Abstract
We examined the effects of a maternal cafeteria diet on skeletal muscle and adipose tissue development in the offspring at weaning. Rats born to mothers fed the cafeteria diet either during gestation alone or during both gestation and lactation exhibited a 25% reduction in muscle cross-sectional area with approximately 20% fewer fibres compared with pups fed a balanced chow diet. Maintaining the cafeteria diet during lactation increased intramuscular lipid content and fat pad weights characterized by adipocyte hypertrophy but not hyperplasia. These pups also had elevated muscle IGF-1, IGF-1 receptor, and PPARγ mRNA levels, which may indicate an attempt to maintain normal insulin sensitivity. The increased adiposity and elevated IGF-1, IGF-1 receptor and PPARγ mRNAs were not seen in the pups rehabilitated to the balanced diet during lactation. However, these pups exhibited reduced muscle cell proliferation (PCNA) with reduced insulin receptor and a trend towards reduced glucose transporter (GLUT)-4 mRNAs when compared with pups fed a balanced chow diet, indicating possible alterations in glucose uptake by muscle tissue. Therefore, rats born to mothers fed a cafeteria diet during gestation alone or during both gestation and lactation exhibited impaired skeletal muscle development and metabolic disorders normally associated with insulin resistance as early as the weaning stage.
Around 15–20% of children are overweight in Western societies and the proportion is predicted to increase (Ogden et al. 2002; Lobstein & Frelut, 2003). Obese children are more likely to remain obese into adulthood (Mossberg, 1989) with a higher incidence of type-2 diabetes, heart disease, cancer and arthritis (Must et al. 1992) leading to a reduced quality of life as well as important economic consequences through healthcare and sick-leave. Obesity and related disorders are generally attributed to high intake of processed food or ‘junk-food’ diets, which contain high amounts of calories, fat, salt and refined sugars, combined with a lack of exercise (Marx, 2002; BBC, 2003). However, there is now growing evidence that obesity and related disorders may be programmed during the fetal life of an individual. Evidence for the fetal programming of obesity and related disorders comes from studies on maternal under-nutrition where it was shown that low birth weight individuals were more likely to develop hyperphagia, diabetes, cardiovascular diseases and a sedentary behaviour into adulthood (Barker, 1993; Barker et al. 1993; Vickers et al. 2001). However, nowadays in Western societies maternal under-nutrition only concerns a small percentage of the population because of abundant food supplies. In contrast, the consumption of high-calorie processed foods predominates in the western world but their influence on fetal development remains to be characterized (Armitage et al. 2005).
In this study, we have investigated the influence of feeding pregnant rats a cafeteria diet, either during gestation alone or during both gestation and lactation, on skeletal muscle and adipose tissue cellularity and on the expression of genes involved in muscle growth and metabolism in the offspring at weaning. The gene expression analyses included genes that constitute the insulin-like growth factor (IGF) system, namely IGF-1, IGF-1 receptor (IGF-1R), IGF-binding protein (BP)-4 and IGFBP-5 (Florini et al. 1996); a marker of cell proliferation, the proliferating cell nuclear antigen (PCNA) (Jaskulski et al. 1988; Johnson & Allen, 1993); a marker of quiescent and activated satellite cells, M-cadherin (Cornelison & Wold, 1997); the myogenic factor myoD; the myogenic inhibitor myostatin (McCroskery et al. 2003); the insulin receptor (InsR); the glucose transporter (GLUT)-4; and two markers of adipocyte differentiation and lipid synthesis, namely the peroxisome proliferator-activated receptor (PPAR)-γ and adipsin (Wilkison et al. 1990; Rieusset et al. 1999; Lapsys et al. 2000).
We present evidence that maternal over-feeding on ‘junk-food’ during pregnancy can have an even greater influence on fetal development than maternal under-nutrition. Rats exposed to a cafeteria diet during gestation and lactation exhibited impaired skeletal muscle development and increased adiposity. Such a diet also induced the onset of cellular and molecular metabolic disorders that are normally associated with insulin resistance and type-2 diabetes in the offspring. These disorders were observed as early as the end of lactation.
Methods
Animals
Eighteen virgin female Wistar rats (230 ± 5 g) purchased from Charles River (Kent, UK) were individually housed with Wistar males in wire-bottomed cages. On the day a copulation plug was found, the females were isolated and assigned to one of three nutritional groups. The control (C) group (n = 6) was given free access to a balanced standard rat chow diet RM3 (SDS Ltd, Betchworth, Surrey, UK) throughout gestation and lactation. The two other groups were given a cafeteria diet (Rothwell & Stock, 1979), which consisted of an ad libitum choice of palatable processed food with a high fat and/or high sugar content including muffins, jam doughnuts, biscuits, cheese, marshmallows, potato crisps and chocolate bars in addition to rat chow (RM3). The general nutritional information for 100 g of RM3 chow, as provided by the manufacturer, was 363.3 kcal (1521.1 kJ), 22.39% proteins, 55.73% carbohydrates including 5.75% sugars, 3.27% fat including 0.70% saturated fat, 15.43% fibres and 0.32% sodium. The average nutritional information for 100 g of cafeteria food given to the rats, as provided by the manufacturer, was 416.5 kcal (1743.8 kJ), 9.17% protein, 47.04% carbohydrates including 24.95% sugars, 19.15% fat including 8.54% saturated fat, 3.02% fibres and 0.38% sodium. The daily amounts of food eaten by the animals were not measured in this study but adult rats given cafeteria style food have been reported to increase their overall energy and fat intake but their protein intake is unchanged while carbohydrate intake is similar or higher compared with rats fed control chow (Esteve et al. 1994; Llado et al. 1995). In one of these two groups of rats, the CDW group (cafeteria diet up to weaning), the cafeteria diet was maintained throughout gestation and lactation (n = 6). In the other, the CDG group (cafeteria diet during gestation), the cafeteria diet was maintained throughout gestation alone followed by rehabilitation to rat chow given ad libitum (RM3) during lactation (n = 6).
