Abstract
We hypothesized that an acute bout of strenuous, non-damaging exercise would increase rates of protein synthesis of collagen in tendon and skeletal muscle but these would be less than those of muscle myofibrillar and sarcoplasmic proteins. Two groups (n = 8 and 6) of healthy young men were studied over 72 h after 1 h of one-legged kicking exercise at 67% of maximum workload (Wmax). To label tissue proteins in muscle and tendon primed, constant infusions of [1-13C]leucine or [1-13C]valine and flooding doses of [15N] or [13C]proline were given intravenously, with estimation of labelling in target proteins by gas chromatography–mass spectrometry. Patellar tendon and quadriceps biopsies were taken in exercised and rested legs at 6, 24, 42 or 48 and 72 h after exercise. The fractional synthetic rates of all proteins were elevated at 6 h and rose rapidly to peak at 24 h post exercise (tendon collagen (0.077% h−1), muscle collagen (0.054% h−1), myofibrillar protein (0.121% h−1), and sarcoplasmic protein (0.134% h−1)). The rates decreased toward basal values by 72 h although rates of tendon collagen and myofibrillar protein synthesis remained elevated. There was no tissue damage of muscle visible on histological evaluation. Neither tissue microdialysate nor serum concentrations of IGF-I and IGF binding proteins (IGFBP-3 and IGFBP-4) or procollagen type I N-terminal propeptide changed from resting values. Thus, there is a rapid increase in collagen synthesis after strenuous exercise in human tendon and muscle. The similar time course of changes of protein synthetic rates in different cell types supports the idea of coordinated musculotendinous adaptation.
For the transfer of force from muscle cells to bone, the proteins of the extracellular matrix (ECM) of muscle and tendon, particularly collagen, are important. However, current knowledge of the responses of the collagen mass (and its turnover) to contractile activity is drawn almost entirely from animal models (Kjaer, 2004). Studies of the adaptive responses of the rates of protein synthesis to exercise have largely so far focused on mixed muscle proteins (Rennie & Tipton, 2000) or myocellular fractions (Balagopal et al. 1997) excluding collagen.
In tendon, indirect evidence of increased Type I collagen turnover in response to exercise has been obtained from measurement of the procollagen I carboxyterminal propeptide (PICP, a synthesis marker) and the carboxyterminal telopeptide of type I collagen (ICTP, a breakdown marker), analysed in dialysis fluid sampled from the human Achilles tendon (Langberg et al. 1999, 2001). In the skeletal muscle of animals, indirect indices of collagen synthesis, e.g. enzyme activity of prolyl 4-hydroxylase (Takala et al. 1983; Han et al. 1999), concentrations of hydroxyproline and collagen (Suominen et al. 1980; Kovanen et al. 1984), and of mRNA for collagens Types I, III and IV (Han et al. 1999; Koskinen et al. 2001b), all increase in response to exercise. For human beings, information on collagen concentration and indirect indices of collagen formation in muscle and the changes in response to acute exercise is sparse, being limited to activities of enzymes of collagen synthesis (Suominen & Heikkinen, 1975; Suominen et al. 1977) and tissue concentration of hydroxyproline (Hellsten et al. 1996). Hitherto, there has been no information on the response to exercise of the collagen synthesis, directly measured, in human tendon, although it is recognized that habitual exercise can thicken the Achilles tendon (Rosager et al. 2002).
Recently some of the present authors have developed a method for the direct measurement of human tissue collagen synthesis using flooding doses of stable isotope labelled proline (Babraj et al. 2002, 2005a, b) to overcome the ‘precursor problem’ (see papers referred to for details of the technical approach). The method has already been applied to studies of human muscle collagen synthesis in human muscle fibres of different types (Mittendorfer et al. 2005) ands the effects of eccentric and concentric exercise on mixed muscle collagen (Moore et al. 2005). However, there is no information available on the relationship between the collagen synthetic responses in skeletal muscle and tendon. Mechanotransduction via the ECM and the subsequent increased synthesis of collagen from fibroblasts are probably dependent upon an interplay between mechanical load, integrins (Ingber et al. 1994; Chiquet et al. 2003) and growth factors, insulin-like growth factor I (IGF-I) (Adams, 2002) and transforming growth factor β (TGF-β) (Heinemeier et al. 2003) released systemically or locally. Whereas we recently showed TGF-β rises immediately after exercise in human connective tissue (Heinemeier et al. 2003), no information exists with regards to tissue IGF-1. We aimed, in the work described here, to fill the previous gaps.
We studied the responses of the synthesis of tendon collagen and muscle collagen (probably mainly perimysial and endomysial) and non-collagen (myofibrillar and sarcoplasmic) proteins in human quadriceps muscle. We predicted that after an acute bout of strenuous, non-damaging exercise, the rates of synthesis of skeletal muscle non-collagen protein would respond more rapidly, and to a larger degree, than muscle or tendon collagen protein synthesis, and that local changes in IGF-I and IGF binding proteins might help to explain the responses. We were also keen to see to what extent the changes in an indirect measure of collagen synthesis, the concentration of PINP (procollagen type I N-terminal propeptide), in dialysate sampled from muscle and peritendinous fluid, would mirror changes in directly measured collagen synthesis.
Methods
Subjects
Two groups (group I, n = 8; group II, n = 8) of young healthy men were recruited to the study (group I: 25 ± 1 years, 186 ± 9 cm, 76 ± 8 kg; group II: 29 ± 1 years, 181 ± 9 cm, 76 ± 6 kg (means ±s.d.)). All were non-smoking, not taking medication, and free of anatomical and metabolic disorders as judged by history and routine medical examination. The subjects gave informed consent to a protocol adhering to the Declaration of Helsinki, and approved by the Ethics Committee of Copenhagen and Frederiksberg Communities.
Study design
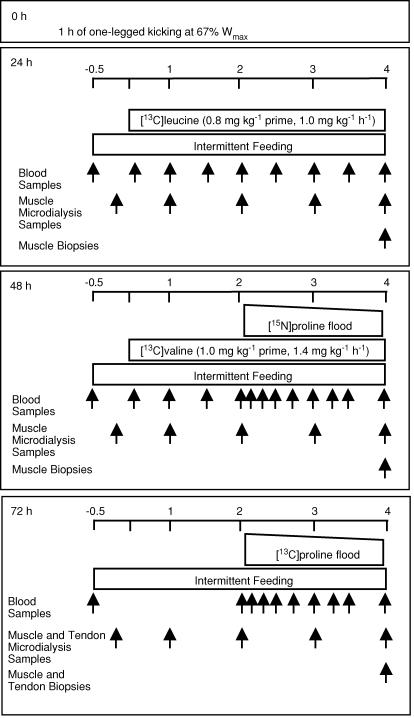
Initially we designed a study to obtain as much information as possible about the responses to strenuous exercise of muscle and tendon collagen protein synthesis (probed using a flooding dose of labelled proline (Babraj et al. 2002, 2005a, b)) in relation to myocellular protein synthesis (probed using our accustomed methods (Smith & Rennie, 1996) with constantly infused labelled leucine or valine). The different tracers were to be used to allow us to make repeated measurements without interference from the previously used labelled amino or imino acid tracers. We thus recruited subjects (group I) to carry out a protocol in which one leg would be exercised, the other rested, and samples taken at 24, 48 and 72 h post exercise. Muscle collagen was to be measured at rest and after exercise at 48 and 72 h while tendon collagen synthesis was only measured at rest and 72 h using two differently labelled variants of proline (see Fig. 1). We decided to measure tendon collagen synthesis at 72 h, a decision based upon the results of our previous studies in which concentrations of indirect markers of synthesis of Type I collagen in microdialysis fluid from around the Achilles tendon had been found to peak 72 h after exercise (Langberg et al. 1999) and because we were constrained ethically as to the number of tendon biopsies to one per patellar tendon. We also arranged to make repeated measures (at 24, 48 and 72 h) of muscle myocellular protein synthesis using leucine and valine tracers (Smith et al. 1998), and expected also to use the labelled proline probes to measure myocellular synthesis.
