Abstract
The purpose of this study was to examine the effects of increased fat availability induced by growth hormone (GH) administration on the oxidative metabolism during exercise. Seven well-trained males (age 25 ± 2 years (mean ±s.e.m.); peak oxygen consumption 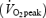 : 62 ± 1 ml min−1 kg−1 (completed four randomised trials: 120 min bicycling at 55%
: 62 ± 1 ml min−1 kg−1 (completed four randomised trials: 120 min bicycling at 55% 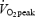 4 h after receiving either 7.5 IU (2.5 mg) GH or placebo (Plc), and during rest after receiving either GH or Plc. In all studies a standardized meal was given 2 h after GH or Plc injection. GH administration resulted in an ∼60-fold increase in serum GH concentration at rest (P < 0.0001) and during exercise (P < 0.0001). The increase in serum GH was followed by an increase in circulating glycerol at rest (8%, P < 0.0001). When combined with exercise the increase in plasma glycerol was more pronounced (GH: 716% of baseline versus Plc: 328%, P < 0.0001). However, this increase in fat mobilization did not increase fat oxidation during exercise (indirect calorimetry). In conclusion, GH administration combined with aerobic exercise increased lipolytic parameters substantially more than exercise alone, but did not further augment whole body fat oxidation.
4 h after receiving either 7.5 IU (2.5 mg) GH or placebo (Plc), and during rest after receiving either GH or Plc. In all studies a standardized meal was given 2 h after GH or Plc injection. GH administration resulted in an ∼60-fold increase in serum GH concentration at rest (P < 0.0001) and during exercise (P < 0.0001). The increase in serum GH was followed by an increase in circulating glycerol at rest (8%, P < 0.0001). When combined with exercise the increase in plasma glycerol was more pronounced (GH: 716% of baseline versus Plc: 328%, P < 0.0001). However, this increase in fat mobilization did not increase fat oxidation during exercise (indirect calorimetry). In conclusion, GH administration combined with aerobic exercise increased lipolytic parameters substantially more than exercise alone, but did not further augment whole body fat oxidation.
Compared with the limited capacity of the human body to store carbohydrate (CHO), endogenous fat depots are large and represent a vast source of fuel for exercise. However, fat oxidation is limited, and especially during intense exercise CHO remains the major fuel for oxidative metabolism. GH is a powerful lipolytic hormone and the metabolic effects of its administration in the resting condition have been studied extensively (Møller et al. 1990a, 1992a, b, 1993; Copeland & Nair, 1994; Lange et al. 2000). The general finding in these resting studies is an enhanced fat oxidation rate after acute or prolonged GH administration, and a decrease in fat mass after prolonged GH administration. These findings support the idea that lipid availability up-regulates lipid oxidation and down-regulates CHO oxidation, in line with the Randle Cycle (Randle et al. 1994). Based on this theory, it can be hypothesized that the relative fat oxidation rate during submaximal exercise would be enhanced, when fat availability was increased by GH administration in the hours before exercise, and this could potentially increase time to exhaustion in a submaximal exercise session by postponing glycogen depletion in the muscle and liver. In contrast, others have suggested a crossover exists between fat and carbohydrate oxidation during exercise, with little influence of fat availability upon the fat oxidation during exercise (Brooks & Mercier, 1994).
Growth hormone (GH) is listed as a doping drug by the World Anti-Doping Agency. Even though evidence of beneficial effects of GH administration on healthy individuals is sparse, abuse of GH among elite athletes seems to be widespread (Rennie, 2003). Unfortunately, as far as we are aware, knowledge of the metabolic effects of acute GH administration during endurance exercise is limited to two studies (Lange et al. 2002; Irving et al. 2004). No change in the substrate oxidation pattern was observed in these studies, which supports the idea that in working human muscle, glycolysis dominates and down-regulates fatty acid entry into the TCA cycle, as described by Brooks & Mercier (1994). However, based on these studies, no final conclusion about the effect of GH administration on substrate utilization could be made, since the increase in availability of fatty acids may not have been enhanced sufficiently to have an effect (Irving et al. 2004), or the calculation of respiratory exchange ratio may have been confounded by an increase in metabolic acidosis (Lange et al. 2002).
The purpose of this study was to provide information about the regulation of carbohydrate and fat oxidation during exercise in a situation where fat availability was elevated by GH administration.
Methods
Subjects
Eight healthy, well-trained young males were enrolled in the study. The study protocol was approved by the Ethics Committee for Medical Research in Copenhagen (KF 01-004/01) and by the Danish National Board of Health (journal number: 2612-1592). Prior to inclusion, informed written and oral consent was obtained from each subject according to the Declaration of Helsinki. Each subject underwent a medical evaluation, including routine blood tests. Exclusion criteria were anaemia, metabolic, cardiac and malignant disease, and any kind of medication. Only subjects training regularly on a bicycle were recruited. One subject was excluded because he for some unaccountable reason was not able to complete the exercise trials even through he was a competitive well-trained cyclist. Individual baseline characteristics of the remaining seven subjects are presented in Table 1.
Table 1.
Subject characteristics
| Subject | Age(years) | Height (cm) | Weight (kg) | BMI (kg m−2) |
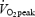 (ml min−1kg−1) (ml min−1kg−1) |
|---|---|---|---|---|---|
| 1 | 25 | 183 | 79.7 | 23.8 | 64 |
| 2 | 33 | 180 | 73.8 | 22.8 | 61 |
| 3 | 30 | 186 | 86.1 | 24.9 | 58 |
| 4 | 18 | 190 | 81.5 | 22.6 | 65 |
| 5 | 25 | 186 | 69.0 | 19.9 | 68 |
| 6 | 23 | 173 | 69.0 | 23.1 | 60 |
| 7 | 24 | 175 | 64.5 | 21.1 | 60 |
| Mean ±s.e.m. | 25 ± 2 | 182 ± 2 | 74.8 ± 3.0 | 22.6 ± 0.6 | 62 ± 1 |
BMI, body mass index.
Study design
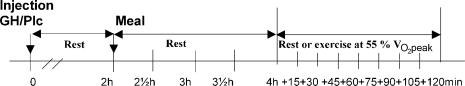
The study was designed as a randomized, placebo-controlled, double-blinded crossover study comparing the effects of a single injection of GH and placebo (Plc) on metabolic parameters during subsequent resting or 2 h bicycling at a moderate intensity. The study design is presented in Fig. 1.