The litters used in this study contained between 10 and 15 live pups at birth to limit birth weight variations due to litter sizes; outsized litters were not included. All animals were given free access to water and were maintained in a light, temperature and humidity controlled environment (14: 10 h light–dark cycles; 20 ± 2°C; 45% relative humidity). The weights of mothers and pups were recorded every morning. On the 21st day post partum the pups were killed by CO2 inhalation. The semitendinosus muscles were dissected and flash frozen in freezing isopentane for subsequent histological analyses. The gastrocnemius muscles were frozen in liquid nitrogen for gene expression analyses and the perirenal fat depots (Krakower et al. 1988) were fixed in buffered formalin (BDH, UK). Histological analyses of muscle and adipose tissues were performed in six and five litters per group, respectively. The results for each litter were the average of six pups per litter and litter results rather than individuals were analysed statistically. The gene expression analyses were performed in six litters per group and in one pup per litter chosen randomly while excluding the smallest and largest littermates at birth. All samples were collected within 10–15 min of kill and were stored at −80°C until further analyses except for the formalin-fixed fat pads, which were stored at room temperature.
Histology
The semitendinosus muscles were sectioned (10 μm) in the mid-belly region and stained with toluidine blue, haematoxylin and eosin (H&E), Hoechst 33258 (Sigma, UK) and oil red O for determination of muscle cross-sectional area, fibre number, nuclei number and intramuscular lipid accumulation, respectively.
The fat pads were fixed in buffered formalin (BDH, UK) and were processed for wax embedding in a Shandon Citadel 2000 automatic tissue processor (Shandon Scientific Ltd, UK). The wax blocks were then mounted and 6 μm thick sections were cut using a Microm HM360 microtome (Microm international, UK). Sections were stained with H&E for subsequent measurements.
The Kontron image analysis software (Zeiss, Germany) was used to determine adipocyte sizes and numbers in adipose tissue as well as intramuscular lipid content and muscle fibre and nuclei numbers per muscle cross-sectional area. Adipocyte sizes and numbers were measured in five different microscopic frames chosen randomly such that between 160 and 400 cells were measured from each sample. Intramuscular fat area was measured in the whole semitendinosus cross-sectional area. Muscle fibres and nuclei were counted in five microscopic frames per section chosen randomly which represented 7–10% of the whole muscle cross-sectional area.
RNA isolation
Total RNA was extracted from the gastrocnemius muscles using Tri-Reagent (Sigma, UK) and dissolved in Sigma pure water (Sigma, UK). The total RNA was then treated with 1 unit per μg total RNA of RQ1 RNase-free-DNase (Promega, Southampton, UK) to prevent possible chromosomal DNA contamination. Spectrophotometry was used to quantify and check the purity (260/280 nm ratio) of the total RNA (Gene Spec I, Naka Instruments, Japan). The integrity of the RNA was checked by formaldehyde gel electrophoresis and visualization of intact 18S and 28S ribosomal RNA bands under UV light.
Reverse transcription
One microgram of total RNA from each sample was reverse transcribed using the Ominiscript RT kit (Qiagen, Crawley, UK) with 5 pmol of random hexamer primers (Invitrogen, Paisley, UK) and 0.5 units of RNAse inhibitor (Roche, Switzerland) in a 20 μl reaction volume. The reverse transcription of all samples used in each subsequent real time PCR run was carried out simultaneously using the same master-mix to prevent variability in the reverse transcription efficiency between samples.
Real time (RT) quantitative PCR
The real-time PCR analyses were performed using a protocol developed by Owino et al. (2001) and Hameed et al. (2003), as previously described (Bayol et al. 2004). All primers and mRNA accession numbers used for the real-time PCR are listed in Table 1. The primers were designed using the Primer-3 Web-Software (Whitehead Institute for Biomedical Research, MA, USA), such that the sequences amplified overlapped two different exons. All primers were synthesized by MWG-Biotech (Germany). Real-time PCR were performed in the Opticon 2 light cycler (MJ Research Inc, MA, USA) with 2 μl cDNA product from the reverse transcription reaction using SyBR green detection (Qiagen, UK). The relative concentrations of the target sequences in control and overfed samples were calculated by the Opticon 2 Lightcycler software (MJ Research Inc., MA, USA) from a standard curve created with serial dilutions of standard DNA (target sequence of interest), which were included with each run. For each transcript analysis, the standards and samples were run simultaneously on the same 96-well PCR plate using the same SyBR green master-mix. Each standard and sample was run in duplicate on the same PCR plate and the average of each duplicate value was used for subsequent statistical analyses. Each real-time PCR run was carried out in duplicate to check reproducibility of the results. The relative concentrations of target sequences in each run were expressed as numbers of copies and were normalized to one microgram of total RNA. All PCR products were checked for specificity and purity from a melting curve profile performed by the lightcycler software at the end of each run. The PCR products were further checked for size and specificity by agarose gel electrophoresis. They were subsequently sequenced by ABC (Advanced Biotechnology Centre, Imperial College London, UK) and checked by BLAST analyses (NCBI, USA) to ensure homology of the amplification products to the mRNA of interest.
