Abstract
The vestibular organs can feed perceptual processes that build a picture of our route as we move about in the world. However, raw vestibular signals do not define the path taken because, during travel, the head can undergo accelerations unrelated to the route and also be orientated in any direction to vary the signal. This study investigated the computational process by which the brain transforms raw vestibular signals for the purpose of route reconstruction. We electrically stimulated the vestibular nerves of human subjects to evoke a virtual head rotation fixed in skull co-ordinates and measure its perceptual effect. The virtual head rotation caused subjects to perceive an illusory whole-body rotation that was a cyclic function of head-pitch angle. They perceived whole-body yaw rotation in one direction with the head pitched forwards, the opposite direction with the head pitched backwards, and no rotation with the head in an intermediate position. A model based on vector operations and the anatomy and firing properties of semicircular canals precisely predicted these perceptions. In effect, a neural process computes the vector dot product between the craniocentric vestibular vector of head rotation and the gravitational unit vector. This computation yields the signal of body rotation in the horizontal plane that feeds our perception of the route travelled.
As we move about in the world, perhaps walking, perhaps in a vehicle, sensory experiences allow us to build a picture or internal representation of the route that we travel. The vestibular organs of the inner ear, which respond to acceleration of the head, provide sensation that feeds this representation (Mergner et al. 1991; Ivanenko et al. 1997; Glasauer et al. 2002). The problem is that these sensory organs are fixed in the skull, which means that the same whole-body movement will evoke a different pattern of vestibular firing for every different alignment of the head relative to the body. Walking around a corner with the head bent forward looking at the ground and walking the same corner looking up at a signpost will produce very different vestibular signals. The information about the route travelled is encoded in head coordinates. This means that the vestibular signal cannot simply be read off to give the route but must be transformed to take account of the head orientation in space. The vestibular signal also may contain vertical plane acceleration information that is unrelated to the route, meaning that the components of the vestibular signal that are relevant to progression across the terrain must be extracted from the total signal. The present experiments investigate a process that solves these problems of route reconstruction from vestibular information.
Natural vestibular activation requires moving the head in space, but this inevitably activates other sensory receptors making it difficult to tease out the vestibular contribution. This problem can be circumvented by electrically stimulating human vestibular nerves to evoke a signal of virtual head motion without creating a real movement. A small, direct current is passed between electrodes placed behind the ears to modulate the firing of vestibular afferents (Fitzpatrick & Day, 2004). A number of lines of evidence suggest that this galvanic vestibular stimulation (GVS) causes a change in semicircular canal afferent firing (Goldberg et al. 1982; Day & Cole, 2002; Fitzpatrick et al. 2002; Schneider et al. 2002; Wardman et al. 2003; Cathers et al. 2005). Because the electrical stimulus produces the same pattern of afferent firing irrespective of the head's orientation, the signal evoked from the semicircular canal afferents should mimic a head rotation about an axis that is fixed in the skull. Using GVS, we spin the head, ‘virtually’, about a skull-fixed axis and measure the subject's perception of body spin about an earth-fixed vertical axis. By having the subject adopt different head pitch orientations we change the angle between the virtual-spin and vertical-reference axes. Here, we use this GVS technique to investigate the vestibular transformation and extraction problems outlined above. We demonstrate a neural process that, in effect, computes a vector dot product between the craniocentric vestibular vector of head rotation and the gravitational unit vector to create a signal of body rotation in the horizontal, terrestrial plane. Furthermore, by modelling the GVS signal, we are able to compare the empirically determined brain process with a mathematically ideal process, thus revealing how GVS modulates the afferent signal from the semicircular canals.
Methods
Two experiments are described here. The first asks (i) whether a vestibular signal of head rotation, without a contribution from any other sensory source, produces a perception of whole body rotation, and (ii) how alignment of the head relative to the body affects this perception. The second experiment determines whether the perceptual effects scale with the intensity of the vestibular stimulus.
Seven female and three male subjects, aged 24–49, participated after giving informed consent. The institutional human research ethics committee approved the procedures and the study conformed to the Declaration of Helsinki. Subjects were presented with two sensations of rotation. One was a real rotation of the whole body about a vertical axis. The other was a virtual rotation about a head-referenced axis, evoked by electrical stimulation of semicircular canal afferents. The two axes were aligned differently by placing the head at different pitch angles. Subjects reported their perceived rotation about the reference vertical axis when the real and virtual rotations were presented either on their own or together.
Set-up
Real rotation
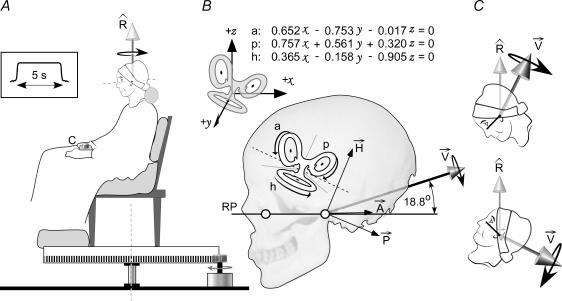
Subjects sat in a chair fixed to a rotating platform (Fig. 1A). The chair was positioned so that the midpoint of the subject's interaural line, regardless of head pitch, coincided with the vertical axis of rotation. A servomotor rotated the chair at one of seven velocities (±10, ±5, ±2, 0 deg s−1; positive clockwise) for 5 s (velocity profile in Fig. 1A). These velocities were chosen because they are of similar magnitude to the expected rotation signal that is evoked by galvanic vestibular stimulation. Small transient wobbles of the platform were superimposed at the start and stop of the platform rotation, including the zero-rotation trials, to make initial and final acceleration cues identical for all chair velocities. Subjects wore blindfolds and heard white noise through earphones to mask cues about rotation and orientation. The chair was padded to minimize sensory cues about rotation from the trunk and legs.
Figure 1. The experiment and model.
A, subjects are rotated at constant velocity for 5 s about a vertical axis (vector Rˇ). At the end of that rotation they use the control (c) to drive themselves back to the starting point. B, planar equations describe the orientation in the skull of the anterior (a), posterior (p) and horizontal (h) semicircular canals in Cartesian coordinates (x, y, z) relative to Reid's plane (RP). The canals are polarized by the orientation of their receptor cells so that the galvanic stimulus will produce a specific rotation signal from each (vectors A⃗, P⃗, H⃗). The 6 canal vectors add to produce a resultant (V⃗) that is directed backwards and elevated 18.8 deg above RP. This direction of V⃗ applies for cathode-left, anode-right stimulation. Opposite polarity reverses the direction. C, when the head is tilted, Rˇ is fixed in terrestrial coordinates but V⃗ changes direction.
Galvanic vestibular stimulation
Bipolar, binaural galvanic vestibular stimulation (GVS) was delivered from a constant-current source to electrodes (3 cm2 AgCl) adhered behind the ears. The three stimulation conditions were anode-right (R+), anode-left (L+), or no stimulus. The stimulus current was a 5 s plateau, smoothed at the beginning and end to reduce sensations that accompany transient currents (current profile in Fig. 1A). Some subjects could feel small cutaneous paraesthesia at the electrodes with the 1.0 mA stimulus but not with the 0.5 mA stimulus.
Virtual rotation
We previously presented a model of the effect of GVS on semicircular canal input to the brain (Fitzpatrick & Day, 2004). Briefly, stimulation of the afferent nerves from each semicircular canal will change their firing rate in a way that would normally signal rotation in the plane of the canal. A net rotation signal from the three canals (Fig. 1B; h, a, p) is the vector sum of the three canal rotations (Fig. 1B; A⃗, P⃗, H⃗). GVS increases the firing frequency of afferents on the cathodal side and decreases firing frequency on the anodal side. Thus, the vector sum of the signals evoked by anodal stimulation on the left side is directed backwards, upwards and laterally (Fig. 1B, V⃗). Cathodal stimulation of the mirrored canals on the right side would produce the same sagittal plane vector but an oppositely directed lateral component. Summing the vectors from both sides leaves a net vector in the mid-sagittal plane, the lateral components having cancelled. Thus, in this experiment, by changing the pitch orientation of the head in space we can change the angle between the vertical axis of real body rotation and the axis of virtual head rotation produced by GVS to range from collinear to orthogonal (Fig. 1C). Furthermore, if each canal is weighted equally, the specific orientation of the resultant vector should be identical to the angle calculated from the planar orientations of the canals provided by Blanks et al. (1975), i.e. inclined up and back by 18.8 deg from Reid's plane, a stereotaxic plane defined by the lines joining the inferior orbital margin and the external auditory meatus on each side (Reid's plane; RP joining open circles in Fig. 1B).