Figure 1. Schedule of application of stable isotope infusions and biopsy sampling in Group I (n = 8) for the examination of the time course in change of muscle contractile and collagen and tendon collagen protein.
Time scale is in hours with the initiation of tracer infusion at time 0. Not shown in the figure is the skin biopsy obtained prior to isotope infusion.
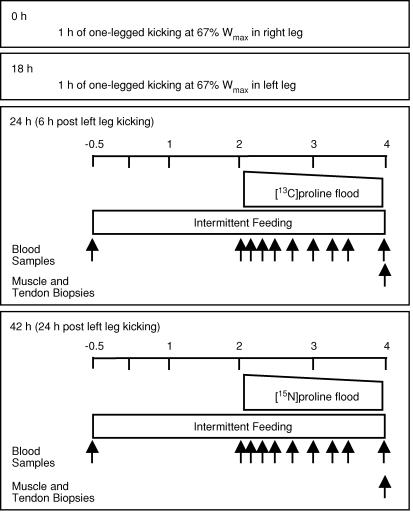
From initial studies at rest, it turned out, surprisingly, that for muscle collagen and non-collagen proteins, the values obtained at rest with [15N]- or [13C]proline administered as a bolus or [13C]leucine and its ketoacid [13C]α-ketoisocaproate administered as a constant infusion in the same state, were identical (Babraj et al. 2005a). We confirmed this result in the initial analysis of tissue from the present study and extended it to include exercised muscle probed with proline or leucine tracers. Thus we were able to obtain values of the synthetic rates of the protein classes of interest in appropriate biopsies taken on all days of investigation at rest and after exercise. Realizing this and the fact that resting protein synthetic rates remained constant at all times after exercise we designed a second supplementary study to provide a more detailed description of the time course of any possible changes. To achieve this, we recruited subjects in group II who did the one-legged kicking exercise in each leg at staggered times, so that we were able to make measurements of tendon and muscle collagen protein synthesis (as well as of myocellular synthesis) at 6, 24 and 42 h after exercise.
We also arranged for all the studies to be done in the fed state because we knew from previous work that the responses to exercise of muscle protein synthesis are maximal in the fed state (Rennie & Tipton, 2000) but also to minimize whole metabolic variations, especially of availability of amino acids and hormones. We accordingly adopted the oral intermittent feeding approach, which results in an increased but steady availability of amino acids in blood (Rennie et al. 1982; Moore et al. 2005).
We chose the one-legged kicking exercise model as one being sufficiently strenuous to be likely to evoke a measurable response in myocellular and ECM protein turnover, as we certainly achieved. Recently it has become clear that modes of muscle contraction other than resistance exercise can result in increases in muscle protein synthesis, with increases being reported in endurance type activities (Carraro et al. 1990; Sheffield-Moore et al. 2004; Short et al. 2004). We did not set out to measure net accretion of protein or protein degradation as this would have been technically impossible with current methods.
Investigative protocols
Two weeks prior to the study, each subject visited the laboratory to determine his workload maximum (Wmax) on a one-legged modified Krogh ergometer. After 5 min warming up without resistance, the subject began one-legged kicking (35 kicks per minute) for 3 min at 0.5 kg resistance; this was increased by 0.5 kg every 3 min until the subject could no longer maintain the cadence. The workload for each subject's subsequent 1 h exercise bout was defined as 67% of this Wmax (Figs 1 and 2). The subjects were instructed to avoid physical activity and to maintain their habitual diet (as confirmed by 5-day diet diaries) for the 2 days before, and during, all testing days. On the test days the fasted subjects received a commercial clinical nutrient drink (Semper, Fredriksberg, DK: 15% protein, 64% carbohydrate and 21% fat) in doses every 30 min over the subsequent 4 h. The drink provided the equivalent of 1.4 × basal metabolic rate (as estimated from the subject's fat-free mass determined by anthropometry (Cunningham, 1982). The feeding was started with a double dose to bring the subjects rapidly into a fed state. No tracer was added to the oral feed. All subjects were ‘tracer virgins’ allowing assumption of basal enrichment of tissue proteins at natural abundance values.
Figure 2. Schedule of application of stable isotope infusions and biopsy sampling in Group II (n = 6) for determination of the time course in change of muscle contractile and collagen and tendon collagen protein.
Time scale is replicated from Group I. Not shown in the figure is the skin biopsy obtained prior to isotope infusion. Please note that the 42 h protocol was only performed on 3 subjects for the purposes of obtaining 42 h measurements and to ascertain the feasibility of performing repeated tendon biopsies. Although the 42 h measurements fall in line with the time course, the reader is encouraged to use discretion in their interpretation.
Biopsies were taken in resting and exercised legs in subjects in group I 24, 48 and 72 h after exercise (Fig. 1) whereas in group II biopsies were taken 6, 24 and 42 h after exercise (Fig. 2). We took biopsies at 42 h in three subjects and although the results are consistent with our other observations, the uncertainty in their value is greater than for the other observations. All studies contained measurement of rates of protein synthesis by different stable isotope labelled probes with additional measurements of IGF-I and IGFBP-3 and IGFBP-4 in group I, and tissue histology in group II.
For the details and underlying assumptions of the methodology employed to measure tissue protein synthesis we refer to our previously published reviews and original reports (Smith & Rennie, 1996; Babraj et al. 2002, 2005a, b).
Measurement of tissue protein synthesis
Upon arrival, two cannulae were inserted into veins on opposite forearms for tracer infusion and blood sampling. A blood sample was then obtained for measurement of background tracee isotope enrichments, insulin and creatine kinase. Prior to the administration of the flooding dose of labelled proline, a skin biopsy (10 mg) was obtained from the back, just below the waist, under local anaesthetic (lidocaine, 1%). This was for measurement of the tissue natural abundance of 13C and 15N in collagen proline thereby eliminating the need for a pre-infusion basal biopsy of the tendon or muscle. Tracers were then administered (as per protocols) as continuous infusions of [1-13C]leucine (0.8 mg kg−1 prime, 1.0 mg kg−1 h−1 continuous) or [1-13C]valine (1.0 mg kg−1 prime, 1.4 mg kg−1 h−1 continuous), or as a flooding dose of [15N]proline or [1-13C]proline (1–1.5 g labelled proline + 2.5–3 g unlabelled proline, 4 g total) administered over 3 min. In group II investigations, no infusions of leucine or valine tracer were used as we had then discovered that proline was adequate for collagen and non-collagen proteins. All tracers were obtained from Cambridge Isotope Laboratories (Woburn, MA, USA), were chemically pure, > 99 atoms% in 13C or 15N, and were certified to be sterile and without pyrogens. The tracers were dissolved on the morning of the infusion in 0.9% NaCl using a sterile technique and then passed through a 0.20 μm filter (Bie and Bernstein, Rødovre, Denmark). Blood samples were drawn at 10–30 min intervals for determination of labelling (tracer/tracee ratio) of amino and imino acids in plasma. Biopsies of patella tendon (∼10 mg) and vastus lateralis muscle (50–100 mg) of both legs were taken using, for tendon, a 16 G Monopty biopsy instrument (Bard Inc., Covington, GA, USA) under ultrasound guidance, and for muscle a Bergström needle with suction after previously preparing incision sites with local anaesthetic (lidocaine, 1%). When more than one muscle biopsy was required it was taken 1 cm distal to a previous biopsy to avoid possible influences of inflammation. Biopsies were cleared of external adipose tissue and blood, frozen in liquid nitrogen, and stored at −80°C for subsequent analysis.