Table 1.
Primers in the 5′-to 3′ direction used for the real time PCR analyses
| Primer | Sequence 5′-to 3′ | Product size bp | Accession number |
|---|---|---|---|
| IGF-1 forward | GCTTGCTCACCTTTACCAG | 300 | M17335 |
| IGF-1 reverse | AAGTGTACTTCCTTCTGAGTCT | — | — |
| IGF-1R forward | CATGCAGGAGTGTCCATCAG | 194 | NM_052807 |
| IGF-1R reverse | CTCGCCGGATGTTAATAAGC | — | — |
| IGFBP-4 forward | CAGTGCCCATGATCACAG | 236 | X81582 |
| IGFBP-4 reverse | TGTTTGGGGTGGAAGTTG | — | — |
| IGFBP-5 forward | GATCGCAGAAAGAAGCTGAC | 272 | NM_012817 |
| IGFBP-5 reverse | GTCCACACACCAGCAGATGC | — | — |
| PCNA forward | CTTAGCACTAGTATTTGAAGCACC | 143 | NM_022381 |
| PCNA reverse | GTGCAAATTCACCAGATGG | — | — |
| M-cadherin forward | ATGTGCCACAGCCACATCG | 238 | M74541 |
| M-cadherin reverse | TCCATACATGTCCGCCAGCC | — | — |
| MyoD forward | ACTACAGCGGCGACTCAGAC | 208 | M84176 |
| MyoD reverse | GTGGAGATGCGCTCCACTAT | — | — |
| Myostatin forward | GCTCTTTGGAAGATGACGATT | 99 | AF019624 |
| Myostatin reverse | CATTTGGGCTTTCCATCC | — | — |
| InsR forward | ATCTCCTGGGATTCATGCTG | 196 | M29014 |
| InsR reverse | TACTGGGTCCAGGGTTTGAG | — | — |
| GLUT4 forward | CTTGGGTTGTGGCAGTGAG | 217 | D28561 |
| GLUT4 reverse | AGGACCAGTGTCCCAGTCAC | — | — |
| PPARγ forward | CCCTGGCAAAGCATTTGTAT | 222 | AB011365 |
| PPARγ reverse | ACTGGCACCCTTGAAAAATG | — | — |
| Adipsin forward | CCTACATGGCTTCAGTGCAA | 204 | M92059 |
| Adipsin reverse | CCGGGTGAAGCACTACACTT | — | — |
Statistical analyses
Statistical analyses were performed using SPSS v. 12.0 for Windows (SPSS Inc., IL, USA). All results were analysed by the Levene's test for homogeneity of variance and differences between the C, CDG and CDW groups were analysed by one-way ANOVA followed by either Tukey's or the Games-Howell post hoc test when Levene's values were greater than 0.05 or below 0.05, respectively. Results were considered statistically significant when P < 0.05 and were considered as trends when 0.05 ≤P≤ 0.10.
Ethical considerations
The animal work was approved by the Royal Veterinary College Ethics and Welfare committee and was carried out under the UK Animals (Scientific Procedures) Act 1986.
Results
Animals and tissue weights
Results in Table 2 show that the cafeteria diet did not affect the body weight of pups at birth (C vs. CDG, P= 0.58; CDG vs. CDW, P= 0.99; C vs. CDW, P= 0.67) or at weaning (C vs. CDG, P= 0.34; CDG vs. CDW, P= 0.86; C vs. CDW, P= 0.62). Similarly, the daily postnatal growth rates were not affected (C vs. CDG, P= 0.45; CDG vs. CDW; P= 0.82, C vs. CDW, P= 0.79). The cafeteria diet did not affect the weight of the gastrocnemius muscle at weaning (C vs. CDG, P= 0.92; CDG vs. CDW, P= 0.37; C vs. CDW, P= 0.21) (Table 2); however, maintaining the cafeteria diet during both gestation and lactation induced an increase in the perirenal fat pad weight in the CDW group compared with both the C and CDG groups (C vs. CDG, P= 0.84; CDG vs. CDW, P= 0.004; C vs. CDW, P= 0.001).
Table 2.
Body and tissue weights of 21-day-old rat pups born to mothers fed either chow ad libitum during both gestation and lactation (C), the cafeteria diet during gestation followed by chow ad libitum during lactation (CDG) or the cafeteria diet during both gestation and lactation (CDW)
| C | CDG | CDW | |
|---|---|---|---|
| Body weight at birth | 6.64 ± 0.23 | 6.33 ± 0.16 | 6.38 ± 0.23 |
| Body weight at weaning | 56.56 ± 2.21 | 50.81 ± 1.46 | 52.85 ± 4.03 |
| Daily gth rate | 2.33 ± 0.10 | 2.11 ± 0.06 | 2.22 ± 0.18 |
| Gastrocnemius muscle | 0.17 ± 0.01 | 0.16 ± 0.01 | 0.13 ± 0.01 |
| Perirenal fat pad | 0.50 ± 0.09a | 0.59 ± 0.05a | 1.22 ± 0.17b |
Results are weight (g) shown as means ±s.e.m.; n = 6 litters per group; different letters indicate statistically significant differences (P < 0.05) by ANOVA followed by Tukey's post hoc analysis.
Results also showed that on the 21st day of pregnancy the body weights of mothers fed the cafeteria diet (456.2 ± 11.5 g, mean ±s.e.m.) tended to be increased compared with mothers fed the control diet (422.6 ± 9.5 g) (P= 0.076). At weaning the maternal body weights were comparable between the three groups examined (data not shown).
