Abstract
Trafficking of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors (AMPARs) at synapses has been suggested to play an important role in the expression of synaptic plasticity. Both the regulated and the constitutive trafficking of synaptic AMPARs are thought to involve the insertion and removal of receptors by means of an exocytotic and endocytotic process, respectively. In contrast, N-methyl-d-aspartate (NMDA) receptors (NMDARs), which are colocalized with AMPARs at excitatory synapses, appear to be much less dynamic. Here, we present evidence supporting the idea that synaptic AMPARs turn over through a constitutive endocytotic process and that glutamate application greatly enhances this turnover of AMPARs. The glutamate-induced internalization of AMPARs requires a rise in postsynaptic Ca2+. The AMPAR internalization is mimicked by latrunculin A, a drug that selectively depolymerizes actin and is blocked by jasplakinolide, a drug which stabilizes actin filaments. The rate of endocytosis is not altered by glutamate application, whereas a clear enhancement is observed with insulin application. We propose a model in which the glutamate-induced dissociation of AMPARs from their anchor on the postsynaptic membrane involves actin depolymerization, which allows the released AMPARs to segregate from the NMDARs and diffuse to a presumably perisynaptic site, where they become available to an endocytotic machinery and are selectively internalized.
Recent evidence suggests that trafficking of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors (AMPARs) at synapses plays an important role in the expression of synaptic plasticity (1–5). Furthermore, it has been proposed that AMPARs are constitutively cycling in and out of the synapse on a rapid time scale (2, 4, 6–8). This constitutive cycling appears to play a role in activity-dependent trafficking of AMPARs, because disruption of the cycling occludes expression of long-term depression in hippocampal slices (2, 6). Based on results with inhibitors of exocytosis and endocytosis, it appears that both the regulated (6, 9–12) and the constitutive (6, 7, 13) trafficking of synaptic AMPARs involve the insertion and removal of receptors by an exocytotic and endocytotic process, respectively.
Glutamate induces disappearance of AMPARs from synaptic sites on a rapid time scale (14). This change is selective to AMPARs, in that synaptic N-methyl-d-aspartate (NMDA) receptors (NMDARs) remain intact, and is accompanied by a reduction in the frequency of AMPAR miniature excitatory postsynaptic currents (mEPSCs) (14). Recently, it has been shown that glutamate induces internalization of AMPARs by a clathrin-dependent pathway (10).
The sequence of events required for internalization is still unclear. Because AMPARs are clustered in the postsynaptic density (PSD) and are intermingled with NMDARs (15–17), presumably the selective internalization of AMPARs requires the release of AMPARs from their anchors at the PSD, so that they can be segregated before their internalization. In the current study, we used glutamate as the stimulus to study the mechanism of AMPAR endocytosis. We sought to answer two questions: (i) Can glutamate disassociate AMPARs from their anchoring to the postsynaptic membrane? (ii) Does glutamate enhance the rate of endocytosis? We found that the Ca2+ influx associated with glutamate application is required for internalization, and we present evidence that depolymerization of actin filaments caused by this rise in intracellular Ca2+ releases synaptic AMPARs to the endocytotic machinery. Finally, we were unable to detect any gross change in the rate of endocytosis following the application of glutamate. These results suggest a model in which glutamate receptor activation increases the concentration of Ca2+ in the dendritic spine, causing actin depolymerization. This releases AMPARs from their anchoring to the PSD and allows access of the receptors to a presumably perisynaptic endocytotic pathway.
Materials and Methods
Neuronal Culture and Immunocytochemistry.
Hippocampal cultures were prepared as described (14) and were used for experimentation 2–3 wk after plating. Surface AMPARs were labeled with an antibody against an extracellular epitope (amino acid 271–285) of the rat GluR1 subunit (Oncogene Research, Boston, MA) for 15 min at 37°C, at a concentration of 5 μg/ml. After a quick wash, neurons were incubated with 100 μM glutamate for 15 min, unless otherwise noted. Cells were then washed and fixed with 4% paraformaldehyde and 4% sucrose in PBS for 20 min at room temperature. AMPARs on the surface were labeled with anti-rabbit Cy3-conjugate for 1 h at room temperature. Then, cells were permeabilized with 0.25% Triton for 5 min, followed by incubation with anti-rabbit Cy2-conjugate to visualize AMPARs inside the cells.
To measure uptake of transferrin (Tf), cells were incubated with Texas Red-conjugated Tf (50 μg/ml; Molecular Probes) for 5 or 10 min. After thorough washing, cells were imaged immediately. The intensity of fluorescent puncta was measured and averaged for each cell. To visualize the synaptic spines, cells were permeabilized and incubated in PBS containing 10% BSA for 1 h to block nonspecific binding. They were then incubated with rhodamine-conjugated phalloidin (Rd-phalloidin; 1:10,000 dilution; Molecular Probes) for 1 h at room temperature.
Fluorescence Microscopy.
