Abstract
Penile erection is dependent on the nitric oxide (NO)/cGMP-dependent protein kinase I (PKGI) pathway. One important target of PKGI in smooth muscle is the large-conductance, calcium-activated potassium (BK) channel, which upon activation hyperpolarizes the smooth muscle cell membrane, causing relaxation. Relaxation of arterial and corpus cavernosum smooth muscle (CCSM) is necessary to increase blood flow into the corpora cavernosa that leads to penile tumescence. We investigated the functional role of BK channels in the corpus cavernosum utilizing a knock-out mouse lacking the Slo gene (Slo−/−) responsible for the pore-forming subunit of the BK channel. Whole-cell currents were recorded from isolated CCSM cells of Slo+/+ and Slo−/− mice. Iberiotoxin-sensitive voltage- and [Ca2+]-activated K+ currents, the latter activated by local transient calcium releases (calcium sparks), were present in Slo+/+ CCSM cells, but absent in Slo−/− cells. CCSM strips from Slo−/− mice demonstrated a four-fold increase in phasic contractions, in the presence of phenylephrine. Nerve-evoked relaxations of precontracted strips were reduced by 50%, both in strips from Slo−/− mice and by blocking BK channels with iberiotoxin in the Slo+/+ strips. Consistent with the in vitro results, in vivo intracavernous pressure exhibited pronounced oscillations in Slo−/− mice, but not in Slo+/+ mice. Furthermore, intracavernous pressure increases to nerve stimulation, in vivo, were reduced by 22% in Slo−/−mice. These results indicate that the BK channel has an important role in erectile function, and loss of the BK channel leads to erectile dysfunction.
Penile erection is a haemodynamic event that occurs in response to the release of NO from both parasympathetic nonadrenergic-noncholinergic nerves and from the vascular endothelium (Holmquist et al. 1991; Andersson & Wagner, 1995; Hedlund et al. 1999; Mizusawa et al. 2001). During erection, the CCSM relaxes, mainly through parasympathetic stimulation, and an increase in blood inflow induces a rapid increase in intracavernous pressure (ICP). After ejaculation or cessation of stimuli, parasympathetic dominance decreases, and sympathetic tonic discharge causes contraction of the smooth muscle in the arterioles and around sinusoids leading to reduced arterial flow, reopening of venous channels and a drop in ICP.
The majority of NO-relaxing effects are mediated through cGMP (Holmquist et al. 1991; Burnett et al. 1992; Schmidt et al. 1993; Andersson & Wagner, 1995). cGMP acts as a modifying agent on ion channels, phosphodiesterases, and protein kinases. Precontracted CCSM strips from mice lacking cGMP-dependent protein kinase I (PKGI) do not relax during nerve stimulation (Hedlund et al. 2000), supporting the central role of PKGI in the corpus cavernosum (CC). PKGI phosphorylates numerous proteins, including ion channels and pumps known to reduce intracellular calcium concentration [Ca2+]i (Lincoln & Cornwell, 1993). It has been shown, in particular, that PKGI activates BK channels (Robertson et al. 1993; Alioua et al. 1998) which hyperpolarize smooth muscle cell membranes, and thus oppose muscle contraction.
Blocking the BK channel with tetraethylammonium ions (TEA) and charybdotoxin led to an increase in phenylephrine-induced contractions of CCSM strips in vitro, whereas the BK-channel opener NS1619 reduced these contractions (Spektor et al. 2002). In aged or diabetic rats, intracavernous injection of cDNA encoding the human BK channel led to a reversal of erectile dysfunction (Melman et al. 2003; Christ et al. 2004). These studies support the idea that elevating BK-channel expression can restore erectile function following age- or disease-induced decline. However, the effect of a lack of BK channel activity on erectile function is not known, and the role of BK channels in nerve-induced CC relaxation is also not known. To examine these issues from in vitro cellular and tissue to in vivo levels we used a mouse model with targeted deletion of the pore-forming α-subunit (Slo) of the BK channel (Meredith et al. 2004; Thorneloe et al. 2005).
In our previous report (Meredith et al. 2004), we noticed that only 5% of the male mice lacking the BK channel were able to sire a litter of pups. We hypothesize that this could be a consequence of impaired erectile function due to an increased contractility of the CCSM. The aim of the current study is to elucidate the role of the BK channel in erectile function by performing in vitro contraction experiments and in vivo studies using cavernous nerve electrostimulation and intracavernous pressure recording on BK channel knock-out (Slo−/−) mice.
Methods
Tissue preparation
Slo−/− mice were generated as previously described (Meredith et al. 2004). All the procedures performed in the course of this study were approved by the Office of Animal Care Management at the University of Vermont. Adult male mice (10–20 weeks old; ∼30 g bodyweight) were killed with intraperitoneal injection of sodium pentobarbital (150 mg kg−1) followed by thoracotomy. For in vitro studies and immunohistochemistry, the penis was removed and immediately placed in ice-cold dissection solution (DS; (mm): 80 monosodium glutamate, 55 NaCl, 6 KCl, 10 glucose, 10 N-2-hydroxyethylpiperazine-N-2-ethanesulphonic acid (Hepes), 2 MgCl2, pH 7.3 adjusted with NaOH).
Immunohistochemistry
Whole penises from Slo+/+ and Slo−/− mice were immersion-fixed with ice-cold 4% formaldehyde in phosphate buffered saline (PBS; pH 7.4) at 4°C for 1 h followed by rinsing in PBS and freezing in Tissue-Tek OCT compound (Sakura Finetek Inc., Torrance, CA, USA). Cryosections of 10 µm thickness were cut, mounted on glass slides and air dried. For immunohistochemistry, the sections were incubated with anti-BK antibody (1: 1000; Alomone Laboratories Ltd, Jerusalem, Israel) followed by application of the Vectastain ABC Elite Kit (Vector Laboratories, Burlingame, CA, USA) and staining with the DAB Enhanced Liquid Substrate System (Sigma). Data analysis was performed with an Olympus BX50 light microscope equipped with an Optronics Magna Fire digital camera.