Histological analysis of fat pads
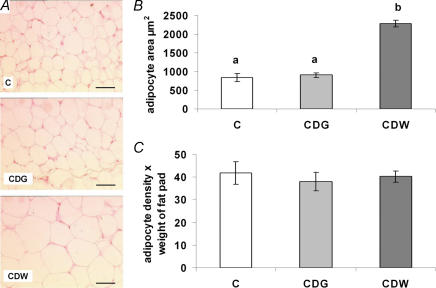
To determine the cellular nature of the increased perirenal fat pad weight, we performed histological analyses (H&E) of this tissue. Figure 1A and B) shows a dramatic adipocyte hypertrophy in the CDW group which was confirmed by image analyses (Fig. 1B)). The average adipocyte area was increased in the CDW group (2289.6 ± 84.3μm2) compared with C and CDG groups (C vs. CDG, P= 0.84; CDG vs. CDW, P < 0.001; C vs. CDW, P < 0.001) but was comparable in the C (837.0 ± 107.8μm2) and CDG groups (906.7 ± 63.7μm2). The adipocyte density (number of adipocytes per screen) was decreased in the CDW group (32.8 ± 1.5 adipocytes per screen) compared with both C (81.0 ± 9.3 adipocytes per screen) and CDG (72.5 ± 4.8 adipocytes per screen) groups but there were no differences between the C and CDG groups (C vs. CDG, P= 0.60; CDG vs. CDW, P= 0.002, C vs. CDW, P < 0.001). Because white adipose tissue is mostly composed of adipocytes, we estimated the relative number of adipocytes in each fat pad by multiplying adipocyte densities by fat pad weights. Results showed that the relative number of adipocytes was similar in the three nutritional groups (C vs. CDG, P= 0.72; CDG vs. CDW, P= 0.47; C vs. CDW, P= 0.90) (Fig. 1C)), indicating that the three diets did not influence adipocyte differentiation in the fat pads of the offspring. Therefore, the increased fat pad weight observed in the CDW group (Table 2) was caused by increased accumulation of lipids within adipocytes (hypertrophy) rather than by increased adipocyte number (hyperplasia).
Figure 1. A maternal cafeteria diet induces adipocyte hypertrophy in the offspring.
Histological analyses of perirenal fat pads of 21-day-old rat pups born to mothers fed either chow ad libitum during gestation and lactation (C, □), the cafeteria diet during gestation followed by chow ad libitum during lactation (CDG, ░) or the cafeteria diet during gestation and lactation (CDW, ▒). A, H&E stained 6 μm thick sections from each nutrition group. B, adipocyte area results. C, an estimation of adipocyte numbers. Results are means ±s.e.m., n = 5 litters; different letters indicate statistically significant differences (P < 0.05), by ANOVA followed by Tukey's post hoc analysis. Scale bars are 50 μm.
Intramuscular fat analysis
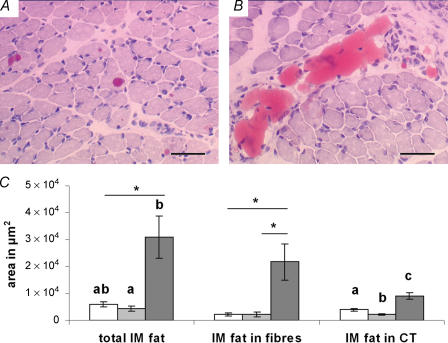
Microscopic analyses of oil red O stained muscle sections enabled a distinction to be made between intramuscular lipids localized within fibres (Fig. 2A)) and the lipids localized in the connective tissue (adipocytes) within muscle (Fig. 2B)). Results (Fig. 2C)) showed that pups from the CDW groups exhibited an increased total lipid accumulation per muscle cross-sectional area compared with the CDG group (P= 0.04). The same trend was observed between the CDW and the C groups (P= 0.05) but the total intramuscular lipid accumulation was comparable between the C and CDG groups (P= 0.41). The oil red O stain analyses also showed that the levels of lipids accumulated within muscle fibres were comparable between the C and CDG groups (P= 0.99) but there was a trend towards an increased accumulation in the CDW group compared with both the C (P= 0.07) and the CDG (P= 0.07) groups (Fig. 2C)). The amount of intramuscular lipids localized within the connective tissue was increased in the CDW group compared with both the C (P= 0.02) and CDG groups (P= 0.005) and was also increased in the C group compared with the CDG group (P= 0.03) (Fig. 2C)). Results also showed that the C, CDG and CDW groups stored 34.0%, 50.1% and 70.7% of their total intramuscular lipids within muscle fibres, respectively, indicating a greater proportion of intramyocellular lipids accumulation in the CDW group.
Figure 2. A maternal cafeteria diet promotes intramuscular fat accumulation in the offspring.
Histological analyses (oil red O) of intramuscular (i.m.) lipid accumulation in the semitendinosus muscles of 21-day-old rat pups born to mothers fed either chow ad libitum during gestation and lactation (C, □), the cafeteria diet during gestation followed by chow ad libitum during lactation (CDG, ░) or the cafeteria diet during gestation and lactation (CDW, ▒). A, lipids accumulated within muscle fibres (CDW group). B, lipids accumulated in the connective tissue (C group). C, quantified results from image analyses. Results are means ±s.e.m., n = 6 litters; different letters indicate statistically significant differences (P < 0.05); *0.05 ≤P≤ 0.10, by ANOVA followed by Games-Howell post hoc analysis. Scale bars are 50 μm.
