrotoxin to block active GABAA receptors. Spontaneity was manifest by the generation of an outward current superimposed on the holding current. This current was not observed for the wild-type α1β2 or α1β2γ2 receptors. The extent of spontaneity was established by examining the maximum outward current induced by picrotoxin (IPTX) which was summed with the maximum current activated by a saturating concentration of GABA (IGABA,max). The level of spontaneous receptor activation was quantified according to:
Molecular modelling
The transmembrane domains of GABAA receptor α1, β2 and γ2 subunits were aligned with those of nACh receptors using Clustal W (Thompson et al. 1994) and a homology model based on the structure of Torpedo nACh receptor transmembrane domains (Miyazawa et al. 2003; PDB accession code, 1oed) was generated using Deep View (Guex & Peitsch, 1997). Deep View was used to dock the model transmembrane domains with Ernst and colleagues' (Ernst et al. 2003) model of the GABAA receptor ligand binding domain, which is based upon the crystal structure of the acetylcholine binding protein (AChBP; PDB accession code, 1i9b; Brejc et al. 2001). All images were generated with POV-Ray.
Results
External alkaline pH and recombinant α1β2 and α1β2γ2 GABAA receptors
The effect of alkaline pH on GABAA receptors was assessed by comparing GABA concentration–response relationships for both α1β2 and α1β2γ2 GABAA receptors at pH 7.4 and 8.4 (Fig. 1A and B). For either receptor construct, considering the entire curves, there appeared to be no significant shift in the concentration-dependence or in the maximum response to GABA when the pH was increased. Accordingly, the GABA EC50s for α1β2 (means ± s.e.m.; pH 7.4, 2.9 ± 0.3 μm; pH 8.4, 2.3 ± 0.2 μm; P > 0.05; n = 24) and α1β2γ2 (pH 7.4, 3.4 ± 0.2 μm; pH 8.4, 2.6 ± 0.1 μm; P > 0.05; n = 22) receptors remained unaffected. However, close inspection of the lower segment of the concentration response curves at pH 8.4 revealed a small, leftward shift tendency that was apparent only at the lowest concentrations of GABA (EC10-40) for both receptor constructs (Fig. 1C and D).
Figure 1. Alkaline pH and the GABA concentration response relationships for α1β2 and α1β2γ2 GABAA receptors.
A and B, GABA concentration–response curves for α1β2 (A) and α1β2γ2 (B) GABAA receptors (n = 8–32 cells). Peak GABA currents were measured at pH 7.4 (○) and 8.4 (▴) and then normalized to the maximum GABA response at pH 7.4 (= 1). In this and other figures, all points represent the mean ± s.e.m. The insets illustrate sample GABA-activated currents induced by applying 10 μm GABA (continuous lines) at the indicated external pH. Calibration bars represent: 500 pA, 2 s (A) and 1000 pA, 2 s (B). C and D, expansions of the GABA curves shown by the boxed regions in A and B for both α1β2 (C) and α1β2γ2 (D) at pH 7.4 (○) and 8.4 (▴).
Modulation at alkaline pH is dependent on GABA concentration
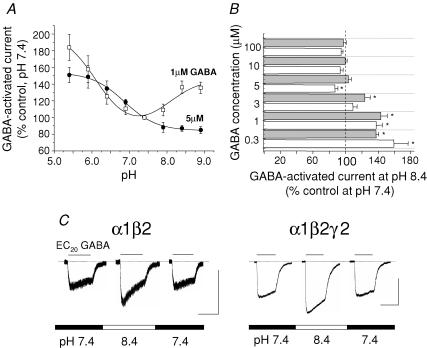
To further examine the dependence of pH modulation on low GABA concentrations, pH titration profiles were constructed for responses to 1 and 5 μm GABA using the α1β2 receptor (Fig. 2A). For 1 μm GABA, which is near the EC20, the titration was complex revealing a potentiation of the GABA-activated current in both acidic and alkaline pH relative to pH 7.4, with apparent pKa values of 6.1 ± 0.22 and 7.7 ± 0.38, respectively (Fig. 2A; n = 14). By increasing the GABA concentration to 5 μm, alkaline pH had a small inhibitory effect on the GABA response for α1β2 receptors, as reported for α1β1 (Krishek et al. 1996), with the titration curve now described by a single pKa of 6.9 ± 0.04 (Fig. 2A; n = 13). The effect of alkaline pH was examined further using 0.3–100 μm GABA applied to αβ and αβγ receptors at pH 7.4 and 8.4; however, only with 0.3–3 μm GABA were clear potentiations observed (P < 0.05; n = 12; Fig. 2B and C).
Figure 2. Modulation of α1β2 and α1β2γ2 receptors by alkaline pH is dependent on low GABA concentrations.
A, pH titration profiles for α1β2 receptors determined for 1 μm (□) and 5 μm (•) GABA-activated responses (n = 4–12 cells). Data were normalized to the responses evoked by 1 or 5 μm GABA at pH 7.4 (= 100%). B, bar graph of GABA-activated current determined at different GABA concentrations for α1β2 (white bars) and α1β2γ2 (grey bars) receptors at pH 8.4 (n = 4–12). *P < 0.05 compared with the control response (= 100%) determined at pH 7.4 for the corresponding GABA concentration. C, GABA-activated currents induced by 1 μm (EC20) GABA at the indicated external pH for α1β2 and α1β2γ2 GABAA receptors. Calibration bars represent: 400pA, 2 s.
As both types of GABAA receptor were regulated in a similar manner by alkaline pH, it was conceivable that the underlying molecular mechanism(s) involved identical residues. To facilitate their identification using site-directed mutagenesis, we targeted residues whose pKa values were similar to those determined from the pH titrations. For the α1β2 receptor, the pKa of ∼7.7 implicated cysteine (pKa∼8.3) and/or histidine (pKa∼6) residues. As only external pH excursions affected GABAA receptor function (Krishek et al. 1996), only extracellular residues were considered. On this basis, the two extracellular cysteine residues are probably precluded since they form a disulphide bridge (Amin et al. 1994; Lu, 1997). Although histidines are alternatives, the involvement of H267 in receptor modulation at acidic pH suggested that they may not be involved (Wilkins et al. 2002). Further alternatives included lysines or tyrosines and although their side-chain pKa values are ∼10, these can be considerably lower and within range of the alkaline pH change, depending upon their microenvironment in the receptor protein (Schulte et al. 1999).