Electrophysiology
CCSM cells from Slo+/+ and Slo−/− mice were isolated enzymatically for perforated whole-cell patch clamp recordings at 22°C as previously described (Horn & Marty, 1988; Herrera & Nelson, 2002). The CC was cut into 10–30 pieces and placed in fresh ice-cold DS. Next, the tissue was placed in 2 ml fresh DS (37°C) containing 1 mg ml−1 papain (Worthington Biochemical Corporation, Freehold, NJ, USA) for 25 min. After washing with ice-cold DS, the CC pieces were further incubated with 2 ml DS (37°C) containing 1 mg ml−1 each of collagenase type II and type H, and 100 µm CaCl2 for 20 min. Following enzyme treatment, the tissue was washed repeatedly with fresh ice-cold DS and then stored in this solution on ice. Individual cells were freed from the tissue by passing the tissue pieces through the tip of a fire-polished Pasteur pipette. The external solution for the patch clamp recordings contained the following (mm): 134 NaCl, 6 KCl, 1 MgCl2, 2 CaCl2, 10 glucose, and 10 Hepes, pH 7.4 (adjusted with NaOH), and the pipette contained (mm): 110 potassium aspartate, 30 KCl, 10 NaCl, 1 MgCl2, 0.05 EGTA, 200 g ml−1 amphotericin B, and 10 Hepes, pH 7.2 (adjusted with KOH).
In vitro contraction studies
The tunica albuginea was cut longitudinally, starting at the most proximal point of the CC toward the penile shaft, and the erectile tissue was partially dissected free from the tunica. One strip of tissue (0.3 × 0.3 × 3 mm) was obtained from each CC. The contractility of each isolated CCSM strip was measured using a MyoMED myograph system (MED Associates Inc., Georgia, VT, USA). The strip was mounted in a thermostatically controlled tissue bath containing aerated PSS (mm: 119 NaCl, 4.7 KCl, 24 NaHCO3, 1.2 KH2PO4, 2.5 CaCl2, 1.2 MgSO4, 0.023 EDTA, and 11 glucose; 5 ml volume, 95% O2 and 5% CO2, 37°C) and stretched to a resting tension of 0.1 mN. The contractile responses of the strips were analysed by adding 10 µm phenylephrine to the bath, and force changes were recorded in response to drug application and to electrical field stimulation. Electrical field stimulation was delivered for 2 and 60 s, each at 30 Hz (20 V amplitude, 0.5 ms pulse width, alternating polarity between pulses).
In vivo intracavernous pressure measurement
Mice were anaesthetized with isoflurane followed by intraperitoneal injection of sodium pentobarbital (50 mg kg−1) and placed on a heated blanket. Body temperature and circulatory volume were kept optimal by frequent intraperitoneal administration of body-temperature 0.9% saline solution. The cavernous nerve was identified through a lower midline abdominal incision and its short segment was dissected from the surrounding tissue. Subsequently the base of the penis, enclosed by the striated bulbospongious and ischiocavernous muscles, was exposed through a perineal incision. Using blunt dissection, the ischiocavernous muscle covering the CC was divided on one side and the underlying tunica albuginea was visualized. A 27-gauge needle attached to a polyethylene catheter (PE50) filled with heparinized (100 IE ml−1) 0.9% saline was inserted into the crus of the CC. The catheter was connected to one port of a pressure transducer, while a syringe with heparinized 0.9% saline was attached to the other port of the pressure transducer to allow for regular flushing of the line. By retracting the surrounding tissues, the cavernous nerve was visualized, running on the postero-lateral side of the prostate. Electrostimulation of this nerve was then performed with a bipolar platinum contact electrode. Square-wave pulses were delivered by a Grass stimulator (1.0 ms, 12 Hz, 4 V; Grass Instrument Co., MA, USA). Each period of stimulation lasted ∼ 60 s, and resting intervals of 15–20 min were allowed between the periods of stimulation. The catheter was flushed prior to each period of stimulation to ensure an unclogged line.
Drugs and chemicals
All drugs and chemicals were ordered from Sigma-Aldrich unless stated otherwise. Iberiotoxin (IBTX; Peptides International Inc., Louisville, KY, USA) was used at a final concentration of 100 nm (electrophysiology) or 300 nm (in vitro contraction studies).
Statistical analyses
Results are expressed as means ± s.e.m. where applicable. Comparisons were made with the two-tailed Student's t test (before and after IBTX application were paired, Slo+/+vs.Slo−/− were unpaired).
Results
BK channel α-subunit is present in CCSM from Slo+/+ but not from Slo−/− mice
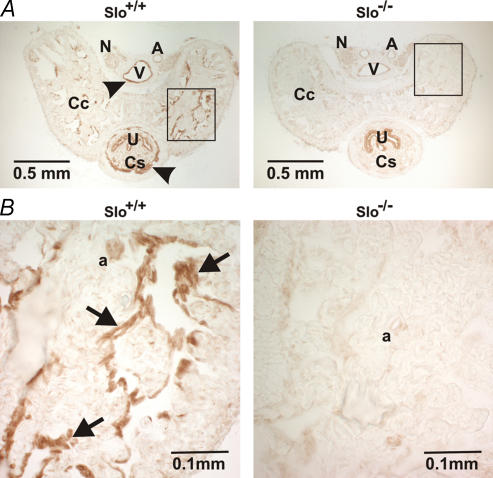
Others have shown that BK channels are expressed in the rat penis (e.g. Archer, 2002), but the exact location of the channel has not been identified. In our study, BK channel-specific immunoreactivity was observed in CCSM as well as in SM of the corpus spongiosum (CS), and in vascular SM of helicine arteries and veins in penis sections from Slo+/+ mice. However, the immunoreactivity was completely absent in penile sections from Slo−/− mice (Fig. 1). No BK channel staining was observed in the nerves of either genotype.
Figure 1. Immunohistochemical detection of BK channels in penile sections from Slo+/+ and Slo−/− mice.
A, cross-sections from a Slo+/+ and a Slo−/− penis stained with a BK channel-specific antibody. B, higher magnification of the rectangles shown in panel A. Specific staining (arrow heads in panel A, and arrows in panel B) was observed in the corpus cavernosum (Cc) smooth muscle, the corpus spongiosum (Cs) smooth muscle and in vascular smooth muscle. Nonspecific staining was observed in the urethra (U). N, nerve; V, vein; A, artery; a, helicine artery.