Labelling of free amino and imino acids
Plasma and tissue proline, valine, leucine, and their ketoacids, α-ketoisocaproate (KIC) and α-ketoisovalerate (KIV), were prepared as previously described and analysed as their t-BDMS derivatives by gas chromatography–mass spectrometry (Schwenk et al. 1984; Babraj et al. 2002, 2005a, b). We did not measure true intracellular free amino acid labelling since in muscle in the fed state the labelling of the keto acids of the branched chain amino acids and the plasma labelling are within 10% (Watt et al. 1991, 1992) and the proline flooding dose equilibrates all free tissue pools so that the plasma proline labelling is likely to give a good indication of the intracellular labelling and probably of the imino-t-RNA labelling (Babraj et al. 2005b). It is not necessary to mechanically separate different fractions of muscle protein because this is done by differential solubility in buffers of different composition (see below).
Extraction of muscle protein fractions and muscle and tendon collagen
Full details have been given elsewhere (Bohéet al. 2001). Briefly, skin (10 mg), tendon (10 mg) and muscle (20–30 mg) were ground in liquid nitrogen to a fine powder and hand-homogenized in a buffer containing 0.15 m NaCl, 0.1% Triton X-100 and 0.02 m Tris-HCl pH 7.4 (Ahtikoski et al. 2001). Next the sample was centrifuged and subjected to differential salt extraction. Briefly, muscle was powdered under liquid nitrogen and extracted with 0.15 m NaCl, 0.1% Triton X-100 and 0.02 m Tris-HCl pH 7.4 (Ahtikoski et al. 2001), centrifuged at 1600 g, at 4°C for 20 min to pellet (in tendon) the collagen and (in muscle), myofibrils and collagen. For muscle samples the supernatant was taken as the sarcoplasmic fraction. KCl (0.7 m) was added to the pellet containing myofibrillar proteins and collagen, centrifuged and the supernatant containing myofibrillar proteins removed; the pellet containing collagen was washed with 0.5 m acetic acid and then acetic acid–pepsin (0.1% w/v), dissolving procollagen and immature collagen and leaving an insoluble collagen pellet. The myofibrillar, soluble and insoluble collagens were hydrolysed in 0.1 m HCl–Dowex H+ slurry at 110°C overnight and the liberated amino and imino acids separated using Dowex 50W-X8 H+ ion-exchange resin.
Previous investigations have established the purity of the muscle subfractions (Bohéet al. 2001). On analysis by PAGE the collagen fraction in muscle and tendon showed protein bands which stained immunochemically for collagen only. Collagen synthesis using proline tracer is assayed as the incorporation of labelled proline into hydroxyproline, which by definition, must be contained in collagen, the only hydroxyproline-containing protein, providing confidence in the robustness of the assay. When we assayed leucine tracer in the muscle collagen in which proline tracer was also assayed, the rates of collagen synthesis were identical, providing further confidence in the specificity and purity of the collagen preparation.
Gas chromatography–combustion–isotope ratio mass spectrometry (GC-C-IRMS)
Isolated protein fractions were hydrolysed in a slurry of 0.05 m HCl–Dowex 50WX8-200 (Sigma Ltd, Poole, UK) (500 μl) at 110°C overnight (Balagopal et al. 1997) and the liberated free amino and imino acids purified eluted from it using 1 m HCI. The amino and imino acids were derivatized as their N-acetyl-n-propyl (NAP) ester (Meier-Augenstein, 1999). NAP amino and imino acids were analysed by capillary GC-C-IRMS (Delta-plus XL, Thermofinnigan, Hemel Hempstead, UK); separation was achieved on a 25 m CP-SIL 19CB column (Chrompack). Leucine, proline and hydroxyproline standards were prepared as their NAP derivatives and analysed by GC-C-IRMS.
Calculations
The rates of tissue protein synthesis were calculated using standard equations (Rennie et al. 1982); thus, for the constant infusion approach fractional protein synthesis (FSR,% h−1) =ΔEm/Ep× 1/t× 100, where ΔEm is the change in enrichment (tracer: tracee) of protein for any two points sampled of leucine, valine or proline (assuming basal tissue labelling to be identical to that at the natural abundance in the initial skin biopsy), Ep is the mean enrichment over time of the precursor for protein synthesis (taken as venous plasma KIC, KIV or proline labelling), and t is the time (h) of tracer incorporation. Labelling of venous KIC and KIV were chosen to represent the labelling of the immediate precursor for muscle non-collagen protein synthesis. We have previously demonstrated that in a number of tissues, values of plasma and tissue-free proline labelling are indistinguishable (Babraj et al. 2002, 2005b) indicating that the flooding dose technique is successful. Therefore, venous plasma proline was taken to represent the labelling of prolyl-t-RNA in tenocyte and fibroblast for the calculation of tendon and muscle collagen synthetic rates.
Measurement of IGF-I and IGF binding proteins and PINP
Systemic IGF-I and IGFBP-3 were measured from serum samples separated by centrifugation (4°C, 10 min, 2000 g) and stored at −80°C. Local tissue fluid concentrations of IGF-I and IGFBP-3 and IGFBP-4 were sampled by tissue microdialysis. High molecular mass cut-off (3000 kDa, membrane length 30 mm, i.d. 0.50 mm) fibres were inserted (under ultrasound guidance as previously described (Langberg et al. 2002)) in the vastus lateralis of the rested and exercised legs 24, 48 and 72 h after exercise. Additionally, 72 h after exercise, a microdialysis fibre was placed in the peritendinous spaces of the rested and exercised patella tendons. The microdialysis relative recovery (as estimated by the loss of [3H]human Type IV collagen calculated by the internal reference method (Scheller & Kolb, 1991)) exceeded 85%. Microdialysis fibres were placed within 2 cm of the appropriate tissue sample sites with analysis dialysis fluid collected in the third and fourth hour after insertion. IGF-I concentration was determined after acid–ethanol extraction with a non-competitive, time-resolved monoclonal immunofluorometric assay as previously described (Frystyk et al. 1995). Serum IGFBP-3 was measured by immunoradiometric assay (Diagnostic System Laboratories, Webster, TX, USA). Small volumes of muscle and tendon dialysates only allowed analysis of IGFBP-3 and IGFBP-4 by Western ligand blotting as previously described (Flyvbjerg et al. 1992). Intra- and interassay coefficients of variation (CVs) were between 5 and 10%, respectively. Dialysate fluid PINP was measured as previously described (Langberg et al. 2001).
Tissue histology
A tendon biopsy was obtained from an additional subject to confirm that the procedure did indeed harvest tendon tissue. The sample was immediately fixed in 2% glutaraldehyde and 0.05 m sodium phosphate buffer (pH 7.2), and stored cold (40°C) for 1–3 days. After three rinses in 0.15 m sodium cacodylate buffer (pH 7.2) the tissue was post-fixed in 1% OsO4 in 0.15 m sodium cacodylate buffer (pH 7.2) for 2 h. The tissue was dehydrated in graded ethanol, transferred to propylene-oxide and embedded in Epon according to standard procedures. Sections were cut with a Reichert-Jung Ultracut E microtome. The sections were examined using a Philips CM 100 transmission electron microscope equipped with a Kodak Slow Scan camera and operated at an accelerating voltage of 80 kV.
Sub-samples of ∼25 mg were separated from muscle biopsies performed 6 and 24 h after exercise, the fibres were visually aligned under low power microscopy and the aligned specimen embedded in Tissue-Tek (Sakura Finetek, Zoeterwoude, the Netherlands) and frozen in pre-cooled isopentane. All samples were stored at −80°C until further analysis. Transverse serial sections of the muscle biopsy samples were cut at 10 μm thickness using a cryostat microtome (Microm, Germany) at −22°C and mounted. Serial sections were stained with haematoxylin–eosin (HE) and immunohistochemically stained for (a) dystrophin (NCL-DYS 2, Novocastra Laboratories, Newcastle, UK), (b) desmin (18-0016, Zymed Laboratories, San Francisco, CA, USA), and (c) fibronectin (NCL-FIB, Novocastra Laboratories, Newcastle, UK). A negative and positive control was included in each staining batch for all antibodies assessed. Sections were analysed with Tema software (v. 1.0, Checkvision, Denmark).
Additional analyses
Serum creatine kinase was determined spectrophotometrically by a routine hospital laboratory method. Plasma insulin was determined in triplicate by an enzyme-linked immunosorbent assay (ELISA, DAKO Ltd,. Cambridge, UK) with a coefficient of variation and sensitivity of 7.5% and 3 pmol l−1, respectively.
Statistics
Results are presented as means ±s.d. Values obtained after exercise were compared with the grand mean of the resting values (which were not identical by any test used) using a one-way analysis of variance (ANOVA). When appropriate, significant differences were identified by the Bonferroni post hoc test. The level of significance was set at P≤ 0.05.
Results
Work rate
The maximal work rates for groups I and II, were 96 ± 17 and 107 ± 8 W. The average workload during 1 h of kicking was 60 ± 9 and 67 ± 3 W (P > 0.05).
Utility of differently labelled amino and imino acids as tracers
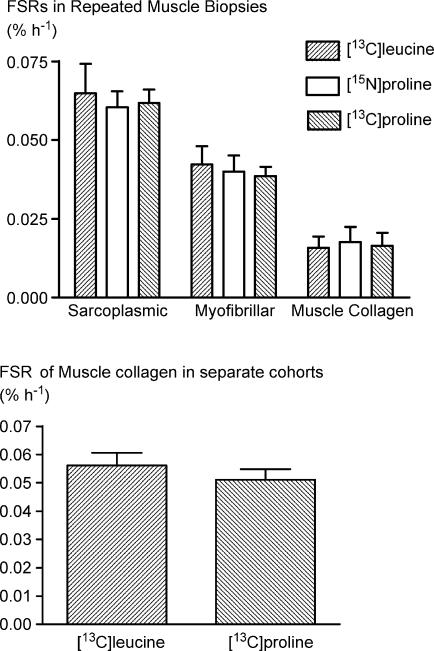
When measured with different probes, the synthetic rates within each class of protein (whether muscle collagen, myofibrillar or sarcoplasmic) of the resting leg were identical irrespective of the use of [13C]leucine, [15N]proline or [13C]proline (Fig. 3, top panel). Furthermore, measurements of muscle collagen synthesis made 24 h after exercise using [13C]leucine or [13C]proline in groups I and II did not differ (Fig. 3, bottom panel).
Figure 3. No difference (P≥ 0.05) between FSR of proteins in repeated rested muscle biopsies on three different days (top) or between 24 h values in two groups (bottom).
Values are the same regardless of tracer used. Values are means ±s.d.
Tendon collagen synthesis and histological appearance
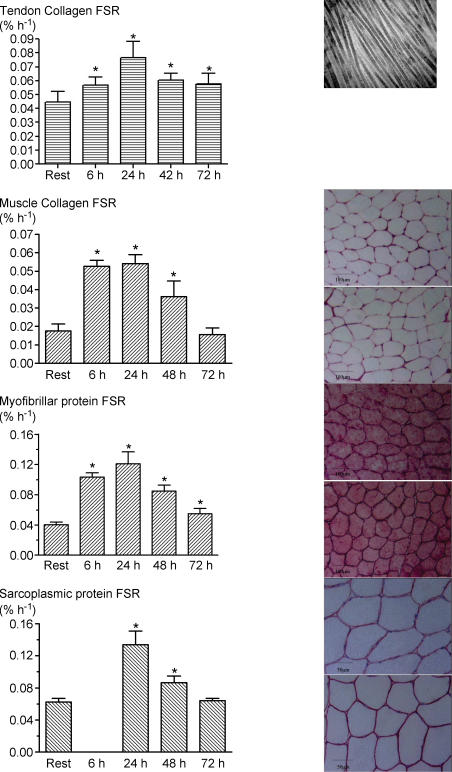
Electron microscopy confirmed that our biopsy showed the normal characteristics of tendon tissue and was distinct in appearance from muscle and bone. The resting rate of tendon collagen synthesis was 0.045 ± 0.003% h−1 (Fig. 4). At 6 and 24 h, the synthetic rates of tendon collagen were increased ∼1.7-fold compared with those in the resting leg. The tendon collagen synthetic rate fell thereafter but was still significantly elevated at 72 h (0.058 ± 0.008% h−1, P≤ 0.05).
Figure 4. Fractional rates of synthesis of: tendon collagen protein (row 1), muscle collagen protein (row 2), myofibrillar protein (row 3), and sarcoplasmic protein (row 4) at rest and after exercise.
Values are means ±s.d.*Significantly different compared with rested leg (P < 0.05). Tendon biopsy electron micrograph (top right) illustrates that the biopsy procedure was sampling tendon tissue as denoted by striated pattern of collagen fibres. Tissue staining of muscle samples at 6 and 24 h with: fibronectin (row 2 right), desmin (row 3 right), and dystrophin (row 4 right). There was no tissue damage as evident by membrane integrity (fibronectin and desmin) and lack of ghost fibres (dystrophin).
Muscle collagen synthesis and muscle histology
Histological staining of the ECM with fibronectin revealed no tissue damage in previously exercise muscle. In the resting leg, the synthetic rate of muscle collagen estimated from three biopsies each in eight subjects was 0.018 ± 0.001% h−1 (Fig. 4). At 6 and 24 h, the rate in the exercised leg was ∼3.5 times greater (∼0.054% h−1) than that in the resting leg and was still elevated 2-fold at 48 h (0.036 ± 0.008% h−1, P≤ 0.05). After 72 h, the differences between the rates in the exercised and resting leg had vanished.
PINP in dialysis fluid in muscle and tendon
No significant changes were seen in the concentrations of PINP in muscle dialysate from values at rest of 4.7 ± 0.7 μg ml−1, but in tendon there was actually a significant fall at 72 h of 23% of the resting value of 13.5 ± 3.1 μg ml−1 (P < 0.01). Thus there was no correspondence between the changes observed in direct and indirect measures of collagen synthesis.
Muscle protein synthesis and tissue appearance
The rates of protein synthesis of the myofibrillar and sarcoplasmic fractions of muscle in the resting leg were 0.040 ± 0.002 and 0.062 ± 0.002% h−1, respectively, values entirely consonant with our previous results for the fed state (Louis et al. 2003; Mittendorfer et al. 2005; Moore et al. 2005). The rates of protein synthesis in the exercised leg increased substantially by 6 h and peaked within 24 h at ∼0.13% h−1 in both myofibrillar and sarcoplasmic fractions (Fig. 4), i.e. increases of 2.8- and 2-fold, respectively. The rates of myofibrillar and sarcoplasmic protein synthesis in the exercised muscle had fallen slightly by 48 h to ∼0.085% h−1 but were still significantly above the rates in the rested leg. By 72 h, the rates of both fractions had decreased to ∼0.06% h−1. In terms of relative increases, muscle collagen and muscle myofibrillar and sarcoplasmic fractions followed similar temporal patterns. Histological staining revealed no tissue damage as a result of exercise as evident by membrane integrity (desmin) and lack of ghost fibres (dystrophin).
