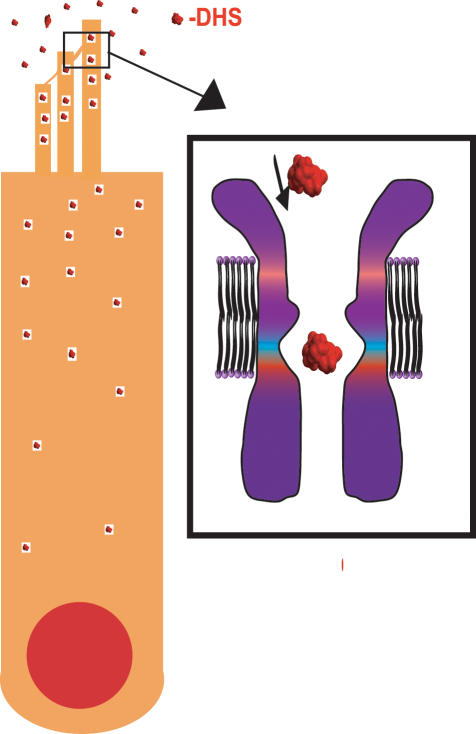
The widely used aminoglycoside antibiotics have the unfortunate side-effect of targeting sensory hair cells of the inner ear, so that treatment often results in permanent hair cell loss. In this issue of The Journal of Physiology, Marcotti et al. (2005) demonstrate that dihydrostreptomycin (DHS) acts as a permeant blocker of the mechanotransducer (met) channel thus allowing DHS to accumulate to toxic levels in these sensory cells.
Aminoglycoside antibiotics such as streptomycin are the most commonly used treatment against gram-negative bacterial infections. Aminoglycosides also target sensory inner ear hair cells by an as yet to be characterized mechanism such that 2–5% of treated patients have irreparable hearing loss. Aminoglycoside toxicity begins with the disarray of hair cell stereocilia leading to their complete disappearance, along with degeneration and death of hair cells. Marcotti et al. (2005) demonstrate that hair cells are targeted because DHS acts as a permeant blocker of the met channel, entering the cell through mechanically gated channels in the stereocilia (Fig. 1), a result consistent with the estimated large size of the met channel pore (Farris et al. 2004). A greater efficacy from the external than the internal face of the channel leads to rapid accumulation of the toxin within the hair cell. If this asymmetrical dose dependence is the result of spatial asymmetries in the free energy spectrum of the pore, as modelled in this paper, then the channel acts as a one-way valve for aminoglycoside entry, promoting intracellular accumulation of DHS. Marcotti et al. calculate that in vivo DHS influx is 0.05 fmol h−1 in the absence of sound stimulation; at this rate it would take 80 s to reach a 1 µm intracellular concentration in an outer hair cell! Lowering external calcium, such as in normal endolymph, enhances DHS permeation of the channel, again promoting intracellular accumulation. Thus, strong evidence is presented that DHS targets hair cells through permeation of met channels.
Figure 1. Schematic representation of proposed mechanism for DHS accumulation in sensory hair cells.
DHS enters through mechanotransducer channels and accumulates because entry requires less energy than exit. The enlarged channel shows DHS size relative to estimated channel size (Farris et al. 2004) showing that indeed the molecule can fit through the channel.
That DHS is a permeant blocker of the met channel explains several other observations regarding aminoglycoside ototoxicity. The incidence of ototoxicity increases with noise exposure, and also in cells with larger transduction currents. Both of these results are consistent with more DHS entering cells where there are more met channels or where the met channels are open for longer periods of time. Mice lacking myosin VII are less sensitive to DHS ototoxicity (Richardson et al. 1997), a result consistent with a permeant channel block because these mutants have met activation curves shifted far to the right, resulting in few open channels at rest. Thus treatments that open met channels increase aminoglycoside toxicity while those closing met channels reduce ototoxicity.