Calcium-activated BK currents are absent in CCSM from Slo−/− mice
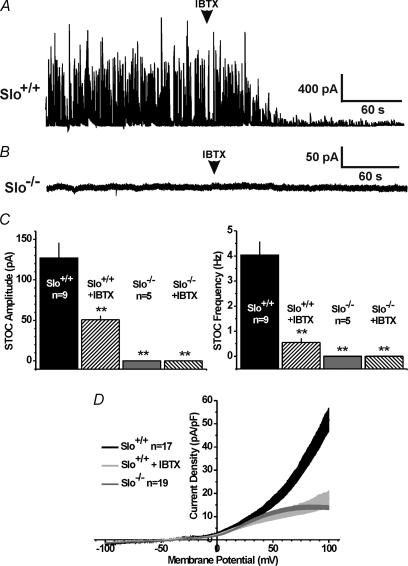
BK channels are activated by elevations in [Ca2+]i, and in particular by local transient calcium release (calcium sparks) through ryanodine receptors in the sarcoplasmic reticulum membrane (Nelson et al. 1995; Perez et al. 1999; Herrera et al. 2001; Mizusawa et al. 2001; Herrera & Nelson, 2002). CCSM cells isolated from Slo+/+ mice exhibited pronounced transient BK currents characteristic of those produced by calcium sparks. These calcium-activated currents, also called spontaneous transient outward currents (STOCs), were largely inhibited by the channel's specific blocker IBTX (Fig. 2A and C). Consistent with our previous findings in cells from urinary bladder smooth muscle (Meredith et al. 2004), STOCs were not detected in CCSM cells isolated from Slo−/− mice (Fig. 2B and C), and IBTX had no effect, consistent with the absence of BK channel expression in Slo−/− mice in general (Meredith et al. 2004), and specifically in CCSM (Fig. 1).
Figure 2. Calcium- and voltage-activated BK currents in Slo+/+ and Slo−/− CCSM cells.
A, transient Ca2+-activated BK currents at 0 mV, in the absence and presence of IBTX, from a Slo+/+ CCSM cell. B, transient Ca2+-activated BK currents at 0 mV, in the absence and presence of IBTX, from a Slo−/− CCSM cell. C, average transient BK current amplitude and frequency from panels A and B(n, number of cells; **P < 0.01 vs.Slo+/+). D, whole-cell 800-ms depolarizing ramp currents from freshly isolated Slo+/+ CCSM cells, in the absence and presence of IBTX, and Slo−/− CCSM cells. The s.e.m. is represented by the width of the curves.
Voltage-activated BK currents are absent in Slo−/− mice
BK channels are activated by both intracellular calcium and membrane depolarization. CCSM cells isolated from Slo+/+ mice exhibited a pronounced voltage-activated BK channel current, measured as the voltage-activated, IBTX-sensitive outward current (Fig. 2D). This very significant outward current was completely absent in CCSM cells from Slo−/− mice.
We further examined the properties of CCSM cells isolated from Slo+/+ and Slo−/− mice. The whole-cell capacitance was not significantly different, indicating similar cell surface areas (Slo+/+ 29.6 ± 2.2 pF, n = 14; and Slo−/− 31.1 ± 1.8 pF, n = 16; P > 0.05).
Membrane depolarization activates a significant K+ outward current in addition to the BK current. Based on the time dependence of this calcium-insensitive voltage-dependent K+ (KV) current, two types of non-BK currents could be identified: a slow delayed rectifier and a fast A-type current (for review see Amberg et al. 2003). Although exceptions exist (e.g. Rudy et al. 1991), delayed rectifier current is sensitive to external TEA, whereas the A-type current is not (e.g. Thompson, 1977). Additionally, A-type current activates and inactivates at very negative membrane potentials with thresholds between −45 and −60 mV and half-inactivation between −50 and −80 mV (Amberg et al. 2003).
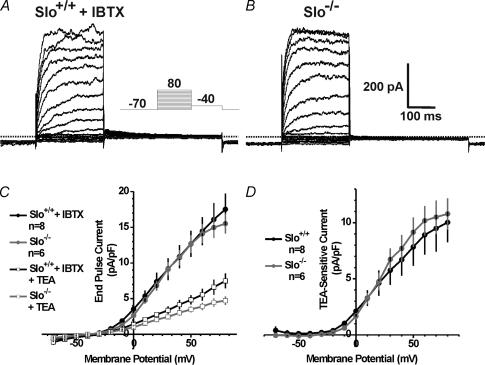
To analyse the KV current present in CCSM cells, a depolarizing step protocol was used to assess voltage-gated and IBTX-resistant K+ current in cells from Slo+/+ and Slo−/− mice (Fig. 3A and B, respectively). Voltage-gated K+ current densities, measured at the end of the 250 ms test pulse, were similar for all voltages (Fig. 3C). In CCSM cells from both Slo+/+ and Slo−/−, and with BK channels blocked with IBTX, end-pulse outward current was reduced by 60% with 5 mm TEA. No difference in the TEA-sensitive current was detected between the two genotypes (Fig. 3C and D).
Figure 3. Voltage-gated potassium currents in Slo+/+ and Slo−/− CCSM cells.
A and B, representative whole-cell currents from a Slo+/+ CCSM cell in the presence of IBTX and a Slo−/− CCSM cell, respectively. Currents were elicited by 250-ms depolarizing pulses (see inset). C, voltage-gated K+ current density and current–voltage relationship for Slo+/+ cells (with IBTX) is the same as for Slo−/− cells. The voltage-gated K+ current in CCSM cells of both genotypes is inhibited with 5 mm TEA. D, average TEA-sensitive current density from Slo+/+ and Slo−/− cells (n, number of cells).