Alkaline pH-induced modulation does not involve histidine or tyrosine residues
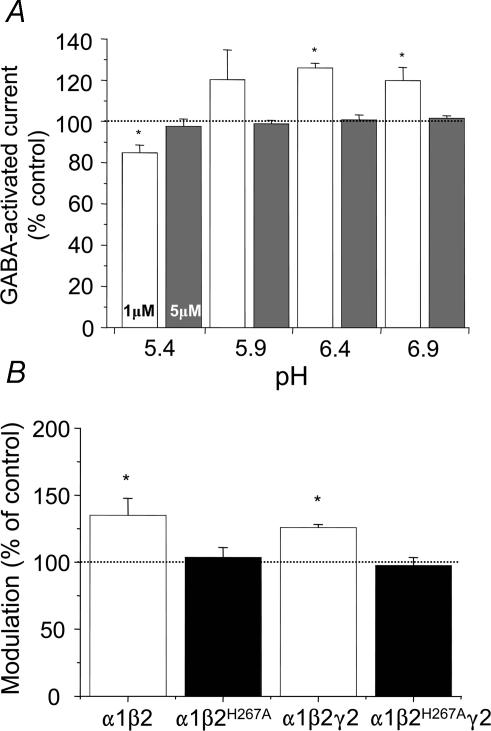
The involvement of histidine and tyrosine residues with the modulation of the GABAA receptor at pH 8.4 was investigated using the covalent modifying reagent, diethylpyrocarbonate (DEPC) (Miles, 1977) and site-directed mutagenesis. The mutation of the previously identified proton-sensitive H267 on the β subunit, forming α1β2H267A and α1β2H267Aγ2 receptors, did not prevent alkaline pH from potentiating the 1 μm (EC20) GABA-activated currents by 55 ± 11% and 36 ± 4% from their control current amplitudes (n = 4–6). This level of potentiation was similar to that observed with the respective wild-type receptors, α1β2 (38 ± 8%) and α1β2γ2 (43 ± 8%; n = 10; P > 0.05), suggesting that H267 was unimportant for the modulation at alkaline pH. On a similar basis, previous mutation experiments suggested that other external histidines were not mediating the alkaline pH modulation (data not shown; Dunne et al. 2002; Wilkins et al. 2002).
As the pH effect was GABA concentration dependent we considered if external tyrosines were crucial for the alkaline pH regulation, particularly Y157 and Y205 in the β subunit, which are thought to reside at the GABA binding site (Cromer et al. 2002; Korpi et al. 2002). These residues were mutated to phenylalanines and separately expressed with wild-type α1 subunits. As previously reported, the sensitivity to GABA for the mutant receptors was reduced (Amin & Weiss, 1993; data not shown) which required a new GABA EC20 (3 μm) to be determined. Exposure of α1β2Y157F and α1β2Y205F to pH 8.4 potentiated the GABA-activated currents by 33 ± 5% and 39 ± 7%, respectively, which is comparable to that observed with the wild-type receptor (n = 5; P > 0.05), suggesting that these GABA binding site tyrosine residues are not responsible for the potentiation at pH 8.4. It appeared unlikely that other external, accessible tyrosines and histidines were involved in the alkaline pH regulation, since applying 1 mm DEPC for 2 min prior to and during intermittent GABA (10 μm) application did not affect the potentiation of 1 μm GABA-activated responses on α1β2 receptors at pH 8.4 (24 ± 6%, n = 5; P > 0.05).
Role of β subunit lysines in alkaline pH modulation of GABAA receptors
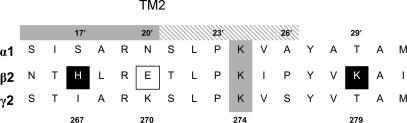
The prospect of lysine residues mediating the effects of alkaline pH was investigated following the reported distortion of their pKa by charged environments in channels (Schulte et al. 1999). Such a charge distortion in the GABAA receptor, by nearby arginines in TM2 or in the Cys loop, might bring lysine pKa values within range of the alkaline pH shift (from 10 to 7.4). The two lysines, K274 and K279, selected for mutation, form part of the β2 subunit TM2/TM2–TM3 linker (Fig. 3), a region associated with ion channel gating (Cromer et al. 2002; Kash et al. 2003). Furthermore, they also lie in close proximity to the proton sensitive H267 (Wilkins et al. 2002). Sequence homology comparisons reveal that K274 is conserved between the isoforms of the α, β and γ subunit families, with the sole exception of γ3, whils, K279 is only present in the β and δ subunit families.
Figure 3. Alignment of the TM2–TM3 linkers for α1, β2 and γ2 subunits.
The lysine at position 274 (in the β2 subunit) is conserved between all three subunits (shaded grey). However, the lysine at position 279 and H267 (black boxes in the β2 subunit) and also E270 (open box) are only conserved between the β subunit family. The grey shaded region denotes the traditional end of TM2 at 20′ and the grey hatched box to 26′ reflects the increased length of TM2 as reported previously (Horenstein et al. 2001). The numbering refers to the mature β2 subunit and the prime numbers are referenced from the cytoplasmic ends of TM2.
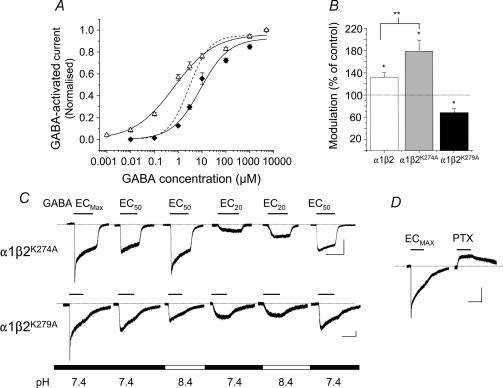
The expression of either α1β2K274A or α1β2K279A resulted in the formation of functional receptors, but the GABA concentration–response curves were displaced to either the right (K274A) or the left (K279A) when compared with the curve for the wild-type α1β2 receptor at pH 7.4 (Fig. 4A). These displacements were reflected in GABA EC50 values (α1β2K274A, 8.6 ± 3.2 μm; α1β2K279A, 0.7 ± 0.2 μm; n = 12) and Hill slopes (α1β2K274A, 0.7 ± 0.18; α1β2K279A, 0.5 ± 0.06; n = 12), compared to their wild-type equivalents (α1β2, EC50, 2.9 ± 0.3 μm; Hill slope, 1.0 ± 0.08; P < 0.05).
Figure 4. β subunit lysines and the potentiation of the GABA-activated current for α1β2 receptors at pH 8.4.