Figure 1. Overall design of each of the four trails.
At 08.00 h the subjects received either Plc or 2.5 mg GH (s.c.). In all four trials subjects rested during the first 4 h after the injection. During the following 2 h they either continued resting or exercised at 55% of 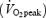 (n = 6) or 65% of
(n = 6) or 65% of 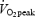 (n = 1). A standardized meal was served 2 h after the injection. Venous blood samples were collected at baseline before the injection (t= 0) and at t= 2 h, 2.5 h, 3 h, 3.5 h, 4 h, 4.5 h (+15), 4.5 h (+30), 4.75 h (+45), 5 h (+60), 5.25 h (+75), 5.5 h (+90), 5.75 h (+105) and 6 h (+120) after the injection. Breath samples were collected during 5 min intervals in each 15 min interval during in the exercise period.
(n = 1). A standardized meal was served 2 h after the injection. Venous blood samples were collected at baseline before the injection (t= 0) and at t= 2 h, 2.5 h, 3 h, 3.5 h, 4 h, 4.5 h (+15), 4.5 h (+30), 4.75 h (+45), 5 h (+60), 5.25 h (+75), 5.5 h (+90), 5.75 h (+105) and 6 h (+120) after the injection. Breath samples were collected during 5 min intervals in each 15 min interval during in the exercise period.
After inclusion, maximal oxygen uptake 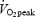 was determined in each individual. The subject then performed the four experimental days in random order. The evening before each trial a standardized meal was served (pasta, meat, sausages and cheese). The size of the meal corresponded to 30% (5.9 ± 0.7 MJ) of estimated total daily energy requirement calculated on the basis of monitoring activity for 3 days. The nutrient composition was carbohydrate, 59 energy (E)%; fat, 21 E%; and protein, 19 E%.
was determined in each individual. The subject then performed the four experimental days in random order. The evening before each trial a standardized meal was served (pasta, meat, sausages and cheese). The size of the meal corresponded to 30% (5.9 ± 0.7 MJ) of estimated total daily energy requirement calculated on the basis of monitoring activity for 3 days. The nutrient composition was carbohydrate, 59 energy (E)%; fat, 21 E%; and protein, 19 E%.
Only light training (< 2 h at low intensity) was allowed 2 days before the experimental days and no physical training was allowed on the day preceding the experimental days. The two exercise trials were separated by 7 days. This time period was chosen to reduce the potential effects of acute GH administration and to keep the fitness level and body composition in the two exercise trials as similar as possible. The two resting experimental days were separated by at least 7 days. Each subject was instructed to continue his habitual training programme and dietary habits during the study period.
Incremental bicycling test 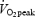
On a separate day after inclusion each subject performed an incremental bicycling test on the subject's own racing bicycle, the rear wheel being replaced by a stationary, magnetically braked bicycle ergometer (Ciclotraining Olympionic, Politecnica 80, Padova, Italy) (Lange et al. 2002). After a 10–15 min warm-up four submaximal workloads of 5 min duration were performed (∼50–80% of 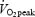 ). The load was increased by 30 W per step corresponding to an increase of ∼3 km h−1 in speed (W = 9.93 × cycling speed (km h−1) − 96.34). After a 10 min rest, the cyclists were told to bicycle to exhaustion within 5 min to determine
). The load was increased by 30 W per step corresponding to an increase of ∼3 km h−1 in speed (W = 9.93 × cycling speed (km h−1) − 96.34). After a 10 min rest, the cyclists were told to bicycle to exhaustion within 5 min to determine 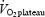 . Respiratory variables (averaged for each 15-s period) were measured continuously through a mouthpiece connected to an automated metabolic cart (AMIS 2001, Innovision, Odense, Denmark). The mean of the three highest 15-s values was recorded as
. Respiratory variables (averaged for each 15-s period) were measured continuously through a mouthpiece connected to an automated metabolic cart (AMIS 2001, Innovision, Odense, Denmark). The mean of the three highest 15-s values was recorded as 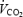 . Heart rate (HR) was measured continuously by a wireless HR monitor (Polar Sport Tester, Polar Electro OY, Kempele, Finland). To ensure that a true
. Heart rate (HR) was measured continuously by a wireless HR monitor (Polar Sport Tester, Polar Electro OY, Kempele, Finland). To ensure that a true 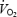 was attained, at least two of the following three criteria had to be fulfilled: (1)
was attained, at least two of the following three criteria had to be fulfilled: (1) 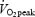 was reached, (2) a HR was within ±5 beats min−1 of the age adjusted maximal HR, and (3)
was reached, (2) a HR was within ±5 beats min−1 of the age adjusted maximal HR, and (3) 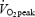 /
/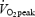 > 1.1. The velocity eliciting 55% of
> 1.1. The velocity eliciting 55% of 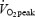 was calculated by linear regression and used in the exercise protocol on the experimental days. On the first exercise experimental day the cadence and gear corresponding to 55% of
was calculated by linear regression and used in the exercise protocol on the experimental days. On the first exercise experimental day the cadence and gear corresponding to 55% of 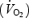 were determined. The same cadence, gear and thus workload were used on the second exercise experimental day.
were determined. The same cadence, gear and thus workload were used on the second exercise experimental day.