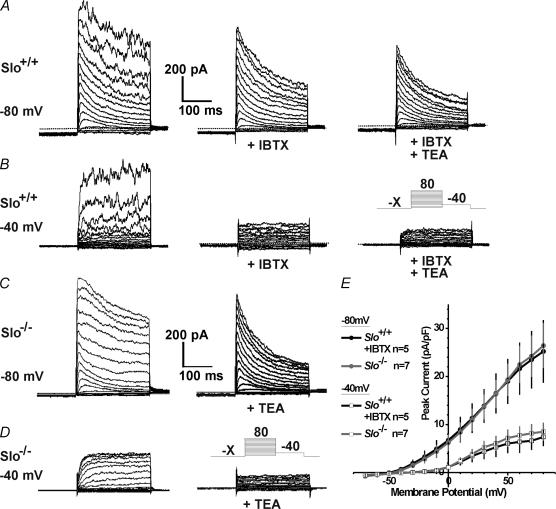
In addition to the TEA-sensitive delayed rectifier KV current, significant IBTX- and TEA-resistant KV current was observed in about 50% of the CCSM cells from both Slo+/+ (12 out of 21 cells) and Slo−/− mice (9 out of 19 cells). This current had a shape characteristic of a fast-activating and rapidly inactivating A-type current. It was activated by holding the membrane potential at −80 mV prior to the test pulses and was completely inactivated at −40 mV (Fig. 4A–D). A-type current densities, measured at the peak of the 250-ms test pulse were similar in Slo+/+ and Slo−/− cells for all voltages when held at −80 or −40 mV (Fig. 4E). Furthermore, in cells with a pronounced A-type current, the delayed rectifier current was smaller than in cells without A-type current, even though the average IBTX-resistant current densities measured at the end of the test pulses were the same in all cells. These data suggest that there may be at least two different groups of SM cells present in the CC of mice: one that has a mainly TEA-sensitive delayed rectifier KV current but little or no TEA-resistant A-type current, and a second group that has less TEA-sensitive delayed rectifier KV current, but pronounced TEA-resistant A-type current (Figs 3 and 4).
Figure 4. A-type potassium currents in CCSM cells.
A and B, representative whole-cell currents from a Slo+/+ CCSM cell in the absence and presence of IBTX or TEA. C and D, representative whole-cell currents from a Slo−/− CCSM cell in the absence and presence of TEA. Currents were elicited by 250-ms depolarizing pulses (see insets; –X, holding membrane potential) after holding the cell at −80 mV (A and C) or −40 mV (B and D). E, average peak current densities from Slo+/+ and Slo−/− cells held at −80 mV and −40 mV (n, number of cells).
CC strips from Slo−/− mice demonstrate enhanced contractility in vitro
To examine the functional role of BK channels in the CC, contractility of isolated CCSM strips from Slo+/+ and Slo−/− mice were examined. In the absence of stimulating agents, neither strips from Slo+/+ mice nor those from Slo−/− mice exhibited spontaneous contractile activity (n = 10), consistent with other studies (Hedlund et al. 2000; Mizusawa et al. 2001). To verify the contractile ability of the strips and to analyse the depolarization-induced contractile force, membrane depolarization was elicited by elevating the external K+ concentration to 60 mm. This leads to an influx of Ca2+ through voltage-dependent Ca2+ channels causing smooth muscle contraction. The observed mean contractile force was not different between Slo+/+ (0.22 ± 0.03 mN, n = 10) and Slo−/− strips (0.24 ± 0.03 mN, n = 10), suggesting no changes in Ca2+ influx or in the subsequent contractile response. This is consistent with recent findings from others (Sausbier et al. 2005), who found that L-type Ca2+ currents in tibial artery smooth muscle were not altered in BK channel KO mice.
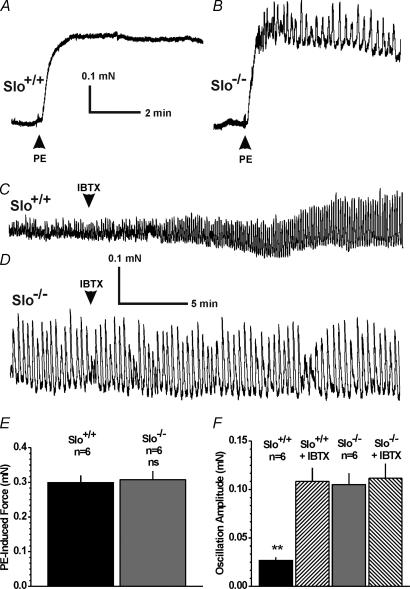
The CC is under sympathetic influence to maintain a contracted, flaccid state. To simulate this situation, the α-adrenergic receptor agonist phenylephrine (10 µm) was applied to induce contraction. The maximum phenylephrine-induced contraction, reached 10–20 s after application, was similar in CCSM strips from Slo+/+ and Slo−/− mice (Fig. 5A, B and E). In addition to elevating force, phenylephrine induced phasic contractions of small amplitude in CCSM strips from Slo+/+ mice. The amplitude of these phasic contractions was increased about four-fold in Slo−/− strips, indicating enhanced contractility (Fig. 5C, D and F). Blocking the BK channel with IBTX in Slo+/+ strips increased phasic contractions about four-fold to the same amplitude observed in Slo−/− strips. IBTX did not influence the oscillation frequency, either in Slo+/+ (Slo+/+ 2.4 ± 0.7 min−1, n = 5, vs.Slo+/+ + IBTX 3.1 ± 1.1 min−1, n = 5; P > 0.05), or in Slo−/− animals (Slo+/+ 0.4 ± 0.04 min−1, n = 5 vs.Slo−/− + IBTX 0.4 ± 0.05 min−1, n = 5; P > 0.05). However, the frequency was significantly different between the two genotypes (P < 0.05). Under unstimulated conditions, i.e. in the absence of phenylephrine, IBTX had no effect on the CCSM strips (n = 4).
Figure 5. In vitro contraction measurements of isolated CCSM strips.
