Abstract
Dexamethasone, a widely clinically used glucocorticoid, increases human skeletal muscle Na+,K+ pump content, but the effects on maximal Na+,K+ pump activity and subunit specific mRNA are unknown. Ten healthy male subjects ingested dexamethasone for 5 days and the effects on Na+,K+ pump content, maximal activity and subunit specific mRNA level (α1, α2, β1, β2, β3) in deltoid and vastus lateralis muscle were investigated. Before treatment, maximal Na+,K+ pump activity, as well as α1, α2, β1 and β2 mRNA levels were higher (P < 0.05) in vastus lateralis than in deltoid. Dexamethasone treatment increased Na+,K+ pump maximal activity in vastus lateralis and deltoid by 14 ± 7% (P < 0.05) and 18 ± 6% (P < 0.05) as well as Na+,K+ pump content by 18 ± 9% (P < 0.001) and 24 ± 8% (P < 0.01), respectively. Treatment with dexamethasone resulted in a higher α1, α2, β1 and β2 mRNA expression in the deltoid (P < 0.05), but no effects on Na+,K+ pump mRNA were detected in vastus lateralis. In conclusion, dexamethasone treatment increased maximal Na+,K+ pump activity in both vastus lateralis and deltoid muscles. The relative importance of transcription and translation in the glucocorticoid-induced regulation of Na+,K+ pump expression seems to be muscle specific and possibly dependent on the actual training condition of the muscle, such that a high Na+,K+ pump maximal activity and mRNA level prior to treatment prevents the transcriptional response to dexamethasone, but not the increase in Na+,K+ pump content and maximal activity.
The Na+,K+ pump is expressed in almost all mammalian cells (Orlowski & Lingrel, 1988). Since human skeletal muscle comprises ∼40% of the total body mass in adults (Clarys et al. 1999) this tissue contains the largest Na+,K+ pump pool in the body. As such, it is clear that skeletal muscle Na+,K+ pumps play a crucial role in regulation of Na+ and K+ homeostasis, and that any changes in skeletal muscle Na+,K+ pump content or activity may have profound effects on the regulation of Na+,K+ balance not only in muscle but in the entire body.
It is well known that treatment with the artificial glucocorticoid dexamethasone induces an increase in skeletal muscle Na+,K+ pump content of 20–60% (Dorup, 1996; Dorup & Clausen, 1997; Djurhuus et al. 2002). However, dexamethasone treatment has surprisingly been demonstrated not to affect maximal Na+,K+ pump activity in rat skeletal muscle, as detetermined by ouabain-suppressible ATPase activity (Thompson et al. 2001). As noted by the authors, that finding may be questioned, since the ouabain-suppressible ATPase activity assay used by Thompson et al. (2001) has been argued to be unreliable when used in muscle homogenates (Fowles et al. 2004), possibly because of a substantial background activity from other highly active ATP-splitting cellular components (Hansen & Clausen, 1988). However, it is also possible that Na+,K+ pump content is changing differently from maximal Na+,K+ pump activity as evidenced by the recent finding that intense exercise depresses maximal Na+,K+ pump activity but not Na+,K+ pump content in human skeletal muscle (Fraser et al. 2002; Aughey et al. 2004; Leppik et al. 2004).
Glucocorticoids are frequently used clinically, i.e. in treatment of rheumatic disease (Lundberg et al. 2004) and in critical care (Han & Sun, 2002). Due to the effect on Na+,K+ pump expression, this treatment may have possible implications for cardiac and skeletal muscle contractility. Therefore, it should be investigated whether dexamethasone, in addition to the known increase in Na+,K+ pump content in human skeletal muscle, affects maximal Na+,K+ pump activity, determined by use of the 3-O-methylfluorescein phosphatase (3-O-MFPase) assay (Fraser & McKenna, 1998; Fowles et al. 2004) which is more sensitive than the previously applied ATPase-assay in rat skeletal muscle (Thompson et al. 2001). Dexamethasone treatment may be hypothesized to elevate Na+,K+ pump content by increased transcription based on the concomitant increase in α2 and β1 mRNA and protein content observed in rat skeletal muscle (Thompson et al. 2001). However, dexamethasone treatment may also enhance translational efficiency as demonstrated for the α-subunits in a cell-free system (Devarajan & Benz, 2000) and by the finding of increases in Na+,K+ pump α1 protein but not mRNA in rat alveolar epithelial cells (Barquin et al. 1997). In addition, the finding of a dexamethasone induced increase in rat skeletal muscle Na+,K+ pump content of 22% and 48% in gastrocnemius and soleus muscle, respectively (Dorup, 1996), suggest that the response to dexamethasone treatment may be muscle specific. It may be hypothesized that a different increase in Na+,K+ pump content between muscle groups could be related to a difference in oxidative potential, since the oxidative potential has been suggested to be related to the expression of the Na+,K+ pump, at least in rat type II fibres (Fowles et al. 2004). In humans, the postural load-bearing vastus lateralis exhibits a higher succinate dehydrogenase activity in sedentary women (Nygaard, 1981) as well as a higher activity of citrate synthase in cross-country skiers (van Hall et al. 2003) when compared with the less active deltoid muscle. Thus, analysis of vastus lateralis and deltoid muscle adaptation to dexamethasone treatment of humans may reveal whether changes in Na+,K+ pump subunit-specific mRNA, protein content and maximal activity are dependent on the investigated muscle group.
One aim of the present study was to examine if a period of dexamethasone treatment induces an increase in Na+,K+ pump activity and content in human skeletal muscle. A second aim was to investigate to what extent two muscles, which are likely to differ in oxidative potential, respond differently to dexamethasone administration with respect to changes in Na+,K+ pump subunit-specific mRNA, as well as Na+,K+ pump content and maximal activity.
Methods
Ten male subjects with a mean age of 25 years (range 21–35 years) and weight of 78 ± 8 kg (s.d.) participated in the study after giving their informed consent. Eight of the subjects had a sedentary lifestyle and two subjects were recreationally active, as judged from self reported activity profiles. The study conforms to the code of Ethics of the World Medical Association (Declaration of Helsinki) and was approved by the Ethics Committee of Copenhagen and Frederiksberg communities.
Protocol
The subjects ingested 2 mg of dexamethasone at 09.00 h and at 20.00 h for 5 days. Before the treatment and ∼11 h after the last ingestion of dexamethasone, a muscle biopsy was obtained from vastus lateralis and the posterior part of the deltoid muscle. On the day before the biopsies, the subjects consumed a standardized meal in the evening, and refrained from physical activity. On the following day, the subjects consumed a standardized breakfast and reported to the laboratory between 08.00 h and 10.00 h. The two biopsy days were separated by exactly 2 weeks.
Muscle biopsies
Muscle biopsies were obtained under local anaesthesia using a Bergström needle with suction. Visible blood, fat and connective tissue were immediately removed and the biopsy was frozen in liquid N2 no more than 30 s after sampling.
Analyses
Maximal Na+,K+ pump activity
Muscle samples (∼25 mg wet wt) were homogenized in homogenate buffer (HB) containing 250 mm sucrose, 2 mm EDTA and 10 mm Tris (pH 7.40) at 0°C for 2 × 20 s and subsequently stored in liquid N2. After thawing, homogenates were diluted 1/5 in cold HB and then freeze-thawed another three times. Muscle Na+,K+ pump activity was determined in quadruplicate using the K+-stimulated 3-O-methylfluorescein phosphatase activity assay (Fraser & McKenna, 1998). The assay medium (AM) in which 3-O-MFPase activity was measured contained 5 mm MgCl2, 1.25 mm EDTA, 100 mm Tris, and an 80 nm 3-O-methylfluorescein standard (pH 7.40). The freeze-thawed, diluted homogenate (30 μl) was incubated in 2.5 ml AM at 37°C for 5 min before addition of 40 μl of 10 mm 3-O-MFP to initiate the reaction. After 60 s, 10 μl of 2.58 m KCl (final concentration, 10 mm) was added to stimulate K+-dependent phosphatase activity, and the reaction was measured for a further 60 s. All assays were performed at 37°C, using continuous stirring, with data sampled at 1 Hz, on a spectrofluorimeter (Aminco Bowman AB2 SLM, Thermospectronic, Madison, WI, USA). Excitation wavelength was 475 nm, and emission wavelength was 515 nm. The 3-O-MFPase activity was calculated from the difference in the linear time versus fluorescence relationship before and after addition of 10 μm KCl (Fraser & McKenna, 1998). All slopes were determined including a minimum of 20 measurements. The maximal Na+,K+ pump activity is expressed relative to the protein content of the homogenate, which was determined spectrophotometrically using serial diluted bovine serum albumin as a standard.
Na+,K+ pump content
Na+,K+ pump content was determined as [3H]ouabain binding site content, as previously described (Norgaard et al. 1983). Briefly, muscle samples of ∼5 mg wet wt were analysed in triplicate. Each specimen was washed for 2 × 10 min in 37°C vanadate buffer (VB) containing 250 mm sucrose, 10 mm Tris, 3 mm MgSO4, and 1 mm NaVO4 (pH 7.2–7.4) followed by incubation for 120 min at 37°C in 2 ml VB and [3H]ouabain (10−6m, 2.0 μCi ml−1). After incubation, the samples were washed 4 × 30 min in ice-cold VB, blotted on filter paper, and weighed. After incubation at 5°C overnight in 0.5 ml of 5% TCA and 0.1 mm ouabain, 2.5 ml of scintillation fluid (Opti-Fluor, Packard) was added before liquid scintillation counting. The content of [3H]ouabain binding sites was expressed as picomoles per gram wet weight.
RNA isolation and reverse transcription
From each sample ∼25 mg wet wt muscle tissue was used for RNA extraction by a modified guanidinium thiocyanate (GT)–phenol–chloroform extraction method (Chomczynski & Sacchi, 1987) as previously described (Pilegaard et al. 2000). Briefly, the samples were homogenized in the GT solution and RNA was extracted by centrifugation after adding sodium acetate pH 4.0, diethylpyrocarbonate-saturated phenol, and chloroform–isoamyl-OH. RNA was precipitated by centrifugation after adding isopropanol and the pellet was rinsed with 75% ethanol, and resuspended in 50 μl nuclease free water containing 0.1 mm EDTA. The RNA quality was verified by detection of 18S and 28S bands using electrophoresis (formaldehyde and ethidium bromide containing 2.5% agarose gel). Furthermore, the absorbance at 260 nm/280 nm ratio was higher than 1.7. The Superscript II RNase H− system and Oligo dT primers were used for reverse transcription of 11 μl RNA following the manufactures protocol (Invitrogen, Carlsbad, CA, USA).
cDNA quantification
In order to circumvent the problems associated with using endogenous controls such as GAPDH as reference gene (Mahoney et al. 2004; Dheda et al. 2004) the amount of RNA–cDNA hybrids (dsHybrids) in each reverse transcribed sample was determined using the PicoGreen reagent (Molecular Probes) and subsequently used for normalization (C. Lundby & H. Pilegaard, unpublished observations). Each sample was analysed in triplicates composed of 2.5 μl RNA:cDNA template, 97.5 μl TE buffer, and 100 μl of 200 × TE-diluted PicoGreen reagent in each well. Using a Fluoroskan Ascent (Thermo Labsystems) the fluorescence emission intensity was measured at 520 nm with excitation at 480 nm. The mean reading of each triplicate analysis was converted to an absolute amount by the use of a standard curve constructed from a serial dilution of bacteriophage lambda DNA standard (Molecular Probes).
Real time PCR
Specific primers and probes were designed for each of the mRNA sequences of interest as previously published (Nordsborg et al. 2005). It was verified that amplification of RNA samples not subjected to reverse transcription did not result in a detectable PCR product within the cycle numbers used for analysis of mRNA expression. Validation of the different PCR product sizes was performed by electrophoresis (ethidium bromide containing 2.5% agarose gel). The ABI 7900 real time PCR system was used for relative quantification. Each reaction was composed of 1 μl of the diluted cDNA and 5 μl of 2 × TaqMan® Universal MasterMix (AmpliTaq Gold DNA Polymerase, AmpErase uracil N-glycosylase, dNTPs with dUTP, buffer components, ROX as passive reference; Applied Biosystems, Foster City, USA). Primers, probe and water were added to give a final reaction volume of 10 μl per well. Triplicate analyses were performed for each PCR reaction. Prior to PCR cycling, incubation at 50°C for 2 min followed by 95°C for 10 min was performed to activate the UNG enzyme and AmpliTaq Gold enzyme, respectively. PCR cycling was performed by heating to 95°C for 15 s followed by 60°C for 60 s. A total of 40 cycles were completed. An arbitrary mRNA amount was calculated from a standard curve constructed using a serial diluted sample.
Statistics and calculations
Expression of β3 mRNA was only detected in four of the samples obtained from the deltoid before treatment. Thus, no statistics are reported for β3 mRNA. Expression of mRNA was calculated as the arbitrary mRNA amount divided by the dsHybrid amount. Before performing the statistical analysis, all mRNA expression data were logarithmic-transformed in order to obtain a normally distributed dataset. A two-way ANOVA with muscle and treatment as variables was performed. Significant main effects were analysed with a Student-Newman-Keuls post hoc test. Gene expression data are reported as geometric means and 95% confidence intervals. Apart from mRNA data, results are reported as the mean ± s.e.m. unless otherwise stated. A two-way ANOVA for repeated measurements followed by a Student-Newman-Keuls post hoc test was used to test for any significant differences in Na+,K+ pump maximal activity or content. The level of significance was set to P < 0.05. All statistical analyses were performed using SigmaStat v. 2.03.
Results
Na+,K+ pump content
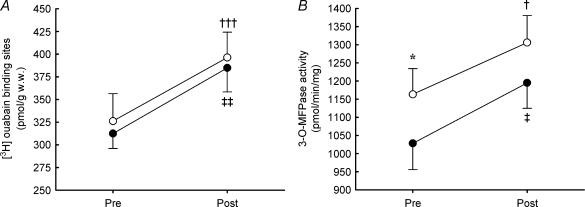
Before dexamethasone treatment the Na+,K+ pump content, determined as [3H]ouabain binding, was 326 ± 30 and 312 ± 17 pmol (g wet wt)−1 in vastus lateralis and deltoid, respectively, with no difference between the muscles (Fig. 1). Treatment with dexamethasone increased Na+,K+ pump content by 18 ± 9% (P < 0.001) in vastus lateralis and by 24 ± 8% (P < 0.01) in deltoid, with no difference between vastus lateralis and deltoid (Fig. 1).
Figure 1. Na+,K+ pump content determined as [3H]ouabain binding sites (A) and Na+,K+ pump activity determined as maximal K+ stimulated 3-O-MFPase activity in vitro (B) in human vastus lateralis (open symbols) and deltoid (filled symbols) muscle before (Pre) and after (Post) dexamethasone treatment (n = 10).
Values are the mean and s.e.m. Significant differences between muscles are denoted by *P < 0.05. Significant difference between pre and post in vastus lateralis are denoted by †P < 0.05; †††P < 0.001, and in deltoid by ‡P < 0.05; ‡‡P < 0.01.
Na+,K+ pump maximal K+ stimulated activity
The maximal K+ stimulated Na+,K+ pump activity in vitro, determined as maximal 3-O-MFPase activity, was 13% higher (P < 0.05) in vastus lateralis compared to deltoid (1163 ± 70 versus 1028 ± 73 pmol min−1 g−1) before dexamethasone treatment (Fig. 1). After the treatment, the maximal K+ stimulated in vitro Na+,K+ pump activity increased by 14 ± 7% (P < 0.05) in vastus lateralis and 18 ± 6% (P < 0.05) in the deltoid, with no difference between the increases in vastus lateralis and deltoid (Fig. 1). After dexamethasone treatment no difference (P = 0.13) in maximal K+ stimulated Na+,K+ pump activity was observed between vastus lateralis and deltoid.
Na+,K+ pump mRNA
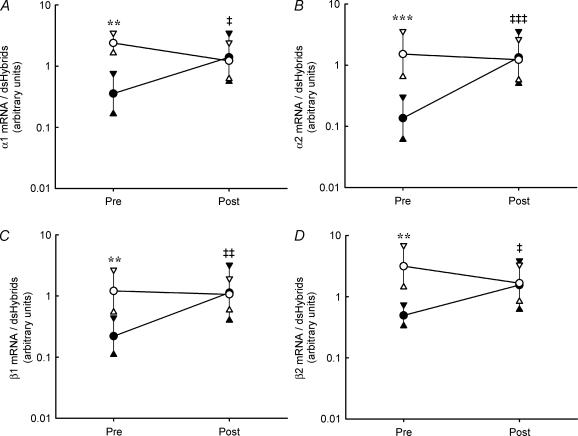
Before dexamethasone treatment, mRNA for all investigated subunits of the Na+,K+ pump was expressed at a higher level in vastus lateralis than in deltoid muscle (Fig. 2) with a difference of ∼7- (P < 0.001), 11- (P < 0.001), 6- (P < 0.01), 6- (P < 0.001) and 5-fold for α1, α2, β1, β2 and β3, respectively. Treatment with dexamethasone resulted in a higher mRNA expression in the deltoid, comprising a ∼3- (P < 0.05), 7- (P < 0.001), 4- (P < 0.01), 3- (P < 0.05) and 5-fold increase in α1, α2, β1, β2 and β3 mRNA, respectively (Fig. 2). No effect of dexamethasone on Na+,K+ pump subunit mRNA expression in vastus lateralis could be detected. After dexamethasone treatment, no differences in Na+,K+ pump subunit specific mRNA expression existed between vastus lateralis and the deltoid.
Figure 2. Na+,K+ pump subunit α1 (A), α2 (B), β1 (C) and β2 (D) mRNA expression in human vastus lateralis (open symbols) and deltoid (filled symbols) muscle before (Pre) and after (Post) dexamethasone treatment (n = 9–10).
Values are geometric means and 95% confidence intervals. Significant differences between muscles are denoted by **P < 0.01; ***P < 0.001. Significant treatment effect in deltoid is denoted by ‡P < 0.05; ‡‡P < 0.01; ‡‡‡P < 0.001. No significant treatment effect existed for any of the subunits in vastus lateralis.
Discussion
The major findings of the present study are that dexamethasone treatment increased Na+,K+ pump maximal activity, as well as content, in a similar way in human deltoid and vastus lateralis muscle and that dexamethasone treatment increased deltoid, but not vastus lateralis, Na+,K+ pump subunit-specific mRNA. Furthermore, the maximal Na+,K+ pump activity and Na+,K+ pump subunit-specific mRNA levels were lower in deltoid than vastus lateralis muscle prior to dexamethasone treatment.
The present study describes for the first time an increase in skeletal muscle maximal Na+,K+ pump activity after dexamethasone treatment of humans, in addition to the well known increase in Na+,K+ pump content (Dorup & Clausen, 1997; Djurhuus et al. 2002). In rat skeletal muscle no increase in maximal Na+,K+ pump activity was detected after dexamethasone treatment (Thompson et al. 2001). The observation of an increase in maximal Na+,K+ pump activity in the present study is most likely related to the inclusion of 10 subjects, the use of a repeated design and the use of the more sensitive 3-O-MFP assay for determination of maximal Na+,K+ pump activity (Hansen & Clausen, 1988; Fowles et al. 2004). The finding of an elevated maximal Na+,K+ pump activity in human skeletal muscle implies that a period of dexamethasone ingestion may have profound effects on the Na+,K+ homeostasis of the human body.
The present study also aimed at detecting possible differences in the response to dexamethasone between two different muscle groups. In the deltoid, Na+,K+ pump α1, α2, β1 and β2 mRNA increased concomitantly with Na+,K+ pump maximal activity and content, leading to the speculation that dexamethasone induced an increased transcription of Na+,K+ pump genes and thereby caused the increased protein expression. This hypothesis is supported by the previous finding of an concomitant increase in Na+,K+ pump α2 and β1 subunit mRNA and protein in rat skeletal muscle after dexamethasone treatment (Thompson et al. 2001). However, Thompson et al. (2001) did not find any change in α1 mRNA and reported a reduced β2 mRNA expression. The apparent discrepancy between the present study and the study by Thompson et al. (2001) may be due to species differences or to the more vigorous dexamethasone treatment regime, which in the study by Thompson et al. (2001) comprised continuous infusion of 0.1 mg kg−1 dexamethasone per day for 14 days compared to the 5 day treatment with 2 × 2 mg (around 0.05 mg kg−1) per day in the present study. It should be noted that α2 and β1 mRNA is by far the most highly expressed in human skeletal muscle (Nordsborg et al. 2005) and therefore the major alterations in Na+,K+ pump mRNA observed in human skeletal muscle is in concordance with the finding in rat skeletal muscle (Thompson et al. 2001). As opposed to the increase in deltoid Na+,K+ pump mRNA, no changes in mRNA expression were observed in vastus lateralis after dexamethasone treatment. Combined with the observed increase in Na+,K+ pump content and maximal activity, the lack of elevated mRNA levels suggests that Na+,K+ pump protein expression was increased by translational regulation in the vastus lateralis. This is in line with the suggestion that dexamethasone can enhance translation of Na+,K+ pump mRNA via a putative glucocorticoid modulatory element in the α subunit structure (Devarajan & Benz, 2000), and the finding that dexamethasone can increase Na+,K+ pump activity and α1 protein expression without concomitant changes in α1 mRNA (Barquin et al. 1997). The muscle-specific response to dexamethasone treatment at the mRNA level can be hypothesized to be dependent on the muscle Na+,K+ pump mRNA and maximal activity levels prior to the treatment, which again may be related to a higher oxidative potential in vastus lateralis compared with deltoid in humans (Nygaard, 1981; van Hall et al. 2003). This hypothesis is based on the fact that contractile activity has the potential to increase both oxidative capacity (Saltin et al. 1976) and Na+,K+ pump mRNA and protein expression (Nordsborg et al. 2005; Nielsen et al. 2004). Thus, a larger contractile activity of the vastus lateralis compared with the deltoid may be the reason for the higher Na+,K+ pump mRNA level and maximal activity found in vastus lateralis prior to dexamethasone treatment. Furthermore, it can be speculated that a high prevailing mRNA level may reduce the mRNA increase to a given stimulus. It appears not to have been caused by a difference in fibre type distribution, since no difference in the ST distribution between deltoid and vastus lateralis was found in a group of physical education students (Tesch & Karlsson, 1985). Likewise, young women had the same relative number of ST, FTa and FTx in vastus lateralis and deltoid (Nygaard, 1981). Only for well-trained athletes has a difference in fibre type distribution between vastus lateralis been observed (Tesch & Karlsson, 1985). Finally, the muscle group-specific regulation may also imply a more profound difference in histology and physiological function between arm and leg muscles.
In summary, the present study demonstrates that dexamethasone treatment increases maximal Na+,K+ pump activity as well as Na+,K+ pump content in human skeletal muscle. Furthermore, the vastus lateralis was found to have a higher level of Na+,K+ pump mRNA than the deltoid, a difference that was eliminated by the selective increase of Na+,K+ pump mRNA in deltoid after dexamethasone treatment. Thus, it is suggested that dexamethasone induced regulation of Na+,K+ pump expression can occur both at the transcriptional and translational level in human skeletal muscle.
Acknowledgments
The present study was supported by two grants from Novo Nordisk Fonden, The Danish Ministry of Culture and The Danish Natural Science Research Council.
References
- Aughey RJ, Gore CJ, Hahn AG, Garnham AP, Clark SA, Petersen AC, Roberts AD, McKenna MJ. Chronic intermittent hypoxia and incremental cycling exercise independently depress muscle in-vitro maximal Na+,K+ATPase activity in well-trained athletes. J Appl Physiol. 2004;98:186–192. doi: 10.1152/japplphysiol.01335.2003. [DOI] [PubMed] [Google Scholar]
- Barquin N, Ciccolella DE, Ridge KM, Sznajder JI. Dexamethasone upregulates the Na-K-ATPase in rat alveolar epithelial cells. Am J Physiol. 1997;273:L825–L830. doi: 10.1152/ajplung.1997.273.4.L825. [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Clarys JP, Martin AD, Marfell-Jones MJ, Janssens V, Caboor D, Drinkwater DT. Human body composition: a review of adult dissection data. Am J Human Biol. 1999;11:167–174. doi: 10.1002/(SICI)1520-6300(1999)11:2<167::AID-AJHB4>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Devarajan P, Benz EJ., Jr Translational regulation of Na-K-ATPase subunit mRNAs by glucocorticoids. Am J Physiol Renal Physiol. 2000;279:F1132–F1138. doi: 10.1152/ajprenal.2000.279.6.F1132. [DOI] [PubMed] [Google Scholar]
- Dheda K, Huggett JF, Bustin SA, Johnson MA, Rook G, Zumla A. Validation of housekeeping genes for normalizing RNA expression in real-time PCR. Biotechniques. 2004;37:112–119. doi: 10.2144/04371RR03. [DOI] [PubMed] [Google Scholar]
- Djurhuus MS, Henriksen JE, Klitgaard NA. Magnesium, sodium, and potassium content and [3H]ouabain binding capacity of skeletal muscle in relatives of patients with type 2 diabetes: effect of dexamethasone. Metabolism. 2002;51:1331–1339. doi: 10.1053/meta.2002.35199. [DOI] [PubMed] [Google Scholar]
- Dorup I. Effects of K+, Mg2+ deficiency and adrenal steroids on Na+, K+-pump concentration in skeletal muscle. Acta Physiol Scand. 1996;156:305–311. doi: 10.1046/j.1365-201X.1996.208000.x. [DOI] [PubMed] [Google Scholar]
- Dorup I, Clausen T. Effects of adrenal steroids on the concentration of Na+-K+ pumps in rat skeletal muscle. J Endocrinol. 1997;152:49–57. doi: 10.1677/joe.0.1520049. [DOI] [PubMed] [Google Scholar]
- Fowles JR, Green HJ, Ouyang J. Na+-K+-ATPase in rat skeletal muscle: content, isoform, and activity characteristics. J Appl Physiol. 2004;96:316–326. doi: 10.1152/japplphysiol.00745.2002. [DOI] [PubMed] [Google Scholar]
- Fraser SF, Li JL, Carey MF, Wang XN, Sangkabutra T, Sostaric S, Selig SE, Kjeldsen K, McKenna MJ. Fatigue depresses maximal in vitro skeletal muscle Na+-K+-ATPase activity in untrained and trained individuals. J Appl Physiol. 2002;93:1650–1659. doi: 10.1152/japplphysiol.01247.2001. [DOI] [PubMed] [Google Scholar]
- Fraser SF, McKenna MJ. Measurement of Na+, K+-ATPase activity in human skeletal muscle. Anal Biochem. 1998;258:63–67. doi: 10.1006/abio.1998.2572. [DOI] [PubMed] [Google Scholar]
- Han YY, Sun WZ. An evidence-based review on the use of corticosteroids in peri-operative and critical care. Acta Anaesthesiol Sin. 2002;40:71–79. [PubMed] [Google Scholar]
- Hansen O, Clausen T. Quantitative determination of Na+-K+-ATPase and other sarcolemmal components in muscle cells. Am J Physiol. 1988;254:C1–C7. doi: 10.1152/ajpcell.1988.254.1.C1. [DOI] [PubMed] [Google Scholar]
- Leppik JA, Aughey RJ, Medved I, Fairweather I, Carey MF, McKenna MJ. Prolonged exercise to fatigue in humans impairs skeletal muscle Na+-K+-ATPase activity, sarcoplasmic reticulum Ca2+ release, and Ca2+ uptake. J Appl Physiol. 2004;97:1414–1423. doi: 10.1152/japplphysiol.00964.2003. [DOI] [PubMed] [Google Scholar]
- Lundberg IE, Grundtman C, Larsson E, Klareskog L. Corticosteroids – from an idea to clinical use. Best Pract Res Clin Rheumatol. 2004;18:7–19. doi: 10.1016/j.berh.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Mahoney DJ, Carey K, Fu MH, Snow R, Cameron-Smith D, Parise G, Tarnopolsky MA. Real-time RT-PCR analysis of housekeeping genes in human skeletal muscle following acute exercise. Physiol Genomics. 2004;18:226–231. doi: 10.1152/physiolgenomics.00067.2004. [DOI] [PubMed] [Google Scholar]
- Nielsen JJ, Mohr M, Klarskov C, Kristensen M, Krustrup P, Juel C, Bangsbo J. Effects of high-intensity intermittent training on potassium kinetics and performance in human skeletal muscle. J Physiol. 2004;554:857–870. doi: 10.1113/jphysiol.2003.050658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordsborg N, Thomassen M, Lundby C, Pilegaard H, Bangsbo J. Contraction induced increases in Na+, K+ -ATPase mRNA levels in human skeletal muscle are not amplified by activation of additional muscle mass. Am J Physiol Regul Integr Comp Physiol. 2005;289:R84–R91. doi: 10.1152/ajpregu.00771.2004. [DOI] [PubMed] [Google Scholar]
- Norgaard A, Kjeldsen K, Hansen O, Clausen T. A simple and rapid method for the determination of the number of 3H-ouabain binding sites in biopsies of skeletal muscle. Biochem Biophys Res Commun. 1983;111:319–325. doi: 10.1016/s0006-291x(83)80154-5. [DOI] [PubMed] [Google Scholar]
- Nygaard E. Skeletal muscle fibre characteristics in young women. Acta Physiol Scand. 1981;112:299–304. doi: 10.1111/j.1748-1716.1981.tb06820.x. [DOI] [PubMed] [Google Scholar]
- Orlowski J, Lingrel JB. Tissue-specific and developmental regulation of rat Na,K-ATPase catalytic alpha isoform and beta subunit mRNAs. J Biol Chem. 1988;263:10436–10442. [PubMed] [Google Scholar]
- Pilegaard H, Ordway GA, Saltin B, Neufer PD. Transcriptional regulation of gene expression in human skeletal muscle during recovery from exercise. Am J Physiol Endocrinol Metab. 2000;279:E806–E814. doi: 10.1152/ajpendo.2000.279.4.E806. [DOI] [PubMed] [Google Scholar]
- Saltin B, Nazar K, Costill DL, Stein E, Jansson E, Essen B, Gollnick D. The nature of the training response; peripheral and central adaptations of one-legged exercise. Acta Physiol Scand. 1976;96:289–305. doi: 10.1111/j.1748-1716.1976.tb10200.x. [DOI] [PubMed] [Google Scholar]
- Tesch PA, Karlsson J. Muscle fiber types and size in trained and untrained muscles of elite athletes. J Appl Physiol. 1985;59:1716–1720. doi: 10.1152/jappl.1985.59.6.1716. [DOI] [PubMed] [Google Scholar]
- Thompson CB, Dorup I, Ahn J, Leong PK, McDonough AA. Glucocorticoids increase sodium pump α2- and β1-subunit abundance and mRNA in rat skeletal muscle. Am J Physiol Cell Physiol. 2001;280:C509–C516. doi: 10.1152/ajpcell.2001.280.3.C509. [DOI] [PubMed] [Google Scholar]
- van Hall G, Jensen-Urstad M, Rosdahl H, Holmberg HC, Saltin B, Calbet JA. Leg and arm lactate and substrate kinetics during exercise. Am J Physiol Endocrinol Metab. 2003;284:E193–E205. doi: 10.1152/ajpendo.00273.2002. [DOI] [PubMed] [Google Scholar]