A, normalized GABA concentration–response curves for α1β2K274A (♦) and α1β2K279A (▵) at pH 7.4. Peak currents were normalized to the maximum responses at pH 7.4 (n = 4–12 cells). The curve for the wild type α1β2 receptor at pH 7.4 is shown as a dashed line for comparison (data from Fig. 1A). B, bar graph of the modulation of EC20 GABA-activated currents at pH 8.4 for α1β2, α1β2K274A and α1β2K279A receptors (n = 4–12) compared with their corresponding control responses at pH 7.4 (= 100%). *P < 0.05 compared with the control response at pH 7.4; **P < 0.05 between the potentiated responses. C, GABA-activated currents induced by ECmax, EC50 and EC20 GABA concentrations applied at the indicated external pH for α1β2K274A and α1β2K279A receptors. D, 1 mm GABA- and 100 μm PTX-activated currents for the α1β2K279A receptor at pH 7.4. All calibration bars represent: 200 pA, 2 s.
Using the appropriate GABA EC20 (1 μm for α1β2 and α1β2K274A and 0.1 μm for α1β2K279A) at pH 8.4, the potentiation of GABA-activated current for α1β2K274A receptors was increased (79 ± 20%; Fig. 4B and C; P < 0.05) compared to that at pH 7.4. In contrast, exposing the α1β2K279A receptor to pH 8.4 abolished the potentiation, revealing a significant inhibition of the GABA response to 78 ± 5% compared to that at pH 7.4 (Fig. 4B and C). Another notable feature of the α1β2K279A receptor was that the deactivation kinetics of the GABA-activated currents were quite slow, a feature not apparent for the α1β2K274A mutant (Fig. 4C). Given the position of the lysines in the TM2–TM3 linker, we investigated whether the receptor was capable of spontaneous activation. Both α1β2K274A and α1β2K279A receptors were exposed to 100 μm picrotoxin (PTX) in the absence of GABA, which would inhibit any spontaneous channel openings (Sigel et al. 1989; Wooltorton et al. 1997). The K274A mutation was unaffected by PTX (n = 5), in contrast to the outward current observed for the α1β2K279A receptor (Fig. 4D), which was in accord with a level of spontaneous gating estimated at 22 ± 1.4% (n = 7: see Methods).
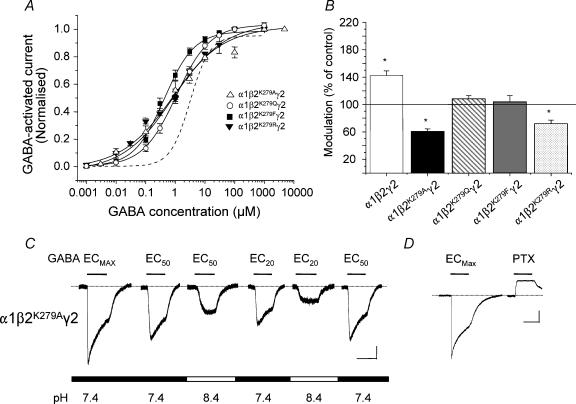
In a similar manner to the α1β2K279A GABAA receptor, the GABA concentration–response curve for α1β2K279Aγ2 at pH 7.4 also reflected an increased GABA potency (Fig. 5A) with the EC50 (0.79 ± 0.22μm; P < 0.05) and Hill slope (0.5 ± 0.06; P < 0.05; n = 8) both significantly reduced in comparison to the α1β2γ2 receptor (EC50 3.4 ± 0.3 μm; Hill slope 1.3 ± 0.2; n = 10). As for the α1β2K279A receptor, the EC20 GABA-activated current for the α1β2K279Aγ2 receptor was now inhibited at pH 8.4 (Fig. 5B and C) and these receptors were also spontaneously active to a level of 26 ± 7% (n = 6; Fig. 5D).
Figure 5. β subunit K279 and the potentiation of the GABA-activated current for α1β2γ2 receptors at pH 8.4.
A, normalized GABA concentration–response curves for the α1β2K279Aγ2, α1β2K279Qγ2, α1β2K279Fγ2 and α1β2K279Rγ2 GABAA receptors at pH 7.4 (n = 5–12 cells). The curve for the wild-type α1β2γ2 receptor at pH 7.4 (dashed line) is taken from Fig 1B. B, bar graph of the modulation of the EC20 GABA-activated current at pH 8.4 for the α1β2γ2, α1β2K279Aγ2, α1β2K279Qγ2, α1β2K279Fγ2 and α1β2K279Rγ2 GABAA receptors compared to their controls at pH 7.4 (= 100%; n = 5–13 cells). *P < 0.05 compared with the control response at pH 7.4. C, GABA-activated currents induced by ECmax, EC50 and EC20 GABA concentrations applied at the indicated external pH for the α1β2K279Aγ2 GABAA receptor. Calibration bars represent: 200pA, 2 s. D, 1 mm GABA- and 100 μm PTX-activated currents at pH 7.4 for the α1β2K279Aγ2 GABAA receptor. Calibration bar represents: 500pA, 2 s.
To ensure that the mutation of lysine to alanine, with its smaller side chain, was not distorting receptor structure and thus indirectly affecting the response to alkaline pH, we mutated K279 to uncharged glutamine (Q), non-polar phenylalanine (F) and basic arginine (R), with their comparable side chain volumes. All three mutant receptors, α1β2K279Qγ2, α1β2K279Fγ2 and α1β2K279Rγ2, were functional with reduced EC50 values (1.08 ± 0.04 μm, 0.42 ± 0.06 μm and 0.88 ± 0.21 μm, respectively; n = 5–16) and Hill slopes (0.77 ± 0.02, 0.85 ± 0.1 and 0.49 ± 0.21, respectively; Fig. 5A) compared to α1β2γ2 (EC50 3.4 ± 0.3 μm; Hill slope 1.3 ± 0.2). Potentiation of the EC20 GABA responses at pH 8.4 was abolished by all three lysine mutations and only for the α1β2K279Rγ2 receptor was the GABA response inhibited in a similar manner to that observed with the K279A mutant (Fig. 5B). In addition, as for the α1β2K279Aγ2 receptor, all the other mutants were spontaneously active with levels of 14 ± 2% (K279Q), 13 ± 4% (K279F) and 34 ± 4% (K279R). Taken overall, these findings suggest that the β subunit K279 was critically important for the potentiation of the current activated by low GABA concentrations at both αβ and αβγ subunit-containing receptors in alkaline pH, as well as a potentially important residue in ion channel gating.