A and B, representative recordings of 10 µm phenylephrine-induced (PE) contractions from a Slo+/+ and a Slo−/− CCSM strip, respectively. C, sample recording from a PE-precontracted Slo+/+ strip of cells in the absence and presence of IBTX. D, sample recording from a PE-precontracted Slo−/− strip of cells in the absence and presence of IBTX. E, average PE-induced contractions from panels A and B. F, average PE-induced oscillations from panels C and D(n, number of CCSM strips from four Slo+/+ and five Slo−/− mice; **P < 0.01 vs.Slo+/+; ns, not significant)
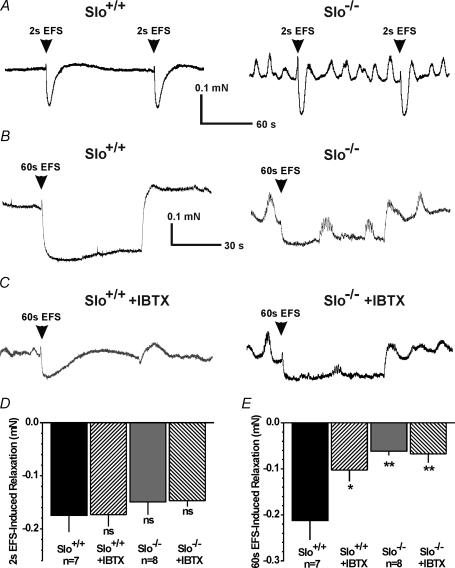
Electrical field stimulation (EFS) has been used to stimulate the nerves that innervate the smooth muscle cells of the CC (Andersson & Wagner, 1995). When stimulated, cavernosal nonadrenergic-noncholinergic nerves release NO, causing relaxation of CCSM. In our studies, electrical field stimulation (lasting 2 s) evoked relaxations in phenylephrine-precontracted strips from Slo+/+ and Slo−/− mice with the same peak values, although Slo−/− strips always exhibited continuous oscillations (Fig. 6A and D). Blocking the BK channel with IBTX did not affect relaxations induced by these brief periods of stimulation.
Figure 6. Electrical field stimulation-induced relaxation of isolated precontracted CCSM strips.
A, sample recordings of EFS-induced relaxations (2-s pulse) from a PE-precontracted Slo+/+ CCSM strip (left trace) and a Slo−/− CCSM strip (right trace). B, examples of EFS-induced relaxations (60-s pulse) from PE-precontracted Slo+/+ (left trace) and Slo−/− (right trace) CCSM strips. C, examples of EFS-induced relaxations (60-s pulse) from PE-precontracted Slo+/+ (left trace) and Slo−/− (right trace) CCSM strips in the presence of 300 nm IBTX. D, average EFS-induced relaxations from the PE-precontracted CCSM strips shown in panel A. E, average EFS-induced relaxations from the PE-precontracted CCSM strips shown in panels B and C(n, number of CCSM strips from five Slo+/+ and six Slo−/− mice; *P < 0.05; **P < 0.01 vs.Slo+/+; ns, not significant).
Prolonged electrical field stimulation (60 s) evoked pronounced relaxations of Slo+/+ strips (Fig. 6B). Blocking BK channels with IBTX significantly reduced (50%) the maximal (60 s) EFS-induced relaxation of CCSM strips from Slo+/+ mice (Fig. 6C). Consistent with the effect of IBTX on Slo+/+ strips, CCSM strips from Slo−/− mice exhibited a similar reduction in relaxation to prolonged EFS and were insensitive to IBTX. In contrast to CCSM strips from Slo+/+ mice, EFS-induced relaxations were not maintained in Slo−/− strips or in Slo+/+ strips in the presence of IBTX (Fig. 6B and C).
Slo−/− mice exhibit enhanced spontaneous pressure fluctuations and decreased erectile response to cavernous nerve stimulation in vivo
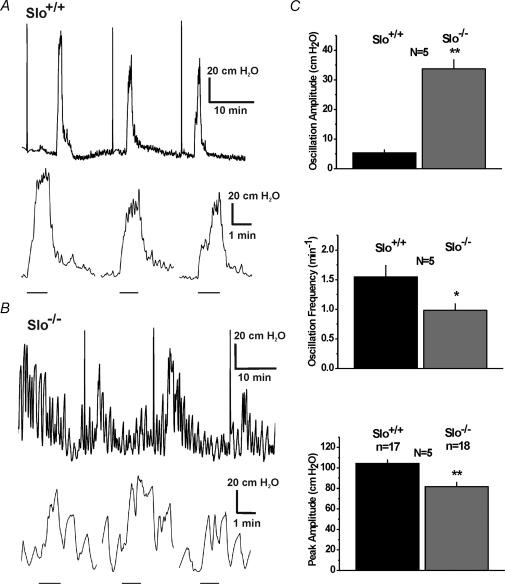
The spontaneous contractions and the reductions in relaxation during nerve stimulation of phenylephrine-precontracted CC strips from Slo−/− mice predict that in vivo intracavernous pressure should fluctuate and that nerve-evoked increases in pressure should be impaired, but not eliminated. To test these predictions, we performed in vivo measurements of the intracavernous pressure in the anaesthetized mouse. The baseline intracavernous pressure under unstimulated conditions did not differ between the two genotypes (Slo+/+ 12.6 ± 1.6 cmH2O (number of mice, n = 5) and Slo−/− 14.0 ± 1.1 cmH2O (n = 5)). Pressure oscillations with small amplitudes were detected in Slo+/+ mice, consistent with the phasic contractions in our in vitro results in the presence of phenylephrine. The amplitude of these pressure oscillations was six-fold greater in Slo−/− mice (Fig. 7).
Figure 7. In vivo intracavernous pressure measurements.
A and B, sample ICP recordings from litter-matched Slo+/+ and a Slo−/− mice, respectively. The upper traces of both panels show three responses to stimulation of the cavernous nerve (the initial brief pulse results from flushing the line prior to stimulation). The lower traces show the same three responses on an expanded time scale, i.e. as higher time-resolution displays of the cavernous nerve stimulation-induced pressure increases. The bars below the traces represent the stimulation period of ∼ 60 s. C, average oscillation amplitude and frequency, and peak amplitude of nerve-induced pressure increases from the experiment shown in panels A and B(n, number of stimulations; N, number of Slo+/+ and Slo−/− mice; *P < 0.05, **P < 0.01).
Electrical stimulation of the cavernous nerve mimics nerve activity under physiological conditions and has been routinely used to investigate erectile function (Sezen & Burnett, 2000; Mizusawa et al. 2001). Nerve stimulation for 60 s induced an increase in intracavernous pressure in both genotypes. The stimulation-induced peak pressures recorded from Slo−/− mice were reduced by 22%, indicating an impaired ability of the CCSM to relax (Fig. 7). In addition, in Slo+/+ mice, the intracavernous pressure remained constant during the entire stimulation period, whereas in Slo−/− mice the intracavernous pressure exhibited pressure oscillations, and gradually fell throughout the course of stimulation (Fig. 7B). Reduced peak intracavernous pressure and inability to maintain pressure in Slo−/− animals indicates an impaired ability of the CCSM to relax during nerve stimulation.