Histological analyses of skeletal muscle
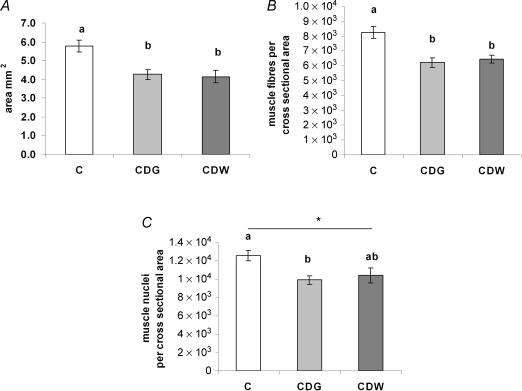
Figure 3A) shows that pups from both the CDG (P= 0.01) and CDW (P= 0.005) groups exhibited a reduction in semitendinosus muscle cross-sectional areas by approximately 25% compared with the C group but there were no differences between the CDG and CDW groups (P= 0.96). The number of muscle fibres per cross-sectional area in the semitendinosus muscle was also reduced in both the CDG (P= 0.002) and CDW (P= 0.005) groups by about 20% compared with the C group (Fig. 3B)), with no differences between the CDG and CDW groups (P= 0.87). The number of nuclei per cross-sectional area was reduced by about 21% in the CDG group compared with the C group (P= 0.03); a similar trend (about 17% reduction) was observed in the CDW versus C groups (P= 0.07) but there were no differences between CDG and CDW groups (P= 0.85) (Fig. 3C)).
Figure 3. A maternal cafeteria diet impairs skeletal muscle development.
Histological analyses of muscle area (A), number of muscle fibres (B) and nuclei per cross sectional area (C) in the semitendinosus muscles of 21-day-old rat pups born to mothers fed either chow ad libitum during gestation and lactation (C, □), the cafeteria diet during gestation followed by chow ad libitum during lactation (CDG, ░) or the cafeteria diet during gestation and lactation (CDW, ▒). Results are means ±s.e.m., n = 6 litters; different letters indicate statistically significant differences (P < 0.05), *0.05 ≤P≤ 0.10 by ANOVA followed by Tukey's post hoc analysis.
Gene expression analyses
The IGF system
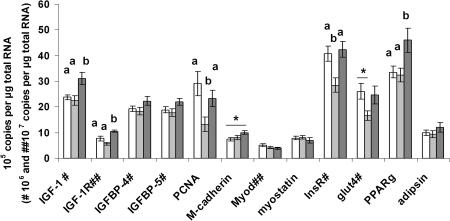
The IGF system is a well-characterized regulator of muscle growth (Florini et al. 1996) and metabolism (Cusi & DeFronzo, 2000). At weaning, the levels of IGF-1 mRNA were increased in the gastrocnemius muscle from the CDW group compared with both C (P= 0.04) and CDG (P= 0.015) groups (Fig. 4)). IGF-1 mRNA levels were similar between C and CDG groups (P= 0.88). A similar transcription profile was observed for IGF-1R, also expressed at higher levels in the CDW group compared with both C (P= 0.02) and CDG (P= 0.001) groups (Fig. 4)) but no differences were seen between the C and CDG groups (P= 0.11). IGFBP-4 (C vs. CDG, P= 0.87; C vs. CDW, P= 0.32; CDG vs. CDW, P= 0.15) and IGFBP-5 (C vs. CDG, P= 0.85; C vs. CDW, P= 0.29; CDG vs. CDW, P= 0.11) mRNA levels were not affected by the three diets examined (Fig. 4)).
Figure 4. A maternal cafeteria diet affects the expression of genes involved in muscle growth and metabolism in the offspring.
Relative gene expression analyses using real-time PCR and SyBR green detection in the gastrocnemius muscles of 21-day-old rat pups born to mothers fed either chow ad libitum during gestation and lactation (C, □), the cafeteria diet during gestation followed by chow ad libitum during lactation (CDG, ░) or the cafeteria diet during gestation and lactation (CDW, ▒). Results are means ±s.e.m., n = 6; different letters indicate statistically significant differences (P < 0.05), *0.05 ≤P≤ 0.10 by ANOVA followed by Tukey's post hoc analysis.
Cell proliferation, satellite cell activity and regulators of myogenesis
Levels of PCNA mRNA, a marker of cell proliferation (Jaskulski et al. 1988), were reduced in offspring from the CDG group compared with the C group (P= 0.02) but there were no differences between CDG vs. CDW (P= 0.16) and CDW vs. C (P= 0.51) (Fig. 4)). Levels of M-cadherin mRNA, a marker of satellite cells (Cornelison & Wold, 1997), tended to be increased in the CDW group compared with the C group (P= 0.06) but there were no differences between the C vs. CDG groups (P= 0.81) and the CDG vs. CDW groups (P= 0.18) (Fig. 3)). mRNA levels for the myogenic regulatory factor MyoD (C vs. CDG, P= 0.29; C vs. CDW, P= 0.18; CDG vs. CDW, P= 0.95) and the myogenic inhibitor myostatin (C vs. CDG, P= 0.96; C vs. CDW, P= 0.72; CDG vs. CDW, P= 0.56) were comparable between the three nutrition groups examined (Fig. 4)).
