Abstract
Cytotoxic T lymphocytes kill targets via secretion of lytic agents including perforin and granzymes. Recently, new methods have been developed to monitor cytotoxic T lymphocyte degranulation. These include detecting the appearance of lysosome-associated membrane protein on the cell's surface, and monitoring decreases in cellular perforin content. We have combined these methods with microscopy and flow cytometry to provide the first analysis of how single cytotoxic T cells degranulate. We used TALL-104 human leukaemic cytotoxic T cells as a model system, and stimulated them with thapsigargin and PMA, soluble agents that mimic the two major signalling pathways activated by T cell receptor cross-linking. Our results indicate that essentially every TALL-104 cell responds to maximal stimulation by releasing about half of its lytic granule complement. This reflects complete release of the contents of half the cell's granules, rather than partial release of the contents of all of the granules. Sub-maximal stimulation reduces both the fraction of cells that respond and the magnitude of single cell responses. We find that individual cells respond to maximal stimulation with a variable latency, and provide evidence that, once it starts, degranulation is a slow process taking tens of minutes.
One powerful mechanism cytotoxic T lymphocytes (CTLs) use to kill virus-infected, tumour, or transplanted target cells is regulated exocytosis of lytic agents such as perforin and granzymes from specialized lytic granules (Berke, 1994, 1995). Normally, killing occurs in several stages. Signalling is initiated via the T cell receptor (TCR) upon contact with an appropriate target, and a complex structure called the immunological synapse forms at the interface between the CTL and the target (Monks et al. 1998; Bromley et al. 2001; Potter et al. 2001). Lytic granules and the CTL's microtubule organizing centre may reorient towards the target (Kupfer et al. 1983; Kupfer & Dennert, 1984; Kuhn & Poenie, 2002) before granules are released at the point of contact with the target, triggering target cell death.
Despite the immunological importance of this mechanism, relatively little is known about the signalling involved. TCR engagement is clearly the primary stimulus for CTL-mediated killing (Berke, 1994, 1995; Griffiths, 1995), triggering activation of protein kinase C (PKC) and increases in intracellular calcium concentration ([Ca2+]i) that are required for fusion of lytic granules with the plasma membrane (Lancki et al. 1987; Takayama & Sitkovsky, 1987; Sitkovsky, 1988). Soluble stimuli that increase [Ca2+]i and activate PKC can therefore be used to stimulate granule exocytosis, bypassing the need for TCR engagement (Lancki et al. 1987; Nishimura et al. 1987; Haverstick et al. 1991; Esser et al. 1998; Lyubchenko et al. 2003). However, how these signals are coupled to exocytosis remains to be explained. Furthermore, it is likely that lytic granule exocytosis stimulated by soluble agents occurs without formation of an immunological synapse or granule/microtubule organizing centre reorientation.
CTL lytic granules are thought to be secretory lysosomes (reviewed in Griffiths & Argon, 1995; Page et al. 1998). The soluble lytic agents are stored in a dense core, while the membrane that encloses the granules includes lysosomal glycoproteins such as lysosome-associated membrane protein-1 (LAMP-1), LAMP-2 and CD63 (Peters et al. 1989). While soluble granule contents are released during exocytosis, the granule membrane proteins become incorporated into the plasma membrane. Recently, new flow cytometric assays have been developed that exploit either decreases in cellular perforin content (perforin destaining; Weren et al. 2004) or incorporation of LAMP into the plasma membrane following exocytosis (Betts et al. 2003; Rubio et al. 2003; Alter et al. 2004; Betts & Koup, 2004) to monitor lytic granule exocytosis. These new methods offer the possibility of examining the exocytic responses of CTLs at the single cell level, and represent an important technical advance. Essentially nothing is known about how individual CTLs respond to stimulation, largely because the standard methods used to study granule exocytosis – BLT-eserase assays (Takayama et al. 1987) or measurements of target cell killing (Lichtenfels et al. 1994) – are population assays which cannot give information about the response of individual CTLs.
In the present study we have used microsocopic and flow cytometric analysis of perforin destaining and LAMP-1 externalization to study CTL granule exocytosis in response to soluble stimuli at the single cell level in a human leukaemic CTL line. We have been able to assemble a novel view of how single CTLs respond to a range of conditions.
Methods
Chemicals and reagents
Salts for physiological solutions were from Sigma-Aldrich (St Louis, MO, USA). Fetal calf serum was from Atlas Biologicals (Fort Collins, CO, USA). Thapsigargin and antiperforin monoclonal antibodies were from Alexis Biochemicals (San Diego, CA, USA). Mouse IgG anti-CD107a (clone H4A3) and a matched isotype control were purchased from BD Biosciences (San Diego, CA, USA). Secondary antibodies were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA, USA).
Cells and solutions
TALL-104 cells were obtained from the American Type Culture Collection (Rockville, MD, USA) and grown in Iscoves's medium supplemented with 10% fetal calf serum (FCS) and 100 IU interleukin (IL)-2. Cells were grown in a humidified incubator at 37°C in 10% CO2. Ringer solution contained (mm): 145 NaCl, 4.5 KCl, 1 MgCl2, 2 CaCl2, 5 Hepes and 10 glucose (pH 7.4 with NaOH). Zero Ca2+ Ringer solution was identical except CaCl2 was replaced with MgCl2 and 1 mm EGTA was added. Ringer solution containing 0.25 and 0.5 mm Ca2+ was made by mixing normal Ringer solution and zero Ca2+ Ringer solution that did not have EGTA added.
BLT esterase assays
Granyzme B released from TALL-104 cells was assayed by measuring hydrolysis of Nα-benzyloxycarbonyl-l-lysine thiobenzyl ester (BLT) essentially as previously described (Takayama et al. 1987). Tall-104 cells were suspended at a density of ∼5 × 107 cells per ml of cell culture medium. Five microliltres of the cell suspension (2.5 × 105 cells) was then pipetted into wells of a microtitre plate. Two hundred microlitres of appropriate experimental solution was added to each well. Following stimulation, cells were centrifuged at 200 g for 0.5 min 50 μl of supernatant was transferred to another 96-well plate, and 150 μl of BLT solution (0.2 mm BLT, and 0.22 mm 5,5′-dithio bis(2-nitrobenzoic acid) (DTNB)) in phosphate-buffered saline (PBS, pH 7.2) was added to the supernatant of each condition and allowed to react for 40 min at room temperature (20–22°C). After removal of the remaining supernatant, 200 μl of 0.1% Triton X-100 in PBS was added to the pellet of each experimental well, and 50 μl of this solution was treated as described above to determine the residual cellular granzyme activity. Absorbance measurements were made with a Bio-Tek Synergy HT-I plate reader (Bio-Tek Instruments, Winooski, VT, USA) read at 410 nm after subtraction of an appropriate blank. The percentage of BLT esterase activity released spontaneously was measured from unstimulated CTLs that were treated identically to stimulated cells and incubated in normal Ringer solution. Release was calculated as absorbancesupernatant/absorbancepellet+ absorbancesupernatant. Spontaneous release was subtracted from percentage release obtained upon stimulation to obtain percentage specific stimulated release.