Discussion
To our knowledge, this is the first in vitro and in vivo study of erectile function performed in mice lacking the BK channel. By using immunohistochemistry, others have shown the presence of key members of the NO/cGMP/PKGI pathway in the CC, e.g. the soluble guanylyl cyclase and PKGI in human CC (Klotz et al. 2000), and PKGI in mouse CC (Hedlund et al. 2000). However, the exact location of the BK channel has not been identified, although it is known that it is expressed in the penis (e.g. Archer, 2002). Using Slo−/− mice as negative controls, we were able to demonstrate that the BK channel is located in CC, CS and vascular SM.
In accordance with our previous study performed on the urinary bladder smooth muscle (Meredith et al. 2004) and the absence of the BK channel immunoreactivity in CC sections, we demonstrated that functional BK currents are absent in single CCSM cells from Slo−/− mice (Fig. 2). Furthermore, there does not appear to be a compensatory up-regulation of TEA-sensitive KV channels (Fig. 3). Additionally, we identified cells with pronounced A-type KV currents, which were resistant to 5 mm TEA (Fig. 4). As with the TEA-sensitive KV current, compensatory up-regulation of TEA-resistant A-type KV current was not observed. Even though a similar A-type current has been observed in human CCSM cells (Christ et al. 1993), the role of this current is unclear as it is usually inactivated at physiological resting membrane potentials. In the current study, we identified two groups of CCSM cells based on the voltage-dependence of the observed K+ currents. Similar results were reported by Malysz et al. (2001) in rabbit CCSM cells. However, although these authors described cells with and without delayed rectifier KV currents, they observed no A-type KV current.
We have observed in vitro that the baseline levels of phenylephrine-induced contractions were similar in Slo+/+ and Slo−/− strips. Significantly, however, lack of the BK channel in Slo−/− strips enhances phenylephrine-induced contractility by increasing phasic contractions above the baseline, and this effect can be mimicked partially in Slo+/+ strips by blocking the BK channel with IBTX. In human CCSM strips, Spektor et al. (2002) observed phasic contractions following BK channel blockade with 10 mm TEA, while 100 mm TEA led to a tonic contraction. Although external TEA blocks BK channels (half block with 0.1 mm; Langton et al. 1991), it also significantly blocks voltage-gated K+ channels at concentrations above 1 mm (e.g. Thorneloe & Nelson, 2003). Spektor et al. (2002) also used the more specific BK channel inhibitor charybdotoxin (which also inhibits intermediate-conductance calcium-activated potassium channels), but did not report oscillations. They described an overall increase in agonist-induced CCSM strip contraction in the presence of TEA or charybdotoxin.
Electrical field stimulation has been shown to induce relaxation in phenylephrine-precontracted strips (Andersson & Wagner, 1995; Hedlund et al. 1999, 2000). In the present study we applied this technique to analyse the relaxation properties of CCSM strips from Slo+/+ and Slo−/− mice. No significant difference and no effect of blocking the BK channel with IBTX were observed during a 2-s pulse. However, IBTX reduced the response to a prolonged, more physiological 60-s pulse by 50% in Slo+/+ strips (Fig. 6). The channel blocker had no effect on Slo−/− strips, which already had a significantly reduced relaxation. This indicates that the absence of BK channel function (Slo−/− or IBTX) impairs 60 s EFS-induced relaxations. The results also suggest a minor role of the BK channel in brief (2 s) EFS-induced relaxation, and a more important role in maintaining the relaxed state. However, the reduced ability of Slo−/− CC strips, or Slo+/+ CC strips plus IBTX, to relax during 60 s electrical field stimulation was less dramatic than in PKGI-deficient mice (Hedlund et al. 2000), suggesting a role for other PKGI targets.
In accordance with the in vitro findings, we show in vivo that BK channel-deficient mice have dramatically increased intracavernous pressure oscillations, consistent with the phasic contractions observed in CCSM strips in the presence of phenylephrine. Furthermore, when compared to Slo+/+, these mice exhibit a blunted increase in the intracavernous pressure with the electrostimulation of the cavernous nerve (Fig. 7). Finally, Slo−/− (but never Slo+/+) mice failed to maintain the elevated intracavernous pressure during nerve stimulation.
In the flaccid state, the CCSM is contracted due to a predominance of adrenergic neural control (Andersson & Wagner, 1995). In this study this state was mimicked in vitro by adding phenylephrine to the organ bath. Our results suggest that BK channel absence leads to unstable CCSM tone, measured as force (in vitro) or pressure (in vivo) oscillations in response to sympathetic stimulation. Sexual stimulation was mimicked in vivo by electrostimulation of the cavernous nerve, inducing primarily parasympathetic nerves. In our study, the nerve stimulation led to a fast CCSM relaxation response, however, the response was significantly reduced in the absence of the BK channel.
The smooth muscle tone in the penis, as in other vascular and nonvascular tissues, depends on the balance between contractile and relaxant factors. Previous studies on vascular and nonvascular smooth muscle indicate that the BK channel has a role as a negative feed-back mechanism to oppose contraction (Nelson & Brayden, 1993; Meredith et al. 2004). As such, altered BK channel behaviour may impair the hyperpolarizing ability of CCSM, altering its reactivity and tone. Our observations provide direct evidence that the BK channel could be a major target of the NO/cGMP/PKG pathway, as mice lacking the BK channel exhibit elevated force oscillations and diminished nerve-evoked relaxations, and erectile dysfunction. However, our study also suggests that there is more than one regulatory mechanism and target involving PKGI, as CC relaxes to brief nerve stimulation in the absence of BK channels or in presence of BK channel blockers. A possible mechanism for the immediate relaxation could be through ATP-sensitive K+ channels, as they are involved in one of the NO mechanisms relaxing cavernous tissue (Insuk et al. 2003).
An alternative NO-independent pathway to increase cavernous pressure has been proposed, involving inhibition of Rho kinase (Chitaley et al. 2001). However, in a rat model of diabetes-associated erectile dysfunction, inhibiting this pathway also led to an improved endothelial NO production (Bivalacqua et al. 2004).