Modulation of α1β2γ2 GABAA receptors by acidic pH at low GABA concentrations
Previously, α1β2γ2 GABAA receptors were considered to be either insensitive to, or inhibited by, acidic pH (Krishek et al. 1996; Huang & Dillon, 1999), contrasting with α1β2 and α1β3 receptors, whose function was potentiated (Krishek et al. 1996; Wilkins et al. 2002; Feng & MacDonald, 2004). This differentiation was attributed to the disruption, by the γ subunit, of either the protonation site, or a transduction mechanism that operates following protonation (Krishek et al. 1996). Given that the pH sensitivity of the α1β2 receptor clearly changed at low GABA concentrations (Fig. 2A), it was conceivable that the α1β2γ2 receptor might behave similarly. Indeed, for α1β2γ2 receptors, 1 μm GABA currents were potentiated at pH 6.4, but at pH 5.4, inhibition was observed when compared to control currents at pH 7.4 (Fig. 6A). The potentiation, at pH 6.4, was comparable to that observed with the α1β2 receptor; moreover, the β2H267A mutation was also just as effective at abolishing the potentiation (Fig. 6B). However, in contrast to the α1β2 receptor, the enhanced GABA response at pH 6.4 for the α1β2γ2 receptor was only observed at low GABA concentrations (1 μm; Fig. 6A). These data demonstrate the importance of βH267 in the modulation of αβγ GABAA receptors by protons, but this is only operative over a defined GABA concentration range.
Figure 6. Role of βH267 in the acidic pH modulation of the GABAA receptor.
A, bar graph representing the pH titration profile of the α1β2γ2 GABAA receptor over the pH range 5.4–6.9, determined for 1 μm (open bars) and 5 μm (grey bars) GABA-activated responses compared to their control responses at pH 7.4 (n = 13). B, bar graph for the modulation of 1 μm GABA-activated responses determined for α1β2, α1β2H267A, α1β2γ2 and α1β2H267Aγ2 GABAA receptors at pH 6.4 (n = 5–10). *P < 0.05 compared with the control responses determined at pH 7.4 (= 100%).
Role of lysine 279 in the regulation by acidic pH
At pH 5.4, the pH sensitivities for α1β2 and α1β2γ2 receptors diverged, with 1 μm GABA currents mediated via the latter now clearly inhibited (Figs 6A and 7A). The β2H267A mutation increased the inhibition of the GABA-activated current at pH 5.4 for the α1β2H267Aγ2 receptor to a level similar to that observed with the α1β2H267A. receptor (Fig. 7A). This complex pH profile of the α1β2γ2 receptor may result from the replacement of a β subunit by a γ subunit in the receptor complex, decreasing the number of H267 protonation sites in the receptor or affecting the transduction mechanism involved at pH 5.4. To examine the first possibility, a histidine residue (I282H) was introduced into the γ2 subunit at the homologous position to H267 in the β2 subunit restoring (as for α1β2) three histidines around the entry to the ion channel, presuming a receptor stoichiometry of 2α: 2β: 1γ (Farrar et al. 1999). A further discrepancy in the charged residue complement within the TM2-TM3 linker was also corrected, by replacing γ2K285 with the homologous β2E270 With these mutations, it was conceivable that the α1β2γ2I282H,K285E receptor might now support a potentiation of the GABA-activated current at pH 5.4. The GABA concentration–response curve for the α1β2γ2I282H,K285E receptor was not different to the wild-type receptor (GABA EC50: α1β2γ2, 3.4 ± 0.3 μm; α1β2γ2I282H,K285E, 3.3 ± 0.3 μm; n = 7; Fig. 7B). However, the double mutation did not allow any potentiation of the EC20 GABA-activated current at pH 5.4, revealing just inhibition to a similar extent to that observed with the wild-type α1β2γ2 receptor (Fig. 7A and C). These data suggested that the provision of three histidine residues around the ion channel in the α1β2γ2 receptor is insufficient for proton-induced potentiation of the GABA-activated current at pH 5.4.
Figure 7. Modulation of the GABAA receptor at pH 5.4 is dependent on K279 in the β2 subunit.
A, bar graph for the modulation of EC20 GABA-activated currents at pH 5.4 for wild-type α1β2 and α1β2γ2 (open bars) and mutant αβγ GABAA receptors, incorporating: β2H267A (black bars), γ2I282H,K285E (HE), γ2I282H,K285E,T294K (HEK), γ2T294K (K) and β2K279Aγ2T294K (AK), all grey bars. *P < 0.05 compared with the respective control responses determined at pH 7.4 (= 100%; n = 5–12). B, normalized GABA concentration–response curves for α1β2γ2I282H,K285E, α1β2γ2I282H,K285E,T294K, α1β2γ2T294K and α1β2K279Aγ2T294K GABAA receptors (n = 4–10). The dashed line represents the curve for the wild-type α1β2γ2 receptor at pH 7.4 (from Fig. 1B). C, GABA-activated currents induced by EC20 GABA applied at the indicated external pH for the α1β2γ2 and mutant αβγ GABAA receptors. See A, for key. Calibration bar represents: 200pA, 1 s.
The second possibility, regarding disruption to the transduction pathway by the γ2 subunit, was addressed by identifying residues that may mediate the potentiation of the GABA current at acid pH. Lysine 279 in the β2 subunit was selected because of its importance for alkaline pH modulation. Indeed, its mutation in α1β2K279A receptors, resulted in much smaller potentiations of 1 or 10 μm GABA-activated currents at pH 5.4 compared to the wild-type receptor (α1β2, potentiation of 94 ± 17% (1 μm GABA) and 91 ± 10% (10 μm), n = 25; α1β2K279A, 15 ± 7% (1 μm) and 41 ± 11% (10 μm), n = 7). These data are in accord with K279 playing a critical role in the potentiation of the GABA-activated current for the α1β2 receptor at pH 5.4 as well as at pH 8.4. As the K279 is normally only present in the β subunit, we introduced a lysine into the γ2 subunit at the homologous position (T294K), together with the previously introduced residues, I282H and K285E, as found in the α1β2 receptor. The GABA concentration–response relationship for the α1β2γ2I282H,K285E,T294K receptor at pH 7.4 was marginally displaced (∼2-fold) with a reduced EC50 (1.7 ± 0.2 μm; n = 10) compared with the wild-type receptor (3.4 ± 0.3 μm; P < 0.05; Fig. 7B). Of importance, the 1 μm (EC20) GABA-activated current at pH 5.4 was now potentiated, contrasting with the inhibition normally observed with the wild-type α1β2γ2 receptor (Fig. 7A and C).