Experimental days
On each experimental day each subject attended the laboratory at 07.45 h, after an overnight fast. At 08.00 h (time = 0) a fasting blood sample was obtained from a cubital vein and, subsequently, 2.5 mg GH or placebo (Norditropin SimpleXx, 10 mg or Placebo for SimpleXx, both Novo Nordisk A/S, Bagsværd, Denmark) was injected anteriorly at the midthigh level. The subject rested for the following 2 h. Subsequently, a second blood sample was obtained and a standardized meal comprising ∼20% of estimated total daily energy requirement (3.9 ± 0.4 MJ) was served to make the experiment applicable to a realistic exercise situation. The ingredients of the breakfast were oatmeal, low-fat milk, hazelnuts, seedless raisins and orange juice (carbohydrate, 62 E%; fat, 24 E%; protein, 14 E%). The breakfast was ingested within 15 min. Each food category was weighed on a scale with a precision of 1 g. During the following 2 h of rest, blood samples were obtained at 30 min intervals (2.5, 3.0, 3.5 and 4.0 h) through an i.v. catheter inserted into a cubital vein. Immediately after the blood sample at 4 h, the subject either continued resting (resting trials) or started bicycling for 2 h at a constant speed eliciting 55% of predetermined 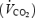 (n = 6) (or 65% of
(n = 6) (or 65% of 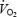 , n = 1) (exercise trials). The low intensity was chosen in order to prevent lactate accumulation and a steady state level of acid/base parameters, which might interfere with indirect calorimetry. A fan was placed in front of the subject during the exercise period to ensure adequate cooling. The room temperature was kept between 21 and 22°C. Eight additional blood samples were obtained at 15 min intervals during these last 2 h of the experimental day (+15, +30, +45, +60, +75, +90, +105 and +120 min). During exercise, whole body oxygen uptake
, n = 1) (exercise trials). The low intensity was chosen in order to prevent lactate accumulation and a steady state level of acid/base parameters, which might interfere with indirect calorimetry. A fan was placed in front of the subject during the exercise period to ensure adequate cooling. The room temperature was kept between 21 and 22°C. Eight additional blood samples were obtained at 15 min intervals during these last 2 h of the experimental day (+15, +30, +45, +60, +75, +90, +105 and +120 min). During exercise, whole body oxygen uptake 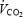 and carbon dioxide production
and carbon dioxide production 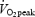 were measured in 5 min intervals (from +10–15, +25–30, +40–45, +55–60, +70–75, +85–90, +100–105 and +115–120 min). Respiratory variables between 2 min 45 s and 4 min 30s in each 5-min period were averaged to estimate
were measured in 5 min intervals (from +10–15, +25–30, +40–45, +55–60, +70–75, +85–90, +100–105 and +115–120 min). Respiratory variables between 2 min 45 s and 4 min 30s in each 5-min period were averaged to estimate 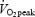 and
and 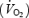 in the respective periods. Appropriate calibrations of the O2 and CO2 sensors and the volume transducer were performed twice before the start of exercise and after 60 min of exercise. HR was registered continuously during exercise.
in the respective periods. Appropriate calibrations of the O2 and CO2 sensors and the volume transducer were performed twice before the start of exercise and after 60 min of exercise. HR was registered continuously during exercise.
On the first experimental day (exercise or resting trial) each subject was allowed to drink water freely. The amount of water and the time schedule were recorded and used on the second experimental day (exercise or resting trial) to ensure identical hydration conditions.
Analytical methods
All blood samples were taken from a cubital vein into sealed vials. The vials for analyses of glycerol, non-esterified fatty acids (NEFA), glucose and lactate contained heparin. After separation by centrifugation, serum was stored at −80°C until analysis, except the samples for determination of the concentration of lactate and glucose, which were analysed on-site immediately after the samples were obtained. Serum GH was determined by time-resolved immunofluorometric assay (TR-IFMA) (Wallac Oy, Turku, Finland). Serum insulin-like growth factor I (IGF-I) was measured in acid–ethanol serum extracts using an in-house monoclonal antibody-based TR-IFMA as previously described (Frystyk et al. 1995). Serum insulin was determined by a TR-IFMA (Wallac Oy, Turku, Finland). The samples for plasma lactate and glucose were analysed using enzymatic colorimetric methods (AB700, Radiometer, Copenhagen, Denmark). Serum NEFA were determined by a colorimetric method (Wako Chemicals, Neuss, Germany). Plasma glycerol was determined spectrophotometrically (Boehringer Mannheim, cat. no. 148270, Diffchamp, Denmark).
Statistical analysis
Data are presented as means ±s.d. The effects of time and treatment (GH or placebo), and time and activity (e.g. rest or exercise) on plasma metabolites, serum and plasma hormones and physiological variables were analysed using a two-way ANOVA with repeated measures (GraphPad Prism version 4.00, GraphPad Software, San Diego, CA, USA). Results from post hoc Student's t test are only indicated in the figures if significant interaction effects were observed. P < 0.05 (two-tailed) was considered statistically significant.
Results
Eight subjects were included in the study protocol but only seven completed all four trials at a speed eliciting 65% of 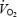 (n = 1) or 55% of
(n = 1) or 55% of 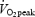 (n = 6). Mean exercise workload was 202 ± 34 and 204 ± 34 W during the GH and Plc trial, respectively (P= 0.51). HR (mean) during the exercise period was significantly higher after GH administration than after Plc administration (148 ± 11 versus 141 ± 13 beats min−1, P < 0.02).
(n = 6). Mean exercise workload was 202 ± 34 and 204 ± 34 W during the GH and Plc trial, respectively (P= 0.51). HR (mean) during the exercise period was significantly higher after GH administration than after Plc administration (148 ± 11 versus 141 ± 13 beats min−1, P < 0.02).
Hormones and metabolites
No differences were observed at baseline (t= 0) in any of the blood parameters between the four study days.
GH, IGF-I and insulin
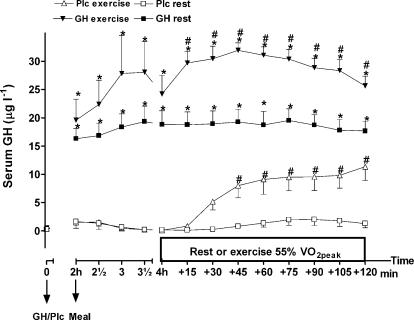
At rest no change in serum GH was observed after injection of Plc (Fig. 2). Exercise increased serum GH significantly, which indicates an increase in the endogenous secretion of GH (activity by time interaction, P < 0.0001; activity, P < 0.001). The increase in serum GH was significant after 30 min of exercise compared to rest in the Plc trials (P < 0.05). After GH administration, serum GH was significantly increased in the resting and exercise trial compared to corresponding placebo trials (GH versus Plc: treatment by time interaction, P < 0.0001; treatment, P < 0.001). After 15 min of exercise and throughout the rest of the experiment serum GH had increased to an even higher level than at the same time points during the resting trial with GH administration (P < 0.05).
Figure 2. Serum GH (μg l−1) after injection of either GH or Plc during exercise (EX) trials and resting (rest) trials.
Values are shown as means ±s.d. (n = 7). GH rest versus Plc rest: treatment by time interaction, P < 0.0001; treatment, P < 0.0001; time, P < 0.0001. GH EX versus Plc EX: treatment by time interaction, P < 0.0001; treatment, P < 0.0001; time, P < 0.0001. *Significant difference between GH and Plc (identical activity, e.g. exercise or rest); #significant difference between EX and rest (identical treatment).