In coronary vascular smooth muscle of aged rats (Marijic et al. 2001; Nishimaru et al. 2004) as well as of humans (Toro et al. 2002), it was shown that the expression of both α- and β-subunits of the BK channel is decreased, leading to an increased risk of coronary artery vasospasm, myocardial ischaemia and infarct in the elderly. To this date, there is no available CCSM study in humans, but evidence exists for age-related erectile dysfunction in rats due to altered BK channel function (Melman et al. 2003). Our results strongly support the idea that the BK channel could be a very important target in treating patients with erectile dysfunction, especially those who do not respond to the currently available drug therapy.
Acknowledgments
We thank Anna-Maria Knorn and Paul Doetsch for mouse breeding and genotyping and Anna-Maria Knorn for assisting with the immunohistochemistry. The work was supported by grants from the NIH (DK-5R01DK053832, HL44455 and 1R01DK065947) and the Howard Hughes Medical Institute.
References
- Alioua A, Tanaka Y, Wallner M, Hofmann F, Ruth P, Meera P, Toro L. The large conductance, voltage-dependent, and calcium-sensitive K+ channel, Hslo, is a target of cGMP-dependent protein kinase phosphorylation in vivo. J Biol Chem. 1998;273:32950–32956. doi: 10.1074/jbc.273.49.32950. [DOI] [PubMed] [Google Scholar]
- Amberg GC, Koh SD, Imaizumi Y, Ohya S, Sanders KM. A-type potassium currents in smooth muscle. Am J Physiol Cell Physiol. 2003;284:C583–C595. doi: 10.1152/ajpcell.00301.2002. [DOI] [PubMed] [Google Scholar]
- Andersson KE, Wagner G. Physiology of penile erection. Physiol Rev. 1995;75:191–236. doi: 10.1152/physrev.1995.75.1.191. [DOI] [PubMed] [Google Scholar]
- Archer SL. Potassium channels and erectile dysfunction. Vascul Pharmacol. 2002;38:61–71. doi: 10.1016/s1537-1891(02)00127-1. [DOI] [PubMed] [Google Scholar]
- Bivalacqua TJ, Champion HC, Usta MF, Cellek S, Chitaley K, Webb RC, Lewis RL, Mills TM, Hellstrom WJ, Kadowitz PJ. RhoA/Rho-kinase suppresses endothelial nitric oxide synthase in the penis: a mechanism for diabetes-associated erectile dysfunction. Proc Natl Acad Sci U S A. 2004;101:9121–9126. doi: 10.1073/pnas.0400520101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett AL, Lowenstein CJ, Bredt DS, Chang TS, Snyder SH. Nitric oxide: a physiologic mediator of penile erection. Science. 1992;257:401–403. doi: 10.1126/science.1378650. [DOI] [PubMed] [Google Scholar]
- Chitaley K, Wingard CJ, Clinton WR, Branam H, Stopper VS, Lewis RW, Mills TM. Antagonism of Rho-kinase stimulates rat penile erection via a nitric oxide-independent pathway. Nat Med. 2001;7:119–122. doi: 10.1038/83258. [DOI] [PubMed] [Google Scholar]
- Christ GJ, Day N, Santizo C, Sato Y, Zhao W, Sclafani T, Bakal R, Salman M, Davies K, Melman A. Intracorporal injection of hSlo cDNA restores erectile capacity in STZ-diabetic F-344 rats in vivo. Am J Physiol Heart Circ Physiol. 2004;287:H1544–H1553. doi: 10.1152/ajpheart.00792.2003. [DOI] [PubMed] [Google Scholar]
- Christ GJ, Spray DC, Brink PR. Characterization of K currents in cultured human corporal smooth muscle cells. J Androl. 1993;14:319–328. [PubMed] [Google Scholar]
- Hedlund P, Alm P, Andersson KE. NO synthase in cholinergic nerves and NO-induced relaxation in the rat isolated corpus cavernosum. Br J Pharmacol. 1999;127:349–360. doi: 10.1038/sj.bjp.0702556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedlund P, Aszodi A, Pfeifer A, Alm P, Hofmann F, Ahmad M, Fassler R, Andersson KE. Erectile dysfunction in cyclic GMP-dependent kinase I-deficient mice. Proc Natl Acad Sci U S A. 2000;97:2349–2354. doi: 10.1073/pnas.030419997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera GM, Heppner TJ, Nelson MT. Voltage dependence of the coupling of Ca2+ sparks to BKCa channels in urinary bladder smooth muscle. Am J Physiol Cell Physiol. 2001;280:C481–C490. doi: 10.1152/ajpcell.2001.280.3.C481. [DOI] [PubMed] [Google Scholar]
- Herrera GM, Nelson MT. Differential regulation of SK and BK channels by Ca2+ signals from Ca2+ channels and ryanodine receptors in guinea-pig urinary bladder myocytes. J Physiol. 2002;541:483–492. doi: 10.1113/jphysiol.2002.017707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmquist F, Stief CG, Jonas U, Andersson KE. Effects of the nitric oxide synthase inhibitor NG-nitro-L-arginine on the erectile response to cavernous nerve stimulation in the rabbit. Acta Physiol Scand. 1991;143:299–304. doi: 10.1111/j.1748-1716.1991.tb09236.x. [DOI] [PubMed] [Google Scholar]
- Horn R, Marty A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. J Generalphysiol. 1988;92:145–159. doi: 10.1085/jgp.92.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insuk SO, Chae MR, Choi JW, Yang DK, Sim JH, Lee SW. Molecular basis and characteristics of KATP channel in human corporal smooth muscle cells. Int J Impot Res. 2003;15:258–266. doi: 10.1038/sj.ijir.3901013. [DOI] [PubMed] [Google Scholar]
- Klotz T, Bloch W, Zimmermann J, Ruth P, Engelmann U, Addicks K. Soluble guanylate cyclase and cGMP-dependent protein kinase I expression in the human corpus cavernosum. Int J Impot Res. 2000;12:157–164. doi: 10.1038/sj.ijir.3900524. [DOI] [PubMed] [Google Scholar]