Metabolism and adiposity
The levels of InsR mRNA were reduced in the CDG group compared with both C (P= 0.03) and CDW (P= 0.01) groups but were not different between the C and CDW groups (P= 0.91) (Fig. 4)). There was a trend towards reduced GLUT-4 mRNA levels in the CDG group compared with the C group (P= 0.08) but GLUT-4 mRNA levels were comparable between C versus CDW groups (P= 0.93) and CDG versus CDW groups (P= 0.16) (Fig. 4)). The levels of PPARγ mRNA were increased in the CDW group compared with both C (P= 0.02) and CDG (P= 0.01) groups with no differences between C and CDG groups (P= 0.96) (Fig. 4)). There were no differences in adipsin mRNA expression between the C, CDG and CDW groups (C vs. CDG, P= 0.97; C vs. CDW, P= 0.56; CDG vs. CDW, P= 0.43) (Fig. 4)).
Discussion
In this study, Wistar rats were fed either a balanced chow diet during gestation and lactation or a cafeteria diet during gestation followed by chow during lactation, or a cafeteria diet during gestation and lactation. The influence of the three diet regimes on the offspring was examined at weaning (21 days post partum). Results showed that the three diets did not influence birth weights, which is consistent with a previous report by Holemans et al. (2004), and postnatal growth rates. However, pups fed the cafeteria diet during gestation and lactation, exhibited a markedly increased adiposity at weaning. The increased adiposity was characterized both by an increase in fat pad weight as well as intramuscular lipid deposition.
Adipose tissue is the site of safe storage of fat and is indispensable for normal metabolic function. A lack of adipose tissue leads to insulin resistance soon after birth (Moitra et al. 1998) and is responsible for accumulation of fat in the ‘wrong places’ (Friedman, 2002; Hegarty et al. 2003). The build up of fat in organs other than adipose tissue is believed to alter the normal function of these organs and leads to insulin resistance and diabetes. Therefore, an increased number of adipocytes would potentially increase an individual's fat storage capacity within adipose tissue and would prevent the accumulation of fat in organs that are poorly designed for this function. We therefore examined whether rats exposed to a cafeteria diet early in life would develop such a protective mechanism and thus examined the cellular nature of the increased fat pad weight observed in the CDW group. Results showed that the marked increased fat pad weight in the rats fed the cafeteria diet throughout gestation and lactation was caused by adipocyte hypertrophy alone and that this marked hypertrophy was not accompanied by increased adipocyte differentiation. Therefore, a protective mechanism involving increased adipocyte number to increase fatty acid storage capacity within adipose tissue did not seem to have taken place in rat offspring at weaning following early exposure to a high calorie diet.
Skeletal muscle is another site of fatty acid storage. However, intramyocellular fat accumulation disrupts normal muscle insulin sensitivity and therefore constitutes a reliable marker of whole-body insulin resistance (Hegarty et al. 2003). Our study shows that pups fed the cafeteria diet during gestation and lactation exhibited an increased intramyocellular fat accumulation. They also exhibited preferential muscular fat accretion within muscle fibres (70.7%) rather than in connective tissue (29.3%) which was almost the reverse situation that was observed in the C group where only 34% of total intramuscular fat was localized within muscle fibres. These results suggest that not only did pups from the CDW group exhibit adipocyte hypertrophy (that was not accompanied by increased adipocyte differentiation) but also they exhibited fat accretion in skeletal muscle fibres, which is known to disrupt the normal metabolic function of this tissue. Therefore rat offspring from mothers fed a cafeteria diet during gestation and lactation exhibited signs of increased propensity for insulin resistance detectable as early as the end of lactation.
The molecular mechanism for intramyocellular fat accretion in offspring from the CDW group may involve PPARγ. PPARγ is a regulator of adipogenesis expressed at high levels in adipocytes (Rieusset et al. 1999) and at lower levels in muscle fibres (Lapsys et al. 2000). In muscle, PPARγ regulates lipid metabolism (Lapsys et al. 2000) and muscle-specific knockout leads to adiposity and insulin resistance (Norris et al. 2003). In this study, the increased intramuscular fat content and preferential accretion within muscle fibres observed in the CDW group correlated with increased muscle PPARγ mRNA. However, PPARγ transcription rate did not correlate with that of adipsin, a marker of adipocyte differentiation that is not expressed in skeletal muscle (Lapsys et al. 2000). Unchanged adipsin levels suggest that the increased intramuscular adiposity in the CDW group was not accompanied by increased intramuscular adipocyte differentiation and thus increased PPARγ levels are likely to be linked to increased lipid synthesis within muscle fibres in the CDW group. It is also interesting to note that pups from the CDW group exhibited both an increased muscle PPARγ transcription and increased adipose tissue weight. A similar correlation has been found in obese humans where it was proposed that increased muscle PPARγ expression was a compensatory mechanism to help maintain normal insulin sensitivity (Kruszynska et al. 1998).
Skeletal muscle not only serves to produce movement and force but is also a major metabolic organ responsible for 70–80% of whole body insulin-stimulated glucose uptake (DeFronzo et al. 1981). Therefore, alterations in skeletal muscle development may influence an individual's long-term ability to exercise and regulate glucose homeostasis. Such alterations have been demonstrated in rats born to nutritionally restricted mothers (Bedi et al. 1982; Prakash et al. 1993; Martin et al. 2000; Vickers et al. 2001; Vickers et al. 2003) but the influence of maternal over-feeding on skeletal muscle development has not been previously investigated. We therefore performed histological analyses of the semitendinosus muscles at weaning. This muscle was chosen because fibres are arranged in parallel to the line of muscle (Roy et al. 1984), and thus counting fibres within the mid belly region is a good indicator of the total number of muscle fibres that constitute this muscle. Results showed that the maternal cafeteria diet induced a marked reduction in the semitendinosus cross-sectional area (approximately 25%) and in the number of fibres (approximately 20%) that formed in this muscle at weaning. Furthermore, the number of muscle fibres was comparable in the CDG and CDW groups suggesting that returning to a balanced diet during lactation did not restore muscle fibre numbers to control levels. In rats, muscle fibre hyperplasia ends around the time of weaning (Rayne & Crawford, 1975). Therefore, there is strong evidence to suggest that the muscle atrophy and fibre hypoplasia observed in weanling rats exposed to the cafeteria diet during gestation will remain into adulthood. In a previous study carried out in our laboratory (Bayol et al. 2004), using the same strain of rats, even a maternal restriction to 40% of ad libitum did not cause a reduction in fibre number in the offspring, suggesting that maternal overfeeding may be more detrimental than severe under-nutrition on skeletal muscle development.