Serum IGF-I did not change during the experimental period of 6 h following either Plc or GH injections (treatment by time interaction, P= 0.17; treatment, P= 0.79) (activity by time interaction, P= 0.14; treatment, P= 0.75).
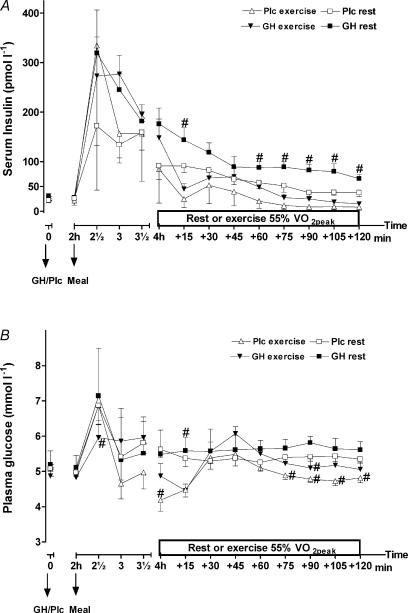
Serum insulin changed over time during all four trials (P < 0.0001) but no overall treatment by time interaction was observed when the resting (P= 0.15, treatment P < 0.01) or exercise trails (P= 0.6, treatment P < 0.07) were analysed separately (Fig. 3A). However, when the results from the exercise period was analysed, a significantly higher insulin level was observed after GH administration (mean: 51.4 ± 25.1 versus 28.9 ± 16.1 pmol l−1, P < 0.001). Also, in the resting trails a significantly higher mean level of serum insulin was observed after GH administration (124.2 ± 24.5 versus 76.1 ± 29.5 pmol l−1, P < 0.0001).
Figure 3. Serum insulin (pmol l−1) (A) and plasma glucose (mmol l−1) (B) after injection of either GH or Plc during exercise (EX) trials and resting (rest) trials.
Values are shown as means ±s.d. (n = 7). A, GH rest versus Plc rest; treatment by time interaction, P= 0.15; treatment, P < 0.01; time, P < 0.0001. GH EX versus Plc EX: treatment by time interaction, P= 0.6; treatment, P= 0.07; time, P < 0.0001. B, GH rest versus Plc rest; activity by time interaction, P= 0.97; treatment, P= 0.49; time, P < 0.0001. GH EX versus Plc EX: activity by time interaction, P= 0.09; treatment, P < 0.05; time, P < 0.0001. *Significant difference between GH and Plc (identical activity, e.g. exercise or rest); #significant difference between EX and rest (identical treatment).
Glycerol and NEFA
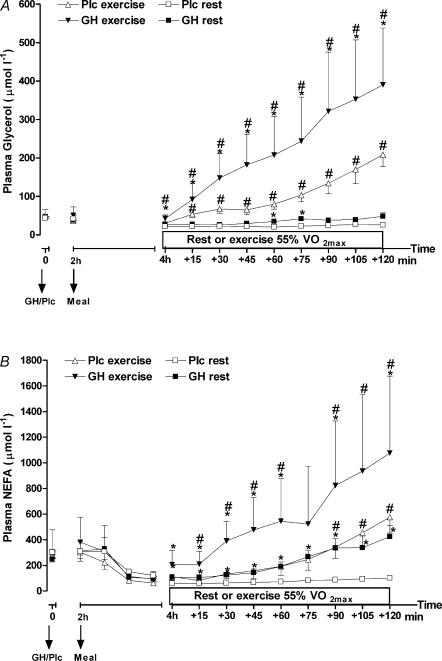
The changes in plasma glycerol are shown in Fig. 4A. Exercise and GH administration had separate stimulatory effects on the plasma glycerol level over time (interaction effects, P < 0.0001). The increase in plasma glycerol during exercise was enhanced by GH administration compared to Plc administration. At the end of the exercise period, plasma glycerol concentration was increased 716 ± 262% above baseline level (P < 0.001) while the increase after Plc administration was less pronounced (328 ± 152%, P < 0.01).
Figure 4. Plasma glycerol (μmol l−1) (A) and plasma non-esterified fatty acids (NEFA) (μmol l−1) (B) after injection of either GH or Plc during exercise (EX) trials and resting (rest) trials.
Values are shown as means ±s.d. (n = 7). A, GH rest versus Plc rest: treatment by time interaction, P < 0.0001; treatment, P < 0.10; time, P < 0.0001. GH EX versus Plc EX: treatment by time interaction, P < 0.0001; treatment, P < 0.01; time, P < 0.0001. B, GH rest versus Plc rest: treatment by time interaction, P < 0.0001; treatment, P < 0.05; time, P < 0.0001. *Significant difference between GH and Plc (identical activity, e.g. exercise or rest); #significant difference between EX and rest (identical treatment).
Plasma NEFA changed significantly over time caused by the ingestion of the meal, GH administration and exercise (Fig. 4B). Plasma NEFA decreased in the period following the meals in all trials, but increased rather quickly again after GH administration. At the start of the exercise period plasma NEFA was no longer different from baseline in the GH trials. Furthermore, GH administration led to a substantially accumulating increase in plasma NEFA immediately after the start of exercise (activity by time interaction, P < 0.0001; treatment, P < 0.05), whereas the response to exercise in the Plc trail was postponed to the late part of the exercise period (activity by time interaction, P < 0.0001). At rest the difference in plasma NEFA between the GH and Plc trial reached significance rather late (5 h 15 min after GH injection), which underlined the interaction between exercise and GH administration.
Glucose and lactate
GH administration did not have any influence on plasma glucose during the resting trials (treatment by time interaction, P= 0.97) (Fig. 3B). When GH administration was combined with exercise, a higher level of plasma glucose (6 ± 7%) was observed, which approached significance (5.32 ± 022 versus 5.04 ± 0.24 mmol l−1; treatment by time interaction, P= 0.09; treatment, P < 0.05). When the data from the exercise period were analysed separately, a significantly higher plasma glucose was observed after GH administration compared to the Plc trial (5.3 ± 0.5 versus 5.03 ± 0.3 mmol l−1, P < 0.002). Additionally, in the same time period during the resting trials, plasma glucose was enhanced after GH administration compared to Plc administration (5.64 ± 0.08 versus 5.36 ± 0.04 mmol l−1, P < 0.0001).