Since the double mutant, α1β2γ2I282H,K285E receptor failed to support any potentiation at acidic pH, we considered that T294K alone might be sufficient to enable potentiation at pH 5.4. The GABA concentration–response curve revealed that the mutant α1β2γ2T294K receptor was similarly sensitive to GABA (GABA EC50 4.2 ± 0.4 μm; n = 7; P > 0.05) when compared to the wild-type receptor (Fig. 7B). Moreover, on exposure to pH 5.4, the 1 μm GABA-activated response was significantly potentiated (53 ± 8%) compared with the control at pH 7.4, approaching the level of potentiation expected for a wild-type α1β2 receptor (103 ± 15%; Fig. 7A and C).
To evaluate if the lysine incorporated into the γ2 subunit was solely responsible for the modulation of the EC20 GABA-activated current in acidic pH, a double mutant α1β2K279Aγ2T294K was examined. As for the α1β2K279A receptor, GABA potency was increased for the α1β2K279Aγ2T294K receptor compared to the wild-type receptor at pH 7.4 (EC50 0.9 ± 0.1 μm; n = 5; P < 0.05; Fig. 7B). In addition, by using 100 μm PTX, this receptor was spontaneous active at a level of 11 ± 1.2% (n = 4); however, at pH 5.4, the EC20 (0.1 μm) GABA-activated response was still inhibited, thus appearing no different from the wild-type and α1β2γ2I282H,K285E receptors (Fig. 7A and C). These data suggest that three copies of the lysine, each one at position 279 in the β subunits or the homologous position in the γ subunit, are required for modulation of the GABAA receptor at pH 5.4, and that inclusion of a lysine only in the γ subunit is in sufficient.
Discussion
This study reveals that the regulation of GABAA receptor function can change over quite small excursions in external pH. It establishes that the concentration of GABA activating the receptor is critical for pH regulation and also identifies a vital lysine residue, located in the TM2–TM3 linker in the β subunit, which is critical for the potentiation of GABA-activated currents at alkaline pH. Furthermore, for the potentiation observed at acidic pH, lysine residues must be present, not only in the GABA binding β subunits but also in the fifth subunit of the pentamer, be it a β or γ subunit, which is the only subunit not directly involved in GABA binding. These results are entirely in accord with K279 playing a vital role in the pH modulation of GABAA receptors as well as being an important residue in GABAA receptor activation.
GABA concentration dependence of pH regulation
The regulation by external pH of GABA-activated currents is dependent on the receptor subunit composition, but even so, it is clear that the pH sensitivities of neuronal GABAA receptors do not always correlate with the behaviour expected from recombinant receptor studies, particularly at alkaline pH (Robello et al. 1994; Krishek et al. 1996; Pasternack et al. 1996; Zhai et al. 1998; Huang & Dillon, 1999; Wilkins et al. 2002). Another factor that may affect alkaline pH regulation is the GABA concentration. In acutely isolated hippocampal neurones, using high GABA concentrations (500 μm), alkaline pH reduced the GABA current amplitude, whereas for low GABA concentrations (5 μm) pH 8.4 increased the current amplitude (Pasternack et al. 1996). In contrast, for spinal neurones, pH dependent modulation was abolished at high GABA concentrations (300 μm), whilst at lower concentrations (10 μm), alkaline pH increased the GABA current amplitude (Li et al. 2003). Using fast perfusion techniques, the potentiation of responses to low GABA concentrations in alkaline pH was thought to reflect increases in the agonist binding rate thus promoting channel opening (Mozrzymas et al. 2003), whilst at higher GABA concentrations, the reduced current amplitude was thought to be due to rapid desensitization. These findings are broadly in agreement with the single channel analyses of cerebellar granule cell GABAA receptors (< 7 DIV) (Krishek & Smart, 2001) and recombinant α3β2γ2 subunit receptors (Huang & Dillon, 1999). In these studies, alkaline pH excursions increased channel open probabilities, without any change in the open time distributions, but reduced the long shut times causing the channel opening frequencies to increase. In the present study, alkaline pH induced a very small leftward shift in the lower part (< EC50) of the GABA concentration–response curves for α1β2 and α1β2γ2 GABAA receptors. This shift was more clearly resolved by pH titration using low GABA concentrations. Under these conditions, both α1β2 and the α1β2γ2 GABAA receptors were affected by pH 8.4 in a similar manner possibly via the same specific molecular mechanism.
Molecular mechanism underlying alkaline pH modulation
The nature of the residues that are involved in the alkaline pH regulation and where they are located has remained elusive. Following a pH titration of the α1β2 wild-type receptor, several residues were identified as prospective candidates. However, in the β subunit, H267, initially considered due to its regulatory role at acidic pH (Wilkins et al. 2002), and Y157 and Y205, which are located in the GABA binding site (Amin & Weiss, 1993; Cromer et al. 2002), played no role in the modulation of the GABAA receptor at pH 8.4. However, the modulation of GABAA receptors in alkaline pH has been reported to involve the GABA binding site with Y205 and F64 in the β2 subunit being involved (Huang et al. 2004). Whilst in our study, Y205 appears to play no role in the alkaline pH modulation, and the excessive decrease in GABA potency for the F64 mutant makes it very difficult to assess its impact in this context. In addition, the observation that αβ GABAA receptors are inhibited in acidic pH (Huang et al. 2004) is also at variance with other groups that have reported potentiation based on the protonation of H267 (Wilkins et al. 2002; Feng & MacDonald, 2004). Furthermore, the use of DEPC, on active or quiescent GABAA receptors, suggested that all other accessible histidines and tyrosines were most likely not involved in pH regulation, leaving only lysine residues as possible candidates.
The β subunit lysines selected for mutation, K274 and K279, form part of the TM2–TM3 linker and lie close to H267 in the ion channel. Lysine 274 is conserved between all of the GABAA receptor subunits and may play an important role in GABAA receptor gating (Sigel et al. 1999; Kash et al. 2003; Kash et al. 2004). Notably, the GABA concentration–response curve for α1β2K274A was laterally displaced to higher GABA concentrations, as reported for the α1β2K274Aγ2 receptor (Sigel et al. 1999); however, this same mutation accentuated the potentiation of the GABA current by alkaline pH. The alternative lysine, K279, is only present in β subunits and its substitution in either α1β2K279A or α1β2K279Aγ2 receptors ablated the alkaline pH potentiation of the GABA-activated current, revealing inhibition.