The marked muscle fibre hypoplasia observed in the cafeteria fed pups was not, however, accompanied by a reduction in the gastrocnemius muscle weight at weaning. We cannot fully explain this discrepancy. However at weaning, rats have only acquired around a quarter of their adult sizes and their muscles are small; it is therefore possible that changes in muscle weights may be more marked when the animals are fully grown.
The muscle atrophy and fibre hypoplasia observed in the CDG group was accompanied by reduced numbers of nuclei when compared with pups fed a balanced diet and a similar trend was observed in the CDW group. Therefore maternal overfeeding reduced the overall rate of muscle cell proliferation in the offspring from gestation to weaning. The transcription profile of PCNA, a protein required for cell proliferation (Jaskulski et al. 1988), directly correlated with muscle nuclei numbers at weaning in the three groups examined, suggesting that the reduced muscle cell proliferation was continuing at day 21 post partum in the pups exposed to the cafeteria diet. Reduced muscle cell proliferation at weaning may lead to long-term reduced muscle growth potential in the pups exposed to the cafeteria diet and possibly a reduced ability to exercise and impaired glucose homeostasis.
Results also showed a reduction in InsR mRNA levels in the CDG group compared with the C group and the same trend was observed for the glucose transporter GLUT-4 mRNA. InsR and GLUT4 are key regulators of insulin-stimulated glucose uptake into muscle. Muscle-specific ablation of GLUT4 induces insulin resistance whereas InsR ablation has more subtle effects on glucose homeostasis because of the compensatory role of IGF-1R (Minokoshi et al. 2003). However, double inactivation of InsR and IGF-1R in muscle induces severe insulin resistance (Fernandez et al. 2001). The reduced InsR levels in the CDG rats were not accompanied by simultaneously increased IGF-1R mRNA levels to compensate for the lack of InsR. This suggests a possible reduced glucose uptake by muscle tissue in the CDG rats, which may also explain the reduced muscle cell proliferation observed in this group.
The IGF-1 system is a key regulator of muscle growth via satellite cell activation and protein synthesis (Barton-Davis et al. 1999). Upon activation, satellite cells proliferate, and some daughter cells fuse with the adjacent fibres and thus donate their nuclei while others return to quiescence to replenish the pool of satellite cells. Quiescent and activated satellite cells express M-cadherin while MyoD is only detected in activated satellite cells (Cornelison & Wold, 1997) and myostatin inhibits satellite activation (McCroskery et al. 2003). Most IGF-1 actions in muscle tissue are mediated through the IGF-1R and are locally modulated by the insulin-like growth factor binding proteins. The two major IGFBPs in muscle are IGFBP-4 and IGFBP-5 and their respective roles are linked to muscle cell proliferation and myotube differentiation (Ewton & Florini, 1995). In this study, offspring from the CDW group exhibited a marked increase in both IGF-1 and IGF-1R mRNA suggesting that the increased IGF-1 signal in this group would be further enhanced by an increase in both ligand and receptor. However, the increased IGF-1 signal in the CDW group was not accompanied by simultaneous marked changes in PCNA, M-cadherin, MyoD, IGFBP-4, IGFBP-5 and myostatin mRNAs; furthermore muscle atrophy was observed in this group. Therefore, the increased IGF-1 signal in the CDW group did not result in increased muscle growth at weaning and did not trigger the up-regulation of genes involved in muscle growth, which is not consistent with the well characterized mitotic and myotrophic properties of IGF-1. This suggests that either the intracellular pathway downstream of IGF-1R leading to muscle growth is impaired or that the increased IGF-1 signal is linked to a biological function other than muscle growth in the CDW group. Since IGF-1 also exhibits insulin-like metabolic properties and systemic injections of IGF-1 can improve insulin sensitivity in diabetic patients (Cusi & DeFronzo, 2000), it is possible that the increase in muscle IGF-1 and IGF-1R observed in pups from the CDW group may be linked to the regulation of muscle insulin sensitivity via autocrine and paracrine actions.
This study shows that rats born to mothers fed a cafeteria diet during gestation and lactation exhibited muscle atrophy and fibre hypoplasia accompanied by intramuscular lipid accumulation and increased adipose tissue weight. These cellular characteristics were accompanied by increased muscle IGF-1, IGF-1R and PPARγ mRNA levels consistent with possible molecular adaptations to maintain normal insulin sensitivity. The increased adiposity was prevented when pups were rehabilitated to a balanced diet during lactation but the muscle atrophy and fibre hypoplasia remained. These animals also exhibited reduced muscle InsR levels which were not compensated by increased IGF-1R and a trend towards reduced GLUT4 expression indicating a possible reduction in glucose uptake by muscle tissue. Therefore, when compared with offspring born to mothers fed a balanced diet, rats fed a maternal cafeteria diet during gestation exhibited impaired myogenesis as well as cellular and molecular metabolic adaptations that are normally associated with the onset of insulin resistance, as early as the end of lactation.