An MRC1024 laser-scanning confocal microscope (Bio-Rad) attached to a Nikon upright microscope was used to image the distribution of AMPARs. A thin optical section was obtained by using a half-open confocal aperture. The image plane was selected so that it focused on the middle of a cell (as evidenced by the presence of nucleus), and excluded signals from above and below the focal plane (which could represent signal from the surface). Laser power of 1–30% was used, depending on the intensity of the labeling. The 488-nm and 594-nm lines of the argon-krypton laser were used for excitation, and band-pass filters were used for emission. A 60× oil-immersion objective (NA 1.4) was used. For time-lapse experiments involving FM1–43, cells were first incubated in external solution containing 1 μM FM1–43 at room temperature for 5 min so that the edge of the cell body could be clearly resolved and a proper image plane through the middle of a cell could be selected. Then, FM1–43 was perfused at 37°C and images were taken every 60 s. A charge-coupled device camera (Hamamatsu, Middlesex, NJ) with a 60× oil-immersion objective (NA 1.4) affixed to a Zeiss inverted microscope was used for other image acquisition. When comparisons between groups of cultures/fields were made, the exposure time was kept constant.
Analysis.
METAMORPH imaging software (Universal Imaging, Media, PA) was used for all image analysis. To quantify receptors on the cell surface, pixel intensity of the fluorescent puncta on the surface was measured and averaged for each cell. The pixel intensity inside the entire cell body, except the nuclear region, was measured to quantify the internalized receptors.
Electrophysiology.
mEPSCs were recorded at room temperature from 10- to 14-day-old cultured neurons by using an Axopatch-1D amplifier (Axon Instruments, Foster City, CA) with low-resistance patch pipettes (3–7 MΩ). Pipette solutions contained 145 mM K⋅gluconate, 8 mM NaCl, 10 mM Hepes, 0.2 mM EGTA, 2 mM MgATP, and 3 mM NaGTP, adjusted to pH 7.3 with 1 M KOH. For recording pure AMPAR-mediated mEPSCs, the control extracellular perfusion solution contained 140 mM NaCl, 3.5 mM KCl, 10 mM Hepes, 20 mM glucose, 2 mM CaCl2, 2 mM MgCl2, 100 μM picrotoxin, and 1 μM tetrodotoxin, and was perfused at a speed of 0.2–0.3 ml/min. For recording dual component mEPSCs mediated by both AMPARs and NMDARs, the perfusion solution contained no MgCl2 and instead 20 μM glycine was included. Cells were held at −70 mV, and currents were low-pass filtered at 2 kHz and digitally sampled at 5 kHz. Series and input resistances were monitored throughout each experiment by using IGOR PRO software (WaveMetrics, Lake Oswego, OR). mEPSCs were recorded and stored on videotape and analyzed offline with MINI ANALYSIS PROGRAM software (Synaptosoft, Leonia, NJ). Threshold mEPSC amplitude was set at 5 pA for AMPAR mEPSCs and 10 pA for dual component mEPSCs. To examine the effect of latrunculin A (lat A) on AMPAR mEPSCs, a single large coverslip was broken into two: one was used for control recordings and the other was incubated with 20 μm lat A at 37°C for 20 min before recording. mEPSCs were recorded from both groups of cells. The average amplitude and frequency of mEPSCs were calculated from 10-min records. To examine the effect of lat A on dual component mEPSCs, a 10-min baseline was recorded and then 20 μM lat A was perfused for 20 min. mEPSCs recorded during the baseline period and 10 min after wash out of lat A were averaged. The AMPAR component was measured at the peak of the dual component, whereas the NMDAR component was measured as the average amplitude between 100 and 110 ms after the onset of mEPSCs. Application of 100 μM D(-)-2-Amino-5-phosphonovalerate (D-APV) at the end of the recording verified that this measurement accurately reflects pure NMDAR responses (see Fig. 4D).
Figure 4.
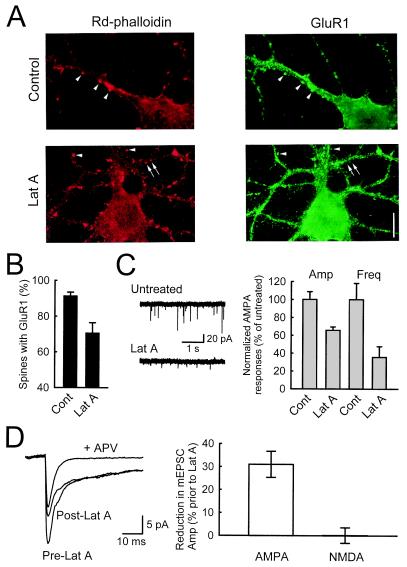
Lat A causes loss of AMPARs from synapses. (A) After cultures were treated with 20 μM lat A for 20 min, dendritic spines colocalized with GluR1 staining can still be seen (arrowheads, red for spines, green for GluR1), but there are also spines without GluR1 staining (arrows). (Scale bar: 10 μm.) (B) The percentage of spines with colocalized GluR1 staining decreases after treatment with lat A, indicating that AMPARs are dissociated from their anchorings in the postsynaptic density (n = 10 and 9 for control and lat A, respectively, from three experiments). (C) A significant reduction in both the amplitude and frequency of AMPAR mEPSCs is observed in cultures treated with lat A. Sample AMPAR mEPSCs are shown in Left for untreated and lat A-treated cultures, whereas population data from 11 cells are shown in the Right. (D) Reduction in AMPAR mEPSCs occurs in the absence of changes in NMDAR mEPSCs. Dual component mEPSCs were collected, and averaged responses from the same cell are shown in the Left. After perfusion with lat A, a significant reduction in the peak amplitude is observed, but there is no change in the slow component (n = 5 cells). Pharmacological isolation of AMPAR mEPSC with APV shows that the peak is mainly due to AMPAR activation, whereas the slow component is exclusively mediated by NMDARs.