Protocol
In each trial, a brief tone from the earphones signalled that the outward rotation was about to start. Another signalled the end of the rotation, indicating that the subject was to return the chair to the start position using a manual rotary switch that drove the chair left or right at a fixed speed of 3.5 deg s−1. The return speed was different from any outward speed so that time could not be used as a cue. Subjects could adjust the end position if they thought they had undershot or overshot the desired target and were asked to indicate when they were finished.
Experiment 1
Subjects performed 105 trials, one of each combination of five head positions, seven rotation velocities and three galvanic stimuli. The five head positions were distributed between as far forward and as far back as comfortable, with the middle position such that Reid's plane was approximately horizontal. Subjects flexed or extended both the neck and lower spine to attain these positions, where an adjustable, horizontal, padded bar attached to the rotating platform supported the head. The order of head positions was block-randomised between subjects. The different velocities and galvanic stimuli were fully randomised.
Experiment 2
To see how the size of the virtual rotation affected perception, subjects were tested with five galvanic stimuli (+R and +L at 0.5 and 1.0 mA, and no stimulus), the seven velocities as above, but only two head positions (furthest forward and backward), giving a total of 70 trials.
Measurement and analysis
The angular difference between the start position and the final return position was measured for each trial from the motor encoder. A digital photograph of the head in profile was taken against a vertical line for each head position, and head pitch was measured as the inclination of Reid's plane to the vertical (RP, Fig. 1).
To determine whether head pitch or rotation velocity systematically biased subjects' return responses, data of trials in which no galvanic stimulus was applied were examined by two-factor ANOVA for head angle and velocity (Student-Newman-Keuls pairwise multiple comparison). The perceived rotations of Experiment 2 were analysed by two-factor ANOVA for stimulus intensity and head pitch.
Inspection of the perceived rotations revealed a sinusoidal function of head pitch. To average results across subjects, we normalized the amplitude of the perceived responses by fitting a sinusoidal function for each subject (rotation = βsin[pitch − α], least-squares for α and β). The amplitude (β) was used to scale the subject's responses to unity. The same function was then fitted to the pooled normalized data from all subjects to estimate the phase angle (α) at which no rotation was perceived.
Results
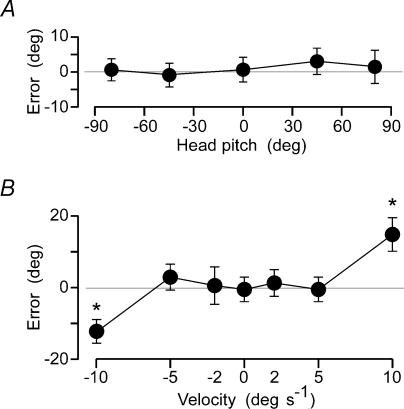
Without the added vestibular stimulus, subjects could return to the start position with a mean absolute error of 7.6 deg for single trials. This was tested with the head supported at five different pitch angles, and this had no effect on the mean return error (Fig. 2A). At all speeds other than the fastest tested here (≤ 5 deg s−1) there was no significant bias in the return position. At the fastest speed (10 deg s−1) subjects undershot the starting point by a mean of 13.6 deg (Fig. 2B). However, as this bias was symmetrical for left and right rotations, it introduced no net bias in the overall result obtained when the galvanic vestibular stimulus was applied.
Figure 2. Perceived body rotation without GVS.
Mean perceived rotation for the group (±2 s.e.m.) is shown for trials in which no GVS was applied. Each graph shows the difference between the start and final positions (Error). A, subjects returned to within a few degrees of the start position and this was not affected by head pitch. B, at speeds less than 5 deg s−1 outward velocity did not affect the accuracy of returning to the start. At 10 deg s−1 they stopped short of the start point.