Acknowledgments
The authors wish to thank Doiran Jones, Tanya Hopcroft and Antoine Fauconneau for their help with the histological analyses. The authors are also grateful to Helen Smith for her help with the image analyses and to the Biological Services Unit for their advice and help with the animal work. This work was funded by the Wellcome Trust.
References
- Armitage JA, Taylor PD, Poston L. Experimental models of developmental programming; Consequences of exposure to an energy rich diet during development. J Physiol. 2005;565:3–8. doi: 10.1113/jphysiol.2004.079756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ. The intrauterine origins of cardiovascular disease. Acta Paediatrsupplement. 1993;82(Suppl. 391):93–99. doi: 10.1111/j.1651-2227.1993.tb12938.x. [DOI] [PubMed] [Google Scholar]
- Barker DJ, Hales CN, Fall CH, Osmond C, Phipps K, Clark PM. Type 2 (non-insulin-dependent) diabetes mellitus, hypertension and hyperlipidaemia (syndrome X): relation to reduced fetal growth. Diabetologia. 1993;36:62–67. doi: 10.1007/BF00399095. [DOI] [PubMed] [Google Scholar]
- Barton-Davis ER, Shoturma DI, Sweeney HL. Contribution of satellite cells to IGF-I induced hypertrophy of skeletal muscle. Acta Physiol Scand. 1999;167:301–305. doi: 10.1046/j.1365-201x.1999.00618.x. [DOI] [PubMed] [Google Scholar]
- Bayol S, Jones D, Goldspink G, Stickland NC. The influence of undernutrition during gestation on skeletal muscle cellularity and on the expression of genes that control muscle growth. Br J Nutr. 2004;91:331–339. doi: 10.1079/BJN20031070. [DOI] [PubMed] [Google Scholar]
- BBC. BBC news report. 2003 http://news.bbc.co.uk/go/pr/fr/-/1/hi/health/3242674.stm.
- Bedi KS, Birzgalis AR, Mahon M, Smart JL, Wareham AC. Early life undernutrition in rats. 1. Quantitative histology of skeletal muscles from underfed young and refed adult animals. Br J Nutr. 1982;47:417–431. doi: 10.1079/bjn19820053. [DOI] [PubMed] [Google Scholar]
- Cornelison DD, Wold BJ. Single-cell analysis of regulatory gene expression in quiescent and activated mouse skeletal muscle satellite cells. Dev Biol. 1997;191:270–283. doi: 10.1006/dbio.1997.8721. [DOI] [PubMed] [Google Scholar]
- Cusi K, DeFronzo R. Recombinant human insulin-like growth factor I treatment for 1 week improves metabolic control in type 2 diabetes by ameliorating hepatic and muscle insulin resistance. J Clin Endocrinol Metab. 2000;85:3077–3084. doi: 10.1210/jcem.85.9.6827. [DOI] [PubMed] [Google Scholar]
- DeFronzo RA, Jacot E, Jequier E, Maeder E, Wahren J, Felber JP. The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes. 1981;30:1000–1007. doi: 10.2337/diab.30.12.1000. [DOI] [PubMed] [Google Scholar]
- Esteve M, Rafecas I, Fernandez-Lopez JA, Remesar X, Alemany M. Effect of a cafeteria diet on energy intake and balance in Wistar rats. Physiol Behav. 1994;56:65–71. doi: 10.1016/0031-9384(94)90262-3. [DOI] [PubMed] [Google Scholar]
- Ewton DZ, Florini JR. IGF binding proteins-4-5 and -6 may play specialized roles during L6 myoblast proliferation and differentiation. J Endocrinol. 1995;144:539–553. doi: 10.1677/joe.0.1440539. [DOI] [PubMed] [Google Scholar]
- Fernandez AM, Kim JK, Yakar S, Dupont J, Hernandez-Sanchez C, Castle AL, et al. Functional inactivation of the IGF-I and insulin receptors in skeletal muscle causes type 2 diabetes. Genes Dev. 2001;15:1926–1934. doi: 10.1101/gad.908001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florini JR, Ewton DZ, Coolican SA. Growth hormone and the insulin-like growth factor system in myogenesis. Endocr Rev. 1996;17:481–517. doi: 10.1210/edrv-17-5-481. [DOI] [PubMed] [Google Scholar]
- Friedman J. Fat in all the wrong places. Nature. 2002;415:268–269. doi: 10.1038/415268a. [DOI] [PubMed] [Google Scholar]
- Hameed M, Orrell RW, Cobbold M, Goldspink G, Harridge SD. Expression of IGF-I splice variants in young and old human skeletal muscle after high resistance exercise. J Physiol. 2003;547:247–254. doi: 10.1113/jphysiol.2002.032136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegarty BD, Furler SMYeJ, Cooney GJ, Kraegen EW. The role of intramuscular lipid in insulin resistance. Acta Physiol Scand. 2003;178:373–383. doi: 10.1046/j.1365-201X.2003.01162.x. [DOI] [PubMed] [Google Scholar]
- Holemans K, Caluwaerts S, Poston L, Van Assche FA. DIET-induced obesity in the rat: a model for gestational diabetes mellitus. Am J Obstet Gynecol. 2004;190:858–865. doi: 10.1016/j.ajog.2003.09.025. [DOI] [PubMed] [Google Scholar]
- Jaskulski D, de Riel JK, