Abstract
The glutamatergic granule cells of the dentate gyrus transiently express GABAergic markers after seizures. Here we show that when this occurs, their activation produces (i) GABAA receptor-mediated synaptic field responses in CA3, with the physiological and pharmacological characteristics of mossy fibre transmission, and (ii) GABAA receptor-mediated collateral inhibition. Control hippocampal slices present, on stimulation of the dentate gyrus, population responses in stratum lucidum, which are blocked by ionotropic glutamate receptor antagonists. By contrast, in slices from rats subjected to seizures in vivo, dentate activation additionally produces GABAA receptor-mediated field synaptic responses in the presence of glutamate receptor antagonists. One-dimensional current source density analysis confirmed the spatial coincidence of the glutamatergic and GABAergic dendritic currents. The GABAA receptor-mediated field responses show frequency-dependent facilitation and strong inhibition during activation of metabotropic glutamate receptors. In the presence of glutamate receptor blockers, a conditioning pulse delivered to one site of the dentate gyrus inhibits the population synaptic response and the afferent volley provoked by the activation of a second site, in a bicuculline-sensitive manner. In accordance with this, antidromic responses evoked by mossy fibre activation were enhanced by perfusion of bicuculline. Our results suggest that, for GABA receptor-dependent field potentials to be detected, a considerable number of boutons of a well-defined GABAergic pathway should simultaneously release GABA to act on a large number of receptors. Therefore, putative GABA release from the mossy fibres acts on pre- and postsynaptic sites to affect hippocampal activity at the network level after seizures.
The presence of the markers of the GABAergic phenotype in the glutamate-releasing granule cells (Crawford & Connor, 1973) of the dentate gyrus (DG) suggests that GABA could be synthesized, vesiculated and released from the same neurones (Gutiérrez, 2003, 2005). These markers are highly expressed during development, down-regulated and barely detectable in the adult rat, but reappear after strong excitation, especially after seizures (Sandler & Smith, 1991; Schwarzer & Sperk, 1995; Sloviter et al. 1996; Lehman et al. 1996; Ramírez & Gutiérrez, 2001; Gómez-Lira et al. 2002; Maqueda et al. 2003; Gutiérrez et al. 2003). Accordingly, cellular electrophysiological studies have compellingly shown that mossy fibre (MF) activation produces, in addition to monosynaptic glutamate receptor (GluR)-mediated responses, monosynaptic GABAAR-mediated synaptic potentials/currents in their target cells, pyramidal cells and interneurones of CA3, when the GABAergic markers are highly expressed (Gutiérrez 2000, 2002; Walker et al. 2001; Gutiérrez & Heinemann, 2001; Gutiérrez et al. 2003; Bergersen et al. 2003; Romo-Parra et al. 2003; Kasyanov et al. 2004). Although direct proof of co-release of glutamate and GABA from the MF can only be obtained by paired recordings from interconnected neurones, the available immunohistochemical, biochemical, molecular biological and electrophysiological evidence strongly suggests that this is the case (Gutiérrez, 2003, 2005).
Because in adult rats subjected to seizures in vivo, but not in naive rats, MF stimulation provokes monosynaptic glutamatergic and GABAergic responses in their target cells of CA3, we hypothesized that the GABAergic transmission via the MF should influence area CA3 as a coordinated aggregate. Thus, if the MF constitutes a GABAergic pathway, then its selective activation in the presence of GluR blockers would evoke GABAAR-mediated population responses in CA3 in the rats subjected to seizures, but not in control rats. These responses should meet the described physiological and pharmacological criteria that identify them as responses of MF origin.
In agreement with our hypothesis, we show here that stimulation of the DG in slices obtained from rats subjected to seizures produces GluR-mediated responses and, in the presence of GluR blockers, GABAAR-mediated population responses in CA3. In the naive adult rat, where no GABAergic markers are expressed in the granule cells, GABAergic population responses are undetected, despite bulk stimulation of the molecular layer of the DG. The pharmacologically isolated MF GABAergic population responses show frequency potentiation and are strongly depressed by the activation of group III mGluRs. Besides these postsynaptic effects, we show that synaptically released GABA from the MF also acts on presynaptic GABAA receptors located in parallel MFs (Ruiz et al. 2003) to produce collateral inhibition. Thus, GABA released from the MF pathway exerts a functionally significant postsynaptic action on the hippocampal region CA3 and, together with glutamate, presynaptically regulates further neurotransmitter release from the MFs themselves.
Methods
For our experiments we used adult Wistar rats of 230 g weight. Seizures were produced either by electrical kindling, which produces a permanent epileptic state, or by a single i.p. injection of pentylenetetrazol, which is a model of seizures in the absence of an underlying permanent epileptic state (PTZ, 60 mg kg−1; Gutiérrez, 2000). In vivo kindled animals were surgically implanted, under ketamine anaesthesia (60 mg kg−1i.p.), with a stimulating electrode in the amygdala and stimulated as described (Gutiérrez, 2000) until five consecutive full-blown seizures were induced. All experimental procedures were approved by the Committee on Ethical Animal Research of our institution. The rats were decapitated under deep ether anaesthesia, the brains were rapidly dissected and combined entorhinal cortex–hippocampus slices (400 μm) were cut with a vibroslicer (Campden Instruments) submerged in oxygenated artificial cerebrospinal fluid (ACSF) at 4°C. After slicing, the preparations were placed in a liquid-to-air interface recording chamber and constantly perfused (1 ml min−1) with ACSF containing (mm): NaCl, 124; KCl, 3; NaH2PO4, 1.25; MgSO4, 2; CaCl2, 2 or 0; NaHCO3, 26; glucose, 10; pH 7.35 at 34°C. Slices of dorsal and ventral hippocampus were used without distinction, as no differences with respect to MF GABAergic transmission have been detected. In a series of experiments (see Results), an in vitro kindling-like protocol was used in slices obtained from naive rats, and consisted of three trains of 0.1 ms pulses at 100 Hz, with a duration of 1 s, and an inter-train interval of 1 min delivered every 15 min, as previously described (Gutiérrez, 2002).
We recorded (AxoClamp 2B amplifier; Axon Instruments) extra- and intracellular responses from area CA3b to DG stimulation and extracellular responses from DG to MF antidromic stimulation. We used borosilicate microelectrodes of 8–12 MΩ filled with NaCl (155 mm) and of 70–90 MΩ filled with potassium acetate (2 m) for extracellular and intracellular recordings, respectively. To evoke synaptic responses in CA3, stimulation (pulse duration 0.1 ms) was provided at 0.05 Hz over the molecular layer of the DG (Henze et al. 1997) and over the stratum radiatum (SR) of the CA3 region, to stimulate commissural–associational fibres (Vogt & Regehr, 2001), with bipolar glass-insulated platinum wire electrodes (25 μm). Antidromic responses to MF stimulation were recorded from the granular layer of the DG. For each experiment, we determined input/output curves and the stimulation intensity was fixed at a value that evoked 60% of the maximal field response during control ACSF perfusion (Gutiérrez, 2002), unless stated otherwise in the Results section. In all experiments on slices from PTZ-treated and kindled rats, pirenzepine (10 μm) was added to the ACSF to avoid contamination by the seizure-induced cholinergic input (Gutiérrez & Heinemann, 2001; Romo-Parra et al. 2003). The drugs used were diluted in the ACSF: the NMDA receptor antagonist (dl)-2-amino-5-phosphonovaleric acid (APV; 30 μm; Tocris Cookson); the non-NMDA receptor antagonist 6-nitro-7-sulfamoyl-benzo(f)quinoxaline-2,3-dione (NBQX; 10 μm; Tocris Cookson); the M1-acetylcholine receptor antagonist pirenzepine (10 μm; RBI); the GABAA receptor antagonists bicuculline methiodide (20 μm; Sigma) or picrotoxin (100 μm; Sigma); the group III metabotropic glutamate receptor (mGluR) agonist l(+)-2-amino-4-phosphonobutyric acid (l-AP4; 1 μm; Tocris Cookson). The afferent volley was isolated at the end of the experiments by the simultaneous perfusion of GluR antagonists and bicuculline or of Ca2+-free ACSF.
The electrophysiological signals were acquired with the program pCLAMP8 (Axon Instruments). The extracellular recordings were exported and analysed with a specially designed program written in MATLAB 6.1 (MathWorks, Inc.). This program computes averages of sets of six successive field responses throughout the experiment and then carries out the point-to-point subtraction of the synaptic responses obtained under the different pharmacological conditions. Thus, we isolated the glutamatergic component by subtracting the responses with and without GluR antagonists, whereas the GABAergic component was isolated by subtracting the responses obtained in the presence of GluR antagonists plus bicuculline (or during perfusion of Ca2+-free ACSF) from those obtained in the presence of GluR antagonists (see Fig. 1). The area under the curve of the pharmacologically isolated synaptic components was then calculated. The effect of l-AP4 on the isolated GABAergic response was routinely tested, prior to bicuculline perfusion, to confirm the MF origin of the GABAergic response.
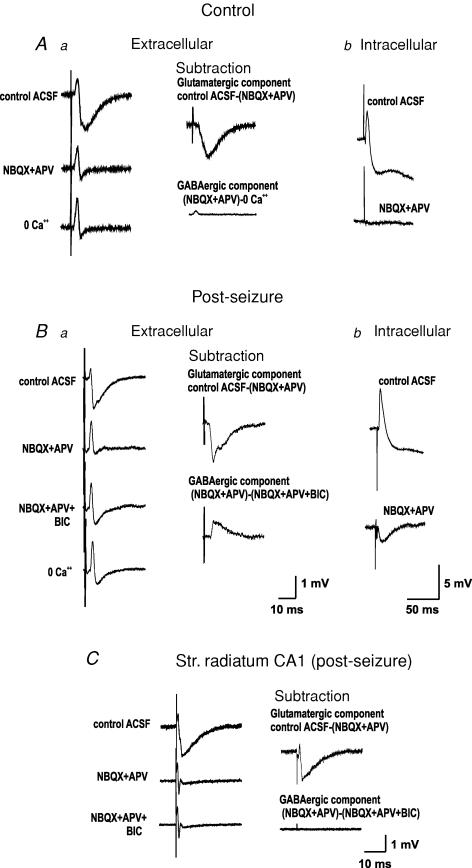
Figure 1. Stimulation of the dentate gyrus evokes identifiable glutamate receptor and GABAA receptor-dependent field potentials in CA3.
Aa, in control adult rats, perfusion of GluR antagonists blocks the synaptic components of the population responses of stratum lucidum to mossy fibre (MF) stimulation, isolating the fibre volley. Perfusion of a calcium-free ACSF does not change the latter. The glutamatergic component can then be isolated by digitally subtracting the field responses obtained prior to and after perfusion of GluR antagonists, whereas the GABAergic component, which is not present, can be isolated by the subtraction of the responses prior to and after addition of bicuculline or calcium-free ACSF. Ab, intracellular recordings show that the EPSP/IPSP sequence observed in control ACSF is blocked by GluR antagonists. Ba, by contrast, in adult rats subjected to kindling-like stimulation in vitro to several kindled seizures or to a single pentylenetetrazol (PTZ)-induced seizure, GluR antagonists seem to block the synaptic response in SL but, upon perfusion of bicuculline, a change in the morphology of the response reveals a bicuculline-sensitive component. This can be isolated by digital subtraction of the responses in the presence and absence of bicuculline. Perfusion of a calcium-free ACSF does not change the isolated afferent volley. Bb, accordingly, intracellular recordings show that MF stimulation in the presence of GluR antagonists produces a monosynaptic IPSP. C, the GABAAR-mediated field potential is restricted to CA3, as it cannot be produced in stratum radiatum of CA1 by Schaffer collateral stimulation. All traces are an average of 5–10 responses. The calibration bars of the extracellular and intracellular recordings of panel A are noted in Ba and Bb, respectively.
One-dimensional current source density (CSD) analysis (Freeman & Nicholson, 1975) was carried out in six slices from three control rats and in nine slices from three rats subjected to PTZ-induced seizures in control ACSF, during perfusion of GluR antagonists and during perfusion of GluR + GABAR antagonists. Field responses (averages of 10) to MF activation were recorded sequentially along the pyramidal cell axis (total distance 600 μm), from stratum pyramidale (SP) to SR at 20 μm intervals (Δx). The depth of the recording electrode was adjusted to maximize the peak amplitude of the recorded field potential (usually 150–250 μm under the slice surface). The averaged field responses obtained in each site were exported to a specially designed program written in MATLAB 6.1 and CSD analysis was computed. This program performs a smoothing procedure through a Savitzky-Golay filter (unweighted linear least-squares fit with a polynomial of third degree) without attenuation of data features. Fixed point current source density was then computed from the field potentials by calculating the second spatial derivative of a 4-element Taylor series expansion and expressed in arbitrary units proportional to actual current densities:
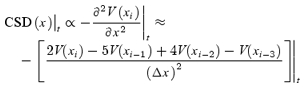 |
(1) |
where V is voltage, t is time and Δx is the distance between adjacent recording sites (fixed interval of 20 μm). We assume that conductivity is not a function of space and that it remains relatively constant during the responses to DG stimulation. Consequently, the slice was treated as homogeneous and isotropic and current source densities were expressed in arbitrary units proportional to actual current densities. To obtain the glutamatergic currents, the currents calculated during perfusion of GluR blockers were subtracted point-to-point from those obtained in control ACSF. For the isolation of the GABAergic currents, the currents calculated during perfusion of GluR blockers + bicuculline were subtracted point-to-point from those obtained during perfusion of GluR antagonists. This procedure is a valid means of isolating the pharmacologically treated currents as the recorded extracellular current density represents the algebraic sum of all currents (Kwan & Murphy, 1974; Mitzdorf, 1985). For an unbiased determination of the peaks of the current sources and sinks, we used a wavelet-based peak detector algorithm that extracts the maximum current values from the laminar profile.