Results
Glutamate Causes Internalization of AMPA Receptors.
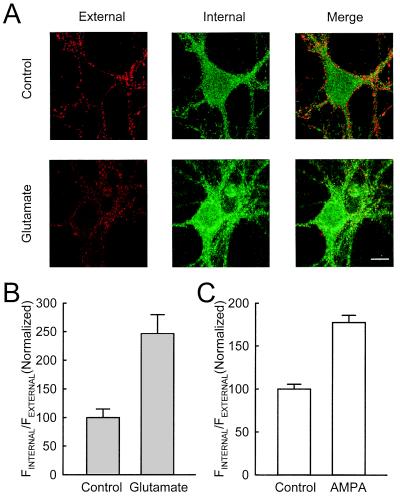
Two approaches were used to ensure that signals inside the cell actually reflect internalized AMPARs in the cytoplasm: saturation labeling of the surface receptors under nonpermeabilizing conditions and the use of thin confocal optical sections. In cells treated with glutamate, there is less AMPAR fluorescent staining on the cell surface, and more internal staining, compared with untreated cells (Fig. 1A). This agrees with previous results (10), indicating that glutamate causes AMPARs to move from the surface to the interior of the cells. We quantified this change by calculating the ratio of fluorescence intensity inside the cells versus those of puncta on the surface (Rin/Rout) and used this ratio as an index for the degree of internalization, so that comparisons could be made across cells and experiments. This ratio is significantly higher for cells treated with glutamate than for cells not treated with glutamate (Fig. 1B). A similar effect is seen when AMPA is applied (Fig. 1C). The above result is unlikely to be the result of antibody binding directly triggering AMPAR internalization, because this would require that glutamate selectively enhances antibody-driven internalization of AMPARs.
Figure 1.
Glutamate and AMPA enhanced internalization of AMPARs. (A) Detection of internalization of AMPARs. In untreated cultures, clear staining of GluR1 is seen on the surface, along the edge of the cell body and dendrites (red), whereas the staining inside the cell body is more homogeneous and weak (green). This distribution is altered by incubation with glutamate: staining becomes weaker on the surface, but stronger inside the cell body that occupies the perinuclear region. (Scale bar: 10 μm.) (B) Quantification of internalization of AMPARs. Ratio of the fluorescence intensity inside the cell body vs. on the surface was calculated and used as an index for internalization. The results were normalized to the control group. In the groups treated with glutamate, a higher ratio is obtained (for control, n = 19 cells from 6 experiments; for glutamate, n = 16 cells from 6 experiments). (C) A similar change is observed when AMPA is applied (for control, n = 18 cells from 5 experiments; for AMPA, n = 19 cells from 5 experiments).
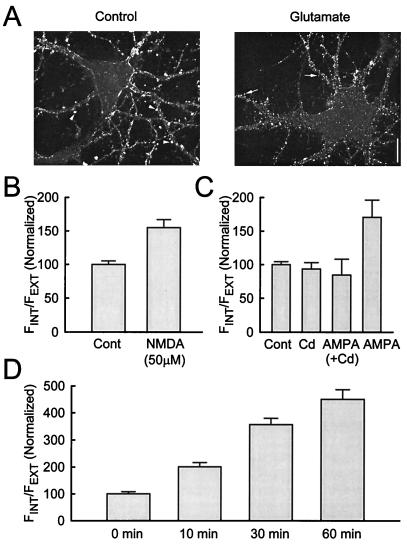
We next examined whether glutamate application causes a reduction in the number of surface synaptic AMPARs. We focused on the staining of GluR1 puncta along the dendrites with the shape resembling that of dendritic spines, because these puncta are highly colocalized with a presynaptic marker (either synaptophysin or SV2; data not shown) and hence represent synaptic AMPARs. In nontreated cultures (15- to 21-day-old), fluorescent staining reveals large, round, and high-intensity dendritic puncta (Fig. 2A). In contrast, the staining in glutamate-treated cultures is weaker and occupied smaller areas, consistent with a decrease in synaptic AMPARs. This apparent internalization of synaptic AMPARs is also seen with NMDA application (Fig. 2B), suggesting that Ca2+ influx through NMDARs may trigger internalization. Ca2+ influx also appears to be required for the AMPA-induced internalization of AMPARs. Blockade of voltage-gated Ca2+ channels with 100 μM Cd2+ blocks the internalization of AMPARs, whereas Cd2+ alone has little effect (Fig. 2C). These results suggest that Ca2+ influx through either NMDARs or voltage-gated Ca2+ channels is the trigger for AMPAR internalization.