Immunocytochemistry and flow cytometry
For flow cytometry, cells were first washed with Normal Ringer solution and then stimulated as indicated in the conditions described. Anti-LAMP and isotype control antibodies were concentrated 20-fold from the stock provided by the manufacturer using Millipore Amicon columns with a 5000 molecular weight cutoff (Millipore, Bedford, MA, USA), then diluted 1: 50 before use. Cells were fixed in 0.2% paraformaldehyde after processing. Flow cytometry was done on a Coulter Cytomics FC500 (Beckman Coulter, Miami, FL, USA). FlowJo software (TreeStar, Ashland, OR, USA) was used to analyse data offline. For multicolour experiments, data were acquired without hardware compensation. Unstained and single-colour controls were run, and data were compensated off-line using FlowJo.
For immunocytochemistry experiments, cells were treated as described above, fixed, and then allowed to settle on coverslips. Prolong antifade reagent (a hardening mounting medium from Molecular Probes, Eugene, OR, USA) was then applied. For experiments with fixed and permeabilized cells, CALTAG's Fix/Perm kit was used according to the manufacturer's protocol (CALTAG laboratories, Burlingame, CA, USA). In experiments analysing internalization of LAMP-1 antibody, FITC-labelled goat antimouse secondary antibodies (1: 50) were added in PBS with 1% BSA for 40 min at 37°C.
Fluorescence imaging
Image acquisition was performed on two systems. For the experiments to analyse patterns of granule release, a system built around a Nikon Diaphot 300 inverted microscope (Nikon Inc.) equipped for epifluorescence was used. Slidebook software (Intelligent Imaging Innovations, Denver, CO, USA) was used to control the hardware and to deconvolve images. All other images were acquired with a Zeiss LSM 510 laser scanning confocal microscope at the UCHSC Light Microscopy facility with a 63× Apochromat oil immersion objective. Bright-field images were acquired by collecting scattered 488 nm light in a third channel. Images were processed with LSM 5 Image Browser software.
Results
Defining granule exocytosis stimulated by agents that bypass the TCR in TALL-104 cells
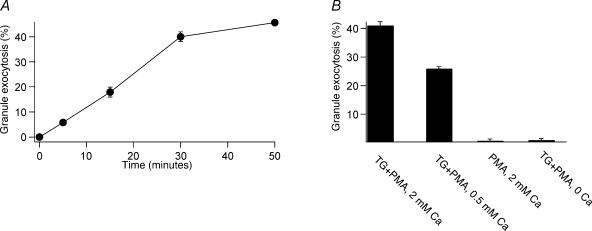
We used BLT-esterase assays, which detect the release of granzyme from populations of CTLs (Takayama et al. 1987), to define patterns of TCR-independent granule exocytosis in TALL-104 human leukaemic CTLs (Cesano & Santoli, 1992). Our previous work indicates that granule exocytosis in TALL-104 cells exhibits many of the properties characteristic of CTL granule exocytosis, including dependence on Ca2+ influx and polarized target-directed exocytosis of lytic granules (Lyubchenko et al. 2001), as well as dependence on ERK mitogen-activated protein kinases (Fierro et al. 2004). TALL-104 cells thus seem to be a good model of CTL function. Figure 1A shows that stimulation of TALL-104 cells with thapsigargin (TG) and phorbol myristate acetate (PMA) in the presence of 2 mm Ca2+o triggers time-dependent lytic granule exocytosis. PMA is a diacylglycerol analogue that activates PKC. TG increases [Ca2+]i by blocking SERCA ATPases (Thastrup et al. 1989, 1990), emptying ER Ca2+ stores and activating Ca2+ release-activated Ca2+ (CRAC) channels (Zweifach & Lewis, 1993; Lewis, 1999, 2001), which probably mediate TCR-stimulated Ca2+ influx in CTLs (Zweifach, 2000). Our results indicate that exocytosis triggered with TG + PMA is a slow process, and was more or less complete after 50 min.
Figure 1. Patterns of TCR-independent secretion in TALL-104 cells measured with BLT- esterase assays.
A, time course of secretion stimulated by 1 μm TG and 50 nm PMA in the presence of 2 mm extracellular calcium. B, granule secretion elicited by different stimulation conditions 50 min after stimulation. All data are mean ±s.e.m. for three experiments performed in triplicate.
We assessed the ability of a variety of stimulation protocols to elicit granule exocytosis measured at 50 min (Fig. 1B). Compared to stimulation with TG + PMA in 2 mm extracellular Ca2+ (Ca2+o), stimulation with TG + PMA in the presence of 0.5 mm Ca2+o caused reduced granule exocytosis, while stimulation with PMA alone in the presence of 2 mm Ca2+o or stimulation with TG + PMA in the complete absence of Ca2+o did not cause granule exocytosis. For all the experiments that follow, we consider stimulation of cells with TG and PMA in the presence of 2 mm Ca2+o for 50 min as causing maximal exocytosis.
Confirming that LAMP-1 externalization can be used to monitor granule exocytosis in TALL-104 cells
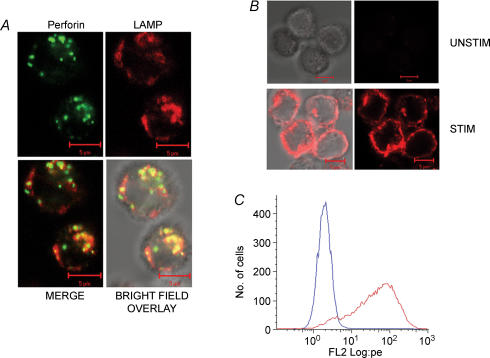
We next confirmed that LAMP externalization can be used to monitor granule exocytosis in TALL-104 cells. We first determined that, in unstimulated cells, LAMP-1 exhibits a cellular localization consistent with its residence in the membrane of lytic granules. As expected based on previous work, we found that there was an association between LAMP-1 staining and perforin staining in unstimulated cells (Fig. 2A). LAMP-1 staining was more diffuse than perforin staining, which may be consistent with the idea that LAMP resides in granule membranes while perforin is concentrated in dense cores.
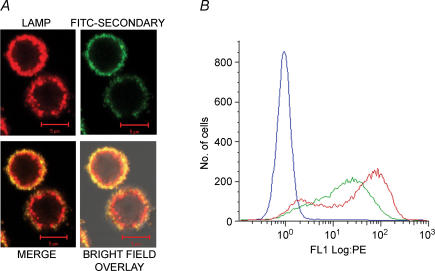
Figure 2. LAMP colocalizes with perforin in unstained cells and is exposed to the extracellular solution during stimulation.