The GABA concentration dependence of the alkaline pH modulation could conceivably be induced by the deprotonation of K279, with this reaction being less likely at high GABA concentrations, perhaps because of restricted access of protons to K279. However, the pKa of the lysine side chain is ∼10 and would therefore be unlikely to act as a proton acceptor at physiological pH or indeed at pH 8.4, as we would expect little change in its ionization state (99.7% or 97.5% positively charged, respectively). For lysine to act as a proton acceptor, the pKa would have to shift into the neutral pH range. Such a perturbation (from 10 to 7) can occur but depends upon the close proximity of other basic residues (Schulte et al. 1999). If such a pKa shift occurred in the GABAA receptor, then, increasing the pH to 8.4 would cause a much greater loss of H+ from the amine group of the lysine, leaving 3.8% of the side-chain positively charged compared with 28.5% at pH 7.4. Interestingly, by altering the charge and size of the side chain at position 279, alkaline pH modulation was prevented, suggesting that the structure of the lysine side chain was critical for potentiation at alkaline pH.
Location of K279 using molecular modelling
An emerging consensus of Cys-loop receptor gating suggests that TM1, 3 and 4 form a rigid structure from which the channel lining the TM2 domain is suspended. The TM1–TM2 and TM2–TM3 linkers act as hinges to allow rotation of TM2 during channel opening, with the TM2–TM3 linker also contacting the ligand-binding extracellular domain (Absalom et al. 2004). The importance of the TM2–TM3 linker for receptor activation is demonstrated by mutating the conserved lysines, K279 (α subunit) and K274 (β subunit). Both residues are thought to interact with acidic residues in loops 2 and 7 of the extracellular domains (AChBP nomenclature; Kash et al. 2003; Kash et al. 2004). Homology modelling, using the electron microscopic images of nicotinic acetylcholine receptors (Miyazawa et al. 2003), suggests that K279 in the TM2–TM3 linker is ideally situated to either interact with the N-terminal domains, or influence the conformational changes that follow agonist binding (Fig. 8). Whilst the mutation of βK279 to aspartate (Kash et al. 2004) did not affect coupling with the N-terminal domain, this residue may participate in channel gating since both α1β2K279A and α1β2K279Aγ2 receptors are spontaneously active. Although alkaline pH modulation of GABAA receptors requires a lysine residue at βK279 it is unaffected by the γ subunit and thus apparently oblivious to the homologous residue in the fifth subunit in the pentamer. By contrast, lysine residues are required in both the β and γ subunits (as well as H267 in the β subunit) for modulation by acid pH (see below). Whether pH changes can alter the protonation of K279 cannot be determined at present, but it is conceivable that the differential structural dependence of these pH effects may be caused by the receptor adopting different open conformations in acid compared to alkaline pH.
Figure 8. Molecular model of the GABAA receptor β2 and γ2 subunits.
A, molecular model showing the β2 subunit (orange) and the γ2 subunit (green) for the GABAA receptor. Both TM2, which lines the receptor channel, and the TM2–TM3 linker, are shown in yellow, with the residues involved in pH modulation space filled. B, magnified view of the area involved in pH modulation. In the β2 subunit; K279 (red), H267 (light yellow) are thought to be either directly involved in protonation, or form part of an allosteric pH modulatory mechanism; whilst K274 (pink) and E270 (dark yellow) are probably not involved. In the γ2 subunit, residues in the homologous positions to those in the β subunit are also shown.
Protonation and the α1β2γ2 receptor
The potentiation of the GABA-activated current, at pH 6.4, for low GABA concentrations on α1β2γ2 receptors, was similar to that observed with the α1β2 receptor but at pH 5.4, the potentiation was absent. The introduction of β subunit residues, H267 and E270, into homologous positions in the γ2 subunit for α1β2γ2 receptors failed to potentiate the GABA-activated current at pH 5.4. These data implied that an allosteric mechanism may be required to augment the response at pH 5.4 and this was seemingly achieved by the mutation γ2T294K. Overall, three lysines were required for potentiation of the GABA-activated current at pH 5.4 on α1β2γ2 receptors (one in each β subunit and one in the mutated γ2 subunit). Interestingly, GABA currents of GABAA receptors containing the δ subunit are also potentiated by acidic pH (Feng & MacDonald, 2004; Krishek et al. 1996). These δ subunits also have a lysine residue (K292) (Shivers et al. 1989) in the homologous position to K279 in the β subunit. The pivotal role that this lysine residue plays, not only in the alkaline pH modulation but also at acidic pH, emphasizes that it is unlikely to be a target for protonation per se, but most probably participates as a key component in allosteric modulation. Clearly, the TM2–TM3 linker, is not just involved in the gating of the GABAA receptor, but is a vital component in acidic and alkaline pH-induced modulation.
Physiological relevance
The importance of the pH regulation of GABAA receptor function, as described in this study, may become physiologically relevant under conditions where low GABA concentrations occur, for example around extrasynaptic receptors where basal GABA concentrations are estimated in the low micromolar range (hippocampus, 0.3–1 μm, M. Mortensen & T. G. Smart, unpublished observations; 0.8–3 μm, Lerma et al. 1986; 0.1 μm, Petrini et al. 2004). Under these conditions, minor external pH excursions of only 0.2 pH units would be sufficient to affect GABAA receptor function causing subtle effects to the level of tonic inhibition and neuronal excitability which could become more apparent during pathological events. Furthermore, studies investigating the changes in extracellular pH during ischaemia, hypothermia and brain trauma have shown that the extracellular pH of the brain can alter by approximately 1 pH unit which will be expected to impact on the function of GABAA receptors (Kraig et al. 1987; Hoffman et al. 1996; Hoffman et al. 1999; Anderson & Meyer, 2002).
References
- Absalom NL, Lewis TM, Schofield PR. Mechanisms of channel gating of the ligand-gated ion channel superfamily inferred from protein structure. Exp Physiol. 2004;89:145–153. doi: 10.1113/expphysiol.2003.026815. [DOI] [PubMed] [Google Scholar]
- Amin J, Dickerson IM, Weiss DS. The agonist binding site of the γ-aminobutyric acid type A channel is not formed by the extracellular cysteine loop. Mol Pharmacol. 1994;45:317–323. [PubMed] [Google Scholar]
- Amin J, Weiss DS. GABAA receptor needs two homologous domains of the β-subunit for activation by GABA but not by pentobarbital. Nature. 1993;366:565–569. doi: 10.1038/366565a0. [DOI] [PubMed] [Google Scholar]
- Anderson RE, Meyer FB. Protection of focal cerebral ischemia by alkalinization of systemic pH. Neurosurgery. 2002;51:1256–1265. doi: 10.1097/00006123-200211000-00022. [DOI] [PubMed] [Google Scholar]
- Brejc K, van Dijk WJ, Klaassen RV, Schuurmans M, van Der OJ, Smit AB, Sixma TK. Crystal structure of an ACh-binding protein reveals the ligand-binding domain of nicotinic receptors. Nature. 2001;411:269–276. doi: 10.1038/35077011. [DOI] [PubMed] [Google Scholar]
- Brickley SG, Cull-Candy SG, Farrant M. Development of a tonic form of synaptic inhibition in rat cerebellar granule cells resulting from persistent activation of GABAA receptors. J Physiol. 1996;497:753–759. doi: 10.1113/jphysiol.1996.sp021806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickley SG, Cull-Candy SG, Farrant M. Single-channel properties of synaptic and extrasynaptic GABAA receptors suggest differential targeting of receptor subtypes. J Neurosci. 1999;19:2960–2973. doi: 10.1523/JNEUROSCI.19-08-02960.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickley SG, Farrant M, Cull-Candy SG. Apparent heterogeneity of extrasynaptic GABA receptors in granule cells of the rat cerebellum. J Physiol. 1995;487.P:52–53. [Google Scholar]
- Brickley SG, Farrant M, Swanson GT, Cull-Candy SG. CNQX increases GABA-mediated synaptic transmission in the cerebellum by an AMPA/kainate receptor-independent mechanism. Neuropharmacology. 2001;41:730–736. doi: 10.1016/s0028-3908(01)00135-6. [DOI] [PubMed] [Google Scholar]
- Chen JC, Chesler M. Extracellular alkalinization evoked by GABA and its relationship to activity-dependent pH shifts in turtle cerebellum. J Physiol. 1991;442:431–446. doi: 10.1113/jphysiol.1991.sp018801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JC, Chesler M. Modulation of extracellular pH by glutamate and GABA in rat hippocampal slices. J Neurophysiol. 1992;67:29–36. doi: 10.1152/jn.1992.67.1.29. [DOI] [PubMed] [Google Scholar]
- Cromer BA, Morton CJ, Parker MW. Anxiety over GABAA receptor structure relieved by AChBP. Trends Biochem Sci. 2002;27:280–287. doi: 10.1016/s0968-0004(02)02092-3. [DOI] [PubMed] [Google Scholar]
- Dunne EL, Hosie AM, Wooltorton JR, Duguid IC, Harvey K, Moss SJ, Harvey RJ, Smart TG. An N-terminal histidine regulates Zn2+ inhibition on the murine GABAA receptor β3 subunit. Br J Pharmacol. 2002;137:29–38. doi: 10.1038/sj.bjp.0704835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Brauchart D, Boresch S, Sieghart W. Comparative modeling of GABAA receptors: limits, insights, future developments. Neuroscience. 2003;119:933–943. doi: 10.1016/s0306-4522(03)00288-4. [DOI] [PubMed] [Google Scholar]
- Farrar SJ, Whiting PJ, Bonnert TP, McKernan RM. Stoichiometry of a ligand-gated ion channel determined by fluorescence energy transfer. J Biol Chem. 1999;274:10100–10104. doi: 10.1074/jbc.274.15.10100. [DOI] [PubMed] [Google Scholar]
- Feng HJ, MacDonald RL. Proton modulation of α1β3δ GABAA receptor channel gating and desensitization. J Neurophysiol. 2004;92:1577–1585. doi: 10.1152/jn.00285.2004. [DOI] [PubMed] [Google Scholar]
- Guex N, Peitsch MC. SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis. 1997;18:2714–2723. doi: 10.1002/elps.1150181505. [DOI] [PubMed] [Google Scholar]
- Hoffman WE, Charbel FT, Edelman G, Ausman JI. Brain tissue oxygen pressure, carbon dioxide pressure, and pH during hypothermic circulatory arrest. Surg Neurol. 1996;46:75–79. doi: 10.1016/0090-3019(96)00049-3. [DOI] [PubMed] [Google Scholar]
- Hoffman WE, Charbel FT, Gonzalez-Portillo G, Ausman JI. Measurement of ischemia by changes in tissue oxygen, carbon dioxide, and pH. Surg Neurol. 1999;51:654–658. doi: 10.1016/s0090-3019(99)00011-7. [DOI] [PubMed] [Google Scholar]
- Horenstein J, Wagner DA, Czajkowski C, Akabas MH. Protein mobility and GABA-induced conformational changes in GABAA receptor pore-lining M2 segment. Nat Neurosci. 2001;4:477–485. doi: 10.1038/87425. [DOI] [PubMed] [Google Scholar]
- Huang RQ, Chen Z, Dillon GH. Molecular basis for modulation of recombinant α1β2γ2 GABAA receptors by protons. J Neurophysiol. 2004;92:883–894. doi: 10.1152/jn.01040.2003. [DOI] [PubMed] [Google Scholar]
- Huang RQ, Dillon GH. Effect of extracellular pH on GABA-activated current in rat recombinant receptors and thin hypothalamic slices. J Neurophysiol. 1999;82:1233–1243. doi: 10.1152/jn.1999.82.3.1233. [DOI] [PubMed] [Google Scholar]
- Kaila K. Ionic basis of GABAA receptor channel function in the nervous system. Prog Neurobiol. 1994;42:489–537. doi: 10.1016/0301-0082(94)90049-3. [DOI] [PubMed] [Google Scholar]
- Kaila K, Paalasmaa P, Taira T, Voipio J. pH transients due to monosynaptic activation of GABAA receptors in rat hippocampal slices. Neuroreport. 1992;3:105–108. doi: 10.1097/00001756-199201000-00028. [DOI] [PubMed] [Google Scholar]
- Kash TL, Dizon MJ, Trudell JR, Harrison NL. Charged residues in the β2 subunit involved in GABAA receptor activation. J Biol Chem. 2004;279:4887–4893. doi: 10.1074/jbc.M311441200. [DOI] [PubMed] [Google Scholar]
- Kash TL, Jenkins A, Kelley JC, Trudell JR, Harrison NL. Coupling of agonist binding to channel gating in the GABAA receptor. Nature. 2003;421:272–275. doi: 10.1038/nature01280. [DOI] [PubMed] [Google Scholar]
- Korpi ER, Grunder G, Luddens H. Drug interactions at GABAA receptors. Prog Neurobiol. 2002;67:113–159. doi: 10.1016/s0301-0082(02)00013-8. [DOI] [PubMed] [Google Scholar]