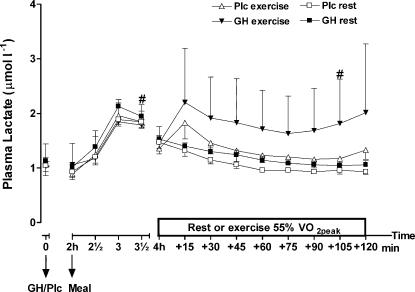
A significant increase in plasma lactate was observed during the exercise trial when GH was administrated compared to GH administration alone (interaction effect, P < 0.001; treatment, P < 0.05) (Fig. 5). Furthermore, when the data from the exercise period were analysed separately, plasma lactate was significantly higher after GH administration compared to Plc administration (exercise trials, 1.8 ± 0.3 versus 1.3 ± 0.2 µmol l−1, P < 0.0001). Similarly, a significant interaction between treatment and time was observed when the results from the last 2 h of the resting trials were analysed (P < 0.05) (resting trials, 1.2 ± 0.0 versus 1.1 ± 0.1 µmol l−1, P < 0.0001).
Figure 5. Plasma lactate (μmol l−1) after injection of either GH or Plc during exercise (EX) trials and resting (rest) trials.
Values are shown as means ±s.d. (n = 7). GH rest versus Plc rest; treatment by time interaction, P= 0.99; treatment, P= 0.12; time, P < 0.0001. GH EX versus Plc EX: treatment by time interaction, P= 0.41; treatment, P= 0.09; time, P < 0.0001. #Significant difference between EX and rest (GH trials).
Respiratory measurements
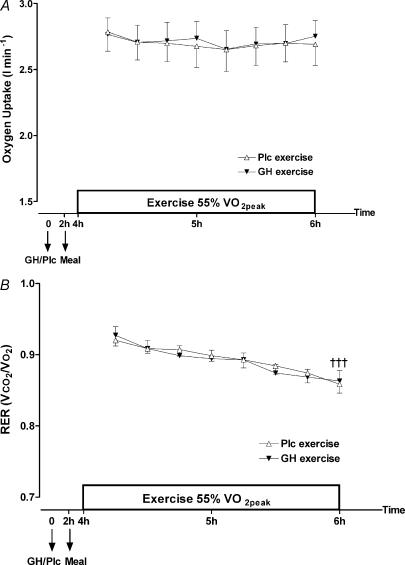
Oxygen uptake 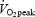 and calculated RER values are illustrated in Fig. 6. During exercise
and calculated RER values are illustrated in Fig. 6. During exercise 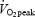 did not change (P= 0.9995), nor did GH administration have an effect (treatment by time interaction, P= 0.98). However, an increase in fat oxidation was observed over time in the exercise period, indicated by a decrease in RER (P < 0.001). However, GH administration did not change this decrease (treatment by time interaction, P= 0.99).
did not change (P= 0.9995), nor did GH administration have an effect (treatment by time interaction, P= 0.98). However, an increase in fat oxidation was observed over time in the exercise period, indicated by a decrease in RER (P < 0.001). However, GH administration did not change this decrease (treatment by time interaction, P= 0.99).
Figure 6. Oxygen uptake (l min−1) (A) and respiratory exchange ratio (RER) (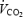 /
/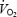 ) (B) during exercise 4–6 h after injection of GH or Plc.
) (B) during exercise 4–6 h after injection of GH or Plc.
Values are shown as means ±s.d. (n = 7). A, oxygen uptake: treatment by time interaction, P= 0.98; treatment, P= 0.52; time, P= 1.00. B, RER: treatment by time interaction, P= 0.99; treatment, P= 0.64; ††† time, P < 0.001.
Discussion
The main purpose of the present study was to examine if an increased availability of fatty acids induced by GH administration prior to exercise would change the substrate oxidation pattern during subsequent moderate intensity exercise in trained individuals. We expected that increased accessibility of fatty acids would increase the fat oxidation rate during exercise, as it has been observed at rest in earlier human studies after acute GH administration (Møller et al. 1990a, 1992a, b, 1993; Bak et al. 1991; Jørgensen et al. 1994; Lange et al. 2000), or when the availability of fatty acids has been enhanced by intravenous infusion of lipids (Intralipid) with heparin (Romijn et al. 1995; Odland et al. 1998). However, this hypothetical relationship could not be confirmed by the present results. Although GH administration increased plasma NEFA substantially during exercise, it had no effect on fat oxidation during exercise. This is in contrary to the Randle Cycle (Randle et al. (1994), but perfectly in line with the crossover concept of Brooks & Mercier (1994), which describes that during exercise glycolysis in human skeletal muscles dominates and down-regulates fatty acid entry into the TCA cycle, probably by acting on CPT1 via malonyal-CoA or by other means (Brooks & Mercier 1994).
As reported in the literature (Sutton & Lazarus, 1976; Jenkins, 1999), exercise by itself increased the endogenous level of serum GH. GH administration increased serum GH significantly more compared to similar time points in the placebo trials, and during exercise a further increase in serum GH was observed compared to the resting trial with GH administration. When the results for the first 4 h of the experiment were analysed separately, no difference in serum GH between the two trials with GH administration was observed (activity by time interaction, P= 0.28; treatment, P < 0.20). However, even though the protocols for the first 4 h of the experiments were identical, serum GH seemed to reach a higher level before the exercise period during GH–exercise compared to GH–rest. This may be related to a higher stress level before the forthcoming exercise period.
A significantly higher level of plasma glycerol was observed right before exercise after GH administration compared to the corresponding resting trial with GH administration. This also supports the idea that knowledge of the forthcoming exercise may have affected the secretion of GH, and thereby fat metabolism. Similar to the changes observed in serum GH, plasma glycerol increased during exercise in the Plc trial, and increased to an even higher level after GH administration. An earlier study showed that the fractional extraction of glycerol by the splanchnic tissue decreases during exercise (Van Hall et al. 2002). Still, the uptake of glycerol by the splanchnic tissue increases during exercise, and the marked increase in glycerol in the present study is probably not explained by a decrease in the clearance of glycerol from the bloodstream, but more reasonably by the well-established lipolytic effect of GH administration (Bak et al. 1991; Møller et al. 1990a, b, 1992b). This assumption is strengthened by the observation of a simultaneous accumulation of NEFA in the blood in the present study. This indicates that GH administration counteracted the insulin-mediated reduction in plasma NEFA normally seen after ingestion of carbohydrates, even though the level of the antilipolytic hormone, serum insulin, was enhanced.