A, colocalization between perforin and LAMP-1 in unstimulated cells. TALL-104 cells were fixed, permeabilized and stained with FITC-labelled antiperforin antibodies (shown in green) and PE-labelled anti-LAMP-1 antibodies (shown in red). Also shown are merge and bright-field overlay images in the bottom row. Yellow spots represent colocalized pixels. Scale bar is 5 μm. B, stimulation-dependent LAMP-1 staining in intact cells. In the presence of LAMP-1-PE antibody, TALL-104 cells were either incubated for 50 min in Normal Ringer solution (Unstim) or stimulated with TG + PMA (Stim). Shown are bright field and fluorescence images of both conditions. Scale bar is 5 μm. C, histograms of flow cytometry data showing an increase in LAMP-1 staining due to stimulation. TALL-104 cells were left untreated (blue trace) or stimulated with TG + PMA (red trace). Both were incubated in the presence of the LAMP-1 antibody.
Next, we confirmed that stimulation with TG and PMA, which causes robust granule exocytosis (Fig. 1), causes externalization of LAMP. In these experiments, cells were stimulated in the presence of a PE-labelled anti-LAMP-1 monoclonal antibody. Under these conditions, staining with the anti-LAMP antibody would only be expected if LAMP becomes exposed to the surface as a result of exocytosis. Figure 2B shows fluorescence images and a bright field overlay of unstimulated and stimulated cells, and Fig. 2C is a histogram of fluorescence intensities of stimulated and unstimulated cells measured via flow cytometry after gating on forward and side scatter. Stimulation causes an approximately two log-unit increase in LAMP-1-PE staining intensity, and the data indicate that the majority of cells (> 80%) responded. There was no increase in fluorescence intensity when cells were stimulated in the presence of a PE-labelled isotype control (data not shown), confirming that staining is specific for LAMP-1. Inspection of the images suggests that LAMP-1 staining is associated with the plasma membrane in stimulated cells (Fig. 2B).
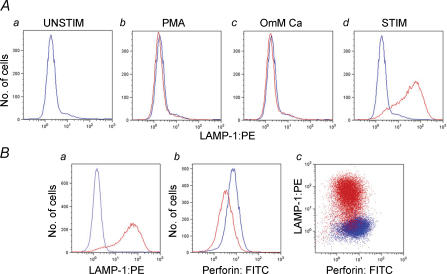
As a further test of the utility of LAMP externalization as a measure of granule exocytosis, we examined LAMP externalization using flow cytometry under similar conditions that were used to elicit granule exocytosis as assessed with BLT-esterase assays in Fig. 1 (Fig. 3A). The results presented in Fig. 3A are in good agreement with those in Fig. 1B, suggesting that granule exocytosis as assessed with BLT-esterase assays is associated with externalization of LAMP. Note that data showing the intensity of LAMP-1 staining in cells stimulated in the presence of 0.5 mm Ca2+o are shown in Fig. 6.
Figure 3. LAMP exposure correlates with lytic granule exocytosis.
A, histograms of LAMP-1 staining following different stimulation protocols measured using flow cytometry. In the presence of LAMP-1-PE antibody, TALL-104 cells were incubated in Normal Ringer solution (a), stimulated with PMA alone (b), stimulated with TG + PMA in the absence of extracellular Ca2+ (c) or stimulated with TG + PMA in Normal Ringer solution (d). In a–d, the unstimulated condition (a) is replotted in blue and the experimental condition is in red. B, correlation between LAMP-1 staining and perforin destaining. In the presence of PE-conjugated LAMP-1 antibody, TALL-104 cells were left untreated or were stimulated for 50 min with TG + PMA in Normal Ringer solution, then fixed and permeabilized and stained with Perforin-FITC antibody. Unstimulated cells are shown in blue, and stimulated cells are shown in red. a shows an increase in LAMP-1 staining upon stimulation, b shows a decrease in perforin staining upon stimulation, and c is a plot of LAMP versus perforin staining for stimulated and unstimulated cells. Note the inverse correlation between perforin staining and LAMP staining in the stimulated cells.
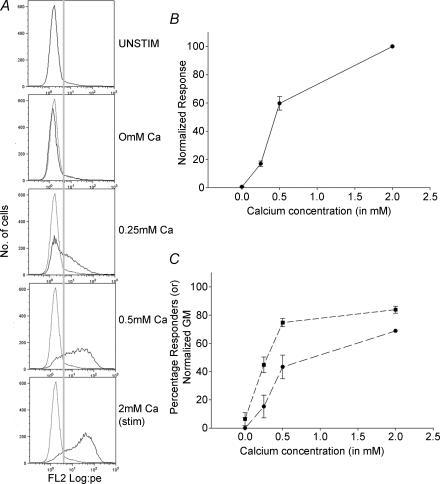
Figure 6. Analysis of the Ca2+ dependence of CTL granule exocytosis.
Varying the stimulation intensity alters both the amplitude of single-cell responses and the number of cells that respond. A, histograms of LAMP-1-PE staining intensity measured using flow cytometry. The cells were stimulated with TG + PMA in the presence of 0 mm, 0.25 mm, 0.5 mm and 2 mm extracellular calcium for 50 min. Dotted traces are the unstimulated condition and the continuous traces are the experimental conditions. The grey line indicates the gate (fluorescence value) considered to separate responders from non-responders. B, the overall response of cells increases with increasing calcium concentrations. The data are normalized to the 2 mm extracellular calcium condition. C, analysis of the fraction of cells responding and the mean amplitude of their responses. Responders were selected by gating against the unstimulated condition. Percentage responders is plotted with squares, and the amplitude of their responses (geometric mean of fluorescent intensity) is plotted with circles.
As a final means of confirming that LAMP staining correlates with granule exocytosis, we investigated the correlation between LAMP externalization and perforin destaining at the single-cell level using two-colour flow cytometry (Fig. 3B). We stimulated cells in the presence of the PE-labelled anti-LAMP-1 antibody, then fixed them and permeabilized them and stained them with a FITC-labelled antiperforin mAb. Consistent with our expectations based on the results of BLT esterase experiments, stimulation with TG and PMA in the presence of 2 mm Ca2+o resulted in a ∼50% decrease in mean cellular perforin content. Importantly, we found that there was an inverse correlation between LAMP staining and perforin staining in the stimulated cells. Those cells that had the lowest perforin content following stimulation tended to be the most brightly stained for LAMP.
Taken together, the results of Figs 2 and 3 provide strong confirmation for the idea that LAMP externalization can be used to estimate the extent of lytic granule exocytosis.