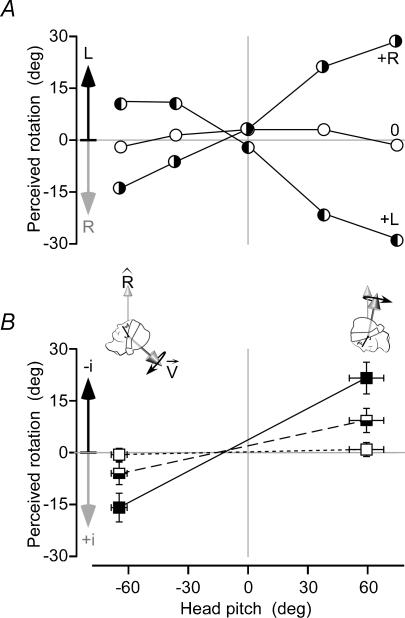
Applying GVS during the outward journey evoked a perception of rotation that was reflected in the pattern of errors that subjects made in returning to the start position. The sign of the error reversed when the stimulus polarity was reversed (Fig. 3A), but most importantly, the error changed with head position. A stimulus that created a perception of body rotation in one direction with the head tilted forwards created the opposite perception with the head tilted backwards and no perception at an intermediate position in which the head was approximately upright (Fig. 3A). This phenomenon was observed at all chair speeds. Even when the chair remained stationary during the ‘outward journey’ subjects reported a perceived rotation by actually moving the chair in the ‘return journey’ to their perceived start. The magnitudes of these effects increased with GVS intensity. Comparing the mean errors at the forward and backward head positions for which the perceptions of the virtual rotation were greatest (Fig. 3B), both head pitch and GVS intensity produced highly significant effects (Pitch × Intensity F2,54= 59.1, P < 0.001). Effects of GVS intensity within pitch levels were highly significant (P < 0.001 for each pitch by post hoc comparison), the perceived rotation being approximately 1.5 times greater with 1 mA than 0.5 mA and no illusory rotation perceived at 0 mA. There were significant effects of head pitch within GVS intensity levels of 0.5 mA and 1.0 mA (P < 0.001 for each), with no effect of head pitch at 0 mA (P = 0.67).
Figure 3. Perceived body rotation with GVS.
A, perceived rotation about the earth-vertical axis (Rˇ) produced by vestibular stimulation for the 5 head positions for 1 subject. Positive head pitch is bent forward (inset figures). With no stimulus (0), the subject realigned herself to within 4 deg of initial orientation. With cathode-left, anode-right stimulation (+R) she perceived rotation to the left but, with the head pitched back, the perceived rotation was to the right. Reversing stimulus polarity (+L) reversed these perceptions. B, group mean perceived rotation (±2 s.e.m.) is shown for stimuli of 0 mA (open squares), ±0.5 mA (semi-filled squares) and ±1.0 mA (filled squares). Responses are normalized to show perceived rotation towards the cathode (−i) as positive values.
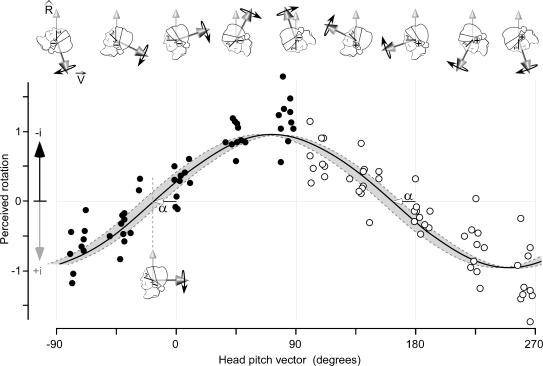
These results show that vestibular signals are interpreted with reference to spatial information about head orientation to provide for a perception of route travelled. The precise relationship between subjects' illusory movement and their head orientation provides important information about this process. To understand the relationship we compared our empirical data with an ideal process. Using our model of the GVS-evoked signal (see Methods), we calculated the perceptual effect that GVS would have assuming that only the component of the virtual head rotation signal in the direction of the vertical reference axis contributes to the perception of the route travelled. This calculation, which predicts a sinusoidal function phase-shifted by −18.8 ± 8.0 deg (95% confidence limits based on the variation of the canal planes from Blanks et al. (1975), is shown as the grey band in Fig. 4. The phase angle is the theoretical position at which the virtual rotation axis is orthogonal to the vertical reference axis (lower inset head in Fig. 4). We have plotted our empirical perceptual data on the same graph as the theoretical data. Anatomical constraints allowed us to study head positions that spanned only 180 deg of pitch but, because anodal and cathodal stimulation evoke signals in opposite directions, we could reverse the direction of the virtual rotation by changing stimulus polarity. This enabled us to plot the perceived rotation across the entire 360 deg orientation of the virtual-rotation axis. To optimize the estimate of this relationship, data from all subjects are combined after first normalizing each subject's contribution to allow for between-subject differences in the absolute size of the perceptual illusion. These pooled data (Fig. 4) show that the perceived rotation is a cyclic function of the angle of the axis of virtual head rotation, coinciding with the calculation based on our model. The continuous curve is the sinusoid of best fit for these data (Y = βsin[X − α]; r2= 0.83). This fit yielded a phase angle: α = −16.4 deg (s.e.m.= 2.8). Our psychophysical data therefore agree remarkably well with the model's prediction of −18.8 deg phase angle.
Figure 4. Perceived GVS-evoked body rotation and model prediction.
Perceived rotation has been normalized for each subject so that the amplitude of the least squares estimate (β, see text) is unity. The data of all subjects are pooled here. The black points were obtained with the anode right stimuli, the white with the anode left. Perceived rotation towards the cathodal electrode (−i) is upward. Zero head pitch (also 180 deg) is the pitch at which Reid's plane is horizontal (black line on face). The continuous line is the least-squares fit: βsin[pitch −α]. The head angle (α) at which the vestibular stimulus produces no perception of rotation is such that Reid's plane is tilted backwards by 16.4 deg. The shaded area shows the 95% confidence limits of the unit rotation vector (dark arrow Vˇ) calculated from anatomical data of the canal planar equations (Fig. 1).
Discussion
From the pattern of the illusory whole-body movement evoked by galvanic vestibular stimulation in this study we can draw several conclusions. First, the net GVS signal represents a virtual rotation of the head about a mid-sagittal axis elevated by approximately 16 deg relative to Reid's plane. Second, vestibular signals from the semicircular canals are transformed according to head orientation with respect to gravity for the perceptual process of route reconstruction. Finally, those components of the total vestibular signal that are not relevant to the perceptual task are disregarded.
To ensure subjects' perceptions of real movement originated predominantly from the semicircular canals, the axis of rotation passed through the middle of the head (Fitzpatrick et al. 2002). Several lines of evidence indicate that GVS modulates the spontaneous firing of the semicircular canal nerves (Goldberg et al. 1982; Day & Cole, 2002; Fitzpatrick et al. 2002; Schneider et al. 2002; Wardman et al. 2003; Cathers et al. 2005). We have proposed that GVS evokes a net signal of head rotation through neural processes that vectorially sum the activity from the six individual semicircular canals (Fitzpatrick & Day, 2004). This model is based on the known effects that GVS has on the firing of single vestibular afferents in animals and the anatomical orientation of the six human semicircular canals (Blanks et al. 1975). We calculated that the rotation axis is directed backwards and slightly upwards (Fitzpatrick & Day, 2004), which was confirmed by the balance responses evoked by GVS in standing subjects (Cathers et al. 2005). The electrical stimulus mimics head rotation about an axis that is fixed in skull coordinates because it produces the same pattern of vestibular nerve firing irrespective of the head's orientation (Fig. 1B). It may also be considered independent of real rotations that occur at the same time; the effects of real rotations and GVS on semicircular canal nerve firing summate linearly when applied together (Lowenstein, 1955). Note that possible GVS effects on otolith afferents have not been included in this model. These are more difficult to predict with certainty from theoretical and anatomical considerations but, with present knowledge, we believe that such effects are likely to be small relative to semicircular canal effects (Fitzpatrick & Day, 2004). This view is supported by (i) the negligible size of the GVS-evoked otolith balance response compared with the canal response (Cathers et al. 2005) and (ii) the near-perfect correspondence between the theoretical phase angle (−18.8 deg) and the measured perceptual phase angle (−16.4 deg).
Our model provides a clue to the computational processes involved in transforming the raw vestibular input into perceptual signals. The model assumes two computations. The first is intrinsic to the vestibular system whereby signals from each of the six semicircular canals are summated vectorially to yield a net rotation vector in craniotopic co-ordinates. The second refers this head-fixed vector to an earth-fixed co-ordinate frame. This process extracts the component of the vestibular signal that lines up with the reference vertical rotation axis, and this represents the perceptual signal of self-motion on the ground. In mathematical terms, this is equivalent to calculating the dot product of the vestibular rotation vector and the reference axis unit vector (Rˇ · V⃗). An ideal process of this sort would produce a perceptual illusion that describes a phase-shifted sinusoid as a function of head-in-space angle. The phase shift arises from the non-zero angle between the axis of virtual head rotation and the axis in Reid's plane that was selected arbitrarily to measure head pitch. The close correspondence between the empirical data and the model output is strong evidence that the brain performs an analogous computation.
The brain must know of the angle between the head and the vertical to perform such a computation. In our simplified experimental situation in which the body is rotated and not linearly accelerated, the computation could be performed entirely from vestibular signals. Thus, the otolith organs provide the gravitational vertical vector, and the semicircular canals provide the rotational vector. Because these signals coexist in the same coordinate frame, the dot product could be performed directly between the two signals without any other information. This computation could involve the rostral vestibular nuclei, for example, as they contain a large proportion of neurones that receive convergent inputs from otolith organs and semicircular canals (Dickman & Angelaki, 2002). In general, however, head rotations occur together with linear accelerations, in which case the otolith organs no longer signal the gravitational vertical vector but the net gravito-inertial acceleration vector. Therefore, it is likely that non-vestibular somatosensory estimates of head orientation contribute to the computation. A neural network that transforms vestibular signals on the basis of somatosensory signals has been identified in the brainstem and cerebellum of cats and monkeys. Within the vermis, Purkinje cells respond to vestibular signals of head movement and this response is modulated by sensory signals from the neck (Manzoni et al. 1999). Similar neck modulation of vestibular signals is present for neurones within the fastigial nucleus (Kleine et al. 2004). In both cases the cerebellar output appears to be referenced to trunk rather than head coordinates. The dense sensory innervations of the neck muscles and joints and their cuneo-cerebellar projection indicate that they provide particularly important information. However, in our experiment the entire spine was bent to achieve the range of head orientations suggesting that proprioceptive signals from all body segments between the head and the reference point to the external world contribute to the transformation.
‘Head direction’ cells within forebrain and midbrain structures discharge with specific horizontal head orientations in an allocentric reference frame, regardless of whole-body orientation or other behaviours (Taube et al. 1990; Taube, 1995; Stackman & Taube, 1997; Robertson et al. 1999; Leutgeb et al. 2000). This directional specificity does not require visual input (Blair & Sharp, 1996; Knierim et al. 1998) but can critically rely on vestibular input (Stackman & Taube, 1997; Stackman et al. 2002). ‘Place cells’ within the hippocampus code for spatial location and are similarly highly dependent on vestibular information (Stackman et al. 2002; Russell et al. 2003). Thus, it is likely that vestibular information feeds a complex network that is the neural substrate of a 2D map across the terrestrial surface. For vestibular information to contribute accurately to updating this map requires the vestibular signal to be projected onto the 2D horizontal surface, irrespective of head orientation and without contamination from route-unrelated vestibular activity as the animal moves. Thus, as in the present experiments, it would be equivalent to calculating the momentary dot product of the vestibular signal and the directional unit vector of gravitational vertical. Such a process is necessary for perceptual processes of the human brain to reconstruct mentally a route of a journey from non-visual sensory information.
Acknowledgments
Supported by the NHMRC of Australia and the MRC of Great Britain.
References
- Blair HT, Sharp PE. Visual and vestibular influences on head-direction cells in the anterior thalamus of the rat. Behav Neurosci. 1996;110:643–660. doi: 10.1037//0735-7044.110.4.643. [DOI] [PubMed] [Google Scholar]
- Blanks RH, Curthoys IS, Markham CH. Planar relationships of the semicircular canals in man. Acta Otolaryngol. 1975;80:185–196. doi: 10.3109/00016487509121318. [DOI] [PubMed] [Google Scholar]
- Cathers I, Day BL, Fitzpatrick RC. Otolith and canal reflexes in human standing. J Physiol. 2005;563:229–234. doi: 10.1113/jphysiol.2004.079525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day BL, Cole J. Vestibular-evoked postural responses in the absence of somatosensory information. Brain. 2002;125:2081–2088. doi: 10.1093/brain/awf212. [DOI] [PubMed] [Google Scholar]
- Dickman JD, Angelaki DE. Vestibular convergence patterns in vestibular nuclei neurons of alert primates. J Neurophysiol. 2002;88:3518–3533. doi: 10.1152/jn.00518.2002. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick RC, Day BL. Probing the human vestibular system with galvanic stimulation. J Appl Physiol. 2004;96:2301–2316. doi: 10.1152/japplphysiol.00008.2004. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick RC, Marsden J, Lord SR, Day BL. Galvanic vestibular stimulation evokes sensations of body rotation. Neuroreport. 2002;13:2379–2383. doi: 10.1097/00001756-200212200-00001. [DOI] [PubMed] [Google Scholar]
- Glasauer S, Amorim MA, Viaud-Delmon I, Berthoz A. Differential effects of labyrinthine dysfunction on distance and direction during blindfolded walking of a triangular path. Exp Brain Res. 2002;145:489–497. doi: 10.1007/s00221-002-1146-1. [DOI] [PubMed] [Google Scholar]
- Goldberg JM, Fernandez C, Smith CE. Responses of vestibular-nerve afferents in the squirrel monkey to externally applied galvanic currents. Brain Res. 1982;252:156–160. doi: 10.1016/0006-8993(82)90990-8. [DOI] [PubMed] [Google Scholar]
- Ivanenko YP, Grasso R, Israel I, Berthoz A. The contribution of otoliths and semicircular canals to the perception of two-dimensional passive whole-body motion in humans. J Physiol. 1997;502:223–233. doi: 10.1111/j.1469-7793.1997.223bl.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleine JF, Guan Y, Kipiani E, Glonti L, Hoshi M, Buttner U. Trunk position influences vestibular responses of fastigial nucleus neurons in the alert monkey. J Neurophysiol. 2004;91:2090–2100. doi: 10.1152/jn.00849.2003. [DOI] [PubMed] [Google Scholar]
- Knierim JJ, Kudrimoti HS, McNaughton BL. Interactions between idiothetic cues and external landmarks in the control of place cells and head direction cells. J Neurophysiol. 1998;80:425–446. doi: 10.1152/jn.1998.80.1.425. [DOI] [PubMed] [Google Scholar]
- Leutgeb S, Ragozzino KE, Mizumori SJ. Convergence of head direction and place information in the CA1 region of hippocampus. Neuroscience. 2000;100:11–19. doi: 10.1016/s0306-4522(00)00258-x. [DOI] [PubMed] [Google Scholar]
- Lowenstein O. The effect of galvanic polarization on the impulse discharge from sense endings in the isolated labyrinth of the thornback ray (Raja clavata) J Physiol. 1955;127:104–117. doi: 10.1113/jphysiol.1955.sp005241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzoni D, Pompeiano O, Bruschini L, Andre P. Neck input modifies the reference frame for coding labyrinthine signals in the cerebellar vermis: a cellular analysis. Neuroscience. 1999;93:1095–1107. doi: 10.1016/s0306-4522(99)00275-4. [DOI] [PubMed] [Google Scholar]
- Mergner T, Siebold C, Schweigart G, Becker W. Human perception of horizontal trunk and head rotation in space during vestibular and neck stimulation. Exp Brain Res. 1991;85:389–404. doi: 10.1007/BF00229416. [DOI] [PubMed] [Google Scholar]
- Robertson RG, Rolls ET, Georges-Francois P, Panzeri S. Head direction cells in the primate pre-subiculum. Hippocampus. 1999;9:206–219. doi: 10.1002/(SICI)1098-1063(1999)9:3<206::AID-HIPO2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Russell NA, Horii A, Smith PF, Darlington CL, Bilkey DK. Long-term effects of permanent vestibular lesions on hippocampal spatial firing. J Neurosci. 2003;23:6490–6498. doi: 10.1523/JNEUROSCI.23-16-06490.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider E, Glasauer S, Dieterich M. Comparison of human ocular torsion patterns during natural and galvanic vestibular stimulation. J Neurophysiol. 2002;87:2064–2073. doi: 10.1152/jn.00558.2001. [DOI] [PubMed] [Google Scholar]
- Stackman RW, Clark AS, Taube JS. Hippocampal spatial representations require vestibular input. Hippocampus. 2002;12:291–303. doi: 10.1002/hipo.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stackman RW, Taube JS. Firing properties of head direction cells in the rat anterior thalamic nucleus: dependence on vestibular input. J Neurosci. 1997;17:4349–4358. doi: 10.1523/JNEUROSCI.17-11-04349.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taube JS. Head direction cells recorded in the anterior thalamic nuclei of freely moving rats. J Neurosci. 1995;15:70–86. doi: 10.1523/JNEUROSCI.15-01-00070.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taube JS, Muller RU, Ranck JB., Jr Head-direction cells recorded from the postsubiculum in freely moving rats. I. Description and quantitative analysis. J Neurosci. 1990;10:420–435. doi: 10.1523/JNEUROSCI.10-02-00420.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardman DL, Day BL, Fitzpatrick RC. Position and velocity responses to galvanic vestibular stimulation during standing. J Physiol. 2003;547:293–299. doi: 10.1113/jphysiol.2002.030767. [DOI] [PMC free article] [PubMed] [Google Scholar]