Abstract
Knowledge of macromolecular distribution volumes is essential in understanding fluid transport within normal and pathological tissues. In this study in vivo we determined the distribution volumes of several macromolecules, including one monoclonal antibody, in tumours and tested whether charges associated with the tumour extracellular matrix influence their available volumes. Steady state levels of the monoclonal antibody trastuzumab (Herceptin) (pI = 9.2), IgG (pI = 7.6) as well as native (pI = 5.0) and cationized albumin (pI = 7.6) were established in rats bearing dimethylbenzanthracene (DMBA)-induced mammary tumours by continuous infusion using osmotic minipumps. After a 5–7 day infusion period, the rats were nephrectomized and the extracellular volume was determined with 51Cr-labelled EDTA. Plasma volumes were measured with 125I-labelled human serum albumin or rat IgM in a separate series. Steady state concentrations of probes were determined in the interstitial fluid that was isolated by centrifugation from tumours or by post mortem wick implantation in the back skin. Calculations were made for interstitial fluid volume (Vi), along with the available (Va/Vi) and excluded (Ve/Vi) relative interstitial volume fractions. The Ve/Vi for the positively charged trastuzumab in tumours averaged 0.29 ± 0.03 (n = 16), a value which was significantly lower than the corresponding one for IgG of 0.36 ± 0.02 (n = 16). Native albumin was excluded from 38% of the tumour interstitial fluid, whereas cationization of albumin reduced the excluded volume by ∼50%. Our experiments suggest that the tumour interstitium acts as a negatively charged matrix and is an important factor in determining the macromolecular distribution volume.
The interstitium is a crowded microenvironment where the presence of substances such as collagen and glycosaminoglycans (GAGs) limits the space accessible to plasma proteins and other macromolecules, a phenomenon called volume exclusion (Bert & Pearce, 1984). In all tissues the magnitude of the excluded volume has important consequences in the dynamics of transcapillary exchange. Due to exclusion, the effective protein concentration in the interstitium is significantly higher than the value that would be estimated if it were assumed that all the fluid in the interstitium was available. In tumours, studies of exclusion phenomena are of special interest since the interstitium represents a major barrier to drug delivery (Jain, 1997). Moreover, the presence of charged matrix components that are markedly increased during tumour development, e.g. hyaluronan (see Toole, 2004), could potentially influence even further the tissue distribution of charged macromolecular species via electrostatic repulsion forces.
Previous reports have shown that macromolecular charge affects transvascular passage in tumours and that positively charged macromolecules extravasate (Dellian et al. 2000) and accumulate (Krasnici et al. 2003) more rapidly in the tumour interstitium. In a recent study, Pathak et al. (2005) described a MRI method to study extravasation and tissue distribution of albumin in tumours for a period up to 140 min. To attain a steady state concentration of a macromolecular probe requires a significantly longer equilibration period in most tissues including tumours (Reed & Wiig, 1983), but the steady state distribution of macromolecules in the fluid bathing the tumour cells and the potential effects of charges on such distribution have never been studied in vivo.
Modification of the net charge of a macromolecular species in order to deliver it selectively to pathological target sites along with the development of methodologies that allow for direct interstitial sampling are important investigative tools for cancer therapy. We have previously developed a continuous infusion method that allows for measuring the available and excluded volume of plasma proteins and other macromolecules in vivo (Wiig et al. 1992; Wiig & Tenstad, 2001), and wanted to optimize and test its applicability in tumour physiology. A fundamental requirement for using of this method is a reliable estimation of a probe concentration in the interstitial fluid. This was made possible by a recently described centrifugation method for isolation of tumour interstitial fluid (Wiig et al. 2003a). We asked two questions, whether the extracellular matrix excludes monoclonal antibodies and plasma proteins from a substantial part of the tumour interstitial fluid in vivo, and whether the fixed negative charges in the matrix influence the distribution volume of macromolecular species. If the tumour interstitium per se is a major barrier for the uptake of macromolecular therapeutic agents, we would also expect that the excluded volume of macromolecular probes would be high compared to normal tissue. To investigate these issues we established steady state concentrations of the monoclonal antibody trastuzumab (Herceptin) and other macromolecular probes of different sizes and charges by continuous infusion, and measured their respective concentrations in tumour interstitial fluid.
Methods
Experimental animals
The experiments were performed in anaesthetized female Sprague-Dawley rats, 208–255 g, fed on a standard laboratory diet. Mammary tumours were induced by gavage of 16 μg dimethylbenzanthracene (DMBA), as described in detail previously (Wiig et al. 2003a). The rats had free access to food and water prior to any experimental procedure. Anaesthesia was induced by intraperitoneal injection of a mixture of Hypnorm/Dormicum and pentobarbital as specified below. While under anaesthesia, the body temperature of these animals was maintained constant at 37 ± 1°C by using a heating lamp. All animal experiments were conducted in accordance with the regulation of the Norwegian State Commission and with preapproval from the ethical committee of the University of Bergen.
Substances
Trastuzumab (Herceptin, Roche), a recombinant humanized IgG-1 monoclonal antibody against the human epidermal growth factor 2 (HER-2), was purchased from the University Hospital pharmacy. Rat IgG (fraction V), rat IgM and rat serum albumin (RSA) (fraction V) were obtained from Sigma.
Preparation of probes
The isoelectric point of trastuzumab and IgG was determined by capillary electrophoresis (ProteomlabT PA800, Beckman Coulter, Inc., Fullerton, CA, USA) using an isoelectric focusing kit (eCAPT cIEF 3-10).
Charge modified rat serum albumin, cRSA, was prepared by a method slightly modified from that described by Hoare & Koshland (1967). The principle of this method is based on activation of carboxyl groups within a protein by 1-ethyl-3-(3-dimethylaminopropyl carbodiimide) hydrochloride (EDC) and their subsequent amidation by ethylenediamine (free base), as described in detail elsewhere (Wiig et al. 2003b). Briefly, 150 mg RSA was dissolved in 15 ml of 0.133 m glycine methyl ester (Sigma). The pH of the mixture was adjusted to 4.75 by adding either HCl or NaCl as needed. The ‘cationization’ was started by adding 5 ml 0.04 m EDC (Sigma) to the reaction mixture. The level of cationization depends on the reaction time, i.e. the protein becomes more positive as a function of reaction time. All reactions were conducted at 20°C while stirring continuously.
We aimed at a neutral or slightly positive pH. Based on previous experience, such a pH value could be obtained from a reaction time of 45 min (Wiig et al. 2003b). Thus, the reaction was stopped by removing aliquots at 45 min and adding an equal volume of 4 m sodium acetate buffer to the solution. After the charge modification the albumin solutions were dialysed against distilled water for at least 24 h before radiolabelling.
The pI values of RSA and cRSA were determined by isoelectric focusing on a commercially available mini-gel system (CBS Scientific, Del Mar, USA). The isoelectric focusing gels (Novex, pH 3–10) were run for 1 h at 100 mV, 1 h at 200 mV and 0.5 h at 500 mV at constant temperature (18°C). The gels were fixed for 60 min in a solution of 12 g trichloracetic acid and 3.5 g sulfosalilicylic acid in 100 ml distilled water and then stained with Novex colloidal blue. The pH gradient profile was determined with marker proteins (Pharmacia Biotech Broad pI Calibration Kit, pI = 3.5–9.3). Native RSA had an average pI of 5.0 whereas the slightly positive albumin, cRSA, had an average pI of 7.6. We verified by autoradiography whether the radioactive label had a charge distribution that corresponded to that observed from the isoelectric focusing gel (Wiig et al. 2003b).
Radiolabelling of the probes
All probes were labelled with 125I or 131I by Iodo-Gen. Briefly, 5 mg 1,3,4,6-tetrachloro-3,6-diphenylglycouril (Sigma-Aldrich Co., product number T0656) was dissolved in 5 ml chloroform and 0.1 ml of this solution was dispersed in a 1.8 ml Nunc vial (Nunc-Kamstrup, Roskilde, Denmark). A film of the virtually water insoluble Iodo-Gen was formed in the Nunc vial by allowing the chloroform to evaporate to dryness under nitrogen. Then 1.5 mg of protein dissolved in 1 ml 0.05 m phosphate-buffered saline (PBS) solution, pH 7.5, containing 10 MBq 125I or 131I (Institute for Energy Technology, Kjeller, Norway) and 15 μl 0.01 m NaI was added, and the iodinating tube gently agitated for 10 min before the reaction was terminated by removing the protein solution. Unincorporated iodine isotope accounting for 5–10% of the total radioactivity as estimated by trichloroacetic acid (TCA) precipitation was removed by dialysing the tracer against 1 litre of 0.9% saline containing 0.02% azide. The stock solution was stored in the dark at 4°C and dialysed for at least 24 h before use.
Measurement of distribution volumes
The present method used for determination of available and excluded volume in vivo is based on reaching steady-state tracer, or probe, concentrations in the extracellular compartment by a slow continuous infusion of radiolabelled macromolecules using an implantable osmotic pump. The labelled probes were infused for 5 or 7 days at a constant flow rate of 1 μl h−1 with an implantable Alzet osmotic pump (model 2001, capacity 200–220 μl), as described in detail previously (Wiig et al. 1992). Briefly, the day before implantation, the pump was filled with the mixture of the isotope solution where the total radioactivity had been adjusted to about 4 MBq ml−1 and then incubated overnight at 37°C in a beaker containing 0.02% sodium azide in 0.9% NaCl solution. The following morning, the rat was anaesthetized intraperitoneally with a 1: 1 mixture of Hypnorm (fentanyl/fluanisone)/Dormicum (midazolam), 2.5 ml kg−1. A bolus of 25 μl solution was administered through the syringe into the left jugular vein and the catheter was connected with the previously incubated pump. The pump was then inserted into the interscapular region and the incision was closed with wound clips. The rat was monitored until recovery under a heat lamp and then transferred to a cage. All rats recovered from anaesthesia within a couple of hours after pump implantation. They were allowed free access to food and water for the remaining duration of the experiment. Daily blood samples taken from the tail vein starting two days after pump implantation verified that the tracer level was stable during the experiment (Wiig et al. 1992; Gyenge et al. 2003).
At the final day of the experiment, the rat was anaesthetized with pentobarbital, 50 mg kg−1 intraperitoneally, and both kidney pedicles were ligated via flank incisions. Then ∼0.15 MBq 51Cr-labelled EDTA was injected through a PE 50 catheter inserted in the right jugular vein for measurement of extracellular fluid volume. After a 51Cr-labelled EDTA equilibration time of 120 min, plasma and tissue were sampled. Tumours were bisected and one part used for determination of radioactivity whereas the other was used for isolation of interstitial fluid (see below).
To measure interstitial distribution, it is necessary to correct for the plasma volume, which was measured in a separate series of experiments. Tumour vessels have been shown to be leakier than normal tissue capillaries (Jain, 1997). If the intravascular tracer leaks out of the vessels during the equilibration phase, the intravascular volume will be overestimated. To see if such leakage occurred, we measured plasma volume using labelled rat serum albumin (see above) and the even larger molecule IgM (Mr∼900 000) (fraction V, Sigma). Before labelling, the IgM was purified by HPLC. The isolated fraction was dialysed against distilled water and freeze-dried. After resuspension in labelling buffer, the purified IgM was labelled as described above. To determine plasma volume, a mixture of 125I-labelled IgM and 131I-labelled RSA (∼0.3 MBq) was injected i.v. after anaesthesia in six rats and allowed 5 min of equilibration. At the end of this period, a ∼0.5 ml blood sample was taken by cardiac puncture and the rat killed by injection of KCl. Tissue samples were taken for determination of radioactivity as described for the continuous infusion experiments.
Isolation of interstitial fluid
Tumour interstitial fluid was obtained by centrifugation as detailed previously (Wiig et al. 2003a). Briefly, the anaesthetized rat was immediately transferred to an incubator kept at room temperature (20–24°C) and 100% relative humidity. Tumours were excised, flushed with saline to remove blood from the surface, blotted gently with tissue paper to remove excess saline and transferred to 2-ml centrifuge tubes used for isolation of tumour fluid. The centrifuge tubes were provided with a basket of nylon mesh with pore size ∼15 μm × 20 μm designed to keep the sample up from the bottom of the tube (Aukland, 1991). The tube was immediately capped, reweighed and spun at 240 g (1500 r.p.m.) in an Eppendorff 5417R centrifuge placed in a cold room at 4°C and then immediately brought back into the incubator. In a previous study we justified the optimal centrifugation speed that provides representative samples of interstitial fluid for this type of tumours (Wiig et al. 2003a). Tumour fluid accumulated at the bottom was collected in graduated glass capillaries that were closed immediately for later determination of radioactivity or protein concentration by HPLC (see below). Typical tumour fluid samples were 2–15 μl.
To compare the tracer distribution volume in tumour tissue with that in skin, interstitial fluid was isolated from back subcutis using dry wicks implanted for 20 min post mortem (Wiig et al. 1992). Fluid isolated from back subcutis was assayed for radioactivity and protein concentration as described for tumours.
Determination of radioactivity
Samples were counted in an LKB gamma counter (model 1282 Compugamma) using window settings of 530–690 keV for 51Cr, 700–860 keV for 131I and 120–320 keV for 125I. Standards were counted in every experiment, and spillover as well as background and decay during the period of measurement were automatically corrected for.
Elution of isotope from tissue
To be able to determine the concentration of tracer in the interstitial fluid we need to be able to account for the amount bound to cells and other tissue components. Thus all individual samples were analysed and corrected for tracer binding to the tissue by measuring ‘free’ and ‘bound’ tracer. We achieved this by eluting the free tracer in the extracellular fluid as detailed (Wiig & Tenstad, 2001; Gyenge et al. 2003). After mincing and determination of radioactivity, tissue samples were eluted in 10 ml 0.9% saline solution containing 0.02% sodium azide during agitation at room temperature for 24 h. After centrifugation, the supernatant was removed, a new aliquot of 10 ml saline–azide solution was supplemented and the agitation procedure repeated. After another 24 h of elution, the individual supernatant-free tissues were counted again and corrected for isotope decay. The fraction of tracer remaining in the tissue after elution represents non-specific binding, and such binding was corrected for in all calculations of distribution volumes.
Characterization of iodine-labelled isotopes
HPLC analyses were performed in order to evaluate whether 125I or 131I was incorporated in other plasma proteins or if the isotope changed during the infusion period (Gyenge et al. 2003). Samples of final plasma from rats (i.e. plasma collected at the end of the experiment after the 5th or 7th day of infusion) were subjected to chromatographic separation in a Superose 12 HR 10/30 size exclusion gel chromatography column with an optimal separation range of 10–300 kDa. This column had a void volume 7.8 ml as estimated with blue dextran, and total column volume 22 ml as estimated with acetone. The elution solution used was a 0.005 m phosphate buffer in 0.15 m NaCl, pH 7.4. The successive collection fractions exiting the column were counted in the gamma-counter.
Measurement of IgG and albumin concentration
The Superose 12 HR 10/30 column described above was also used for measurement of concentration of IgG and albumin in tumour fluid isolated by centrifugation, wick fluid from back skin, and plasma. About 1 μl isolated fluid in a total volume of 100 μl eluent (sodium phosphate buffer, 0.15 m, pH 7.4) was injected onto the HPLC system using a Gilson 234 autoinjector (200 μl loop). Constant flow of 1 ml min−1 was obtained by a Spectreseries P2000 pump (Thermo separation products) and the protein concentration in the elution fluid was measured by UV detection at 280 nm (Spectraseries UV100). The UV signal was digitalized, sampled at 2 Hz and analysed on a computer using ChromoQuest (version 2.51, ThermoQuest Corporation, the Netherlands).
Immunohistochemistry
DMBA tumours were processed for demonstration of possible cross-reaction to the HER2-receptor as part of the routine at the Haukeland University Hospital. Only a weak reaction was observed (data not shown) suggesting a low amount of receptors available for binding of trastuzumab.
Calculations
The extracellular, interstitial and plasma volumes in tumours and skin and the corresponding available and excluded volumes were calculated as previously described (Gyenge et al. 2003; Wiig et al. 1992) and by replacing wick fluid with centrifugate for tumours. The available (Va) and excluded (Ve) volumes for the macromolecular probes are given as fractions of the corresponding interstitial fluid volume (Vi).
Statistics
Data are given as the mean ± 1 s.e.m., and were compared with Student's t test or one-way analysis of variance (ANOVA) and with Tukey's test for multiple comparisons. Differences were accepted as statistically significant at the P < 0.05% level.
Results
Tracer characteristics
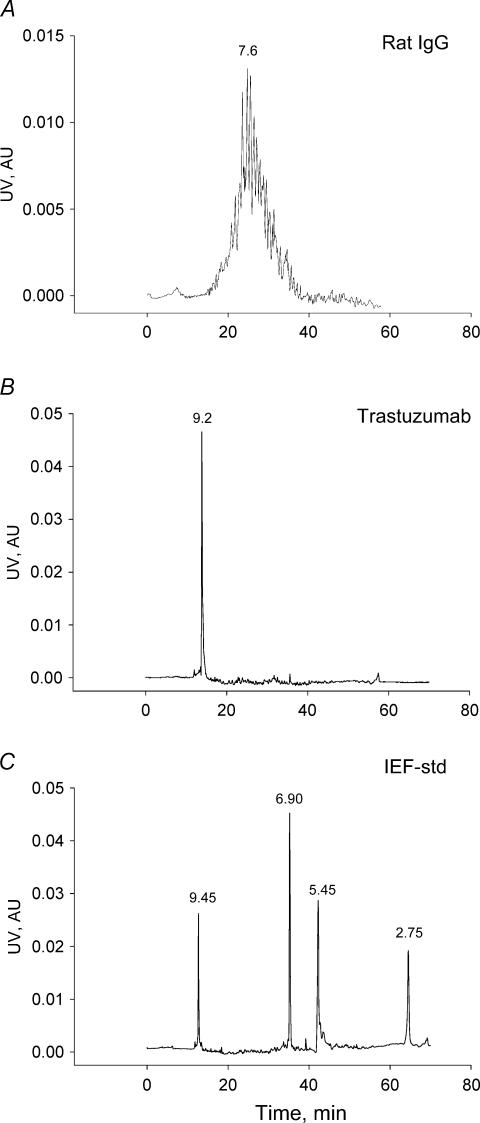
As is evident from Fig. 1, the monoclonal antibody trastuzumab had a slightly positive pI of 9.2. Rat IgG was close to neutral with an average pI of 7.6.
Figure 1. pI of immunoglobulins as determined by capillary electophoresis.
A, pherogram for rat IgG, showing a heterogeneous distribution of pI values for the various isoforms that together constitute the IgG molecule. Average pI indicated on curve. B, the charge distribution of trastuzumab was very homogeneous with a single peak corresponding to a pI of 9.2. C, relevant isoelectric focusing standards. AU, arbitary units.
We were able to ‘titrate’ the charge of rat serum albumin in order to cancel out the charge effect, and produced a probe with neutral or slightly positive pI. The pI of the native RSA was 5.0, whereas cationization for 45 min produced RSA (cRSA) with an average pI of 7.6 (Gyenge et al. 2003).
Establishment of steady-state levels of tracers
The relative concentrations of all tracers in plasma sampled at day 2, 4 and 6 were normalized to the corresponding concentration in final plasma at the end of the infusion period. All plasma tracer concentrations remained stable during the experimental period. In agreement with our previous experience (Wiig et al. 1992; Gyenge et al. 2003), none of the average consecutive relative concentrations differed significantly from the final value of 1.0. The amount of free label, 125I or 131I, in final plasma was less than 1% as determined by column chromatography, with no tendency to rise with increasing infusion times.
We terminated the experiments at 120 and 168 h and measured the corresponding plasma equivalent distribution volumes for the probes to test whether steady-state tissue concentration was attained at these times. Volumes estimated at 120 h did not differ significantly from the corresponding numbers found at 168 h (P > 0.05 for all comparisons), suggesting that that steady state conditions were obtained for all tracers in both types of tissues studied. Therefore data obtained at 120 and 168 h infusion time have been pooled.
IgG and albumin concentration and mass
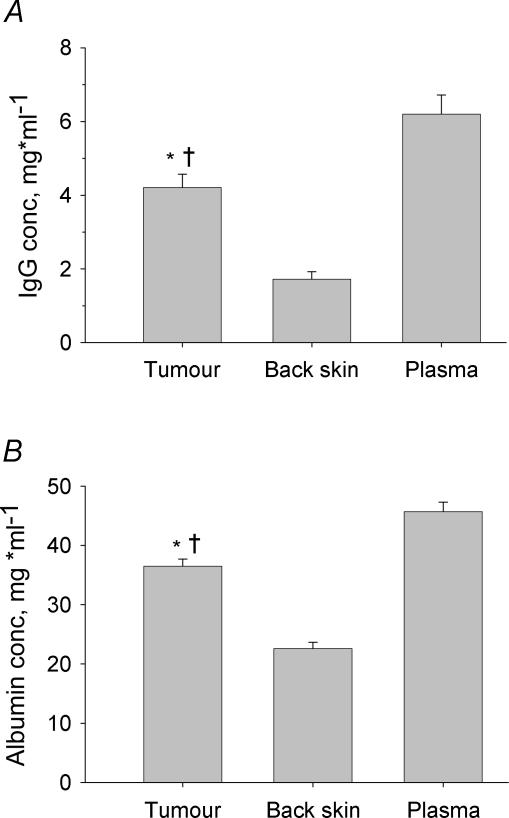
The concentrations of IgG and albumin in plasma as well as interstitial fluid isolated from tumours and skin were quantified by HPLC. As evident from Fig. 2A, tumour fluid IgG averaged 4.2 mg ml−1 (n = 16), significantly lower than the corresponding concentration in plasma of 6.2 mg ml−1 (n = 16), both significantly higher than the IgG concentration in back skin wick fluid of 1.7 mg ml−1 (n = 12). The mean concentration of albumin in tumour fluid was 36.5 mg ml−1 (n = 18), significantly different from the corresponding concentrations of 45.7 (n = 20) and 22.6 mg ml−1 (n = 16) in plasma and back skin interstitial fluid, respectively (Fig. 2B).
Figure 2. Concentration of IgG and albumin in tissue fluids and plasma.
Concentration in tumour and back skin interstitial fluid and plasma of IgG (A) and albumin (B) in tumour and back skin as determined by HPLC. The concentration of IgG as well as albumin was significantly higher in tumour than in back skin but significantly lower than in plasma. The significantly higher concentration of IgG and albumin in tumour compared to back skin suggest less selective capillaries and/or less efficient lymph drainage in the former organ. Error bars: 1 s.e.m.*P < 0.05 when compared to plasma and †P < 0.01 when compared to back skin using ANOVA.
The plasma equivalent volumes for all probes suggested that a steady state was reached at 120 and 168 h of infusion. To substantiate these observations, we compared the specific activities of labelled IgG and RSA in plasma, tumour and back skin interstitial fluid after tracer infusion. For IgG, the specific activity of tumour centrifugate and back skin wick fluid relative to plasma averaged 1.06 ± 0.11 (n = 12) and 1.01 ± 0.09 (n = 12), respectively. The corresponding numbers for albumin were 1.05 ± 0.07 (n = 8) and 0.99 ± 0.11 (n = 8). None of these values differed significantly from 1.0, showing that steady states were established.
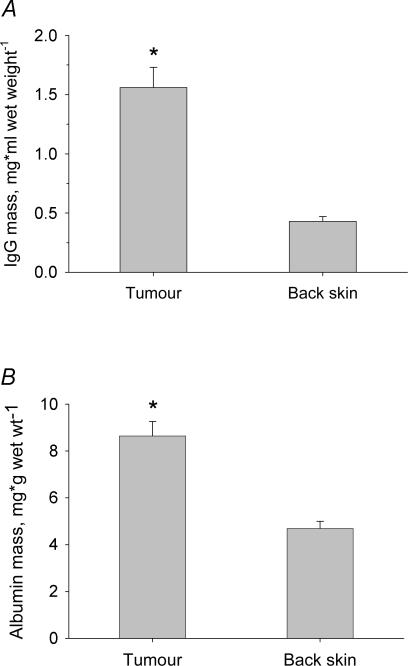
The specific activity of IgG and albumin was used to calculate their extravascular amounts (Fig. 3). In tumour, IgG averaged 1.56 mg (g wet weight)−1, while the amount in back skin was only 0.43 mg (P < 0.001) (Fig. 3A). Albumin averaged 8.6 mg (g wet weight)−1 in tumour, which was significantly higher than the 4.7 mg (g wet weight)−1 found in back skin (Fig. 3B) (P < 0.001).
Figure 3. Content of IgG and albumin in tumours and back skin.
Content of IgG (A) and albumin (B) in tumour and back skin as calculated from the specific activity and tissue counts of tracers. The content of IgG as well as albumin was significantly higher in tumour than in back skin. Error bars: 1 s.e.m.*P < 0.01 when compared to back skin using t tests.
Tissue elution experiments
The tracers were more easily extracted from tumours than from back skin. The extraction fractions of trastuzumab and IgG in tumours were 0.93 ± 0.01 (n = 16) and 0.89 ± 0.01 (n = 16) (P < 0.01), respectively, with corresponding fractions of 0.77 ± 0.05 (n = 12) and 0.70 ± 0.05 (n = 12) (P < 0.01), in back skin. More specifically, the low amount of binding of trastuzumab suggests no cross-reaction to rat HER-2 receptors, in agreement with the results from immunohistochemistry. Charge modification caused albumin to be less elutable than the native substance. Thus for tumours (n = 18), the extracted fractions were 0.92 ± 0.01 and 0.94 ± 0.01 for cRSA and RSA (P < 0.05), respectively, with corresponding numbers for back skin (n = 12) of 0.81 ± 0.02 and 0.92 ± 0.01 (P < 0.001). The extraction fractions of 51Cr-EDTA were 97–99% for all tissues.
Fluid distribution volumes
To correct for the intravascular fraction of tracer, local plasma volumes were determined using 131I-labelled human serum albumin (HSA) and 125I-labelled IgM (n = 6). In tumours, the intravascular volume averaged 19.4 ± 3.5 and 18.6 ± 2.7 μl (g wet wt)−1 as estimated with IgM and HSA, respectively (P = 0.847), suggesting that during the time needed for intravascular volume determination, there was no significant extravasation of the smaller albumin molecule.
The interstitial fluid volumes (Vi) in tumours (n = 34) and back skin (n = 24) averaged 0.37 ± 0.01 and 0.42 ± 0.01 ml (g wet wt)−1, respectively.
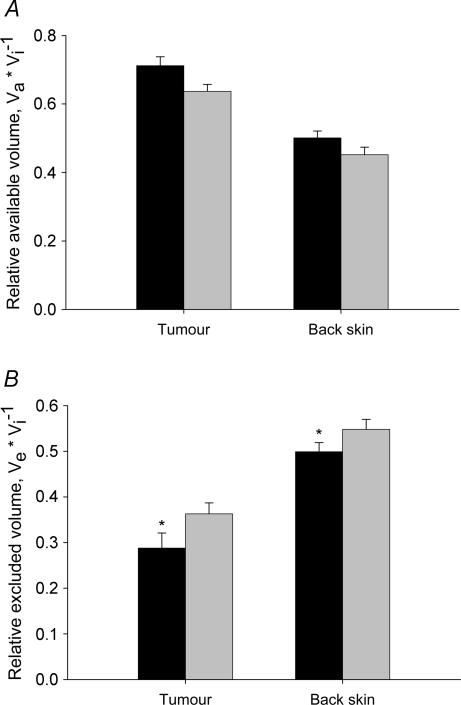
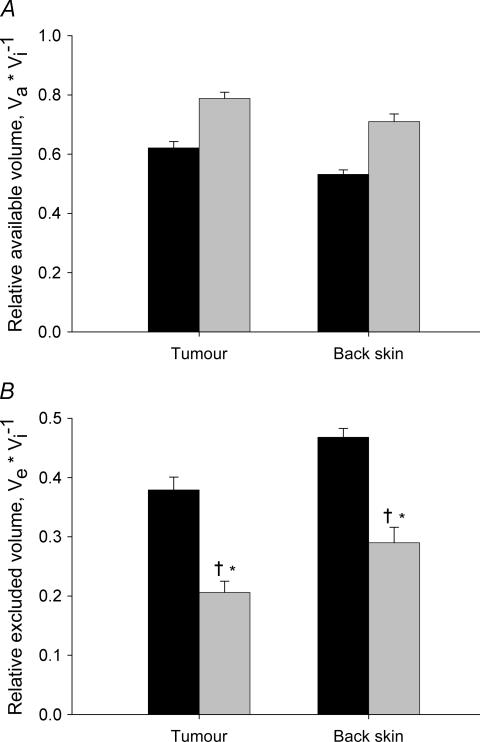
In Fig. 4 we have compiled data for relative available and excluded volumes for trastuzumab and IgG. We observe that the distribution volumes of the probes were higher in tumour than in back skin, and that there was a charge effect that resulted in a larger distribution volume for the more positively charged trastuzumab (Fig. 4A).
Figure 4. Relative available and excluded interstitial volumes of immunoglobulins.
A, relative available volume of trastuzumab (pI = 9.2) (black bars) and IgG (pI = 7.6) (shaded bars) in tumour and back skin. Error bars: 1 s.e.m.B, relative excluded volume for the same substances and tissues as described in A, using the same symbols. The more positive trastuzumab had a significantly lower excluded volume compared to the more neutral IgG. Error bars: 1 s.e.m.*P < 0.05 when compared to IgG in corresponding tissue.
The relative excluded volume (Ve) for these probes was calculated as Ve= 1 −Va/Vi, and these data are displayed in Fig. 4B. Ve for IgG in tumours averaged 0.36, whereas the more positively charged trastuzumab had a significantly lower average excluded volume fraction of 0.29 (P < 0.05). Although numerically different, the same pattern was observed in back skin, where the excluded fractions of IgG and trastuzumab were 0.55 and 0.50, respectively (P < 0.05).
The distribution volumes for the two IgG species suggested that there was a charge effect on macromolecular distribution in tumours. To explore this effect further, we studied the distribution of native and cationized albumin with an even larger difference in pI. A more pronounced charge effect was found for the albumin probes (Fig. 5), supporting our initial hypothesis. In tumour, the average Ve for native albumin was 0.38, whereas the corresponding number for cRSA was 0.21 (P < 0.001) (Fig. 5B). A lower Ve for the cationized probe was also found in back skin, averaging 0.47 and 0.29 for RSA and cRSA, respectively (P < 0.001). All volumes for back skin differed significantly from the corresponding volumes in tumour.
Figure 5. Relative available and excluded interstitial volumes of native and cationized albumin.
A, relative available volume of native rat serum albumin (RSA) (pI = 5.0) (black bars) and cationized RSA (cRSA) (pI = 7.6) (shaded bars) in tumour and back skin. Error bars: 1 s.e.m.B, relative excluded volume for the same substances and tissues as described in panel A, using the same symbols. For albumin, charge neutralization reduced the relative excluded volume fraction in both tissues, most prominent in tumour. *P < 0.001 when compared to native albumin and †P < 0.01 when compared to IgG in corresponding tissue (cf. Fig. 4).
Characteristics of the tracers in plasma
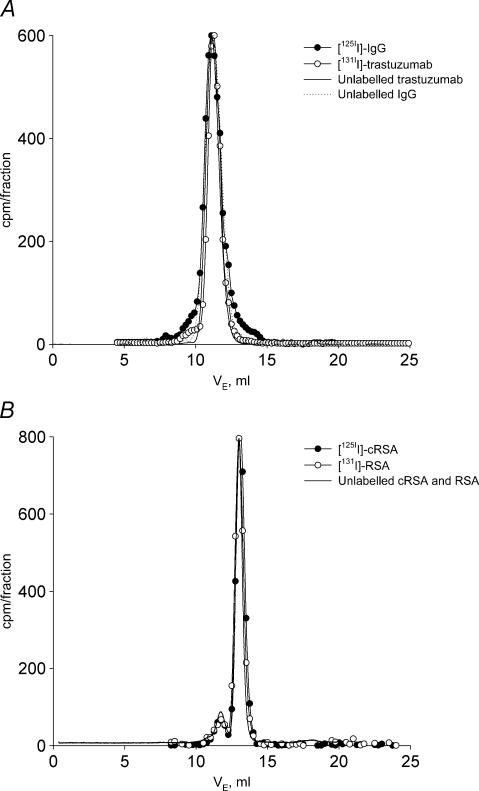
The radiolabelled tracers (trastuzumab, IgG, RSA and cRSA) did not change in plasma for up to 168 h. This was shown by the nearly identical elution patterns of tracers sampled from plasma at the end of the infusion period, from the stock tracer solutions or, from their respective unlabelled protein stock solutions (Fig. 6A and B). There was no change in molecular size, e.g. due to degradation or aggregation of the probes. The slight deviation in elution patterns for native and radiolabelled proteins in plasma was also present when compared to freshly labelled stock solution (data not shown) and thus not caused by their stay in plasma for up to 168 h.
Figure 6. HPLC of plasma after 168 h of tracer infusion.
A, radioactivity in successive collections of eluate from a Superose 12 HR 10/30 size exclusion gel chromatography column after application of plasma sampled after 168 h of infusion. •, 125I-labelled IgG; ○, 131I-labelled trastuzumab. Superimposed on these curves is the UV-signal from native, unlabelled IgG purified from Cohn fraction V rat IgG (dotted line) and unlabelled trastuzumab (continuous line). There was no change in molecular size, e.g. due to degradation or aggregation of the probes. The slight deviation in elution patterns for native and radiolabelled proteins in plasma was also present when compared to freshly labelled stock solution (data not shown) and thus not caused by their stay in plasma for up to 168 h. B, similar data as shown in panel A for cationized rat serum albumin (•, 125I-labelled cRSA) and native rat serum albumin (○, 131I-labelled RSA). UV-signal for the native probes is shown by the continuous line. The tendency of albumin to dimer and/or aggregate formation, as evident by the peak (always less than 10%) eluting before albumin was also present in the elution pattern for the native substances. The optimal separation range of the column is 10–300 kDa, void volume is 7.8 ml as estimated with blue dextran, and total column volume is 22 ml as estimated with acetone.
The peak in the pherogram with a lower elution volume than albumin suggests that albumin dimers or aggregates were present (always less than 10% of area). This peak was also present with the same relative area in the elution pattern for the native substances, showing that this phenomenon was not a consequence of the stay of labelled tracer in plasma.
Discussion
Quantitative information regarding the interstitial distribution volume of macromolecular probes along with investigations relevant to the manner in which the fixed negative charges of the interstitium influence this volume in solid tumours are of importance for understanding tumour fluid exchange. Additionally, with the emergence of new therapeutic tools, such as monoclonal antibodies, quantitative studies of parameters related to transport and distribution of macromolecular probes with varied hydrodynamic sizes and net charges are of special importance for cancer therapy. Complementary work to ours (Dellian et al. 2000; Krasnici et al. 2003) has stressed the importance of molecular charge in transcapillary transport in solid tumours and shown that the positively charged molecules extravasate faster into solid tumours compared with the same-size compounds bearing neutral and negative charges. Here we studied the effect of size and charge from the interstitial perspective. Thus we put forward a methodology in vivo where we compared pairs of macromolecules having the same molecular weights but different net charges and quantified their available volumes in a tumour model for which we have previously measured matrix components and transcapillary fluid balance parameters.
One of the probes chosen, trastuzumab, is a humanized monoclonal antibody that targets the extracellular domain of HER-2 (erbB2). Although traditionally trastuzumab is used as a target treatment for HER-2 positive carcinomas, it often fails to be effective in a large number of receptor positive tumours. A better understanding of its distribution in the interstitial matrix is a prerequisite of gaining an insight as to whether this drug has a significant available volume, i.e. it is able to reach a large population of pathological cells. In this study, we used trastuzumab strictly as a probe, i.e. it meets our requirements with respect to size and charge but it is not involved in binding with the receptor. The tracer dose infused corresponds to ∼2.5 pmol per day. Based on therapeutic recommendations for receptor positive humans a 250 g rat should be given ∼1 μmol trastuzumab per day. Thus, even if the DMBA tumours were receptor positive, our dose would not have any therapeutic effect. We explored our experimental situation further by screening for possible cross-reaction to the HER-2 receptor and corrected for receptor as well as unspecific binding. Although trastuzumab has been found to bind nearly irreversibly to its receptors (Flessner et al. 2005), the fact that this monoclonal antibody was more elutable than rat IgG further supports our assumption that receptor binding was not a complicating factor in our study. We then compared this monoclonal antibody with other probes to further explore the size and charge effect on macromolecular distribution volumes in tumours.
We found that trastuzumab (148 kDa) is excluded from 29% of the tumour interstitial fluid phase. By comparison native RSA (70 kDa) is excluded from ∼40% of the interstitial phase, while neutralization of its charge results in a further 50% reduction of excluded volume fraction. Thus, we showed here that, the hydrodynamic size of a macromolecule along with the charge associated with the fixed interstitial constituents affects strongly the effective volume distribution of a probe. Accordingly, when considering the concentration of macromolecular therapeutic agents in tumours, both the steric and the electrostatic exclusion component have to be considered.
The procedure used for determining interstitial distribution and exclusion of native and charge modified albumin has been evaluated in detail in previous papers (Wiig et al. 1992; Gyenge et al. 2003), and therefore only specific aspects related to tumours are discussed here. It is essential that a steady-state condition with respect to tracers is attained at the time of terminating the experiment. Our finding of similar plasma equivalent fluid distribution volumes at 120 and 168 h infusion time suggests that this condition was met. Furthermore, in a previous study where we followed the uptake of radiolabelled albumin in tumours we found that a steady-state tissue concentration was reached ∼48 h after the start of continuous tracer infusion (Reed & Wiig, 1983) supporting the assumption that the requirements of a steady-state condition was met. Also, the finding that the specific activities of native IgG and albumin in interstitial fluid relative to that of the corresponding probe in plasma was not different from 1.0 substantiates this assumption. The same test regarding relative tracer concentrations could not be performed for the non-native species trastuzumab or cRSA, present in trace amounts only. Since both these species are more positively charged than their native counterpart and it has been shown that positive macromolecules extravasate faster than negative species (Dellian et al. 2000; Krasnici et al. 2003), it may be assumed that steady-state was reached for these non-native substances too.
Isolation and sampling of interstitial fluid was done by an optimized centrifugation method as described in our recent study (Wiig et al. 2003a). Because of the use of a centrifugation methodology and the possibility of a pressure gradient from central to peripheral regions of tumours (Wiig et al. 1982; Jain, 1997) one may question whether a resultant bulk fluid flow in this direction might cause macromolecular sieving. Although macromolecular sieving could take place in some normal tissues (Aukland et al. 1997), we have previously demonstrated that this phenomenon does not occur in tumours (Wiig et al. 2003a) and thus validated the method for this tissue type. Interstitial fluid and tissue samples were collected from peripheral poles of tumours while central regions where necrotic areas might affect tracer uptake were avoided. Accordingly, our exclusion data are representative of peripheral tumour regions. A lower pH in the tumour interstitium (Jain, 1997) may result in a less negative charge of native albumin in tumours compared to that of, e.g. skin. Nonetheless, the charge difference between two pair probes within the tumour interstitium is maintained, and thus they are suitable for quantification of charge effects on available and excluded volume.
One of our hypotheses was that if the interstitium was a significant transport barrier, we would find a high excluded volume fraction for the probes used. Surprisingly, the excluded volumes for all probes used were lower in tumour tissue than in back skin, suggesting that the interstitium only partly restricts the uptake of macromolecules in this tumour model. The macromolecular distribution in a large fraction of the interstitial fluid phase is further supported by our finding of a high concentration and mass of IgG and albumin in tumour tissue. The low excluded volume fraction may of course be related to our model per se since a significantly higher fraction was found in vitro in a fibrosarcoma model discussed below (Krol et al. 1999). There are, however, other studies showing that microvascular clearance of macromolecules into tumours is high, thereby indirectly supporting our present data. In a recent study Flessner et al. (2004) measured clearance of trastuzumab to SKOV3 and OVCAR3 tumours implanted into peritoneum, and found a clearance two to three times that observed in muscle. In an even more recent study they measured uptake of trastuzumab and IgG delivered intraperitoneally as a function of tumour interstitial fluid pressure (Flessner et al. 2005). To their surprise, they observed no effect of reducing the tumour interstitial fluid pressure, and concluded that the barrier to immunoglobulin delivery was the microenvironment itself. Although this conclusion is in contrast to ours, it should be remembered that their tumour models and delivery route were different from ours, calling for additional studies using other models.
Quantification of distribution volumes of macromolecular probes in tumours is important from a clinical perspective but it has been proven to be a difficult task in vivo. Only a few studies have addressed this topic directly. Krol et al. (1999), conducted an in vitro study where they measured available volume fractions for dextrans and bovine serum albumin (BSA) in fibrosarcomas. These authors estimated a tumour interstitial fluid fraction of 0.50 using inulin and an available volume fraction of 0.1 for BSA. As per their study, albumin had access to only 20% of the interstitial fluid volume, i.e. about one-third of the value we determined in our in vivo experiments. In a study using a tracer kinetic method, O'Connor & Bale (1984) estimated a concentration of IgG in interstitial fluid of 50% that in plasma. These authors assume in their calculations an available volume fraction of 1.0. Our study as well as that of Krol et al. (1999) (see above) shows that such a choice is not correct. Furthermore, radiolabelled tracer IgG may bind unspecifically to tissue (Flessner & Schwab, 1996; Wiig & Tenstad, 2001), and affect rate constants and the estimated tissue IgG concentration, making this interpretation of the data inaccurate.
The available and excluded volumes obtained in this study can be interpreted in terms of tumour interstitial structure and organization by use of data for collagen, hyaluronan and total glycosaminoglycan (GAG) content measured in DMBA-induced tumours (Wiig et al. 2003a). In skin or more specifically dermis, by virtue of its high content, the neutral collagen (Li & Katz, 1976) is the main excluding agent (Bert & Pearce, 1984; Aukland & Reed, 1993) although the effect of GAGs is not negligible (Comper & Laurent, 1978; Wiig et al. 2003b). However, due to a lower content of collagen in tumours than in skin (Netti et al. 2000; Wiig et al. 2003a) and owing to an increased content of charged hyaluronan and other GAG species in this tissue type (Toole, 2004), the exclusion contribution of the latter becomes apparent. We can quantify the specific exclusion effect of the various extracellular matrix components by using as an example albumin for which in vitro data are available. We showed in this study that, the native albumin was excluded from 38% of the total interstitial fluid volume of 0.38 ml (g wet wt)−1, i.e. has an exclusion volume of 0.14 ml (g wet wt)−1. If we use the exclusion value of highly organized collagen measured by Bert & Pearce (1984), i.e. 1.6 ml (g collagen)−1, and consider the collagen content of DMBA-induced tumours measured as 4.6 mg (g wet wt)−1, we can calculate that collagen will contribute 0.01 ml (g wet wt)−1 of the total excluded volume. Assuming that 1 mg of hyaluronan will exclude 0.05 ml g−1 of fluid (Laurent, 1964), then the total hyaluronan content measured for DMBA tumours of 1.92 mg (g wet wt)−1 (Wiig et al. 2003b) provides an exclusion volume of 0.10 ml (g wet wt)−1, i.e. 10 times the exclusion value provided by collagen. Furthermore, content of uronic acid measured in DMBA-induced tumours and calculations analogous to those provided in a previous paper (Wiig et al. 2003b) show that proteoglycans will exclude an additional fluid of 0.04 ml (g wet wt)−1. It can be clearly shown that a mere summation of all exclusion volumes provided by the matrix components provides the value we obtained in this study, i.e. 0.14 ml (g wet wt)−1. Albumin charge neutralization almost halved the excluded volume fraction. The more positively charged trastuzumab had a significantly higher distribution volume than IgG, a probe with the same molecular weight.
Previous in vivo and in vitro studies have clearly showed a strong dependency between the macromolecular charge and the available distribution volume of a probe in normal tissues (Parker et al. 1985; Wiig & Tenstad, 2001; Gyenge et al. 2003; for a reviewed perspective see also Taylor & Parker, 2003). In this study we put forward an appropriate methodology to investigate the dependency between the charge of a probe and its available distribution volume in tumour interstitia and used this approach to investigations related to monoclonal antibodies. All data presented here show the significant effect the macromolecular hydrodynamic size and charge along with the fixed charges associated with the tumour matrix components have on the distribution volume in tumours. Although other attempts to address this topic have been inconclusive (Krol et al. 2003; Eikenes et al. 2004), our data from in vivo studies suggest that either the GAGs or the net charge of the, for example, monoclonal antibodies to be delivered may be targeted to increase the available volume and drug uptake in tumours. These phenomena are of considerable importance when the actual effective concentration in the tumour interstitial fluid of neutral and charged macromolecular therapeutic agents is to be considered.
Acknowledgments
Financial support from The Research Council of Norway, Locus on Circulatory Research, University of Bergen and EU Integrated Project Angiotargeting (Contract no. 504743) is gratefully acknowledged. Expert technical assistance from Odd Kolmannskog and Gerd Signe Salvesen is gratefully acknowledged. We thank Dr Rolf Bjerkvig for constructive comments to the manuscript, and Dr Lars Akslen for help with the immunohistochemistry.
References
- Aukland K. Distribution volumes and macromolecular mobility in rat tail tendon interstitium. Am J Physiol. 1991;260:H409–H419. doi: 10.1152/ajpheart.1991.260.2.H409. [DOI] [PubMed] [Google Scholar]
- Aukland K, Reed RK. Interstitial-lymphatic mechanisms in the control of extracellular fluid volume. Physiol Rev. 1993;73:1–78. doi: 10.1152/physrev.1993.73.1.1. [DOI] [PubMed] [Google Scholar]
- Aukland K, Wiig H, Tenstad O, Renkin EM. Interstitial exclusion of macromolecules studied by graded centrifugation of rat tail tendon. Am J Physiol. 1997;273:H2794–H2803. doi: 10.1152/ajpheart.1997.273.6.H2794. [DOI] [PubMed] [Google Scholar]
- Bert JL, Pearce RH. The interstitium and microvascular exchange. In: Renkin EM, Michel CC, editors. Handbook of Physiology, section 2, The Cardiovascular System, vol IV, Microcirculation. American Physiological Society, Bethesda, MD, USA: 1984. pp. 521–547. [Google Scholar]
- Comper WD, Laurent TC. Physiological function of connective tissue polysaccharides. Physiol Rev. 1978;58:255–315. doi: 10.1152/physrev.1978.58.1.255. [DOI] [PubMed] [Google Scholar]
- Dellian M, Yuan F, Trubetskoy VS, Torchilin VP, Jain RK. Vascular permeability in a human tumour xenograft: molecular charge dependence. Br J Cancer. 2000;82:1513–1518. doi: 10.1054/bjoc.1999.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eikenes L, Bruland OS, Brekken C, Davies Cde L. Collagenase increases the transcapillary pressure gradient and improves the uptake and distribution of monoclonal antibodies in human osteosarcoma xenografts. Cancer Res. 2004;64:4768–4773. doi: 10.1158/0008-5472.CAN-03-1472. [DOI] [PubMed] [Google Scholar]
- Flessner MF, Choi J, Credit K, Deverkadra R, Henderson K. Resistance of tumor interstitial pressure to the penetration of intraperitoneally delivered antibodies into metastatic ovarian tumors. Clin Cancer Res. 2005;11:3117–3125. doi: 10.1158/1078-0432.CCR-04-2332. [DOI] [PubMed] [Google Scholar]
- Flessner MF, Choi J, He Z, Credit K. Physiological characterization of human ovarian cancer cells in a rat model of intraperitoneal antineoplastic therapy. J Appl Physiol. 2004;97:1518–1526. doi: 10.1152/japplphysiol.00305.2004. [DOI] [PubMed] [Google Scholar]
- Flessner MF, Schwab A. Pressure threshold for fluid loss from the peritoneal cavity. Am J Physiol. 1996;270:F377–F390. doi: 10.1152/ajprenal.1996.270.2.F377. [DOI] [PubMed] [Google Scholar]
- Gyenge CC, Tenstad O, Wiig H. In vivo determination of steric and electrostatic exclusion of albumin in rat skin and skeletal muscle. J Physiol. 2003;552:907–916. doi: 10.1113/jphysiol.2003.049379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoare DG, Koshland DE., Jr A method for the quantitative modification and estimation of carboxylic acid groups in proteins. J Biol Chem. 1967;242:2447–2453. [PubMed] [Google Scholar]
- Jain RK. The Eugene M. Landis Award Lecture 1996. Delivery of molecular and cellular medicine to solid tumors. Microcirculation. 1997;4:1–23. doi: 10.3109/10739689709148314. [DOI] [PubMed] [Google Scholar]
- Krasnici S, Werner A, Eichhorn ME, Schmitt-Sody M, Pahernik SA, Sauer B, Schulze B, Teifel M, Michaelis U, Naujoks K, Dellian M. Effect of the surface charge of liposomes on their uptake by angiogenic tumor vessels. Int J Cancer. 2003;105:561–567. doi: 10.1002/ijc.11108. [DOI] [PubMed] [Google Scholar]
- Krol A, Dewhirst MW, Yuan F. Effects of cell damage and glycosaminoglycan degradation on available extravascular space of different dextrans in a rat fibrosarcoma. Int J Hyperthermia. 2003;19:154–164. doi: 10.1080/02656730210166519. [DOI] [PubMed] [Google Scholar]
- Krol A, Maresca J, Dewhirst MW, Yuan F. Available volume fraction of macromolecules in the extravascular space of a fibrosarcoma: implications for drug delivery. Cancer Res. 1999;59:4136–4141. [PubMed] [Google Scholar]
- Laurent TC. The interaction between polysaccharides and other macromolecules. 9. The exclusion of molecules from hyaluronic acid gels and solutions. Biochem J. 1964;93:106–112. doi: 10.1042/bj0930106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li ST, Katz EP. An electrostatic model for collagen fibrils. The interaction of reconstituted collagen with Ca++, Na+, and Cl. Biopolymers. 1976;15:1439–1460. doi: 10.1002/bip.1976.360150802. [DOI] [PubMed] [Google Scholar]
- Netti PA, Berk DA, Swartz MA, Grodzinsky AJ, Jain RK. Role of extracellular matrix assembly in interstitial transport in solid tumors. Cancer Res. 2000;60:2497–2503. [PubMed] [Google Scholar]
- O'Connor SW, Bale WF. Accessibility of circulating immunoglobulin G to the extravascular compartment of solid rat tumors. Cancer Res. 1984;44:3719–3723. [PubMed] [Google Scholar]
- Parker JC, Gilchrist S, Cartledge JT. Plasma-lymph exchange and interstitial distribution volumes of charged macromolecules in the lung. J Appl Physiol. 1985;59:1128–1136. doi: 10.1152/jappl.1985.59.4.1128. [DOI] [PubMed] [Google Scholar]
- Pathak AP, Artemov D, Ward BD, Jackson DG, Neeman M, Bhujwalla ZM. Characterizing extravascular fluid transport of macromolecules in the tumor interstitium by magnetic resonance imaging. Cancer Res. 2005;65:1425–1432. doi: 10.1158/0008-5472.CAN-04-3682. [DOI] [PubMed] [Google Scholar]
- Reed RK, Wiig H. Interstitial albumin mass and transcapillary extravasation rate of albumin in DMBA-induced rat mammary tumours. Scand J Clin Laboratory Invest. 1983;43:503–512. [PubMed] [Google Scholar]
- Taylor AE, Parker JC. Intersitial exluded volumes: the effect of charge. J Physiol. 2003;553:333. doi: 10.1113/jphysiol.2003.053595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toole BP. Hyaluronan: from extracellular glue to pericellular cue. Nat Rev Cancer. 2004;4:528–539. doi: 10.1038/nrc1391. [DOI] [PubMed] [Google Scholar]
- Wiig H, Aukland K, Tenstad O. Isolation of interstitial fluid from rat mammary tumors by a centrifugation method. Am J Physiol Heart Circ Physiol. 2003a;284:H416–H424. doi: 10.1152/ajpheart.00327.2002. [DOI] [PubMed] [Google Scholar]
- Wiig H, DeCarlo M, Sibley L, Renkin EM. Interstitial exclusion of albumin in rat tissues measured by a continuous infusion method. Am J Physiol. 1992;263:H1222–H1233. doi: 10.1152/ajpheart.1992.263.4.H1222. [DOI] [PubMed] [Google Scholar]
- Wiig H, Kolmannskog O, Tenstad O, Bert JL. Effect of charge on interstitial distribution of albumin in rat dermis in vitro. J Physiol. 2003b;550:505–514. doi: 10.1113/jphysiol.2003.042713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiig H, Tenstad O. Interstitial exclusion of positively and negatively charged IgG in rat skin and muscle. Am J Physiol Heart Circ Physiol. 2001;280:H1505–H1512. doi: 10.1152/ajpheart.2001.280.4.H1505. [DOI] [PubMed] [Google Scholar]
- Wiig H, Tveit E, Hultborn R, Reed RK, Weiss L. Interstitial fluid pressure in DMBA-induced rat mammary tumours. Scand J Clin Laboratory Invest. 1982;42:159–164. [PubMed] [Google Scholar]