Figure 2.
Further characterization of glutamate-induced internalization of AMPARs. (A) Glutamate reduces AMPARs at the synapse. Large and strong punctate staining of GluR1 is seen in the untreated culture (arrowheads), whereas small and weaker puncta are observed in cultures treated with glutamate (arrows). The staining of GluR1 in untreated cultures is mainly located at extrusions from the dendrites, resembling dendritic spines. Similar results were obtained in five other fields in three experiments. (Scale bar: 10 μm.) (B) NMDA causes internalization of AMPARs (n = 20 cells and 19 cells for control and 50 μM NMDA, respectively, from 6 experiments). (C) AMPA-induced internalization of AMPARs requires Ca2+ influx through voltage-gated Ca2+ channels. Internalization of AMPARs by AMPA is blocked by treating the cultures with 100 μM Cd2+. Cd2+ alone does not affect internalization (n = 18 cells, 18 cells, 10 cells, and 11 cells for control, Cd2+, AMPA (+Cd2+), and AMPA respectively, from three experiments). (D) Constitutive turnover of AMPARs. Cultures were incubated with Fab fragments of GluR1 antibody for the periods indicated, then quickly washed, fixed, and labeled in the same way to visualize the distribution of AMPARs. More internalization is observed after longer incubation, indicating a gradual internalization of surface AMPARs (n = 20 cells, 20 cells, 20 cells, and 17 cells for 0 min, 10 min, 30 min, and 60 min, respectively, from five experiments).
In addition to this activity-dependent internalization of AMPARs, there is physiological evidence for a constitutive turnover of AMPARs (2, 6, 7, 13). Consistent with these results, AMPARs labeled with Fab fragments of the GluR1 antibody shifted from the surface to the interior of the cell over time (Fig. 2D). The use of Fab fragments makes it unlikely that the internalization is triggered by the crosslinking of bound antibodies.
Depolymerization of Actin Filaments Causes Internalization of AMPARs.
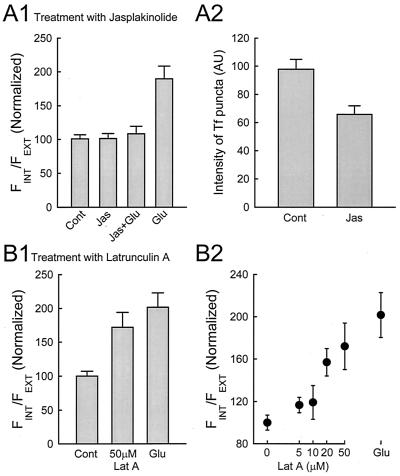
Next, we addressed possible mechanisms underlying the internalization of AMPARs. One possibility is that Ca2+ influx triggers dissociation of AMPARs from their anchoring at the postsynaptic membrane. In particular, elevated intracellular [Ca2+] ([Ca2+]i), is known to depolymerize actin (18), and, in addition, drugs that selectively depolymerize actin filaments cause the disappearance of AMPARs from synapses (19) and a reduction in mEPSC amplitude (20). These results suggest that AMPARs may be anchored to the postsynaptic membrane by actin filaments. To examine this notion in more detail, we applied jasplakinolide (jas), a drug which stabilizes actin filaments (21), to see whether it affected the internalization of AMPARs. Incubation with jas alone did not cause any significant change in internalization of AMPARs compared with the control, but it blocked the effect of glutamate (Fig. 3A1). In addition to preventing the dissociation of AMPARs from the postsynaptic membrane, jas might also affect endocytosis as suggested by Lamaze et al. (22). We tested this possibility by examining the effect of jas on the uptake of Tf. Jas does cause a reduction in the uptake of Tf (Fig. 3A2); however, it is unlikely that this modest reduction in endocytosis can fully account for the blockade of internalization of AMPARs caused by glutamate. We next examined the effect of lat A, a drug that depolymerizes actin, on the internalization of AMPARs. Incubation with lat A caused a clear internalization of AMPARs, in a dose-dependent manner (Fig. 3B). This result supports previous findings suggesting that actin depolymerization, which is triggered by a rise in [Ca2+]i, may be the underlying initiating mechanism for the observed internalization of AMPARs.
Figure 3.