The increased availability of NEFA in the present study after GH administration did not increase the use of fat as a fuel in energy metabolism, even though the dose of GH (29–39 µg kg−1 or 173–380 µg (kg fat mass)−1) was in the same range as in the studies at rest in the literature, where increases in fat oxidation have been observed (Møller et al. 1990a, 1992a, b, 1993; Bak et al. 1991; Jørgensen et al. 1994; Lange et al. 2000). Three explanations seem reasonable: (1) the method used to detect changes in fat oxidation was not sensitive enough, (2) the elevated metabolic rate during exercise limited the use of fat as a fuel, and/or (3) the increased availability of glucose after the meal in the present study overrode the effect of the increased availability of fatty acids.
Theoretically, absence of difference in calculated fat oxidation rate after GH administration could be due to a negative confounding effect of an enhanced level of lactate in the blood. An increase in lactate and thereby H+ can disturb the validity of the calculation of substrate oxidation due to an enhanced excretion of CO2 in the lungs, and thereby lead to an overestimation of RQ. A minor but significantly higher level of plasma lactate was in fact observed in the present study after GH administration compared to the corresponding Plc trial. Nevertheless, hyperventilation was not noticed during the respiratory measurements (data not shown). Presumably, this minor difference in lactate between the Plc and GH trial did not have a confounding effect on the respiratory measurements.
The observed unchanged substrate oxidation pattern during exercise in the present study is in agreement with the findings in the literature where lipolysis has been stimulated by acute GH administration (Lange et al. 2002; Irving et al. 2004). The exercise in the previous studies was performed at a higher intensity. Our results add to the knowledge about effects of acute GH administration during exercise by presenting results for exercise at a moderate intensity, which in theory should have improved the possibilities for using fat as a fuel. Since the skeletal muscles in trained subjects are able to take up and utilize a greater fraction of available fatty acids in the blood (Kiens et al. 1993), our present findings would probably not have been different, in favour of a higher fat oxidation, if untrained subjects rather than trained athletes had been recruited. This argument is supported by the study by Irving et al. (2004), where no change in fat oxidation was observed in untrained individuals after acute GH administration.
In the postabsorptive state at rest, it is well established that acute GH administration has an inhibitory effect on uptake and oxidation of glucose in skeletal muscle, despite GH administration causing hyperinsulinaemia and impairment of the ability of insulin to suppress hepatic glucose production (Ho et al. 1996; Bak et al. 1991). In line with this, both plasma glucose and serum insulin reached a significantly higher level in the late part of the present study. In contrast to the studies at rest a carbohydrate-rich meal was served 2 h before exercise. The RER values in the present study were pretty high for exercise at 55% 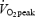 in endurance trained athletes. This is likely to have resulted from the carbohydrate-rich pre-exercise meal 2 h before the exercise period, which may have overridden the effect of an increase in the availability of fatty acids, and thereby counteracted the observed increase in fat oxidation after GH administration at rest in the postabsorptive state (Møller et al. 1990a, 1992a, b, 1993; Bak et al. 1991; Jorgensen et al. 1994; Lange et al. 2000). It is well known that even small differences in serum insulin have great impact on fuel metabolism during exercise under normal circumstances (Montain et al. 1991; Thomas et al. 1991). In addition to the stimulatory effect of insulin on the utilization of carbohydrates, the non-insulin-dependent uptake of glucose triggered by muscle contractions may to a certain degree have compensated for the increase in insulin resistance, and thereby increased the delivery of glucose to the skeletal muscle. Against the argument of a meal-interactive effect being the cause for the disparity between the findings at rest and during exercise after GH administration, Irving et al. (2004) did not observe any change in fat oxidation during exercise in the postabsorptive state. Unfortunately, Irving et al. reported no information about changes in fat availability. Other studies have shown it takes 2–4 h before a significant rise in glycerol and NEFA is observed after GH administration (Møller et al. 1990b, 1992b; Bak et al. 1991; Richelsen, 1997; Gravholt et al. 1999), which was our reason for choosing a delay of 4 h after GH administration before starting the exercise period. In the study of Irving et al. (2004) they initiated the exercise bouts at earlier time points after the GH administration than in the present study, and since the effect of GH administration seemed to get more and more pronounced with time, the increasingly available fatty acids may not have reached a sufficient level to have a detectable stimulatory effect on the uptake and oxidation of fatty acids. In support of this argument, studies have shown an increase in fat oxidation during submaximal exercise in the postabsorptive state when the availability of fatty acids has been enhanced by intravenous infusion of lipids (Intralipid) with heparin (Romijn et al. 1995; Odland et al. 1998). However, the latter argument doesn't explain our results. We cannot rule out that our way of increasing concentrations of fatty acids in the blood by administrating GH might at the same time affect utilization of glucose either directly or indirectly by other related effects of GH administration. Exercise in combination with GH administration may have stimulated the sympathetic nervous system and thereby counteracted any increase in fat oxidation by increasing the rates of the glycogenolysis and glycolysis, and thereby the use of glucose as a fuel in energy metabolism. The higher HR after GH administration supports that GH administration has a stimulatory effect on the sympathetic nervous system. Furthermore, the observed increase in plasma lactate during exercise might be due to accelerated glycolysis, indirectly stimulated by an increase in catecholamines.