CK, insulin, IGF-I, IGFBP-3 and IGFBP-4
Serum CK was 149 ± 92 U l−1 before exercise and 282 ± 143 (24 h), 186 ± 72 (48 h) and 169 ± 46 U l−1 (72 h) after exercise. The 24 h value was significantly (P≤ 0.05) elevated compared with that of rest. Plasma insulin concentration were 32.3 ± 12.4 pmol l−1 before feeding and 51.2 ± 28.8, 56.0 ± 23.6, 64.8 ± 25.1 and 69.8 ± 30.4 pmol l−1 during feeding at 6, 24, 48 and 72 h, respectively (data not shown); there were no significant differences between values of samples collected at any time post exercise. Serum and tissue concentrations of IGF-I, IGFBP-3 and IGFBP-4 (Table 1) did not differ between testing days.
Table 1.
IGF-I, IGFBP-3 and IGFBP-4 in serum and microdialysate from tendon and muscle
| Rest | 24 h | 48 h | 72 h | |
|---|---|---|---|---|
| Serum | ||||
| IGF-I | 223 ± 36 (8) | 227 ± 49 (8) | 224 ± 43 (8) | 232 ± 53 (8) |
| Muscle dialysate | ||||
| IGF-I | 6.3 ± 1.8 (8) | 4.3 ± 1.9 (4) | 5.5 ± 3.0 (8) | 5.0 ± 2.4 (6) |
| IGFBP-3 | 1309 ± 655 (8) | 1271 ± 741 (5) | 988 ± 952 (7) | 589 ± 147 (5) |
| IGFBP-4 | 29.8 ± 11.2 (8) | 22.7 ± 12.6 (5) | 31.3 ± 20.7 (7) | 41.3 ± 22.7 (5) |
| Tendon dialysate | ||||
| IGF-I | 3.3 ± 1.0 (6) | — | — | 3.8 ± 1.2 (8) |
| IGFBP-3 | 155 ± 165 (8) | — | — | 151 ± 180 (8) |
| IGFBP-4 | 9.2 ± 7.1 (8) | — | — | 9.0 ± 6.3 (8) |
Values are means ±s.d. (n). IGF-1 (μg l−1), IGFBP-3 and -4 (pixel intensity).
Plasma amino acid concentrations and labelling
During the period of the measurements of tissue protein synthesis plasma leucine concentrations was steady. Feeding caused an increase in plasma leucine from 130 ± 30 to 213 ± 31 μm at 30 min and the mean value over the period 30–180 min was 212 ± 6 μm; for valine the corresponding values were 242 ± 44 μm rising to 361 ± 20 μm and 366 ± 6 μm over the period. When stable isotope labelled leucine or valine were used as tracers the plasma ketoacid labelling values were steady at tracer: tracee ratios of 4.04 ± 0.50 for KIV and 5.05 ± 0.50 for KIC. The ratio of labelling of amino to keto acids was 1.01 ± 0.1 in each case. The tracer labelling of proline fell linearly with time from ca 20 ± 2 atoms % excess to ca 5 ± 1 atoms % excess over the 2 h of the measurement period (see (Babraj et al. 2005b) for graphical representation). The flooding dose had no effect on the leucine or valine plasma concentrations of enrichments when the probes were administered together (present results and Babraj et al. 2005a).
Discussion
The novel aspects of the present results are that, in response to a bout of strenuous, non-damaging exercise, the rates of directly measured human tendon collagen and skeletal muscle collagen synthesis (i) increased markedly and rapidly after exercise, and (ii) with a time course similar to that of increases in the rates of synthesis of muscle myofibrillar and sarcoplasmic protein. These changes did not coincide with detectable changes in local interstitial concentrations of IGF-I, IGFBP-3 or IGFBP-4, or PINP in tendon or skeletal muscle. The similarity of the time courses for both rates of collagen and non-collagen protein synthesis in the musculotendinous unit suggests that the observed anabolic increases may occur as a result of coordinated responses in muscle and tendon fibroblasts and muscle fibre to mechanical signals communicated via the ECM during contractile activity. The values for protein turnover in the rested legs were steady over time and reproducible with different tracers used for the measurement. Thus, there were no systemic effects of exercise or of repeated muscle biopsies, so that the results obtained should reflect physiological changes in the tissues sampled.
Exercise model
It is acknowledged that the exercise used in our protocols is difficult to classify, and it may be speculated that our changes are due to changes in energy status. However, acute dynamic exercise (Carraro et al. 1990; Sheffield-Moore et al. 2004) has been shown to cause an increase in mixed muscle FSR and bicycle training may increase resting mixed muscle FSR (Short et al. 2004). These changes and the ones we observed are unlikely to be due to a large extent to a decrease in muscle energy status since this would be likely to increase muscle AMP-kinase (AMPK) and inhibit the translational stage of muscle protein synthesis. We have shown in experiments carried out in rat muscle in vitro that an endurance-like pattern of muscle stimulation (10 Hz continuous for 3 h) results in activation of AMPK and much less stimulation of either myofibrillar or sarcoplasmic muscle protein synthesis than does a ‘resistance-like’ pattern (six, 3 s trains of 100 Hz), which was associated with no rise in AMPK but a marked activation of the mTOR precursor TSC2 (Atherton et al. 2005). Further, the increase in mixed muscle FSR is unlikely to be transferable to muscle or tendon collagen, since fibroblasts are unlikely to incur differences in energy status.
Tracer methods
The flooding dose method, originally designed for determining tissue protein synthesis in animals (McNurlan et al. 1979), was adapted by Laurent (Laurent, 1982) to overcome the problems of gaining access to intracellular pools to measure the labelling of the precursor (iminoacyl-t-RNA) for collagen synthesis. The underlying assumption is that flooding equilibrates all free tissue proline pools, enabling an estimate of the labelling of prolyl-t-RNA to be made from measurement of plasma proline labelling. We have presented data elsewhere that this assumption is reasonable and we do not intend to further discuss it here (Babraj et al. 2002, 2005b). In muscle, the application of a flooding dose of proline, like that of other dispensable amino (imino) acids, has no effect on the measurement of muscle protein synthesis simultaneously measured (Smith & Rennie, 1996), suggesting that the application of a proline flood does not artificially stimulate the process of protein synthesis per se, as would a flood of an essential amino acid (Smith et al. 1998). The values we obtained with the various tracers (applied by both continuous infusion and flood) in the rested and exercised leg were internally consistent and consonant with those we have previously reported in similar circumstances (Bohéet al. 2001; Louis et al. 2003; Babraj et al. 2005a; Mittendorfer et al. 2005; Moore et al. 2005), which gives us confidence in the results. It is true that we have no gold standard against which to measure the values for rates of muscle collagen synthesis, but the values obtained with the flooding technique using the differentially labelled versions of proline were identical to each other at rest at 48 and 72 h after exercise. Since fibroblasts are able to transaminate leucine to KIC and vice versa (Wendel & Langenbeck, 1984), KIC enrichment probably accurately reflects the enrichment of the leucine free pool. It also seems reasonable to use the values of leucine incorporation into muscle collagen in the exercised leg to calculate collagen synthesis. This assumption is bolstered by the results of separate studies showing that [13C]leucine and [13C]KIC tracers give identical results for muscle collagen as [13C]- or [15N]proline. On this basis it is explicable that the values obtained with proline were also identical to those obtained by using leucine tracer to measure the rates of synthesis for muscle collagen protein at rest and 24 h after exercise in Group I subjects. These results suggest that in muscle fibroblasts the equilibrium value of intracellular tracer leucine labelling is close to that which would have been obtained by the use of a flooding dose. We could not use valine similarly because valine is too poorly represented in collagen to be assayed by our techniques.
As with muscle collagen, the flooding dose of proline probably allowed us to closely approximate the true precursor enrichment of tendon collagen synthesis, and to determine the extent of the exercise-induced changes. As electron microscopy confirmed the sampling of tendon, it appears that we have made the first direct measurements of rates of tendon and muscle collagen synthesis in human subjects before and after exercise. According to the rapid increases in tendon collagen synthesis (within 6 h), we must conclude that previous indirect measures of collagen synthesis, by the sampling of the markers of collagen synthesis in serum and peritendinous fluid (Langberg et al. 2000; Langberg et al. 2001), underestimated the true rates of synthesis in the first 72 h. In fact we were unable to detect any measurable rise in PINP when we applied the methods previously used successfully in running studies (Langberg et al. 2000, 2001). This may be at least partially because of the measurement of the procollagen peptide of Type I collagen (PINP) rather than of all types of collagen (by incorporation of tracer into hydroxyproline, though the error is likely to be small for either tissue), or to the sluggishness of the escape of PINP from the extracellular space to the sampling sites, and/or to possible degradation of PINP. In any case it seems that the use of the procollagen peptide PINP is problematic as an index of collagen synthesis both in extent and timing.
We chose to perform our study in the fed state in the expectation of maximizing the tissue protein synthetic rates, thus accentuating any potential anabolic effects of exercise and minimizing any changes in amino acid availability. Steady plasma leucine and valine concentrations and labelling and the fact that plasma insulin did not change between the test days suggests that any observed alterations were not the result of variation in nutritional state.
Coordinated responses of myocyte, tenocyte and fibroblast proteins
Our results demonstrate that human tendon and muscle collagen, like muscle contractile proteins, are highly responsive to exercise, presumably via the sensing and chemical transduction of mechanical events. The time course of the observed change was similar in all fractions studied, although tendon collagen and myofibrillar synthetic rates were still slightly elevated 72 h after exercise. The magnitude of the relative increases in synthetic rates was similar among all the protein fractions of muscle studied, indicating that there may be a common mechanical or humoral pathway inducing the anabolic response.
We do not wish to speculate upon the detailed cell-biological mechanisms for the stimulation of protein synthesis. However, the pattern of response to exercise was similar in all the protein classes studied. The cellular tensegrity model advanced by Ingber and others (Ingber et al. 1994; Maniotis et al. 1997) may explain how cells can respond coordinately to mechanical stress through integrins embedded in the plasma membrane and proteins connecting the ECM to those of the cytoskeleton. In this model, mechanical strain induced by muscle contraction could be transduced through cell–matrix interactions, into chemical cellular signals transmitted via appropriate pathways with the result of the simultaneous adaptation and remodelling of all cells (myofibers and fibroblasts) comprising the muscle tissue (Mayer, 2003). This idea is appealing, since for example, increases in the size of muscle fibres would only be fully functional if there were also remodelling of the connective tissue surrounding them, as well as of the tendon connecting the muscle to bone.
The magnitude of maximal change of the rate of collagen synthesis was less in the tendon than muscle (∼1.7 versus (2.8-fold). It was recently inferred that shear strain of intramuscular connective tissue between muscle fascicles of the human quadriceps muscle may reach 140% (Purslow, 2002). Since tensile forces are sensed by cells as strain (Chiquet et al. 2003), the potential for considerable shear strain in the muscle, compared with the lesser shear strain in tendon (Reeves et al. 2003; for discussion see Magnusson et al. 2003), might account for its attenuated response. Even though the response was attenuated, the tendons of habitual runners have different mechanical properties from those of sedentary counterparts (Magnusson et al. 2003) and larger tendons (Rosager et al. 2002), leading one to believe that a shift towards a more positive protein balance is a possibility after exercise.
The present study indicates that the adaptation of the ECM in the musculotendinous unit occurs acutely (i.e. within 6 h and maximally by 24 h) after increased mechanical strain. Histological examination of the muscle and its ECM revealed no tissue damage and, although serum CK was significantly elevated 24 h after exercise, these values were substantially less than those observed in exercise known to cause damage (Koskinen et al. 2001a; Stupka et al. 2001). Therefore, without evidence of inflammation, the rapid (< 6 h) increase in muscle myofibrillar and collagen protein synthesis seems likely to arise from an initial common mechanically induced signal to anabolic pathways, most probably the MAP kinase (Chiquet, 1999) and/or mTOR (Bolster et al. 2003) pathways.
It has also been suggested that IGF-I (and other growth factors) trigger a delayed response (Rennie & Wackerhage, 2003), which, if it occurred, we would have expected to find in our 24, 48 or 72 h measurements. However, we were surprised that there were no significant changes in tissue fluid concentrations in tendon or muscle concentrations of IGF-I or its binding proteins over 72 h after exercise. However, given the relatively few samples at some time points we are at risk of missing an effect through insufficient statistical power. Also, we measured total IGF-I, which does not account for the amount that is actually free and active. A better time resolution of measurement, or assay of additional growth factors (such as TGF-β; Heinemeier et al. 2003) may uncover such a humoral coordination mechanism.
In conclusion, the rates of synthesis of tendon and muscle collagen, and muscle myofibrillar and sarcoplasmic protein all increase after a single acute bout of strenuous, non-damaging exercise. In our hands, the rates of synthesis rose rapidly, peaked at 24 h, and slowly decreased toward resting values 72 h following exercise. The similar time course of the change in both musculotendinous collagen and non-collagen protein synthesis may be due to some common signalling pathway(s) transducing the mechanical stimuli into anabolic events in different cell types.
Acknowledgments
We thank the subjects for their cheerful participation, the fine technical assistance of Annie Hoj, Karen Mathiassen and Kirsten Nyborg, and Drs Peter Magnusson and Klaus Qvortrup for the electron microscopy. This research was supported by The Danish Rheumatism Association, The Copenhagen University Hospital Foundation, The Danish National Research Foundation (504-14), The Medical Research Council of Denmark (22-01-0154) The Wellcome Trust, the UK MRC and BBSRC, World Anti-Doping Agency, Diabetes UK and the Chief Scientist's Office of the Scottish Executive.