Depolymerization of actin filaments causes internalization of AMPARs. (A) Internalization of AMPAR is blocked by prior incubation with jasplakinolide (jas). Cultures were first treated with 2 μM jas for 2 h before they were labeled with GluR1 antibody, and treated with control or glutamate-containing solutions. Glutamate-induced internalization is blocked by jas, whereas jas by itself has no effect (A1, n = 12, 15, 16, and 15 cells for control, jas, jas + Glu, and Glu, respectively, from four experiments). This effect may be due, at least in part, to an inhibition of endocytosis by jas as the uptake of Tf is also reduced (A2, n = 45 and 42 cells for control and jas, respectively, from five experiments). (B) Latrunculin A (lat A) mimics the effect of glutamate on AMPAR internalization. Cultures were incubated with lat A for 1 h at the concentration indicated, and the distribution of AMPAR was evaluated as above. Lat A causes internalization of AMPAR in a dose-dependent manner (n = 19, 19, 21, 15, 14, and 16 cells for control, 5 μM, 10 μM, 20 μM, 50 μM, and glutamate, respectively, from four experiments).
The above results with lat A were obtained under conditions that would be expected to cause substantial depolymerization of actin filaments that, in turn, would be expected to result in the disappearance of dendritic spines (19). To ensure that disruption of spines could not account for the effect of lat A, we incubated cultures with lower concentrations of lat A and for shorter time periods. Under these conditions, spines can still be observed (Fig. 4A, Lower, arrows), and the percentage of spines with GluR1 staining decreases when compared with untreated cells (Fig. 4B). This loss of AMPARs from spines is accompanied by a reduction in the amplitude and frequency of AMPAR mEPSCs (Fig. 4C). Importantly, this change in mEPSC amplitude is selective for the AMPAR component, because when dual component mEPSCs (AMPAR and NMDAR) were recorded, only the AMPAR component is reduced in the presence of lat A (Fig. 4D).
Glutamate Does Not Alter the Rate of Endocytosis.
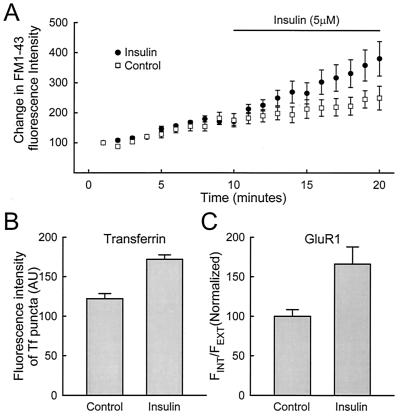
In addition to releasing AMPARs from their anchoring to the postsynaptic membrane, glutamate might also alter the rate of endocytosis by enhancing the efficiency of the endocytotic pathway. Two approaches were used to address this issue. First, we examined the effect of glutamate on the uptake of FM1–43. FM1–43 is a styryl dye that labels the endocytotic compartment if it is present in the extracellular fluid when endocytosis occurs. FM1–43 has been used extensively to study endocytosis of synaptic vesicles (23), as well as internalization in nonneuronal cells (24). Uptake of FM1–43 into the postsynaptic cell can occur in the absence of glutamate, and this uptake is not affected by a subsequent application of high K+, indicating that the labeled compartments are not synaptic vesicles (Fig. 5A1). This uptake is blocked by prior treatment of cells with a hypertonic sucrose solution, consistent with involvement of a clathrin-mediated pathway (Fig. 5A2; refs. 25 and 26). Glutamate has no apparent effect on the uptake rate of FM1–43, as monitored by time-lapse experiments (Fig. 5B). Second, we examined whether uptake of Tf is altered by glutamate application. For the two incubation periods tested, no difference was observed in the presence or absence of glutamate (Fig. 5C). To test the sensitivity of our assay for endocytosis, we next examined the effects of insulin on FM1–43 and Tf uptake. Insulin has been shown to accelerate both endocytosis and exocytosis in nonneuronal cells (27–29) and internalization of AMPARs in neurons (12). Insulin did enhance the uptake of FM1–43 (Fig. 6A) and Tf (Fig. 6B), which was accompanied by an enhanced internalization of AMPARs (Fig. 6C). These results indicate that glutamate, unlike insulin, does not alter the bulk endocytosis in neurons. However, it remains a possibility that the internalization of AMPARs represents a small subset of the overall endocytosis measured with either FM1–43 or Tf, in which case an effect of glutamate on this process would have been missed.
Figure 5.
Glutamate does not alter the rate of endocytosis. (A) FM1–43 labels particles endocytosed through a clathrin-dependent pathway. Incubation of cultures with 1 μM FM1–43 causes distinct labeling around the perinuclear region of the cell body (A1). The particles labeled are not synaptic vesicles because they cannot be released on subsequent depolarization with high K+ solution (A1, 60 mM K+ solution for 1 min, which normally releases all FM1–43 contained in synaptic vesicles, data not shown). Uptake of FM1–43 into the cell body is blocked when cultures were preincubated with hypertonic sucrose solution (A2, 450 mM sucrose solution, total osmolarity was 750 mOsm, treated for 20 min before FM1–43 loading). [Scale bars: 10 μm (A1), 5 μm (A2).] (B) Glutamate does not alter the rate of uptake of FM1–43. The fluorescence intensity of FM1–43 inside the cell body increases roughly linearly with time, and this rate does not differ whether glutamate was present or not. To ensure that the optical section was selected correctly, the fluorescence intensity inside the nucleus was also monitored, which does not change significantly during the same time course (n = 15 cells from 15 experiments). (C) Glutamate does not alter the uptake of Tf. Uptake of Tf was examined with two incubation periods: 5 min or 10 min. In neither case does glutamate alter the uptake significantly. Uptake of Tf depends on a clathrin-mediated pathway because it is severely reduced by prior incubation with hypertonic sucrose solution (n = 26, 24, 29, 29, 14, 16 cells for Tf 5 min, AMPA + Tf 5 min, Tf 10 min, AMPA + Tf 10min, Tf 10 min, and sucrose + Tf 10 min, respectively, from five experiments).