in endurance trained athletes. This is likely to have resulted from the carbohydrate-rich pre-exercise meal 2 h before the exercise period, which may have overridden the effect of an increase in the availability of fatty acids, and thereby counteracted the observed increase in fat oxidation after GH administration at rest in the postabsorptive state (Møller et al. 1990a, 1992a, b, 1993; Bak et al. 1991; Jorgensen et al. 1994; Lange et al. 2000). It is well known that even small differences in serum insulin have great impact on fuel metabolism during exercise under normal circumstances (Montain et al. 1991; Thomas et al. 1991). In addition to the stimulatory effect of insulin on the utilization of carbohydrates, the non-insulin-dependent uptake of glucose triggered by muscle contractions may to a certain degree have compensated for the increase in insulin resistance, and thereby increased the delivery of glucose to the skeletal muscle. Against the argument of a meal-interactive effect being the cause for the disparity between the findings at rest and during exercise after GH administration, Irving et al. (2004) did not observe any change in fat oxidation during exercise in the postabsorptive state. Unfortunately, Irving et al. reported no information about changes in fat availability. Other studies have shown it takes 2–4 h before a significant rise in glycerol and NEFA is observed after GH administration (Møller et al. 1990b, 1992b; Bak et al. 1991; Richelsen, 1997; Gravholt et al. 1999), which was our reason for choosing a delay of 4 h after GH administration before starting the exercise period. In the study of Irving et al. (2004) they initiated the exercise bouts at earlier time points after the GH administration than in the present study, and since the effect of GH administration seemed to get more and more pronounced with time, the increasingly available fatty acids may not have reached a sufficient level to have a detectable stimulatory effect on the uptake and oxidation of fatty acids. In support of this argument, studies have shown an increase in fat oxidation during submaximal exercise in the postabsorptive state when the availability of fatty acids has been enhanced by intravenous infusion of lipids (Intralipid) with heparin (Romijn et al. 1995; Odland et al. 1998). However, the latter argument doesn't explain our results. We cannot rule out that our way of increasing concentrations of fatty acids in the blood by administrating GH might at the same time affect utilization of glucose either directly or indirectly by other related effects of GH administration. Exercise in combination with GH administration may have stimulated the sympathetic nervous system and thereby counteracted any increase in fat oxidation by increasing the rates of the glycogenolysis and glycolysis, and thereby the use of glucose as a fuel in energy metabolism. The higher HR after GH administration supports that GH administration has a stimulatory effect on the sympathetic nervous system. Furthermore, the observed increase in plasma lactate during exercise might be due to accelerated glycolysis, indirectly stimulated by an increase in catecholamines.
In the present study the acute effect of a single injection of GH was studied. Metabolic adaptations to increased use of fat as a fuel during submaximal exercise might occur after a prolonged period of GH administration. Earlier findings support this (Lange et al. 2000). In a randomized controlled study by Lange et al. (2000), GH was administrated for 12 weeks to a group of healthy elderly subjects. Administration of GH combined with a endurance training intervention led to a larger increase in muscle enzymatic oxidative capacity (l-3-hydroxyacyl-CoA dehydrogenase activity and citrate synthase) than in a control group who completed the same training regimen (Lange et al. 2000). In addition, a decrease in fat mass was observed in the GH group, which rationally may be coupled to the increased capacity to use fat as a fuel during exercise and/or at rest in the postabsorptive phase. In particular this long-termed metabolic effect of administrating GH to healthy individuals in both the postabsorptive and the postprandial phases after a meal needs further examination before any final conclusion can be made about effects of GH administration on substrate utilization and oxidation during exercise.
The increase in plasma lactate after GH administration in the present study is in agreement with results from an earlier study from our laboratory where GH was administrated 4 h before exercise (Lange et al. 2002). However, the increase in lactate in our study was rather small, in contrast to marked cumulative changes in the previous study. The difference in response is probably related to lower exercise intensity in the present study. A minor increase in plasma lactate was observed in the last 2 h of the resting trials after GH administration. However, the increase in plasma lactate during exercise was significantly higher, which underlines the influence of energy expenditure on the changes in plasma lactate after GH administration. Irving et al. (2004) found no significant increase in lactate. The disparity may be explained by a shorter exercise duration, a smaller dose of GH administrated, and/or the fact that the subjects in the study by Irving et al. were in the postabsorptive phase while the subjects in the study by Lange et al. (2002) and in the present study ingested a carbohydrate-rich breakfast 2 h before the exercise period. An interaction between the meal and the exercise may have taken place (as discussed earlier). Another explanation for the change in lactate after GH administration in our study in contrast to the study by Irving et al. (2004) could be the difference in the length of the time period from GH administration to the start of exercise. The subjects in the study by Irving et al. may not have reached the level at which the metabolic disturbances induced by GH administration interrupt lactate clearance or production.
In conclusion, no additional increase in relative fat oxidation during exercise at a moderate intensity was observed after acute GH administration to young trained subjects. Unchanged fat oxidation was observed, despite an increase in availability of NEFA for the active skeletal muscles. Thus, under circumstances where a carbohydrate-rich meal is ingested in the hours before exercise, fat oxidation is not limited by the availability of fatty acids induced by acute growth hormone administration. The present findings are in accordance with the view that CHO oxidation has a dominating regulatory role during exercise, even with a substantially enhanced level of fatty acids in the blood.
Acknowledgments
We thank Annie Høj, Kirsten Nyborg and Inga Bisgaard for excellent technical assistance. Novo Nordisk A/S kindly provided GH and placebo. This study was funded by the IMK foundation, the Danish National Research Foundation (504-14), the Danish Medical Research Council (9802636) and by the Aarhus University–Novo Nordisk Centre for Research in Growth and Regeneration (Danish Health Research Council Grants 9600822 and 9700592).