Some structures labelled during stimulation are retained on the cell surface
Previous work indicates that LAMP is rapidly re-internalized following exposure (Betts et al. 2003; Betts & Koup, 2004), necessitating the use of drugs like monensin to prevent quenching of the fluorophore coupled to the anti-LAMP antibody after it gets taken up into acidic intracellular compartments. However, images of stimulated cells like those in Fig. 2B seem to show LAMP-1 staining associated with the plasma membrane. To test whether there are any anti-LAMP-1 labelled structures exposed at the plasma membrane 50 min after stimulation, we performed two kinds of experiment. First, we stimulated TALL-104 cells with TG + PMA in the presence of 2 mm Ca2+o and PE-labelled anti LAMP-1-PE antibody for 50 min, then fixed them and incubated them with a FITC labelled antimouse IgG secondary antibody (Fig. 4A). We expected that internalized structures would not be accessible to the FITC-labelled secondary antibody, and thus would stain with PE only. There was obvious dual-labelling, consistent with the idea that some exposed LAMP is retained on the surface. However, there also appeared to be a population of structures just beneath the plasma membrane that was not accessible to the secondary antibody. These may represent LAMP that had been exposed to the extracellular solution via exocytosis and then reinternalized. Second, we used flow cytometry to compare the fluorescence intensities of cells that were stimulated in the presence of the anti LAMP-1 antibody to cells that were stimulated in the absence of the antibody, then fixed and stained with the antibody (Fig. 4B). We expected that if a significant amount of LAMP-1 was reinternalized after exocytosis, staining would be markedly reduced in the cells that were stimulated in the absence of the antibody. This was not the case. In fact, staining was enhanced in the fixed cells. The reasons for this are unclear, especially in light of the results of Fig. 4A described above suggesting that there may be structures that are inaccessible to the secondary Ab in fixed cells. It may be that the antibody binds better to LAMP-1 after fixation, or has better access to epitopes. Alternatively, if there is some internalization of labelled LAMP-1 after exocytosis as the results of Fig. 4A suggest, then the fluorescence of the fluorophore may be decreased in live cells due to effects of acidic pH in endosomes. Fixation may reverse this.
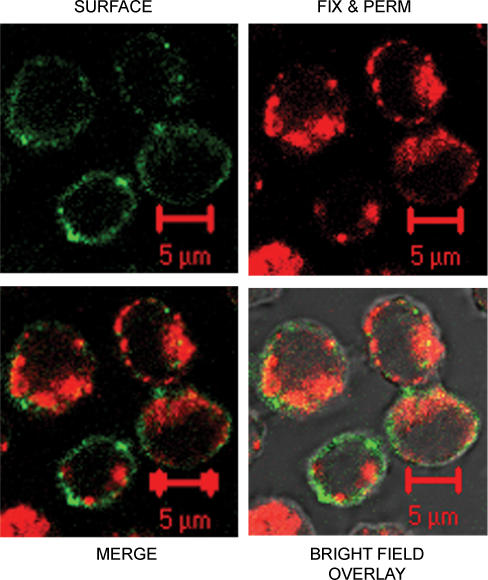
Figure 4. Evidence that LAMP-1 staining is associated with the plasma membrane in stimulated cells.
A, in the presence of LAMP-1-PE antibody, TALL-104 cells were stimulated for 50 min with TG + PMA in the presence of 2 mm extracellular calcium, fixed and stained with FITC-labelled goat-antimouse IgG secondary antibody for 45 min. LAMP-PE staining is shown in red and FITC secondary antibody is shown in green. Also shown are the merge and the bright-field overlay images. Scale bar is 5 μm. B, staining is not reduced if cells are first stimulated and then labelled with the antibody. TALL-104 cells were first stimulated for 50 min with TG + PMA in the presence of 2 mm extracellular calcium, then fixed with 2% PFA in PBS and stained for LAMP-1-PE labelling for 50 min (red), or stimulated in the presence of the antibody (green) or left unstimulated (blue).
Analysing patterns of granule release
The results of Figs 1 and 3B suggest that both granzyme and perforin decrease by ∼50% following stimulation with TG and PMA in the presence of 2 mm Ca2+o. This could be due either to ∼half the cells in the population releasing most of their granules, or due to the majority of cells releasing about half their granules. The results of Fig. 2 with LAMP staining suggest that in fact the majority of cells in the population respond to stimulation. Thus, on average each cell must release about half of its granule contents. In principle, two fundamentally different kinds of single-cell behaviour could account for this. Cells could mobilize all of their lytic granules, but release on average only half the contents of each, or cells could mobilize about half their granules, releasing essentially all of the contents of each.
To distinguish between these possibilities, we performed two kinds of experiment. First, we stained fixed and permeabilized cells with a FITC-labelled antiperforin mAb, and then analysed images to determine whether parameters associated with granule number or with average granule content decreased upon stimulation (Table 1). Z-sections spanning the entire focal depth of cells were acquired, then deconvolved using a fully iterative algorithm. Images were segmented using a threshold set at 10 times the background fluorescence intensity, and the following parameters were computed on a cell-by-cell basis: (1) integrated fluorescence intensity (a measure of total perforin content); (2) the volume (in voxels) occupied by fluorescent structures (a measure of granule number); (3) the estimated number of discrete objects (a measure of the number of granules); and (4) the mean fluorescence of each object (a measure of individual granule perforin content). Note that the algorithm that computes the number of objects counts as a single object structures that are in contact in any focal plane, thus underestimating the true value. Provided the underestimation of the true number of objects is similar in both stimulated and unstimulated cells, comparison of the values will still be informative. In three experiments, stimulation caused a 60–70% decrease in integrated fluorescence intensity, indicating that total cellular perforin content has decreased by this amount, consistent with expectations from flow cytometric analysis of perforin destaining (Fig. 3). Both the volume of fluorescent structures and the estimated number of objects closely correlated with the total fluorescence decrease, but the mean object intensity did not. These results are most consistent with the idea that responses are due to ∼50% of a cell's granules releasing most of their contents, rather than all granules releasing only a fraction. Note that in experiment 3 (Table 1), the integrated fluorescence intensity, volume occupied by fluorescent structures and the estimated number of objects were all more than twice as high as in the other two experiments. These experiments were performed several weeks apart, and it is likely that the difference in the measured parameters reflects a difference in the state of the cells. Critically, though, the change in these parameters upon stimulation is consistent between experiments, regardless of the absolute values.
Table 1.