References
- Adams GR. Autocrine/paracrine IGF-I and skeletal muscle adaptation. J Appl Physiol. 2002;93:1159–1167. doi: 10.1152/japplphysiol.01264.2001. [DOI] [PubMed] [Google Scholar]
- Ahtikoski AM, Koskinen SO, Virtanen P, Kovanen V, Takala TE. Regulation of synthesis of fibrillar collagens in rat skeletal muscle during immobilization in shortened and lengthened positions. Acta Physiol Scand. 2001;172:131–140. doi: 10.1046/j.1365-201X.2001.00848.x. [DOI] [PubMed] [Google Scholar]
- Atherton PJ, Babraj JA, Smith K, Singh J, Rennie MJ, Wackerhage H. Selective activation of AMPK-PGC-1α or PKB-TSC2-mTOR signaling can explain specific adaptive responses to endurance or resistance training-like electrical muscle stimulation. FASEB J. 2005;19:786–788. doi: 10.1096/fj.04-2179fje. [DOI] [PubMed] [Google Scholar]
- Babraj J, Cuthbertson DJ, Rickhuss P, Meier-Augenstein W, Smith K, Bohé J, Wolfe RR, Gibson JN, Adams C, Rennie MJ. Sequential extracts of human bone show differing collagen synthetic rates. Biochem Soc Trans. 2002;30:61–65. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- Babraj JA, Cuthbertson DJR, Smith K, Langberg H, Miller BF, Krogsgaard MR, Kjaer M, Rennie MJ. Collagen synthesis in human musculoskeletal tissues and skin. Am J Physiol Endocrinol Metab. 2005a doi: 10.1152/ajpendo.00243.2005. 10.1152/ajpendo.00243.2005. [DOI] [PubMed] [Google Scholar]
- Babraj JA, Smith K, Cuthbertson DJ, Rickhuss P, Dorling JS, Rennie MJ. Human bone collagen synthesis is a rapid, nutritionally modulated process. J Bone Miner Res. 2005b;20:930–937. doi: 10.1359/JBMR.050201. [DOI] [PubMed] [Google Scholar]
- Balagopal P, Rooyackers OE, Adey DB, Ades PA, Nair KS. Effects of aging on in vivo synthesis of skeletal muscle myosin heavy-chain and sarcoplasmic protein in humans. Am J Physiol. 1997;273:E790–E800. doi: 10.1152/ajpendo.1997.273.4.E790. [DOI] [PubMed] [Google Scholar]
- Bohé J, Low JF, Wolfe RR, Rennie MJ. Latency and duration of stimulation of human muscle protein synthesis during continuous infusion of amino acids. J Physiol. 2001;532:575–579. doi: 10.1111/j.1469-7793.2001.0575f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolster DR, Kubica N, Crozier SJ, Williamson DL, Farrell PA, Kimball SR, Jefferson LS. Immediate response of mammalian target of rapamycin (mTOR)-mediated signalling following acute resistance exercise in rat skeletal muscle. J Physiol. 2003;553:213–220. doi: 10.1113/jphysiol.2003.047019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carraro F, Stuart CA, Hartl WH, Rosenblatt J, Wolfe RR. Effect of exercise and recovery on muscle protein synthesis in human subjects. Am J Physiol Endocrinol Metab. 1990;259:E470–E476. doi: 10.1152/ajpendo.1990.259.4.E470. [DOI] [PubMed] [Google Scholar]
- Chiquet M. Regulation of extracellular matrix gene expression by mechanical stress. Matrix Biol. 1999;18:417–426. doi: 10.1016/s0945-053x(99)00039-6. [DOI] [PubMed] [Google Scholar]
- Chiquet M, Renedo AS, Huber F, Fluck M. How do fibroblasts translate mechanical signals into changes in extracellular matrix production? Matrix Biol. 2003;22:73–80. doi: 10.1016/s0945-053x(03)00004-0. [DOI] [PubMed] [Google Scholar]
- Cunningham JJ. Body composition and resting metabolic rate: the myth of feminine metabolism. Am J Clin Nutr. 1982;36:721–726. doi: 10.1093/ajcn/36.4.721. [DOI] [PubMed] [Google Scholar]
- Flyvbjerg A, Kessler U, Dorka B, Funk B, Orskov H, Kiess W. Transient increase in renal insulin-like growth factor binding proteins during initial kidney hypertrophy in experimental diabetes in rats. Diabetologia. 1992;35:589–593. doi: 10.1007/BF00400489. [DOI] [PubMed] [Google Scholar]
- Frystyk J, Dinesen B, Orskov H. Non-competitive time-resolved immunofluorometric assays for determination of human insulin-like growth factor I and II. Growth Regul. 1995;5:169–176. [PubMed] [Google Scholar]
- Han XY, Wang W, Komulainen J, Koskinen SO, Kovanen V, Vihko V, Trackman PC, Takala TE. Increased mRNAs for procollagens and key regulating enzymes in rat skeletal muscle following downhill running. Pflugers Arch. 1999;437:857–864. doi: 10.1007/s004240050855. [DOI] [PubMed] [Google Scholar]
- Heinemeier K, Langberg H, Olesen JL, Kjaer M. Role of TGF-β1 in relation to exercise-induced type I collagen synthesis in human tendinous tissue. J Appl Physiol. 2003;95:2390–2397. doi: 10.1152/japplphysiol.00403.2003. [DOI] [PubMed] [Google Scholar]
- Hellsten Y, Hansson HA, Johnson L, Frandsen U, Sjodin B. Increased expression of xanthine oxidase and insulin-like growth factor I (IGF-I) immunoreactivity in skeletal muscle after strenuous exercise in humans. Acta Physiol Scand. 1996;157:191–197. doi: 10.1046/j.1365-201X.1996.492235000.x. [DOI] [PubMed] [Google Scholar]
- Ingber DE, Dike L, Hansen L, Karp S, Liley H, Maniotis A, McNamee H, Mooney D, Plopper G, Sims J. Cellular tensegrity: exploring how mechanical changes in the cytoskeleton regulate cell growth, migration, and tissue pattern during morphogenesis. Int Rev Cytol. 1994;150:173–224. doi: 10.1016/s0074-7696(08)61542-9. [DOI] [PubMed] [Google Scholar]
- Kjaer M. Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol Rev. 2004;84:649–698. doi: 10.1152/physrev.00031.2003. [DOI] [PubMed] [Google Scholar]
- Koskinen SO, Hoyhtya M, Turpeenniemi-Hujanen T, Martikkala V, Makinen TT, Oksa J, Rintamaki H, Lofberg M, Somer H, Takala TE. Serum concentrations of collagen degrading enzymes and their inhibitors after downhill running. Scand J Med Sports. 2001a;11:9–15. doi: 10.1034/j.1600-0838.2001.011001009.x. [DOI] [PubMed] [Google Scholar]
- Koskinen SO, Wang W, Ahtikoski AM, Kjaer M, Han XY, Komulainen J, Kovanen V, Takala TE. Acute exercise induced changes in rat skeletal muscle mRNAs and proteins regulating type IV collagen content. Am J Physiol Regul Integr Comp Physiol. 2001b;280:R1292–R1300. doi: 10.1152/ajpregu.2001.280.5.R1292. [DOI] [PubMed] [Google Scholar]
- Kovanen V, Suominen H, Heikkinen E. Collagen of slow twitch and fast twitch muscle fibres in different types of rat skeletal muscle. Eur J Appl Physiol Occup Physiol. 1984;52:235–242. doi: 10.1007/BF00433399. [DOI] [PubMed] [Google Scholar]
- Langberg H, Olesen JL, Gemmer C, Kjaer M. Substantial elevation of interleukin-6 concentration in peritendinous tissue, in contrast to muscle, following prolonged exercise in humans. J Physiol. 2002;542:985–990. doi: 10.1113/jphysiol.2002.019141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langberg H, Rosendal L, Kjaer M. Training-induced changes in peritendinous type I collagen turnover determined by microdialysis in humans. J Physiol. 2001;534:297–302. doi: 10.1111/j.1469-7793.2001.00297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langberg H, Skovgaard D, Asp S, Kjaer M. Time pattern of exercise-induced changes in type I collagen turnover after prolonged endurance exercise in humans. Calcif Tissue Int. 2000;67:41–44. doi: 10.1007/s00223001094. [DOI] [PubMed] [Google Scholar]
- Langberg H, Skovgaard D, Petersen LJ, Bulow J, Kjaer M. Type I collagen synthesis and degradation in peritendinous tissue after exercise determined by microdialysis in humans. J Physiol. 1999;521:299–306. doi: 10.1111/j.1469-7793.1999.00299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent GJ. Rates of collagen synthesis in lung, skin and muscle obtained in vivo by a simplified method using [3H]proline. Biochem J. 1982;206:535–544. doi: 10.1042/bj2060535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis M, Poortmans JR, Francaux M, Hultman E, Berre J, Boisseau N, Young VR, Smith K, Meier-Augenstein W, Babraj JA, Waddell T, Rennie MJ. Creatine supplementation has no effect on human muscle protein turnover at rest in the postabsorptive or fed states. Am J Physiol Endocrinol Metab. 2003;284:E764–E770. doi: 10.1152/ajpendo.00338.2002. [DOI] [PubMed] [Google Scholar]
- McNurlan MA, Tomkins AM, Garlick PJ. The effect of starvation on the rate of protein synthesis in rat liver and small intestine. Biochem J. 1979;178:373–379. doi: 10.1042/bj1780373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson SP, Hansen P, Kjaer M. Tendon properties in relation to muscular activity and physical training. Scand J Med Sci Sports. 2003;13:211–223. doi: 10.1034/j.1600-0838.2003.00308.x. [DOI] [PubMed] [Google Scholar]
- Maniotis AJ, Chen CS, Ingber DE. Demonstration of mechanical connections between integrins, cytoskeletal filaments, and nucleoplasm that stabilize nuclear structure. Proc Natl Acad Sci U S A. 1997;94:849–854. doi: 10.1073/pnas.94.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer U. Integrins: redundant or important players in skeletal muscle? J Biol Chem. 2003;278:14587–14590. doi: 10.1074/jbc.R200022200. [DOI] [PubMed] [Google Scholar]
- Meier-Augenstein W. Applied gas chromatography coupled to isotope ratio mass spectrometry. J Chromatogr A. 1999;842:351–371. doi: 10.1016/s0021-9673(98)01057-7. [DOI] [PubMed] [Google Scholar]
- Mittendorfer B, Andersen JL, Plomgaard P, Saltin B, Babraj JA, Smith K, Rennie MJ. Protein synthesis rates in human muscles: neither anatomical location nor fibre-type composition are major determinants. J Physiol. 2005;563:203–211. doi: 10.1113/jphysiol.2004.077180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore DR, Phillips SM, Babraj JA, Smith K, Rennie MJ. Myofibrillar and collagen protein synthesis in human skeletal muscle in young men after maximal shortening and lengthening contractions. Am J Physiol Endocrinol Metab. 2005;288:E1153–E1159. doi: 10.1152/ajpendo.00387.2004. [DOI] [PubMed] [Google Scholar]
- Purslow PP. The structure and functional significance of variations in the connective tissue within muscle. Comparative Biochem Physiol A, Mol Integr Physiol. 2002;133:947–966. doi: 10.1016/s1095-6433(02)00141-1. [DOI] [PubMed] [Google Scholar]
- Reeves ND, Maganaris CN, Narici MV. Effect of strength training on human patella tendon mechanical properties of older individuals. J Physiol. 2003;548:971–981. doi: 10.1113/jphysiol.2002.035576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennie MJ, Edwards RH, Halliday D, Matthews DE, Wolman SL, Millward DJ. Muscle protein synthesis measured by stable isotope techniques in man: the effects of feeding and fasting. Clin Sci. 1982;63:519–523. doi: 10.1042/cs0630519. [DOI] [PubMed] [Google Scholar]
- Rennie MJ, Tipton KD. Protein and amino acid metabolism during and after exercise and the effects of nutrition. Annu Rev Nutr. 2000;20:457–483. doi: 10.1146/annurev.nutr.20.1.457. [DOI] [PubMed] [Google Scholar]
- Rennie MJ, Wackerhage H. Connecting the dots for mechanochemical transduction in muscle. J Physiol. 2003;553:1. doi: 10.1113/jphysiol.2003.054197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosager S, Aagaard P, Dyhre-Poulsen P, Neergaard K, Kjaer M, Magnusson SP. Load-displacement properties of the human triceps surae aponeurosis and tendon in runners and non-runners. Scand J Med Sports. 2002;12:90–98. doi: 10.1034/j.1600-0838.2002.120205.x. [DOI] [PubMed] [Google Scholar]
- Scheller D, Kolb J. The internal reference technique in microdialysis: a practical approach to monitoring dialysis efficiency and to calculating tissue concentration from dialysate samples. J Neurosci Meth. 1991;40:31–38. doi: 10.1016/0165-0270(91)90114-f. [DOI] [PubMed] [Google Scholar]
- Schwenk WF, Berg PJ, Beaufrere B, Miles JM, Haymond MW. Use of t-butyldimethylsilylation in the gas chromatographic/mass spectrometric analysis of physiologic compounds found in plasma using electron-impact ionization. Anal Biochem. 1984;141:101–109. doi: 10.1016/0003-2697(84)90431-7. [DOI] [PubMed] [Google Scholar]
- Sheffield-Moore M, Yeckel CW, Volpi E, Wolf SE, Morio B, Chinkes DL, Paddon-Jones D, Wolfe RR. Postexercise protein metabolism in older and younger men following moderate-intensity aerobic exercise. Am J Physiol Endocrinol Metab. 2004;287:E513–E522. doi: 10.1152/ajpendo.00334.2003. [DOI] [PubMed] [Google Scholar]
- Short KR, Vittone JL, Bigelow ML, Proctor DN, Nair KS. Age and aerobic exercise training effects on whole body and muscle protein metabolism. Am J Physiol Endocrinol Metabolism. 2004;286:E92–E101. doi: 10.1152/ajpendo.00366.2003. [DOI] [PubMed] [Google Scholar]
- Smith K, Rennie MJ. The measurement of tissue protein turnover. Baillieres Clin Endocrinol Metab. 1996;10:469–495. doi: 10.1016/s0950-351x(96)80651-3. [DOI] [PubMed] [Google Scholar]
- Smith K, Reynolds N, Downie S, Patel A, Rennie MJ. Effects of flooding amino acids on incorporation of labeled amino acids into human muscle protein. Am J Physiol. 1998;275:E73–E78. doi: 10.1152/ajpendo.1998.275.1.E73. [DOI] [PubMed] [Google Scholar]
- Stupka N, Tarnopolsky MA, Yardley NJ, Phillips SM. Cellular adaptation to repeated eccentric exercise-induced muscle damage. J Appl Physiol. 2001;91:1669–1678. doi: 10.1152/jappl.2001.91.4.1669. [DOI] [PubMed] [Google Scholar]
- Suominen H, Heikkinen E. Enzyme activities in muscle and connective tissue of M. Vastus lateralis in habitually training and sedentary 33–70-year-old men. Eur J Appl Physiol Occup Physiol. 1975;34:249–254. doi: 10.1007/BF00999938. [DOI] [PubMed] [Google Scholar]
- Suominen H, Heikkinen E, Parkatti T. Effect of eight weeks' physical training on muscle and connective tissue of the M. vastus lateralis in 69-year-old men and women. J Gerontol. 1977;32:33–37. doi: 10.1093/geronj/32.1.33. [DOI] [PubMed] [Google Scholar]
- Suominen H, Kiiskinen A, Heikkinen E. Effects of physical training on metabolism of connective tissues in young mice. Acta Physiol Scand. 1980;108:17–22. doi: 10.1111/j.1748-1716.1980.tb06495.x. [DOI] [PubMed] [Google Scholar]
- Takala TE, Myllyla R, Salminen A, Anttinen H, Vihko V. Increased activities of prolyl 4-hydroxylase and galactosylhydroxylysyl glucosyltransferase, enzymes of collagen biosynthesis, in skeletal muscle of endurance-trained mice. Pflugers Arch. 1983;399:271–274. doi: 10.1007/BF00652751. [DOI] [PubMed] [Google Scholar]
- Watt PW, Corbett ME, Rennie MJ. Stimulation of protein synthesis in pig skeletal muscle by infusion of amino acids during constant insulin availability. Am J Physiol. 1992;263:E453–E460. doi: 10.1152/ajpendo.1992.263.3.E453. [DOI] [PubMed] [Google Scholar]
- Watt PW, Lindsay Y, Scrimgeour CM, Chien PA, Gibson JN, Taylor DJ, Rennie MJ. Isolation of aminoacyl-tRNA and its labeling with stable-isotope tracers: Use in studies of human tissue protein synthesis. Proc Natl Acad Sci U S A. 1991;88:5892–5896. doi: 10.1073/pnas.88.13.5892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendel U, Langenbeck U. Intracellular levels and metabolism of leucine and alpha-ketoisocaproate in normal and maple syrup urine disease fibroblasts. Biochem Med. 1984;31:294–302. doi: 10.1016/0006-2944(84)90085-1. [DOI] [PubMed] [Google Scholar]