- Langton PD, Nelson MT, Huang Y, Standen NB. Block of calcium-activated potassium channels in mammalian arterial myocytes by tetraethylammonium ions. Am J Physiol. 1991;260:H927–H934. doi: 10.1152/ajpheart.1991.260.3.H927. [DOI] [PubMed] [Google Scholar]
- Lincoln TM, Cornwell TL. Intracellular cyclic GMP receptor proteins. FASEB J. 1993;7:328–338. doi: 10.1096/fasebj.7.2.7680013. [DOI] [PubMed] [Google Scholar]
- Malysz J, Gibbons SJ, Miller SM, Gettman M, Nehra A, Szurszewski JH, Farrugia G. Potassium outward currents in freshly dissociated rabbit corpus cavernosum myocytes. J Urol. 2001;166:1167–1177. [PubMed] [Google Scholar]
- Marijic J, Li Q, Song M, Nishimaru K, Stefani E, Toro L. Decreased expression of voltage- and Ca2+-activated K+ channels in coronary smooth muscle during aging. Circ Res. 2001;88:210–216. doi: 10.1161/01.res.88.2.210. [DOI] [PubMed] [Google Scholar]
- Melman A, Zhao W, Davies KP, Bakal R, Christ GJ. The successful long-term treatment of age related erectile dysfunction with hSlo cDNA in rats in vivo. J Urol. 2003;170:285–290. doi: 10.1097/01.ju.0000063375.12512.6e. [DOI] [PubMed] [Google Scholar]
- Meredith AL, Thorneloe KS, Werner ME, Nelson MT, Aldrich RW. Overactive bladder and incontinence in the absence of the BK large conductance Ca2+-activated K+ channel. J Biol Chem. 2004;279:36746–36752. doi: 10.1074/jbc.M405621200. [DOI] [PubMed] [Google Scholar]
- Mizusawa H, Hedlund P, Hakansson A, Alm P, Andersson KE. Morphological and functional in vitro and in vivo characterization of the mouse corpus cavernosum. Br J Pharmacol. 2001;132:1333–1341. doi: 10.1038/sj.bjp.0703938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson MT, Brayden JE. Regulation of arterial tone by calcium-dependent K+ channels and ATP-sensitive K+ channels. Cardiovasc Drugs Ther. 1993;7(Suppl. 3):605–610. doi: 10.1007/BF00877627. [DOI] [PubMed] [Google Scholar]
- Nelson MT, Cheng H, Rubart M, Santana LF, Bonev AD, Knot HJ, Lederer WJ. Relaxation of arterial smooth muscle by calcium sparks. Science. 1995;270:633–637. doi: 10.1126/science.270.5236.633. [DOI] [PubMed] [Google Scholar]
- Nishimaru K, Eghbali M, Lu R, Marijic J, Stefani E, Toro L. Functional and molecular evidence of MaxiK channel beta1 subunit decrease with coronary artery ageing in the rat. J Physiol. 2004;559:849–862. doi: 10.1113/jphysiol.2004.068676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez GJ, Bonev AD, Patlak JB, Nelson MT. Functional coupling of ryanodine receptors to KCa channels in smooth muscle cells from rat cerebral arteries. J Generalphysiol. 1999;113:229–238. doi: 10.1085/jgp.113.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson BE, Schubert R, Hescheler J, Nelson MT. cGMP-dependent protein kinase activates Ca-activated K channels in cerebral artery smooth muscle cells. Am J Physiol. 1993;265:C299–C303. doi: 10.1152/ajpcell.1993.265.1.C299. [DOI] [PubMed] [Google Scholar]
- Rudy B, Sen K, Vega-Saenz de Miera E, Lau D, Ried T, Ward DC. Cloning of a human cDNA expressing a high voltage-activating, TEA-sensitive, type-A K+ channel which maps to chromosome 1 band p21. J Neurosci Res. 1991;29:401–412. doi: 10.1002/jnr.490290316. [DOI] [PubMed] [Google Scholar]
- Sausbier M, Arntz C, Bucurenciu I, Zhao H, Zhou XB, Sausbier U, Feil S, Kamm S, Essin K, Sailer CA, Abdullah U, Krippeit-Drews P, Feil R, Hofmann F, Knaus HG, Kenyon C, Shipston MJ, Storm JF, Neuhuber W, Korth M, Schubert R, Gollasch M, Ruth P. Elevated blood pressure linked to primary hyperaldosteronism and impaired vasodilation in BK channel-deficient mice. Circulation. 2005 doi: 10.1161/01.CIR.0000156448.74296.FE. (in press) [DOI] [PubMed] [Google Scholar]
- Schmidt HH, Lohmann SM, Walter U. The nitric oxide and cGMP signal transduction system: regulation and mechanism of action. Biochim Biophys Acta. 1993;1178:153–175. doi: 10.1016/0167-4889(93)90006-b. [DOI] [PubMed] [Google Scholar]
- Sezen SF, Burnett AL. Intracavernosal pressure monitoring in mice: responses to electrical stimulation of the cavernous nerve and to intracavernosal drug administration. J Androl. 2000;21:311–315. [PubMed] [Google Scholar]
- Spektor M, Rodriguez R, Rosenbaum RS, Wang HZ, Melman A, Christ GJ. Potassium channels and human corporeal smooth muscle cell tone: further evidence of the physiological relevance of the Maxi-K channel subtype to the regulation of human corporeal smooth muscle tone in vitro. J Urol. 2002;167:2628–2635. [PubMed] [Google Scholar]
- Thompson SH. Three pharmacologically distinct potassium channels in molluscan neurones. J Physiol. 1977;265:465–488. doi: 10.1113/jphysiol.1977.sp011725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorneloe KS, Meredith AL, Knorn AM, Aldrich RW, Nelson MT. Urodynamic properties and neurotransmitter dependence of urinary bladder contractility in the BK channel deletion model of overactive bladder. Am J Physiol Renal Physiol. 2005 doi: 10.1152/ajprenal.00060.2005. 10.1152/ajprenal.00060.2005. [DOI] [PubMed] [Google Scholar]
- Thorneloe KS, Nelson MT. Properties and molecular basis of the mouse urinary bladder voltage-gated K+ current. J Physiol. 2003;549:65–74. doi: 10.1113/jphysiol.2003.039859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toro L, Marijic J, Nishimaru K, Tanaka Y, Song M, Stefani E. Aging, ion channel expression, and vascular function. Vascul Pharmacol. 2002;38:73–80. doi: 10.1016/s0306-3623(02)00128-3. [DOI] [PubMed] [Google Scholar]