Analysis of the effects of stimulation on perforin-containing structures
| No. of cells | Integrated intensity | Volume | No. of objects | Mean intensity | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Experiment | U | S | U | S | % | U | S | % | U | S | % | U | S | % | |
| 1 | Mean | 59 | 71 | 12.2 | 4.1 | 34 | 1120 | 463 | 41 | 24 | 11.9 | 49 | 890 | 745 | 84 |
| s.e.m. | 2.6 | 1.2 | 210 | 118 | 2.3 | 1.6 | 25 | 15.7 | |||||||
| 2 | Mean | 29 | 36 | 9.5 | 3.1 | 32 | 899 | 325 | 40 | 21 | 8.3 | 40 | 972 | 752 | 77 |
| s.e.m. | 2.0 | 1.1 | 20.9 | 8.3 | 3.0 | 1.1 | 51.8 | 26.7 | |||||||
| 3 | Mean | 31 | 50 | 29.0 | 13.2 | 45 | 2970 | 1156 | 47 | 46 | 21.4 | 47 | 954 | 1045 | 110 |
| s.e.m. | 3.9 | 1.8 | 45.5 | 21.4 | 7.2 | 1.7 | 32.1 | 29.2 | |||||||
U = unstimulated, S = stimulated. No. of cells is the number of cells analysed for each condition for that experiment. Integrated intensity is the mean integrated fluorescence given in 104 camera intensity units. Integrated intensity was computed on a cell-by-cell basis, then its mean was calculated. Volume is the volume (in voxels) occupied by structures with fluorescence intensities above the threshold. Volume was determined on a cell-by-cell basis, then its mean was calculated. Mean intensity is the mean fluorescence intensity of all fluorescent structures. Mean intensity was calculated on a cell-by-cell basis, then its mean was determined. s.e.m. is given below each value.% is (stimulated/unstimulated) × 100.
As an independent means of examining this issue, we stimulated cells in the presence of an anti LAMP-1 antibody labelled with one fluorophore, then fixed them and incubated them with an unlabelled anti-LAMP antibody before permeabilizing them and labelling them with a different-coloured anti-LAMP-1 antibody (Fig. 5). The incubation with the unlabelled antibody was performed to ensure that all LAMP that was exposed via exocytosis was antibody-bound, and could not stain with the second labelled antibody. We expected that, if most of a cell's granules released part of their contents, we would observe staining of plasma membrane (and possibly intracellular staining representing reinternalized membrane) labelled with the antibody present during stimulation only. Critically, though, if only a fraction of a cell's granules were mobilized, we would expect to observe a population of intracellular granules labelled exclusively with the antibody applied after fixation and permeabilization. We found that cells treated in this way showed staining with the antibody present during stimulation, consistent with the idea that they had externalized LAMP via exocytosis. Importantly, we clearly observed a population of intracellular granules that labelled only with the second antibody. This result is consistent with the idea that not all of a cell's granules are mobilized during stimulation.
Figure 5. Evidence that secretion represents a fraction of a cell's granules releasing all of their contents.
In the presence of LAMP-1-FITC antibody, TALL-104 cells were stimulated for 50 min with TG + PMA in the presence of 2 mm extracellular calcium, then fixed, permeabilized and stained with LAMP-1-PE antibody. LAMP-1-FITC is shown in green, LAMP-1-PE is shown in red. Bright-field overlay and merge images are also shown. Scale bar is 5 μm.
Exploiting LAMP-1 staining to analyse patterns of exocytosis at the single cell level
The appearance of LAMP-1 on the surface of stimulated cells offers a unique tool to study CTL lytic granule exocytosis at the single cell level, because staining with fluorescent antibodies reveals whether single cells have undergone exocytosis. We have exploited this to analyse the single-cell behaviour underlying granule exocytosis in the presence of Ca2+o < 2 mm, or in the presence of 2 mm Ca2+o at times < 50 min, conditions that as measured using BLT esterase assays result in submaximal exocytosis compared to stimulation for 50 min in the presence of 2 mm Ca2+o. Reduced exocytosis could in principle result from either a decrease in the number of cells that respond, from a decrease in the amplitude of the response of individual cells, or a combination of both.
To assess responses in the presence of reduced Ca2+o, we stimulated the cells with TG + PMA in the presence of 0 mm, 0.25 mm, 0.5 mm and 2 mm Ca2+o (Fig. 6). Figure 6A shows representative flow histograms from one such experiment. As we increased the calcium concentration there was an increase in the normalized response of the cells calculated from the geometric mean of the histograms (Fig. 6B). We analysed these data to determine the fraction of cells that respond at each [Ca2+]o, and the amplitude of their responses. We determined the fraction of cells that responded by setting a gate for all histograms that was based on the unstimulated histogram (grey line). Cells with fluorescence greater than this value were considered to have responded, and the amplitude of responses for these cells was then determined by calculating their geometric mean. Our results show that as [Ca2+]o was increased, both the percentage of cells that respond and the amplitude of their responses increased (Fig. 6C). However, the two parameters did not demonstrate an identical dependence on Ca2+o. At 0.5 mm Ca2+o, the fraction of cells that respond had nearly reached its full value, while the amplitude of responses was only around half of that observed in 2 mm Ca2+o. As is discussed, below, this may be consistent with the existence of multiple Ca2+-dependent steps in granule exocytosis.
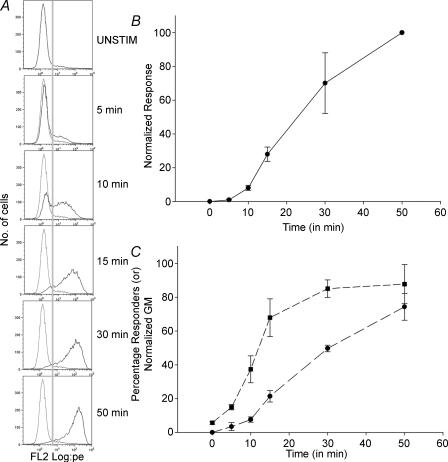
We next assessed the temporal profile of exocytosis (Fig. 7). Cells were stimulated with TG + PMA in the presence of 2 mm extracellular calcium for 5, 10, 15, 30 or 50 min in the absence of antibody, then fixed and stained with LAMP-1-PE antibody for 50 min. This protocol was used because binding of the antibody to LAMP is a relatively slow process, and its kinetics would otherwise distort our results. Figure 7A shows representative histograms from one experiment. Figure 7B show the normalized response of the cells at the different times assayed. These data compare well to the time course of granule exocytosis measured with BLT-esterase assays (Fig. 1A). As was the case for stimulation in the presence of different [Ca2+]o, we analysed the fraction of cells that responded as well as the amplitude of the responses. We found that responses could be detected in some cells as quickly as 5 min after stimulation. However, cells did not respond synchronously. At 10 min after stimulation, only about half the cells had responded. Furthermore, analysis of the amplitude of responses indicates that initial responses were of submaximal amplitude. The amplitude of responses increased slowly over the course of the observation period.
Figure 7. Analysis of the time course of granule exocytosis.
Flow cytometry was used to analyse the time course of granule exocytosis. TALL-104 cells were stimulated with TG + PMA in the presence of 2 mm extracellular calcium for various time intervals, then fixed with 2% PFA in PBS and stained with LAMP-1-PE antibody for 50 min. A, histograms of LAMP-1-PE staining at the indicated times. The dotted traces represent the unstimulated condition, and the continuous traces are the experimental conditions. The grey line indicates the gate (fluorescence value) considered to separate responders from non-responders. B, time course of responses as a function of time normalized to the 50-min time point. C, analysis of the fraction of cells responding and the mean amplitude of their responses. Responders were selected by gating against the unstimulated condition. Percentage responders is plotted with squares, and the amplitude of their responses (geometric mean of fluorescent intensity) is plotted with circles.