- Kraig RP, Petito CK, Plum F, Pulsinelli WA. Hydrogen ions kill brain at concentrations reached in ischemia. J Cereb Blood Flow Metab. 1987;7:379–386. doi: 10.1038/jcbfm.1987.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishek BJ, Amato A, Connolly CN, Moss SJ, Smart TG. Proton sensitivity of the GABAA receptor is associated with the receptor subunit composition. J Physiol. 1996;492:431–443. doi: 10.1113/jphysiol.1996.sp021319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishek BJ, Smart TG. Proton sensitivity of rat cerebellar granule cell GABAA receptors: dependence on neuronal development. J Physiol. 2001;530:219–233. doi: 10.1111/j.1469-7793.2001.0219l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerma J, Herranz AS, Herreras O, Abraira V, Martin DR. In vivo determination of extracellular concentration of amino acids in the rat hippocampus. A method based on brain dialysis and computerized analysis. Brain Res. 1986;384:145–155. doi: 10.1016/0006-8993(86)91230-8. [DOI] [PubMed] [Google Scholar]
- Li YF, Wu LJ, Li Y, Xu L, Xu TL. Mechanisms of H+ modulation of glycinergic response in rat sacral dorsal commissural neurons. J Physiol. 2003;552:73–87. doi: 10.1113/jphysiol.2003.047324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L. Effect of substituting amino acids in extracellular disulfide loop on GABA ρ1 subunit function. Brain Res. 1997;756:1–8. doi: 10.1016/s0006-8993(96)01417-5. [DOI] [PubMed] [Google Scholar]
- McKernan RM, Whiting PJ. Which GABAA -receptor subtypes really occur in the brain? Trends Neurosci. 1996;19:139–143. doi: 10.1016/s0166-2236(96)80023-3. [DOI] [PubMed] [Google Scholar]
- Miles EW. Modification of histidyl residues in proteins by dietheylpyrocarbonate. Meth Enzymol. 1977;47:431–443. doi: 10.1016/0076-6879(77)47043-5. [DOI] [PubMed] [Google Scholar]
- Miyazawa A, Fujiyoshi Y, Unwin N. Structure and gating mechanism of the acetylcholine receptor pore. Nature. 2003;424:949–955. doi: 10.1038/nature01748. [DOI] [PubMed] [Google Scholar]
- Mody I. Distinguishing between GABAA receptors responsible for tonic and phasic conductances. Neurochem Res. 2001;26:907–913. doi: 10.1023/a:1012376215967. [DOI] [PubMed] [Google Scholar]
- Moss SJ, Smart TG. Constructing inhibitory synapses. Nat Rev Neurosci. 2001;2:240–250. doi: 10.1038/35067500. [DOI] [PubMed] [Google Scholar]
- Mozrzymas JW, Zarnowska ED, Pytel M, Mercik K. Modulation of GABAA receptors by hydrogen ions reveals synaptic GABA transient and a crucial role of the desensitization process. J Neurosci. 2003;23:7981–7992. doi: 10.1523/JNEUROSCI.23-22-07981.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternack M, Smirnov S, Kaila K. Proton modulation of functionally distinct GABAA receptors in acutely isolated pyramidal neurons of rat hippocampus. Neuropharmacology. 1996;35:1279–1288. doi: 10.1016/s0028-3908(96)00075-5. [DOI] [PubMed] [Google Scholar]
- Petrini EM, Marchionni I, Zacchi P, Sieghart W, Cherubini E. Clustering of extrasynaptic GABAA receptors modulates tonic inhibition in cultured hippocampal neurons. J Biol Chem. 2004;279:45833–45843. doi: 10.1074/jbc.M407229200. [DOI] [PubMed] [Google Scholar]
- Rabow LE, Russek SJ, Farb DH. From ion currents to genomic analysis: Recent advances in GABAA receptor research. Synapse. 1996;21:189–274. doi: 10.1002/syn.890210302. [DOI] [PubMed] [Google Scholar]
- Robello M, Baldelli P, Cupello A. Modulation by extracellular pH of the activity of GABAA receptors on rat cerebellum granule cells. Neuroscience. 1994;61:833–837. doi: 10.1016/0306-4522(94)90406-5. [DOI] [PubMed] [Google Scholar]
- Schulte U, Hahn H, Konrad M, Jeck N, Derst C, Wild K, Weidemann S, Ruppersberg JP, Fakler B, Ludwig J. pH gating of ROMK (Kir1.1) channels: control by an Arg-Lys-Arg triad disrupted in antenatal Bartter syndrome. Proc Natl Acad Sci U S A. 1999;96:15298–15303. doi: 10.1073/pnas.96.26.15298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivers BD, Killisch I, Sprengel R, Sontheimer H, Kohler M, Schofield PR, Seeburg PH. Two novel GABAA receptor subunits exist in distinct neuronal subpopulations. Neuron. 1989;3:327–337. doi: 10.1016/0896-6273(89)90257-2. [DOI] [PubMed] [Google Scholar]
- Sieghart W. Structure and pharmacology of γ-aminobutyric acidA receptor subtypes. Pharmacol Rev. 1995;47:181–234. [PubMed] [Google Scholar]
- Sigel E, Baur R, Malherbe P, Mohler H. The rat β1-subunit of the GABAA receptor forms a picrotoxin-sensitive anion channel open in the absence of GABA. FEBS Lett. 1989;257:377–379. doi: 10.1016/0014-5793(89)81576-5. [DOI] [PubMed] [Google Scholar]
- Sigel E, Buhr A, Baur R. Role of the conserved lysine residue in the middle of the predicted extracellular loop between M2 and M3 in the GABAA receptor. J Neurochem. 1999;73:1758–1764. doi: 10.1046/j.1471-4159.1999.731758.x. [DOI] [PubMed] [Google Scholar]
- Smart TG. A novel modulatory binding site for zinc on the GABAA receptor complex in cultured rat neurones. J Physiol. 1992;447:587–625. doi: 10.1113/jphysiol.1992.sp019020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart TG, Xie X, Krishek BJ. Modulation of inhibitory and excitatory amino acid receptor ion channels by zinc. Prog Neurobiol. 1994;42:393–341. doi: 10.1016/0301-0082(94)90082-5. [DOI] [PubMed] [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucl Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins ME, Hosie AM, Smart TG. Identification of a β subunit TM2 residue mediating proton modulation of GABA type A receptors. J Neurosci. 2002;22:5328–5333. doi: 10.1523/JNEUROSCI.22-13-05328.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooltorton JR, Moss SJ, Smart TG. Pharmacological and physiological characterization of murine homomeric β3 GABAA receptors. Eur J Neurosci. 1997;9:2225–2235. doi: 10.1111/j.1460-9568.1997.tb01641.x. [DOI] [PubMed] [Google Scholar]
- Zhai J, Peoples RW, Li C. Proton inhibition of GABA-activated current in rat primary sensory neurons. Pflugers Arch. 1998;435:539–545. doi: 10.1007/s004240050550. [DOI] [PubMed] [Google Scholar]