Figure 6.
Insulin increases the rate of endocytosis. FM1–43 and Tf labeling were used to measure the effects of insulin (5 μM) on the rate of endocytosis. An increased uptake of FM1–43 (A: n = 12 cells from 11 experiments) and Tf (B: n = 34 and 25 cells for control and insulin from 5 experiments) is detected, which is accompanied by an enhanced internalization of AMPARs (C: n = 16 and 14 cells for control and insulin, from 3 experiments).
Discussion
We have presented evidence supporting the idea that synaptic AMPARs turn over through a constitutive endocytotic process and that glutamate application greatly enhances this turnover of AMPARs. The glutamate-induced internalization of AMPARs is apparently triggered by Ca2+ influx, because it is also caused by application of NMDA, and is blocked by Cd2+ when AMPA is used. The AMPAR internalization is mimicked by lat A, a drug that selectively depolymerizes actin and is blocked by jasplakinolide, a drug which stabilizes actin filaments, suggesting that the glutamate-induced dissociation of AMPARs from their anchor on the postsynaptic membrane may be because of actin depolymerization. We further show that the efficiency of the endocytotic pathway is not altered by glutamate application, whereas a clear enhancement is observed with insulin application.
To monitor the internalization of AMPARs, we used sequential labeling with secondary antibodies under nonpermeabilizing and permeabilizing conditions and confocal microscopy to visualize the distribution of AMPARs. Judging from the size and distribution of the fluorescent puncta inside the cell body, it is very likely that this staining represents internalized AMPARs inside endosomes (30, 31). It is unclear from the present results whether the visualized internalized AMPARs are being stored temporarily in the cytoplasm before being recycled back onto the surface, or are on their way to being degraded. Because only those AMPARs on the surface before treatment were labeled with antibody, we can only trace the fate of these receptors following stimulation/manipulation. Therefore, we can only conclude that there is a higher internalization rate of AMPARs on stimulation with glutamate. The above results demonstrating a glutamate-induced translocation of AMPARs to the interior of the cell are in agreement with previous results (10).
A number of studies have shown that the cytoskeleton is involved in anchoring neurotransmitter receptors to the postsynaptic membrane. At the neuromuscular junction rapsyn/43-kDa appears to anchor acetylcholine receptors to a subsynaptic cytoskeletal scaffold (32). Actin, in particular, is also important in the stability of surface neuronal nicotinic acetylcholine receptors (nAChRs) on chick ciliary ganglion neurons. A gradual dispersal of nAChR clusters and eventual loss of surface receptors is observed after cell dissociation (33). This loss of surface nAChR is accelerated by incubation with lat A, whereas treatment with jas prevents this loss and maintains the clusters (33). Furthermore, this loss parallels the rundown of nAChR current during whole cell recording, which is postulated to be the result of Ca2+ influx through activated nAChR and subsequent actin depolymerization (34). In cerebellar Purkinje cells, the number of δ glutamate receptor clusters (which are predominantly located on the dendritic spines) is dramatically reduced by incubation with the actin-depolymerizing drugs, cytochalasin D or lat A, suggesting that these receptors are anchored to the actin cytoskeleton (35).
In hippocampal slices, Kim and Lisman (20) observed a reduction in the amplitude of AMPAR EPSCs, but no change in NMDAR EPSCs when cells were perfused with lat B, an actin-depolymerizing drug. In hippocampal culture, incubation with lat A causes a significant reduction in the number of AMPAR-containing spines on pyramidal neurons, suggesting that actin filaments are required for synaptic localization of AMPARs and the maintenance of their clustering (19). Although Allison et al. (19) reported that lat A also caused a decrease in synaptic NMDARs, and a rundown of NMDA whole-cell current has been observed following actin depolymerization (36), our physiological experiments found little change in the NMDAR component, suggesting that, with our treatment, there is a preferential loss of AMPARs, consistent with previous physiological results (20). Furthermore, staining for the NR1 subunit of the NMDAR remains intact following the NMDA-induced loss of actin staining at synapses (21). Importantly, we were able to demonstrate the loss of synaptic AMPARs with short lat A incubation times when spines were still largely intact. This is consistent with previous studies showing that lat A or related drugs do not strongly affect spine morphology on the time scale of a few hours (19, 20, 37). We also found that stabilizing actin filaments with jasplakinolide blocked AMPAR internalization, consistent with the proposal that glutamate induced internalization involves actin depolymerization. However, this result must be treated with caution, because jasplakinolide also inhibited Tf internalization, albeit to a lesser extent.
We also examined the effect of glutamate on the rate of endocytosis, by comparing the uptake rate of FM1–43 and Tf in the absence and presence of glutamate. FM1–43 labels virtually all endocytotic compartments, including the Tf pathway. We have observed consistently that more compartments are labeled by FM1–43 than by Tf (data not shown). Our failure to detect a significant change in the rate of uptake during glutamate application could mean that the endocytotic pathway involved in the internalization of AMPARs represents a small subset of the entire endocytotic pathway labeled with FM1–43. Thus, we tested a more selective endocytotic pathway, the one used by Tf, which has been used widely to assess effects on receptor-mediated endocytosis. Again, we failed to detect any change. On the other hand, insulin, which enhances bulk endocytosis in other cell types, caused a clear enhancement of endocytosis and enhanced internalization of AMPARs, in accord with previous work (12). Our results therefore argue against a glutamate-dependent increase in endocytosis, although it remains possible that the pathway used for the internalization of AMPARs is considerably smaller than that used by Tf.
In summary, our results are in general accord with previous findings, indicating that AMPARs are anchored to the postsynaptic membrane by actin filaments. The precise mechanism for this anchoring is unclear, because a direct interaction between AMPARs and actin has not been reported. We propose a model in which Ca2+ influx at synaptic sites during the application of glutamate untethers synaptic AMPARs, but not NMDARs by depolymerizing actin filaments. This would allow the freed AMPARs to diffuse away from the colocalized NMDARs to a perisynaptic site where they become available to the endocytotic machinery and are selectively internalized. It will be of interest to determine whether long-term depression induced by synaptic stimulation also involves a similar mechanism.
Acknowledgments
We thank Drs. M. Frerking, R. Edwards, and D. Bredt for their comments on the manuscript. R.A.N. is supported by grants from National Institutes of Health and the Bristol–Myers Squibb Co. and is a member of the Keck Center for Integrative Neuroscience and the Silvio Conte Center for Neuroscience Research.
Abbreviations
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid
- AMPAR
AMPA receptor
- NMDA
N-methyl-d-aspartate
- NMDAR
NMDA receptor
- mEPSC
miniature excitatory postsynaptic currents
- Tf
transferrin
- lat A
latrunculin A
- jas
jasplakinolide
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.031573798.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.031573798
References
- 1.Malenka R C, Nicoll R A. Science. 1999;285:1870–1874. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- 2.Luthi A, Chittajallu R, Duprat F, Palmer M J, Benke T A, Kidd F L, Henley J M, Isaac J T, Collingridge G L. Neuron. 1999;24:389–399. doi: 10.1016/s0896-6273(00)80852-1. [DOI] [PubMed] [Google Scholar]
- 3.Lüscher C, Nicoll R A, Malenka R C, Muller D. Nat Neurosci. 2000;3:545–550. doi: 10.1038/75714. [DOI] [PubMed] [Google Scholar]
- 4.Malinow R, Mainen Z F, Hayashi Y. Curr Opin Neurobiol. 2000;10:352–357. doi: 10.1016/s0959-4388(00)00099-4. [DOI] [PubMed] [Google Scholar]
- 5.Scannevin R H, Huganir R L. Nat Rev Neurosci. 2000;1:133–141. doi: 10.1038/35039075. [DOI] [PubMed] [Google Scholar]
- 6.Lüscher C, Xia H, Beattie E C, Carroll R C, von Zastrow M, Malenka R C, Nicoll R A. Neuron. 1999;24:649–658. doi: 10.1016/s0896-6273(00)81119-8. [DOI] [PubMed] [Google Scholar]
- 7.Song I, Kamboj S, Xia J, Dong H, Liao D, Huganir R L. Neuron. 1998;21:393–400. doi: 10.1016/s0896-6273(00)80548-6. [DOI] [PubMed] [Google Scholar]
- 8.Osten P, Srivastava S, Inman G J, Vilim F S, Khatri L, Lee L M, States B A, Einheber S, Milner T A, Hanson P I, Ziff E B. Neuron. 1998;21:99–110. doi: 10.1016/s0896-6273(00)80518-8. [DOI] [PubMed] [Google Scholar]
- 9.Lledo P M, Zhang X, Südhof T C, Malenka R C, Nicoll R A. Science. 1998;279:399–403. doi: 10.1126/science.279.5349.399. [DOI] [PubMed] [Google Scholar]