Discussion
Our results are consistent with previous work demonstrating that LAMP staining is a useful assay of CTL degranulation. This method allows exocytic responses of single CTLs to be monitored, something that is not possible with older methods such as BLT-esterase assays or target cell killing assays, or with the newer perforin destaining measurements. We find that there is so much overlap between levels of perforin in stimulated and unstimulated cells (see Fig. 3B) that it is not possible to determine whether a given cell has responded.
In contrast to previous work (Betts et al. 2003; Betts & Koup, 2004), we found that some LAMP-1 stained structures were still found on the CTL plasma membrane after 50 min of stimulation (Fig. 4). There are several possible explanations for this result. TALL-104 cells respond very rapidly compared to other CTL systems, and it may be that this time interval is not sufficient for the large-scale internalization of LAMP-1 containing structures in TALL-104 cells. Alternatively, it may be that TCR-independent stimulation either does not trigger or inhibits endocytosis. Finally, the explanation may reflect a difference in TALL-104 cells compared to other CTLs.
The primary significance of this work is that we have been able to provide for the first time a view of granule exocytosis at the level of single CTLs. To date, only population behaviour has been assessed. We find that TALL-104 cells release about half their granule contents upon stimulation with TG + PMA in the presence of 2 mm Ca2+o. This partial secretion at the population level results from the majority of cells responding (see for example Fig. 2). Each cell responds by mobilizing about half its granules (Fig. 5 and Table 1), and those granules that are mobilized probably release their contents completely. Thus, individual cells do not appear to respond in an all-or-none manner, but release from the granules that are mobilized is probably all-or-none. This is consistent with the observation of significant LAMP-1 staining retained at the plasma membrane (Fig. 4), and suggests that, after vesicles fuse with the plasma membrane, they collapse fully into it. An alternative, dubbed ‘kiss-and-run’ exocytosis (reviewed in An & Zenisek, 2004), has been inferred to occur in excitable cells. In ‘kiss-and-run’ exocytosis, vesicles do not collapse into the plasma membrane or release all of their contents upon fusion. Instead, release of their cargo probably occurs via a transient fusion pore.
Our analysis of response patterns in the presence of reduced Ca2+ indicates that both the percentage of cells that respond and the amplitude of the responses are modulated. Decreased response amplitude is probably due to a decrease in the number of granules mobilized. Interestingly, the two parameters do not demonstrate an identical dependence on Ca2+o. This may reflect a different dependence on intracellular Ca2+o concentration. One possible explanation for this result is that there may be multiple Ca2+-dependent steps in granule exocytosis. Although it is well known that exocytosis requires Ca2+ influx, little is known about the molecules that participate. Studies with immunosuppressant drugs have implicated the Ca2+/calmodulin-dependent protein phosphatase calcineurin in granule exocytosis (Sitkovsky, 1988; Dutz et al. 1993), and our unpublished observations indicate that FK506 and cyclosporin A inhibit exocytosis in TALL-104 cells (G. A. Wurth and A. Zweifach, unpublished observations) However, in most cases that have been examined, Ca2+-dependent exocytosis involves the participation of synaptotagmins, C2 domain-containing Ca2+- and phospholipid-binding proteins that are required for Ca2+-dependent vesicle fusion. Synaptotagmins form complexes with SNARE proteins, and although the involvement of SNARE proteins in lytic granule exocytosis has not been investigated, recent work has shown that Munc13–4, which is believed to regulate interactions between SNARE proteins, is critical for granule exocytosis (Feldmann et al. 2003). We speculate that lytic granule exocytosis requires both a calcineurin dependent step- perhaps a priming step- as well as interaction of Ca2+ with a synaptotagmin. Each may have its own intrinsic Ca2+-dependence, and if calcineurin is involved in an early step required for vesicles to become fusion-competent, then complex Ca2+-dependent behaviour could be generated.
Analysis of the time course of exocytosis indicates that TALL-104 cells respond to stimulation with TG + PMA with a variable latency of 5–15 min, and that the amplitude of responses for those cells that have responded increases slowly over a period of 5–50 min. That individual cells respond with a variable latency is somewhat surprising. Unlike stimulation via the TCR, in which multiple signalling intermediates are involved, TG and PMA might be expected to activate Ca2+ influx and stimulate PKC in a temporally uniform manner. We have not been able to image increases in [Ca2+]i and labelling with LAMP simultaneously because of slow binding of the anti-LAMP antibody. However, we can roughly estimate the time course of the activation of Ca2+ influx from Ca2+ imaging experiments by subtracting responses obtained in the absence of Ca2+o from those obtained in its presence (Zweifach, 2000). Our unpublished observations suggest that influx starts ∼60 s after the addition of TG in TALL-104 cells, and that Ca2+ increases attributable to influx are maximal after ∼5 min. These results suggest that activation of Ca2+ influx precedes influx by 5–10 min. Too little is known about the signalling underlying granule exocytosis or the molecules involved to interpret these results at present, but future investigations in which responses to a wider variety of conditions are assessed may yield important results, particularly if combined with analysis and perturbation of specific signalling pathways. That the amplitude of the responses increases over a long time suggests that, once it starts, exocytosis is a slow process requiring tens of minutes. This result may also be consistent with the idea that multiple steps are required before granules are competent to undergo fusion.
Although relatively few studies have investigated single-cell exocytosis in haematopoetic cells, essentially all possible response patterns have been observed, depending on the cell type examined and conditions used to stimulate release. Hide et al. (1993) analysed mast cell exocytosis by measuring changes in side-scatter using flow cytometry, and found that the pattern of single-cell response depended upon stimulation conditions. When permeabilized mast cells were stimulated with GTP-γ-S and different Ca2+ concentrations, or were stimulated with different concentrations of the Ca2+ ionophore ionomycin, graded secretion occurred because different numbers of cells responded in an all-or-none fashion. In contrast, when intact cells were stimulated with the secretagogue compound 48/80, graded secretion occurred because individual cells responded in a graded fashion. Boonen et al. (1994) examined responses of electropermeabilized neutrophils, also by measuring changes in side-scatter via flow cytometry. As with mast cells, several different response patterns were observed, depending on the nature of the stimulus. When cells were stimulated with different [Ca2+], monophasic distributions of side-scatter were observed, suggesting that the amplitude of responses rather than the number of cells that responded was modulated. When cells were stimulated with Ca2+ and different concentrations of GTP-γ-S, changes in the number of cells that responded were detected, and cells appeared to respond in an all-or-none fashion. Interestingly, this study also examined the time course of responses to high [Ca2+] and to high [Ca2+] and GTP-γ-S. Cells stimulated with Ca2+ alone appeared to respond in a graded manner, with all cells in the population responding more or less synchronously. In contrast, when stimulated with Ca2+ and GTP-γ-S, cells appeared to respond with varying latency, as multiple peaks in the distribution of side-scattered light were apparent. Finally, MacGlashan et al. (1994) examined basophil responses to stimulation with anti-IgG, and found that graded increases in exocytosis (as measured by the appearance of CD11b on the cell surface) reflected graded changes in the amplitude of response of individual cells.