Aside from the clinical relevance of understanding the nature of aminoglycoside action on hair cells, these compounds have been used to investigate the biophysical properties of the met channel. Focal iontophoretic application of gentamicin, and its subsequent blockage of mechanically sensitive currents, was used to localize the transduction channel to the apical surface of the stereocilia (Jaramillo & Hudspeth, 1991), and to estimate channel gating spring force (Jaramillo & Hudspeth, 1993). The voltage dependence of the block and the rapid reduction in tension suggested that DHS blocks the channel in the open position; however, no time-dependent decrease in current amplitude indicative of an open channel block was observed in the presence of DHS (Ohmori, 1985; Kroese et al. 1989; Ricci, 2002). Previous interpretations of this anomaly in turtle auditory hair cells suggested that channel kinetics were comparable to DHS binding kinetics making the time-dependent component undetectable (Ricci, 2002). In mammals, Marcotti et al. (2005) observe a time-dependent decrease in current amplitude confirming the open channel nature of DHS block, a prerequisite of a permeant blocker, and supporting the hypothesis that met kinetics are faster in mammals than in lower vertebrates, thus allowing this time-dependent decrease in current to be observed.
The interaction between calcium and DHS is also complex. As extracellular calcium is lowered DHS efficacy increases (Kroese et al. 1989; Ricci, 2002). This has been postulated to be a competition between calcium and DHS for a binding site within the channel (Kroese et al. 1989). Alternatively, an indirect interaction due to calcium altering met channel kinetics and changing the channel open probability has been postulated (Ricci, 2002). Marcotti et al. (2005) further quantify the relationship between calcium and DHS predicting a DHS binding site within the pore of 0.8 of the distance into the membrane electric field, different from the location of the calcium binding site (0.5) (Farris et al. 2004) and thus supporting an indirect competition between compounds.
Thus the work presented by Marcotti et al. (2005) has significance both at the clinical and biophysical level, yet questions remain. For example is the different efficacy of DHS when presented internally or externally a function of different channel conformations at positive and negative potentials? On the other hand, it may represent a diffusion gradient into the stereocilia from the soma so that concentrations at the internal face of the channel are underestimated. And secondly, are the interactions between calcium and DHS direct or indirect? They appear not to compete for the same location within the pore but might the interaction be non-competitive or perhaps simply a function of the changing open probability? Given that DHS requires the channel to be open for its action, any perturbations that alter the probability of opening of the channel would be predicted to alter DHS efficacy (like the myosin VII mutant mouse). And finally, will knowing the mechanism of DHS entry into the hair cell allow new therapeutic strategies to alleviate the ototoxic effects by inhibiting this pathway?
References
- Farris HE, LeBlanc CL, Goswami J, Ricci AJ. J Physiol. 2004b;558:769–792. doi: 10.1113/jphysiol.2004.061267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaramillo F, Hudspeth AJ. Neuron. 1991;7:409–420. doi: 10.1016/0896-6273(91)90293-9. [DOI] [PubMed] [Google Scholar]
- Jaramillo F, Hudspeth AJ. Proc Natl Acad Sci U S A. 1993;90:1330–1334. doi: 10.1073/pnas.90.4.1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroese AB, Das A, Hudspeth AJ. Hear Res. 1989;37:203–217. doi: 10.1016/0378-5955(89)90023-3. [DOI] [PubMed] [Google Scholar]
- Marcotti W, van Netten SM, Kros CJ. J Physiol. 2005;567:505–521. doi: 10.1113/jphysiol.2005.085951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmori H. J Physiol. 1985;359:189–217. doi: 10.1113/jphysiol.1985.sp015581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci A. J Neurophysiol. 2002;87:1738–1748. doi: 10.1152/jn.00574.2001. [DOI] [PubMed] [Google Scholar]
- Richardson GP, Forge A, Kros CJ, Fleming J, Brown SD, Steel KP. J Neurosci. 1997;17:9506–9519. doi: 10.1523/JNEUROSCI.17-24-09506.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]