References
- Bak JF, Møller N, Schmitz O. Effects of growth hormone on fuel utilization and muscle glycogen synthase activity in normal humans. Am J Physiol. 1991;260:E736–E742. doi: 10.1152/ajpendo.1991.260.5.E736. [DOI] [PubMed] [Google Scholar]
- Brooks GA, Mercier J. Balance of carbohydrate and lipid utilization during exercise: the ‘crossover’ concept. J Appl Physiol. 1994;76:2253–2261. doi: 10.1152/jappl.1994.76.6.2253. [DOI] [PubMed] [Google Scholar]
- Copeland KC, Nair KS. Acute growth hormone effects on amino acid and lipid metabolism. J Clin Endocrinol Metab. 1994;78:1040–1047. doi: 10.1210/jcem.78.5.8175957. [DOI] [PubMed] [Google Scholar]
- Frystyk J, Dinesen B, Ørskov H. Non-competitive time-resolved immunofluorometric assays for determination of human insulin-like growth factor I and II. Growth Regul. 1995;5:169–176. [PubMed] [Google Scholar]
- Gravholt CH, Schmitz O, Simonsen L, Bülow J, Christiansen JS, Møller N. Effects of a physiological GH pulse on interstitial glycerol in abdominal and femoral adipose tissue. Am J Physiol. 1999;277:E848–E854. doi: 10.1152/ajpendo.1999.277.5.E848. [DOI] [PubMed] [Google Scholar]
- Ho KK, O'Sullivan AJ, Hoffman DM. Metabolic actions of growth hormone in man. Endocr J. 1996;43(Suppl.):S57–S63. doi: 10.1507/endocrj.43.suppl_s57. [DOI] [PubMed] [Google Scholar]
- Irving BA, Patrie JT, Anderson SM, Watson-Winfield DD, Frick KI, Evans WS, Veldhuis JD, Weltman A. The effects of time following acute growth hormone administration on metabolic and power output measures during acute exercise. J Clin Endocrinol Metab. 2004;89:4298–4305. doi: 10.1210/jc.2004-0067. [DOI] [PubMed] [Google Scholar]
- Jenkins PJ. Growth hormone and exercise. Clin Endocrinol (Oxf) 1999;50:683–689. doi: 10.1046/j.1365-2265.1999.00784.x. [DOI] [PubMed] [Google Scholar]
- Jørgensen JO, Pedersen SB, Børglum J, Møller N, Schmitz O, Christiansen JS, Richelsen B. Fuel metabolism, energy expenditure, and thyroid function in growth hormone-treated obese women: a double-blind placebo-controlled study. Metabolism. 1994;43:872–877. doi: 10.1016/0026-0495(94)90269-0. [DOI] [PubMed] [Google Scholar]
- Kiens B, Essen-Gustavsson B, Christensen NJ, Saltin B. Skeletal muscle substrate utilization during submaximal exercise in man: effect of endurance training. J Physiol. 1993;469:459–478. doi: 10.1113/jphysiol.1993.sp019823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange KH, Isaksson F, Juul A, Rasmussen MH, Bülow J, Kjaer M. Growth hormone enhances effects of endurance training on oxidative muscle metabolism in elderly women. Am J Physiol Endocrinol Metab. 2000;279:E989–E996. doi: 10.1152/ajpendo.2000.279.5.E989. [DOI] [PubMed] [Google Scholar]
- Lange KH, Larsson B, Flyvbjerg A, Dall R, Bennekou M, Rasmussen MH, Ørskov H, Kjaer M. Acute growth hormone administration causes exaggerated increases in plasma lactate and glycerol during moderate to high intensity bicycling in trained young men. J Clin Endocrinol Metab. 2002;87:4966–4975. doi: 10.1210/jc.2001-011797. [DOI] [PubMed] [Google Scholar]
- Møller N, Jørgensen JO, Alberti KG, Flyvbjerg A, Schmitz O. Short-term effects of growth hormone on fuel oxidation and regional substrate metabolism in normal man. J Clin Endocrinol Metab. 1990a;70:1179–1186. doi: 10.1210/jcem-70-4-1179. [DOI] [PubMed] [Google Scholar]
- Møller J, Jørgensen JO, Møller N, Christiansen JS, Weeke J. Effects of growth hormone administration on fuel oxidation and thyroid function in normal man. Metabolism. 1992a;41:728–731. doi: 10.1016/0026-0495(92)90312-x. [DOI] [PubMed] [Google Scholar]
- Møller N, Jørgensen JO, Schmitz O, Møller J, Christiansen J, Alberti KG, Ørskov H. Effects of a growth hormone pulse on total and forearm substrate fluxes in humans. Am J Physiol. 1990b;258:E86–E91. doi: 10.1152/ajpendo.1990.258.1.E86. [DOI] [PubMed] [Google Scholar]
- Møller N, Møller J, Jørgensen JO, Ovesen P, Schmitz O, Alberti KG, Christiansen JS. Impact of 2 weeks high dose growth hormone treatment on basal and insulin stimulated substrate metabolism in humans. Clin Endocrinol (Oxf) 1993;39:577–581. doi: 10.1111/j.1365-2265.1993.tb02412.x. [DOI] [PubMed] [Google Scholar]
- Møller N, Schmitz O, Porksen N, Møller J, Jørgensen JO. Dose–response studies on the metabolic effects of a growth hormone pulse in humans. Metabolism. 1992b;41:172–175. doi: 10.1016/0026-0495(92)90147-3. [DOI] [PubMed] [Google Scholar]
- Montain SJ, Hopper MK, Coggan AR, Coyle EF. Exercise metabolism at different time intervals after a meal. J Appl Physiol. 1991;70:882–888. doi: 10.1152/jappl.1991.70.2.882. [DOI] [PubMed] [Google Scholar]
- Odland LM, Heigenhauser GJ, Wong D, Hollidge-Horvat MG, Spriet LL. Effects of increased fat availability on fat–carbohydrate interaction during prolonged exercise in men. Am J Physiol. 1998;274:R894–R902. doi: 10.1152/ajpregu.1998.274.4.R894. [DOI] [PubMed] [Google Scholar]
- Randle PJ, Priestman DA, Mistry SC, Halsall A. Glucose fatty acid interactions and the regulation of glucose disposal. J Cell Biochem. 1994;55(Suppl.):1–11. doi: 10.1002/jcb.240550002. [DOI] [PubMed] [Google Scholar]
- Rennie MJ. Claims for the anabolic effects of growth hormone: a case of the emperor's new clothes? Br J Sports Med. 2003;37:100–105. doi: 10.1136/bjsm.37.2.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richelsen B. Action of growth hormone in adipose tissue. Horm Res. 1997;48(Suppl. 5):105–110. doi: 10.1159/000191338. [DOI] [PubMed] [Google Scholar]
- Romijn JA, Coyle EF, Sidossis LS, Zhang XJ, Wolfe RR. Relationship between fatty acid delivery and fatty acid oxidation during strenuous exercise. J Appl Physiol. 1995;79:1939–1945. doi: 10.1152/jappl.1995.79.6.1939. [DOI] [PubMed] [Google Scholar]
- Sutton J, Lazarus L. Growth hormone in exercise: comparison of physiological and pharmacological stimuli. J Appl Physiol. 1976;41:523–527. doi: 10.1152/jappl.1976.41.4.523. [DOI] [PubMed] [Google Scholar]
- Thomas DE, Brotherhood JR, Brand JC. Carbohydrate feeding before exercise: effect of glycemic index. Int J Sports Med. 1991;12:180–186. doi: 10.1055/s-2007-1024664. [DOI] [PubMed] [Google Scholar]
- Van Hall G, Bülow J, Sacchetti M, Al Mulla N, Lyngsø D, Simonsen L. Regional fat metabolism in human splanchnic and adipose tissues; the effect of exercise. J Physiol. 2002;543:1033–1046. doi: 10.1113/jphysiol.2002.022392. [DOI] [PMC free article] [PubMed] [Google Scholar]