The results presented here constitute a novel view of the exocytic response of individual CTLs, albeit in a leukaemic cell line and in response to unphysiological stimuli. Future work will be required to determine whether responses of primary CTLs are similar. It will also be critical to determine the patterns of single-cell behaviour that underlie responses stimulated via the TCR. Stimulation with TG and PMA probably bypasses important steps in the lytic interaction between CTL and target that occur prior to actual granule exocytosis, including formation of the immunological synapse and reorientation of lytic granules and the microtubule organizing centre to the site of contact with the target cell. We would expect these steps to significantly complicate response patterns, as it is possible that not every cell successfully forms a synapse or reorients its granules when stimulated via the TCR. Furthermore, these processes could proceed at different rates in different cells. Thus we might expect a decrease in the proportion of cells that successfully degranulate and an increased heterogeneity of degranulation time courses when cells are stimulated via the TCR as compared to soluble stimuli. Note that our previous work with TALL-104 cells has provided evidence that calcium influx channels do not exhibit a preferential localization to the site of contact with target cells (Lyubchenko et al. 2001). Thus, if the Ca2+ dependence of granule exocytosis stimulated via the TCR differs from that stimulated by soluble agents, it cannot be due to channel polarization.
Our knowledge of CTL exocytosis lags far behind our understanding of other exocytic cell types. It seems likely that new methods for monitoring granule exocytosis will provide keys to unlock the secrets of killer T lymphocytes.
Acknowledgments
This work was supported by NIH Grant R01 AI054839 (A.Z), and by NIH Training Grant T32 NS07083 (R.A.B). The authors would like to acknowledge the University of Colorado Cancer Center Flow Cytometry Core, the University of Colorado Health Sciences Center Light Microscopy Core Facility for the use of the Zeiss 510 confocal, and would like to thank Mr Steven Fadul for his help.
References
- Alter G, Malenfant JM, Altfeld M. CD107a as a functional marker for the identification of natural killer cell activity. J Immunol Meth. 2004;294:15–22. doi: 10.1016/j.jim.2004.08.008. [DOI] [PubMed] [Google Scholar]
- An S, Zenisek D. Regulation of exocytosis in neurons and neuroendocrine cells. Curr Opin Neurobiol. 2004;14:522–530. doi: 10.1016/j.conb.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Berke G. The binding and lysis of target cells by cytotoxic lymphocytes: molecular and cellular aspects. Ann Rev Immunol. 1994;12:736–753. doi: 10.1146/annurev.iy.12.040194.003511. [DOI] [PubMed] [Google Scholar]
- Berke G. The CTL's kiss of death. Cell. 1995;81:9–12. doi: 10.1016/0092-8674(95)90365-8. [DOI] [PubMed] [Google Scholar]
- Betts MR, Brenchley JM, Price DA, De Rosa SC, Douek DC, Roederer M, Koup RA. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J Immunol Meth. 2003;281:65–78. doi: 10.1016/s0022-1759(03)00265-5. [DOI] [PubMed] [Google Scholar]
- Betts MR, Koup RA. Detection of T-cell degranulation: CD107a and b. Meth Cell Biol. 2004;75:497–512. doi: 10.1016/s0091-679x(04)75020-7. [DOI] [PubMed] [Google Scholar]
- Boonen GJ, De Koster BM, Van Der Keur M, Vansteveninck J, Tanke HJ, Elferink JG. Characterization of exocytosis in electropermeabilized neutrophils by flow cytometric analysis: difference in sensitivity to calcium and guanosine-5′-[gamma-thio]triphosphate. Cytometry. 1994;15:230–236. doi: 10.1002/cyto.990150308. [DOI] [PubMed] [Google Scholar]
- Bromley SK, Burack WR, Johnson KG, Somerslao K, Sims TN, Sumen C, Davis MM, Shaw AS, Allen PM, Dustin ML. The immunological synapse. Annu Rev Immunol. 2001;19:375–396. doi: 10.1146/annurev.immunol.19.1.375. [DOI] [PubMed] [Google Scholar]
- Cesano A, Santoli D. Two unique human leukemic T-cell lines endowed with a stable cytotoxic function and a different spectrum of target reactivity analysis and modulation of their lytic mechanisms. In Vitro Cell Dev Biol. 1992;28A:648–656. doi: 10.1007/BF02631041. [DOI] [PubMed] [Google Scholar]
- Dutz JP, Fruman DA, Burakoff SJ, Bierer BE. A role for calcineurin in degranulation of murine cytotoxic T lymphocytes. J Immunol. 1993;150:2591–2598. [PubMed] [Google Scholar]
- Esser MT, Haverstick DM, Fuller CL, Gullo CA, Bracale VL. Calcium signaling modulates cytolytic T lymphocyte effector functions. J Exp Med. 1998;187:1057–1067. doi: 10.1084/jem.187.7.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmann J, Callebaut I, Raposo G, Certain S, Bacq D, Dumont C, Lambert N, Ouachee-Chardin M, Chedeville G, Tamary H, Minard-Colin V, Vilmer E, Blanche S, Le Deist F, Fischer A, De Saint Basile G. Munc13–4 is essential for cytolytic granules fusion and is mutated in a form of familial hemophagocytic lymphohistiocytosis (FHL3) Cell. 2003;115:461–473. doi: 10.1016/s0092-8674(03)00855-9. [DOI] [PubMed] [Google Scholar]
- Fierro AF, Wurth GA, Zweifach A. Cross-talk with Ca2+ influx does not underlie the role of extracellular signal-regulated kinases in cytotoxic T lymphocyte lytic granule exocytosis. J Biol Chem. 2004;279:25646–25652. doi: 10.1074/jbc.M400296200. [DOI] [PubMed] [Google Scholar]
- Griffiths GM. The cell biology of CTL killing. Curr Opin Immunol. 1995;7:343–348. doi: 10.1016/0952-7915(95)80108-1. [DOI] [PubMed] [Google Scholar]
- Griffiths GM, Argon Y. Structure and biogenesis of lytic granules. Curr Topics Microbiol Immunol. 1995;198:39–58. doi: 10.1007/978-3-642-79414-8_3. [DOI] [PubMed] [Google Scholar]
- Haverstick DM, Engelhard VH, Gray LS. Three intracellular signals for cytotoxic T lymphocyte-mediated killing: independent roles for protein kinase C, calcium influx, and calcium release from intracellular stores. J Immunol. 1991;146:3306–3313. [PubMed] [Google Scholar]