- 10.Carroll R C, Beattie E C, Xia H, Luscher C, Altschuler Y, Nicoll R A, Malenka R C, von Zastrow M. Proc Natl Acad Sci USA. 1999;96:14112–14117. doi: 10.1073/pnas.96.24.14112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Y T, Linden D J. Neuron. 2000;25:635–647. doi: 10.1016/s0896-6273(00)81066-1. [DOI] [PubMed] [Google Scholar]
- 12.Man Y H, Lin J W, Ju W H, Ahmadian G, Liu L, Becker L E, Sheng M, Wang Y T. Neuron. 2000;25:649–662. doi: 10.1016/s0896-6273(00)81067-3. [DOI] [PubMed] [Google Scholar]
- 13.Nishimune A, Isaac J, Molnar E, Noel J, Nash S R, Tagaya M, Collingridge G L, Nakanishi S, Henley J M. Neuron. 1998;21:87–97. doi: 10.1016/s0896-6273(00)80517-6. [DOI] [PubMed] [Google Scholar]
- 14.Lissin D V, Carroll R C, Nicoll R A, Malenka R C, von Zastrow M. J Neurosci. 1999;19:1263–1272. doi: 10.1523/JNEUROSCI.19-04-01263.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kharazia V N, Weinberg R J. Neurosci Lett. 1997;238:41–44. doi: 10.1016/s0304-3940(97)00846-x. [DOI] [PubMed] [Google Scholar]
- 16.Takumi Y, Ramírez-León V, Laake P, Rinvik E, Ottersen O P. Nat Neurosci. 1999;2:618–624. doi: 10.1038/10172. [DOI] [PubMed] [Google Scholar]
- 17.Racca C, Stephenson F A, Streit P, Roberts J D, Somogyi P. J Neurosci. 2000;20:2512–2522. doi: 10.1523/JNEUROSCI.20-07-02512.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bennett J, Weeds A. Br Med Bull. 1986;42:385–390. doi: 10.1093/oxfordjournals.bmb.a072156. [DOI] [PubMed] [Google Scholar]
- 19.Allison D W, Gelfand V I, Spector I, Craig A M. J Neurosci. 1998;18:2423–2436. doi: 10.1523/JNEUROSCI.18-07-02423.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim C-H, Lisman J E. J Neurosci. 1999;19:4314–4324. doi: 10.1523/JNEUROSCI.19-11-04314.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Halpain S, Hipolito A, Saffer L. J Neurosci. 1998;18:9835–9844. doi: 10.1523/JNEUROSCI.18-23-09835.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lamaze C, Fujimoto L M, Yin H L, Schmid S L. J Biol Chem. 1997;272:20332–20335. doi: 10.1074/jbc.272.33.20332. [DOI] [PubMed] [Google Scholar]
- 23.Ryan T A, Smith S J. Neuron. 1995;14:983–989. doi: 10.1016/0896-6273(95)90336-4. [DOI] [PubMed] [Google Scholar]
- 24.Niles W D, Malik A B. J Membr Biol. 1999;167:85–101. doi: 10.1007/s002329900474. [DOI] [PubMed] [Google Scholar]
- 25.Heuser J E, Anderson R G. J Cell Biol. 1989;108:389–400. doi: 10.1083/jcb.108.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Casey C A, Wiegert R L, Tuma D J. Biochem Pharmacol. 1995;49:1117–1123. doi: 10.1016/0006-2952(95)98509-8. [DOI] [PubMed] [Google Scholar]
- 27.Davis R J, Corvera S, Czech M P. J Biol Chem. 1986;261:8708–8711. [PubMed] [Google Scholar]
- 28.Gibbs E M, Lienhard G E, Appleman J R, Lane M D, Frost S C. J Biol Chem. 1986;261:3944–3951. [PubMed] [Google Scholar]
- 29.Miyata Y, Hoshi M, Koyasu S, Kadowaki T, Kasuga M, Yahara I, Nishida E, Sakai H. Exp Cell Res. 1988;178:73–83. doi: 10.1016/0014-4827(88)90379-5. [DOI] [PubMed] [Google Scholar]
- 30.Dunn K W, McGraw T E, Maxfield F R. J Cell Biol. 1989;109:3303–3314. doi: 10.1083/jcb.109.6.3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mukherjee S, Ghosh R N, Maxfield F R. Physiol Rev. 1997;77:759–803. doi: 10.1152/physrev.1997.77.3.759. [DOI] [PubMed] [Google Scholar]
- 32.Glass D J, Yancopoulos G D. Curr Opin Neurobiol. 1997;7:379–384. doi: 10.1016/s0959-4388(97)80066-9. [DOI] [PubMed] [Google Scholar]
- 33.Shoop R D, Yamada N, Berg D K. J Neurosci. 2000;20:4021–4029. doi: 10.1523/JNEUROSCI.20-11-04021.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu Q, Berg D K. J Neurosci. 1999;19:10280–10288. doi: 10.1523/JNEUROSCI.19-23-10280.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hirai H. Eur J Neurosci. 2000;12:563–570. doi: 10.1046/j.1460-9568.2000.00938.x. [DOI] [PubMed] [Google Scholar]
- 36.Rosenmund C, Westbrook G L. Neuron. 1993;10:805–814. doi: 10.1016/0896-6273(93)90197-y. [DOI] [PubMed] [Google Scholar]
- 37.Fischer M, Kaech S, Knutti D, Matus A. Neuron. 1998;20:847–854. doi: 10.1016/s0896-6273(00)80467-5. [DOI] [PubMed] [Google Scholar]