Results
Mossy fibre activation provokes GABAAR-mediated population synaptic responses after seizures
Activation of the MF in hippocampal slices of naive rats provoked field synaptic responses in the stratum lucidum (SL) of area CA3a,b that are completely blocked by the simultaneous perfusion of NBQX (10 μm) and APV (30 μm, n = 15). The additional perfusion of the GABAA receptor antagonist bicuculline (20 μm), or suppressing extracellular Ca2+ from the ACSF, had no further effect on the field response, indicating that the remaining component is the isolated afferent volley (Fig. 1Aa). With intracellular recordings, we observed that MF stimulation provoked an EPSP/IPSP sequence that could be totally blocked by the perfusion of the GluR antagonists (Figs 1 and 2). By contrast, in slices from naive rats subjected to a kindling-like stimulation protocol in the perforant path (n = 6) (Gutiérrez, 2002), from rats having had one pentylenetetrazole (PTZ)-induced seizure (n = 25), or from kindled rats in vivo (n = 8), we confirmed that the perfusion of NBQX plus APV blocked the EPSP isolating a monosynaptic IPSP (Figs 1 and 2), as previously reported (Gutiérrez, 2000; Gutiérrez & Heinemann, 2001). Extracellularly, blockade of GluR resulted in an apparent suppression of field responses but, upon perfusion of bicuculline, the waveform of the responses in SL changed, suggesting the presence of an underlying GABAergic component (Fig. 1Ba; see also Fig. 3). By digital point-to-point subtraction of the responses obtained prior to and after the perfusion of the appropriate antagonists, we were able to isolate the GluR-antagonist-sensitive and the bicuculline-sensitive components. As expected, the extracellular synaptic responses obtained in SL had the opposite polarity to the intracellularly recorded postsynaptic potentials (Fig. 1B). To assess the MF specificity of the GABAA-mediated field potentials, the Schaffer collaterals were stimulated in four slices from animals subjected to PTZ-induced seizures and the same procedure was applied to analyse the responses of SR of CA1. In this region, no bicuculline-sensitive components could be isolated (Fig. 1C).
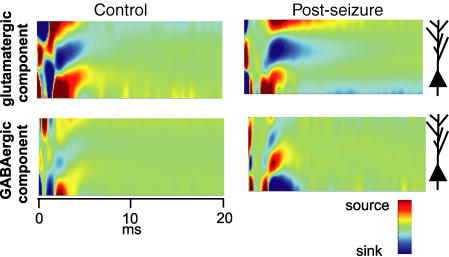
Figure 2. One-dimensional current source density analysis reveals a common origin of the glutamatergic and GABAergic field dendritic currents.
High resolution CSD analysis profiles, presented in the form of contours, were computed for control and post-seizure preparations. The glutamatergic component was computed by point-to-point digital subtraction of the contour maps obtained before and after perfusion of GluR antagonists, and the GABAergic component by subtraction of the maps obtained before and after adding GABAAR antagonist. The GluR-mediated dendritic sink is evident both in control and post-seizure experiments. However, the GABAR-mediated component, which is reflected as a source in the stratum lucidum, can only be detected in post-seizure experiments, whereby the corresponding current sinks are restricted to the proximal stratum radiatum and the pyramidal layer. Note the temporal coincidence of the glutamatergic current sink and GABAergic current source of the post-seizure experiment. Stimulus was applied at time 0 ms.
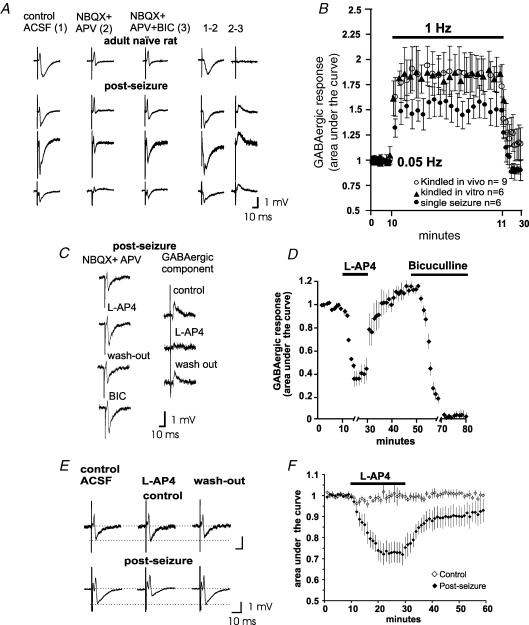
Figure 3. Stimulation of the dentate gyrus produces GABAAR-mediated field potentials in stratum lucidum with the physiological and pharmacological characteristics of MF transmission.
A, field recordings in a slice obtained from a control rat and 3 examples of the recordings obtained after each GABA-response-inducing procedure (kindling-like stimulation in vitro, kindled and PTZ-treated rats; post-seizure). Notice that, despite all responses being obtained in stratum lucidum, the waveforms are somewhat different, depending on the site and depth of the recording electrode in the MF band. The recordings were obtained in control ACSF, and after perfusion of NBQX + APV and NBQX + APV + bicuculline (BIC). The pharmacologically isolated glutamatergic (1–2) and GABAergic (2–3) components of the MF-evoked field synaptic responses can be obtained by digital subtraction. B, plot constructed with the areas under the curve of the isolated GABAergic responses during stimulation at 0.05, 1 and 0.05 Hz in the different protocols used for the induction of the MF GABA response. Frequency facilitation is a characteristic of MF transmission. Each point represents mean ±s.e.m.C, the isolated MF GABAergic component is reversibly depressed by 1 μm of the group III mGluR agonist, l-AP4. D, plot constructed with the results of 6 experiments in PTZ-treated rats, depicting the areas under the curve of the isolated GABAergic component during the effect of l-AP4 and bicuculline. Inhibition by activation of the mGluR is a characteristic of transmission of MF origin. E, MF-evoked field potentials in stratum lucidum of seizing, but not of control rats, are depressed by l-AP4 in normal ACSF. This effect is shown in F for the whole set of experiments in the control and post-seizure group (each n = 6). The plot was constructed with the areas under the curve of the field potentials. Traces in A, C and E are averages of 5–10 responses.
The characteristics of the synaptic components of the extracellular responses recorded in SL were as follows. The glutamatergic component consisted of a negative deflection with an amplitude of 1.8 ± 0.5 mV and a 10–90% slope of 1.1 ± 0.8 mV ms−1 in control animals (n = 8) and an amplitude of 4.6 ± 0.9 mV and a slope of 1.4 ± 0.3 mV ms−1 (n = 16) in seizing rats. The GABAergic component that could only be isolated in slices from rats having had seizures consisted of a positive deflection with an amplitude of 2.1 ± 0.4 mV (n = 16) and a 10–90% slope of 1.9 ± 0.4 mV ms−1 (n = 16). From these measurements, we calculated a ratio of the amplitude of the GABA/glutamate responses of 0.56 ± 0.4, whereas the ratio (GABA/glutamate) of the areas under the curve was 0.61 ± 0.05 (n = 16).
The glutamatergic and GABAergic dendritic currents originate in the stratum lucidum
To explore the origin of each extracellular component, we carried out one-dimensional CSD analysis before and after blockade of ionotropic glutamate and GABA receptors. In slices from control rats, MF activation produced a current sink that was blocked during perfusion of GluR antagonists (Fig. 2). Addition of bicuculline did not change the contour map, as evidenced by digitally subtracting the corresponding contour map from that obtained in the presence of GluR antagonists (Fig. 2; GABAergic component). By contrast, in slices from seizing rats, the point-to-point digital subtraction of the CSD analysis calculated in the presence of GluR and GABAAR antagonists from that obtained in the presence of GluR antagonists isolated a current source (Fig. 2, Post-seizure; GABAergic component). Both the glutamatergic and GABAergic components (sink and source, respectively) were located in SL, at approximately the same distance from the SP. The distance between the peak value of the sink (glutamatergic component) and that of the source (GABAergic component) with respect to the corresponding source/sink (approximately located in the border between the SP and the stratum oriens) for the whole set of experiments was: 273 ± 27 μm (n = 15, including control rats and rats subjected to seizures) for the glutamatergic component and 225 ± 20 μm (n = 6) for the GABAergic component, in slices from rats subjected to seizures. In naive rats (n = 12), the sink of the glutamatergic component peaked at 3.06 ± 0.31 ms and the source at 3.20 ± 0.32 ms, whereas in rats subjected to seizures (n = 6), the GABAergic source peaked at 3.42 ± 0.41 ms and the sink at 3.53 ± 0.37 ms.
GABAAR-mediated population synaptic responses have the characteristics of transmission of MF origin
Because MF transmission undergoes frequency facilitation when the stimulation frequency is changed from 0.05 to 1 Hz (Regehr et al. 1994), we tested the MF-evoked field responses with this stimulation protocol. After pharmacological isolation (Fig. 3A), we measured the area under the curve of the GABAergic field potentials before and after stimulation at 1 Hz. Frequency facilitation was readily produced and, interestingly, it was more pronounced in slices from kindled epileptic rats (185 ± 14%; n = 8) and in in vitro kindled slices (189 ± 19%; n = 6) than in preparations from rats that had a single seizure (153 ± 15%; n = 8; Fig. 3B).
Because MF GABAergic monosynaptic responses of CA3 pyramidal cells are preferentially depressed by the activation of the group III mGluR (Gutiérrez 2000, 2002; Gutiérrez et al. 2003; Romo-Parra et al. 2003; Kasyanov et al. 2004), we tested the effect of l-AP4 (1 μm) on the bicuculline-sensitive MF-evoked field responses recorded in the SL in the presence of NBQX plus APV. l-AP4 reversibly inhibited the GABA-mediated field response in slices from seizing rats (68 ± 11%; n = 10), as assessed by measuring the area under the curve of the isolated GABA component (Fig. 3C and D).
It has been reported that l-AP4 does not alter MF transmission whereas (2S,2′R,3′R)-2-(2′,3′-dicarboxycyclopropyl) glycine (DCG-IV), a group II mGluR agonist, selectively inhibits MF glutamatergic transmission in the rat (Lanthorn et al. 1984; Manzoni et al. 1995; Tong et al. 1996; Min et al. 1998). In view of the sensitivity of the isolated MF GABAergic field responses to l-AP4, we then studied its effects on the field responses obtained in SL, in control ACSF. As expected, the perfusion of a low concentration of l-AP4 (1 μm) in adult control slices was ineffective (Fig. 3E and F). However, in slices where MF GABAergic transmission was expressed, l-AP4 consistently inhibited the MF-evoked compound field responses by 28 ± 5% (n = 4; Fig. 3E and F).
The emergence of MF GABAergic transmission does not produce disinhibition
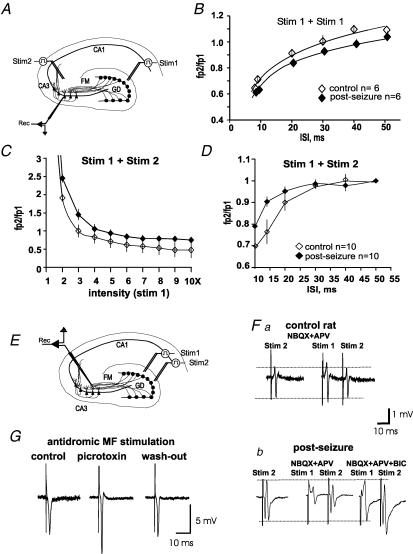
Paired pulse stimulation was applied to the DG (Fig. 4A) at different interstimulus intervals (ISIs) in normal ACSF. Slices obtained from both naive and seizing rats presented feed-forward inhibition at an ISI below 40 ms (Fig. 4B), without statistically significant differences between them. We then assessed the effect of the emergence of MF GABAergic transmission on the response of CA3 to stimulation of commissural–associational (C/A) fibres. Stimulation of the MF 10 ms prior to stimulation of SR of CA3 (where the C/A fibres run; Fig. 4A) produces potentiation or inhibition of the latter, depending on the stimulation intensity of the MF conditioning pulse: the higher the intensity of MF stimulation, the stronger the inhibition of the responses to C/A fibre stimulation. The input–output function is depicted in Fig. 4C, whereby inhibition was slightly decreased (< 20%) in slices of rats subjected to a single seizure compared with those of control rats. On the other hand, we selected the intensity that did not inhibit the responses to C/A stimulation when applied at an ISI of 50 ms, and using this intensity we varied the intervals between the conditioning and the test pulse. We found that inhibition appeared at an ISI below 30 ms and that shortening the interval enhanced inhibition, whereby inhibition was slightly decreased (< 10%) in slices obtained from rats subjected to seizures (Fig. 4D).
Figure 4. MF-evoked GABA release produces collateral inhibition in CA3.
A, schematic drawing showing the sites of stimulation and recording of the experiments depicted in B, C and D. B, paired pulse stimulation of a single set of MFs (Stim 1) produces feed-forward inhibition in control slices and in slices from rats subjected to seizures. C, to explore whether disinhibition of the responses to stimulation of a different input to CA3 occurred in the post-seizure preparations, stimulation of the commissural–associational (C/A) fibres (Stim 2) was preceded (10 ms) by a conditioning pulse in the MF (Stim 1). The responses in CA3 to C/A constant stimulation turned from paired pulse potentiation to paired pulse inhibition, as stimulation in the MF (Stim 1) was increased. Inhibition was slightly increased in control as compared to the post-seizure group. D, in another set of experiments, the stimulation intensity in the MF was kept constant while the interstimulus interval (ISI) (Stim 1 − Stim 2) was shortened. Inhibition of the responses of CA3 to C/A stimulation was increased as the ISI was decreased. Inhibition was slightly increased in control as compared to the post-seizure group; fp, field potential amplitude. E, to confirm MF GABAergic collateral inhibition, two sets of MFs were stimulated with an ISI of 15–20 ms (Stim 1 + Stim 2; see A) in the presence of NBQX + APV. Fa, a test pulse to stimulation site 2 (Stim 2) provoked an afferent volley in a control rat, which was not affected by a preceding afferent volley provoked by site 1 (Stim 1). Fb, by contrast, in seizing rats, the field potential evoked by Stim 2 was strongly inhibited when preceded (15–20 ms) by a conditioning pulse in stimulation site 1 (Stim 1) and this effect was blocked by bicuculline (BIC). G, field responses of the dentate gyrus provoked by antidromic stimulation of the MFs are reversibly enhanced in the presence of picrotoxin (100 μm). Traces in F and G are an average of 6 responses.
The emergence of MF GABAergic transmission produces collateral inhibition
Because it is not possible to assess paired pulse inhibition in the presence of GluR blockers, we decided to assess the action of synaptically released GABA from a set of MFs on the responses evoked by the stimulation of another set by applying the conditioning pulse 15–20 ms before the test pulse (Fig. 4E) in the presence of GluR blockers. In slices from seizing (Fig. 4Fb), but not from control rats (Fig. 4Fa), the conditioning pulse inhibited the response to the test pulse in a statistically significant manner (Student's t test, P < 0.005), by 48 ± 6% (n = 12), as assessed by measuring the area under the curve of the field response. After blocking the synaptic component with bicuculline, the conditioning pulse did not inhibit the second response (afferent volley) provoked by the test pulse. Moreover, the blockade of GABAA receptors with bicuculline increased the amplitude of the afferent volley (see below). The same effects were observed in the presence of the mGluR blocker (+)-α-methyl-4-carboxyphenyl glycine (MCPG), and the GABABR antagonist CGP55845A (not shown). To verify whether a tonic action of GABA was exerted directly on the MF, we obtained antidromic field responses in the DG (in the presence of glutamatergic blockers) by stimulation of the MF and perfused bicuculline or picrotoxin (100 μm; Ruiz et al. 2003). As expected, MF antidromic responses were potentiated by the blockade of GABAAR (Fig. 4G) but no differences were observed between slices from seizing and control rats.
Discussion
Our results show that bulk stimulation of the MF pathway, in the presence of GluR antagonists, is able to provoke a GABAAR-dependent field response in area CA3 after seizures or after strong synaptic stimulation of the DG. By contrast, in slices from naive rats, population synaptic responses are undetected in the presence of GluR antagonists. The GABAAR-mediated field response has the physiological and pharmacological characteristics of transmission of MF origin. Thus, the MF pathway functions as a GABAergic projection in the hippocampus that can alter CA3 population activity after seizures. Moreover, this GABAergic transmission from the MF activates presynaptic GABAA receptors located in other MF axons, producing collateral inhibition. Besides supporting the hypothesis that MFs release GABA, our data provide important insights into the functioning of the DG-to-CA3 projection after seizures.
Our characterization of MF-evoked GABAergic field potentials constitutes a powerful electrophysiological tool with which to study GABAergic transmission in the hippocampus and sets the basis for redefining the role of the MF pathway after seizures. A GABAergic population response can only be detected if GABA is massively released in a highly stratified structure, where the origin of the synaptic responses can be determined. This implies that a considerable number of MF boutons simultaneously release GABA in a localized site (MF connectivity has a very limited convergence; Acsády et al. 1998) that should act on a large number of receptors on the postsynaptic cells in CA3, affecting this region at the network level. Indeed, we show that MF GABAergic field potentials can be recorded in SL, where the MFs contact CA3 pyramidal cell dendrites provoking GABAA receptor-mediated dendritic currents. Thus, the pharmacologically isolated synaptic extracellular components recorded in SL reflect the EPSPs and IPSPs recorded intracellularly. Since field potentials are highly localized within the hippocampus due to its lamellar cytoarchitecture, one-dimensional CSD analysis guarantees acceptable resolution of the sites of sources and sinks. Indeed, the mossy fibre system is considered ‘lamellar’ in the CA3c–b regions (Amaral & Witter, 1989; Acsády et al. 1998) where we recorded. Therefore, the CSD analysis allowed us to determine the coincidence of the site of origin of the excitatory and inhibitory components of the MF input. To reveal the GABAergic dendritic currents, a point-to-point subtraction of the currents recorded in the different pharmacological conditions was used. This procedure is a valid means of isolating the opposed currents as the recorded extracellular current density represents the algebraic sum of all currents (Kwan & Murphy, 1974; Mitzdorf, 1985). The inward dendritic currents (sinks) observed in control ACSF perfusion reflect the depolarizing input from the MF and were restricted to the SL of CA3. The feed-forward inhibitory pathway would produce a hyperpolarization of pyramidal cell dendrites in opposition to the depolarization currents in more distal sites. However, because of its polysynaptic nature, it would come after the depolarization. Interestingly, however, the inhibitory dendritic currents that we observed in slices from rats subjected to seizures, in the presence of GluR antagonists, reflect inhibitory current sources that are also restricted to SL and, thus, coincide both temporally and spatially with the depolarizing currents, suggesting that the glutamatergic and GABAergic signals share the same origin, the MF. Moreover, this notion is supported by the fact that the GABAergic field responses not only occur exclusively after seizures, when the GABAergic markers are expressed in the granule cells, but because recurrent projections and polysynaptic recruitment of interneurones is prevented by the presence of GluR blockers. On the other hand, activation of the interneuronal network would produce inhibitory currents in more distal and proximal sites of the CA3 dendritic tree as their inhibitory terminals do not converge in a single site, as opposed to the isolated MF responses. Interestingly, it has been described in human epileptic resected tissue that, in a subset of subicular neurones, GABAergic inputs are depolarizing, whereby the IPSPs reverse at potentials positive to rest (Cohen et al. 2002). The responses that we recorded in SL seemed to be inhibitory because they had the opposite polarity of the extracellularly recorded glutamatergic component and of the intracellularly recorded IPSPs. Thus, although we cannot rule out changes in chloride extrusion in CA3 pyramidal neurones after seizures (Rivera et al. 2004), the apparent inhibitory integrated response of area CA3 to MF stimulation is in line with a normal, hyperpolarized GABA reversal potential in the pyramidal cells (Gutiérrez & Heinemann, 2001).
One characteristic of MF transmission is that it undergoes frequency facilitation when the stimulus frequency is changed from 0.05 to 1 Hz. We show here that frequency facilitation of the MF GABAergic field responses can be robustly induced; however, it seems to be less pronounced than that observed for MF glutamatergic responses (Salin et al. 1996). This difference seems to correlate with the GABAergic/glutamatergic response ratio that we obtained. Indeed, as previously shown with intracellular and whole-cell recordings (Walker et al. 2001; Gutiérrez, 2002; Romo-Parra et al. 2003; Bergersen et al. 2003), extracellular GABA-dependent responses are less pronounced than glutamate-dependent responses, in about the same proportion as we found for field responses. These physiological data are in agreement and support immunohistological experiments showing that GABA is less concentrated than glutamate in MF boutons (Bergersen et al. 2003). Interestingly, the more pronounced frequency facilitation of the GABAergic responses observed in the kindled preparations in vitro or in vivo than in the preparations from animals having a single seizure also parallels the level of expression of glutamic acid decarboxylase (GAD)67, vesicular GABA transporter (VGAT) mRNA and GABA (Ramírez & Gutiérrez, 2001; Lamas et al. 2001; Gómez-Lira et al. 2002).
Our data show that despite the emergence of MF GABA transmission after induction of hyperexcitability, disinhibition of CA3 does not occur. Interneurones are known to be the primary target of the MFs (Acsády et al. 1998) that, upon stimulation, produce strong feed-forward inhibition. This was shown by applying the conditioning pulse in the MF and the test pulse in two sites that project to CA3: the MF and C/A fibres. The lack of disinhibition upon the emergence of MF GABAergic signalling can be due to the unique physiological compartmentalization of the MF, which continues to effectively excite interneurones to produce a net inhibitory effect on CA3 (Gutiérrez & Heinemann, 2001; Romo-Parra et al. 2003). Additionally, MF GABA release could add a modulatory factor that can be reflected in the variance of inhibitory activity. Indeed, increased variance of inhibitory signals modulates the firing rate of principal cells, whereby the degree of heterogeneity of the GABAergic synaptic inputs to principal cells can powerfully modulate the efficacy of GABAergic inhibition and glutamategic excitation (Aradi et al. 2002; Aradi & Soltesz, 2002).
Importantly, the MF GABAergic field responses are sensitive to l-AP4, a group III mGluR that is present in the rat MF (Ohishi et al. 1993, 1995; Shigemoto et al. 1996, 1997). We have suggested a link of group III mGluRs to GABA release from the MF (Romo-Parra et al. 2003; Gutiérrez, 2003) and additional electrophysiological evidence supporting this view was recently presented (Kasyanov et al. 2004). Thus, the differential activation of presynaptic mGluRs and fast activation of GABAAR (Ruiz et al. 2003) provide an exquisite control of MF output by the MF's own activity. In juvenile rats, activation of group III mGluRs produces a suppression of the strong tonic monosynaptic MF GABAergic input to CA3 (Gutiérrez et al. 2003; Kasyanov et al. 2004). In adult rats, activation of group III mGluRs has no effect unless seizures are provoked. Therefore, the present evidence and that obtained at the cellular level confirm that sensitivity to l-AP4 is a landmark of MF GABAergic transmission. This selectivity is especially important, since it permits the isolation of the contribution of MF GABA in the signal integration of area CA3. Following on from this, it could be suggested that temporal lobe epileptic patients present an up-regulation of group III mGluRs precisely in the DG (Lie et al. 2000) whose excessive activation possibly disrupts MF GABA release.
The finding of GABAA receptors in MFs (Ruiz et al. 2003) suggested that the possible release of GABA from MFs themselves could provoke presynaptic inhibition of collateral MFs. Our results show that this is indeed the case. Activity-dependent autoinhibition of the MF pathway is primarily exerted by glutamate, acting on its metabotropic receptors. High frequency stimulation provokes its spillover, which acts on presynaptic mGluRs and kainate receptors (Min et al. 1998; Vogt & Nicoll, 1999; Schmitz et al. 2001). In accordance with previous studies (reviewed in Gutiérrez, 2003), the present evidence shows that upon higher demands on the system, the GABAergic phenotype of the MF is expressed leading to massive GABA release. This release may exert a strong control of subsequent release by fast activation of presynaptic GABAA receptors. Extending the results of Ruiz et al. (2003), we show that field responses of the DG to antidromic MF stimulation are potentiated by the blockade of GABAARs. Interestingly, although our present results and those of Ruiz et al. show that GABAARs are tonically active, there is no saturation of the receptors as the emergence of MF GABA transmission can still exert collateral inhibition. Thus, postsynaptic dendrites and presynaptic axons alike seem to integrate the input activity in CA3 (Ruiz et al. 2003). In developing rats, fast monosynaptic GABAergic signalling onto CA3 can provide the necessary excitatory drive to activate NMDA receptors (Leinekugel et al. 1997). In the adult, activity-dependent release of GABA from the MF can effectively hyperpolarize CA3 pyramidal cells and it can also have a shunting effect on the incoming glutamatergic signals. This may reflect the emergence of excitation-restraining and neuroprotective actions in response to conditions of extreme activity.
Acknowledgments
M.T. is a doctoral student supported by Consejo Nacional de Ciencia y Tecnología (CONACYT). The authors thank Drs. Uwe Heinemann and Germán Barrionuevo for helpful suggestions during the development of this investigation and José Ayala and Benjamín Muñoz for technical support. Our research is funded by CONACYT and Fundación Miguel Alemán, Mexico.
References
- Acsády L, Kamondi A, Sik A, Freund T, Buzsáki G. GABAergic cells are the major postsynaptic targets of mossy fibres in the hippocampus. J Neurosci. 1998;18:3386–3403. doi: 10.1523/JNEUROSCI.18-09-03386.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral DG, Witter MP. The three-dimensional organization of the hippocampal formation: a review of anatomical data. Neuroscience. 1989;31:571–591. doi: 10.1016/0306-4522(89)90424-7. [DOI] [PubMed] [Google Scholar]
- Aradi I, Santhakumar V, Chen K, Soltesz I. Postsynaptic effects of GABAergic synaptic diversity: Regulation of neuronal excitability by changes in IPSC variance. Neuropharmacology. 2002;43:511–522. doi: 10.1016/s0028-3908(02)00167-3. [DOI] [PubMed] [Google Scholar]
- Aradi I, Soltesz I. Modulation of network behaviour by changes in variance in interneuronal properties. J Physiol. 2002;538:227–251. doi: 10.1113/jphysiol.2001.013054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergersen L, Ruiz A, Bjaalie JG, Kullmann DM, Gundersen V. GABA and GABAA receptors at hippocampal mossy fibre synapses. Eur J Neurosci. 2003;18:931–941. doi: 10.1046/j.1460-9568.2003.02828.x. [DOI] [PubMed] [Google Scholar]
- Cohen I, Navarro V, Clemenceau S, Baulac M, Miles R. On the origin of interictal activity in human temporal lobe epilepsy in vitro. Science. 2002;298:1418–1421. doi: 10.1126/science.1076510. [DOI] [PubMed] [Google Scholar]
- Crawford IL, Connor JD. Localization and release of glutamic acid in relation to the hippocampal mossy fibre pathway. Nature. 1973;244:442–443. doi: 10.1038/244442a0. [DOI] [PubMed] [Google Scholar]
- Freeman JA, Nicholson C. Experimental optimization of current source-density technique for anuran cerebellum. J Neurophysiol. 1975;38:369–382. doi: 10.1152/jn.1975.38.2.369. [DOI] [PubMed] [Google Scholar]
- Gómez-Lira G, Trillo E, Ramírez M, Asai M, Sitges M, Gutiérrez R. Expression of GABAergic transmission in the mossy fibre synapse coincides with the seizure-induced expression of GABA in mossy fibre synaptosomes. Exp Neurol. 2002;177:276–283. doi: 10.1006/exnr.2002.7986. [DOI] [PubMed] [Google Scholar]
- Gutiérrez R. Seizures induce simultaneous GABAergic and glutamatergic neurotransmission in the dentate gyrus-CA3 system. J Neurophysiol. 2000;84:3088–3090. doi: 10.1152/jn.2000.84.6.3088. [DOI] [PubMed] [Google Scholar]
- Gutiérrez R. Activity-dependent expression of simultaneous glutamatergic and GABAergic neurotransmission from the mossy fibres in vitro. J Neurophysiol. 2002;87:2562–2570. doi: 10.1152/jn.2002.87.5.2562. [DOI] [PubMed] [Google Scholar]
- Gutiérrez R. The GABAergic phenotype of the ‘glutamatergic’ granule cells of the dentate gyrus. Prog Neurobiol. 2003;71:337–358. doi: 10.1016/j.pneurobio.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Gutiérrez R. The dual glutamatergic-GABAergic phenotype of the hippocampal granule cells. Trends Neurosci. 2005;28:297–303. doi: 10.1016/j.tins.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Gutiérrez R, Heinemann U. Kindling induces transient fast inhibition in the dentate gyrus – CA3 projection. Eur J Neurosci. 2001;13:1371–1379. doi: 10.1046/j.0953-816x.2001.01508.x. [DOI] [PubMed] [Google Scholar]
- Gutiérrez R, Romo-Parra R, Maqueda J, Vivar C, Ramírez M, Morales MA, Lamas M. Plasticity of the GABAergic phenotype of the ‘glutamatergic’ granule cells of the rat dentate gyrus. J Neurosci. 2003;23:5594–5598. doi: 10.1523/JNEUROSCI.23-13-05594.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henze DA, Urban NN, Barrionuevo G. Origin of the apparent asynchronous activity of hippocampal mossy fibres. J Neurophysiol. 1997;78:24–30. doi: 10.1152/jn.1997.78.1.24. [DOI] [PubMed] [Google Scholar]
- Kasyanov AM, Safiulina VF, Voronin LL, Cherubini E. GABA-mediated giant depolarizing potentials as coincidence detectors for enhancing synaptic efficacy in the developing hippocampus. Proc Natl Acad Sci U S A. 2004;101:3967–3972. doi: 10.1073/pnas.0305974101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan HC, Murphy JT. Extracellular current density analysis of responses in cerebellar cortex to mossy fiber activation. J Neurophysiol. 1974;37:947–953. doi: 10.1152/jn.1974.37.5.947. [DOI] [PubMed] [Google Scholar]
- Lamas M, Gómez-Lira G, Gutiérrez R. Vesicular GABA transporter mRNA expression in the dentate gyrus and in mossy fibre synaptosomes. Mol Brain Res. 2001;93:209–214. doi: 10.1016/s0169-328x(01)00202-9. [DOI] [PubMed] [Google Scholar]
- Lanthorn TH, Ganong AH, Cotman CW. 2-Amino-4-phosphonobutyrate selectively blocks mossy fibre-CA3 responses in guinea pig but not rat hippocampal neurons. Brain Res. 1984;290:174–178. doi: 10.1016/0006-8993(84)90750-9. [DOI] [PubMed] [Google Scholar]
- Lehmann H, Ebert U, Löscher W. Immunocytochemical localization of GABA immunoreactivity in dentate granule cells of normal and kindled rats. Neurosci Lett. 1996;212:41–44. doi: 10.1016/0304-3940(96)12777-4. [DOI] [PubMed] [Google Scholar]
- Leinekugel X, Medina I, Khalilov I, Ben-Ari Y, Khazipov R. Ca2+ oscillations mediated by the synergistic excitatory actions of GABAA and NMDA receptors in the neonatal hippocampus. Neuron. 1997;18:243–255. doi: 10.1016/s0896-6273(00)80265-2. [DOI] [PubMed] [Google Scholar]
- Lie AA, Becker A, Behle K, Beck H, Malitschek B, Conn PJ, Kuhn R, Nitsch R, Plaschke M, Schramm J, Elger CE, Wiestler OD, Blumcke I. Up-regulation of the metabotropic glutamate receptor mGluR4 in hippocampal neurons with reduced seizure vulnerability. Ann Neurol. 2000;47:26–35. [PubMed] [Google Scholar]
- Manzoni O, Castillo PE, Nicoll RA. Pharmacology of metabotropic glutamate receptors at the mossy fibre synapses of the guinea pig hippocampus. Neuropharmacology. 1995;34:965–971. doi: 10.1016/0028-3908(95)00060-j. [DOI] [PubMed] [Google Scholar]
- Maqueda J, Ramírez M, Lamas M, Gutiérrez R. Glutamic acid decarboxylase (GAD)67, but not GAD65, is constitutively expressed during development and transiently overexpressed by activity in the granule cells of the rat. Neurosci Lett. 2003;353:69–71. doi: 10.1016/j.neulet.2003.08.077. [DOI] [PubMed] [Google Scholar]
- Min MY, Rusakov DA, Kullmann DM. Activation of AMPA, kainate, and metabotropic receptors at hippocampal mossy fibre synapses: role of glutamate diffusion. Neuron. 1998;21:561–570. doi: 10.1016/s0896-6273(00)80566-8. [DOI] [PubMed] [Google Scholar]
- Mitzdorf U. Current source-density method and application in cat cerebral cortex: investigation of evoked potentials and EEG phenomena. Physiol Rev. 1985;65:30–100. doi: 10.1152/physrev.1985.65.1.37. [DOI] [PubMed] [Google Scholar]
- Ohishi H, Akazawa C, Shigemoto R, Nakanishi S, Mizuno N. Distributions of the mRNAs for L-2-amino-4-phosphonobutyrate-sensitive metabotropic glutamate receptors, mGluR4 and mGluR7, in the rat brain. J Comp Neurol. 1995;360:555–570. doi: 10.1002/cne.903600402. [DOI] [PubMed] [Google Scholar]
- Ohishi H, Shigemoto R, Nakanishi S, Mizuno N. Distribution of the mRNA for a metabotropic glutamate receptor (mGluR3) in the rat brain: an in situ hybridization study. J Comp Neurol. 1993;335:252–266. doi: 10.1002/cne.903350209. [DOI] [PubMed] [Google Scholar]
- Ramírez M, Gutiérrez R. Activity-dependent expression of GAD67 in the granule cells of the rat hippocampus. Brain Res. 2001;917:139–146. doi: 10.1016/s0006-8993(01)02794-9. [DOI] [PubMed] [Google Scholar]
- Regehr WG, Delaney KR, Tank DW. The role of presynaptic calcium in short-term enhancement at the hippocampal mossy fiber synapse. J Neurosci. 1994;14:523–537. doi: 10.1523/JNEUROSCI.14-02-00523.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera C, Voipio J, Thomas-Crusells J, Li H, Emri Z, Spila S, Payne JA, Minichiello L, Saarma M, Kaila K. Mechanism of activity-dependent downregulation of the neuron-specific K-Cl contransporter KCC2. J Neurosci. 2004;24:4683–4691. doi: 10.1523/JNEUROSCI.5265-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romo-Parra R, Vivar C, Maqueda J, Morales MA, Gutiérrez R. Activity-dependent induction of multitransmitter signaling onto pyramidal cells and interneurons of area CA3 of the rat hippocampus. J Neurophysiol. 2003;89:3155–3167. doi: 10.1152/jn.00985.2002. [DOI] [PubMed] [Google Scholar]
- Ruiz A, Fabian-Fine R, Scott R, Walker MC, Rusakov DA, Kullmann DM. GABAA receptors at hippocampal mossy fibres. Neuron. 2003;39:961–973. doi: 10.1016/s0896-6273(03)00559-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salin PA, Scanziani M, Malenka RC, Nicoll RA. Distinct short-term plasticity at two excitatory synapses in the hippocampus. Proc Natl Acad Sci U S A. 1996;93:13304–13309. doi: 10.1073/pnas.93.23.13304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandler R, Smith AD. Coexistence of GABA and glutamate in mossy fibre terminals of the primate hippocampus: an ultrastructural study. J Comp Neurol. 1991;303:177–192. doi: 10.1002/cne.903030202. [DOI] [PubMed] [Google Scholar]
- Schmitz D, Mellor J, Frerking M, Nicoll RA. Presynaptic kainate receptors at hippocampal mossy fibre synapses. Proc Natl Acad Sci U S A. 2001;98:11003–11008. doi: 10.1073/pnas.191351498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzer C, Sperk G. Hippocampal granule cells express glutamic acid decarboxylase-67 after limbic seizures in the rat. Neuroscience. 1995;69:705–709. doi: 10.1016/0306-4522(95)00348-m. [DOI] [PubMed] [Google Scholar]
- Shigemoto R, Kinoshita A, Wada E, Nomura S, Ohishi H, Takada M, Flor PJ, Neki A, Abe T, Nakanishi S, Mizuno N. Differential presynaptic localization of metabotropic glutamate receptor subtypes in the rat hippocampus. J Neurosci. 1997;17:7503–7522. doi: 10.1523/JNEUROSCI.17-19-07503.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigemoto R, Kulik A, Roberts JD, Ohishi H, Nusser Z, Kaneko T, Somogyi P. Target-cell-specific concentration of a metabotropic glutamate receptor in the presynaptic active zone. Nature. 1996;381:523–525. doi: 10.1038/381523a0. [DOI] [PubMed] [Google Scholar]
- Sloviter RS, Dichter MA, Rachinsky TL, Dean E, Goodman JH, Sollas AL, Martin DL. Basal expression and induction of glutamate decarboxylase and GABA in excitatory granule cells of the rat and monkey hippocampal dentate gyrus. J Comp Neurol. 1996;373:593–618. doi: 10.1002/(SICI)1096-9861(19960930)373:4<593::AID-CNE8>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Tong G, Malenka RC, Nicoll RA. Long-term potentiation in cultures of single hippocampal granule cells: a presynaptic form of plasticity. Neuron. 1996;16:1147–1157. doi: 10.1016/s0896-6273(00)80141-5. [DOI] [PubMed] [Google Scholar]
- Vogt KE, Nicoll RA. Glutamate and γ-aminobutyric acid mediate a heterosynaptic depression at mossy fibre synapses in the hippocampus. Proc Natl Acad Sci U S A. 1999;96:1118–1122. doi: 10.1073/pnas.96.3.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt KE, Regehr WG. Cholinergic modulation of excitatory synaptic transmission in the CA3 area of the hippocampus. J Neurosci. 2001;21:75–83. doi: 10.1523/JNEUROSCI.21-01-00075.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker MC, Ruiz A, Kullmann DM. Monosynaptic GABAergic signaling from dentate to CA3 with a pharmacological and physiological profile typical of mossy fibre synapses. Neuron. 2001;29:703–715. doi: 10.1016/s0896-6273(01)00245-8. [DOI] [PubMed] [Google Scholar]