- Hide I, Bennet J, Pizzey A, Booney G, Bar-Sagi D, Gomperts B, Tatham P. Degranulation of individual mast cells in response to Ca2+ and guanine nucleotides: an all-or-none event. J Cell Biol. 1993;123:585–593. doi: 10.1083/jcb.123.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn JR, Poenie M. Dynamic polarization of the microtubule cytoskeleton during CTL-mediated killing. Immunity. 2002;16:111–121. doi: 10.1016/s1074-7613(02)00262-5. [DOI] [PubMed] [Google Scholar]
- Kupfer A, Dennert G. Reorientation of the microtubule-organizing center and the golgi apparatus in cloned cytotoxic lymphocytes triggered by binding to lysable target cells. J Immunol. 1984;133:2762–2766. [PubMed] [Google Scholar]
- Kupfer A, Dennert G, Singer SJ. Polarizaton of the golgi apparatus and the microtubule-organizing center within cloned natural killer cells bound to their targets. Proc Natl Acad Sci U S A. 1983;80:7224–7228. doi: 10.1073/pnas.80.23.7224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancki DW, Weiss A, Fitch FW. Requirements for triggering of lysis by cytolytic T lymphocyte clones. J Immunol. 1987;138:3646–3653. [PubMed] [Google Scholar]
- Lewis RS. Store-operated calcium channels. Adv Second Messenger Phosphoprotein Res. 1999;33:279–307. doi: 10.1016/s1040-7952(99)80014-7. [DOI] [PubMed] [Google Scholar]
- Lewis RS. Calcium signaling mechanisms in T lymphocytes. Ann Rev Immunol. 2001;19:497–521. doi: 10.1146/annurev.immunol.19.1.497. [DOI] [PubMed] [Google Scholar]
- Lichtenfels R, Biddison WE, Schulz H, Vogt AB, Martin R. CARE-LASS (calcein-release-assay), an improved fluorescence-based test system to measure cytotoxic T lymphocyte activity. J Immunol Meth. 1994;172:227–239. doi: 10.1016/0022-1759(94)90110-4. [DOI] [PubMed] [Google Scholar]
- Lyubchenko TA, Wurth GA, Zweifach A. Role of calcium influx in cytotoxic T lymphocyte lytic granule exocytosis during target cell killing. Immunity. 2001;15:847–859. doi: 10.1016/s1074-7613(01)00233-3. [DOI] [PubMed] [Google Scholar]
- Lyubchenko TA, Wurth GA, Zweifach A. The actin cytoskeleton and cytotoxic T lymphocytes: evidence for multiple roles that could affect granule exocytosis-dependent target cell killing. J Physiol. 2003;547:835–847. doi: 10.1113/jphysiol.2002.033522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGlashan DW, Jr, Bochner BS, Warner JA. Graded changes in the response of individual human basophils to stimulation: distributional behavior of early activation events. J Leukoc Biol. 1994;55:13–23. doi: 10.1002/jlb.55.1.13. [DOI] [PubMed] [Google Scholar]
- Monks CR, Freiberg BA, Kupfer H, Sciaky N, Kupfer A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 1998;395:82–86. doi: 10.1038/25764. [DOI] [PubMed] [Google Scholar]
- Nishimura T, Burakoff SJ, Herrmann SH. Protein kinase C required for cytotoxic T lymphocyte triggering. J Immunol. 1987;139:2888–2891. [PubMed] [Google Scholar]
- Page LJ, Darmon AJ, Uellner R, Griffiths GM. L is for lytic granules: lysosomes that kill. Biochim Biophys Acta. 1998;1401:146–156. doi: 10.1016/s0167-4889(97)00138-9. [DOI] [PubMed] [Google Scholar]
- Peters PJ, Geuze HJ, Van Der Donk HA, Slot JW, Griffith JM, Stam NJ, Clevers HC, Borst J. Molecules relevant for T cell–target cell interaction are present in cytolytic granules of human T lymphocytes. Eur J Immunol. 1989;19:1469–1475. doi: 10.1002/eji.1830190819. [DOI] [PubMed] [Google Scholar]
- Potter TA, Grebe K, Freiberg B, Kupfer A. Formation of supramolecular activation clusters on fresh ex vivo CD8+ T cells after engagement of the T cell antigen receptor and CD8 by antigen-presenting cells. Proc Natl Acad Sci U S A. 2001;98:12624–12629. doi: 10.1073/pnas.221458898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio V, Stuge TB, Singh N, Betts MR, Weber JS, Roederer M, Lee PP. Ex vivo identification, isolation and analysis of tumor-cytolytic T cells. Nat Med. 2003;9:1377–1382. doi: 10.1038/nm942. [DOI] [PubMed] [Google Scholar]
- Sitkovsky MV. Mechanistic, functional and immunopharmacological implications of biochemical studies of antigen receptor-triggered cytolytic T-lymphocyte activation. Immunol Rev. 1988;103:127–160. doi: 10.1111/j.1600-065x.1988.tb00754.x. [DOI] [PubMed] [Google Scholar]
- Takayama H, Sitkovsky M. Antigen receptor-regulated exocytosis in cytotoxic T lymphocytes. J Exp Med. 1987;166:725–743. doi: 10.1084/jem.166.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama H, Trenn G, Sitkovsky MV. A novel cytotoxic T lymphocyte activation assay: optimized conditions for antigen receptor triggered granule enzyme secretion. J Immunol Meth. 1987;104:183–190. doi: 10.1016/0022-1759(87)90502-3. [DOI] [PubMed] [Google Scholar]
- Thastrup O, Cullen PJ, Drobak BK, Hanley MR, Dawson AP. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2+-ATPase. Proc Natl Acad Sci U S A. 1990;87:2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thastrup O, Dawson AP, Scharff O, Foder B, Cullen PJ, Drobak BK, Bjerrum PJ, Christensen SB, Hanley MR. Thapsigargin, a novel molecular probe for studying intracellular calcium release and storage. Agents Actions. 1989;27:17–23. doi: 10.1007/BF02222186. [DOI] [PubMed] [Google Scholar]
- Weren A, Bonnekoh B, Schraven B, Gollnick H, Ambach A. A novel flow cytometric assay focusing on perforin release mechanisms of cytotoxic T lymphocytes. J Immunol Meth. 2004;289:17–26. doi: 10.1016/j.jim.2004.01.028. [DOI] [PubMed] [Google Scholar]
- Zweifach A. Target-cell contact activates a highly selective capacitative calcium entry pathway in cytotoxic T lymphocytes. J Cell Biol. 2000;148:603–614. doi: 10.1083/jcb.148.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweifach A, Lewis RS. Mitogen-regulated Ca2+ current of T lymphocytes is activated by depletion of intracellular Ca2+ stores. Proc Natl Acad Sci U S A. 1993;90:6295–6299. doi: 10.1073/pnas.90.13.6295. [DOI] [PMC free article] [PubMed] [Google Scholar]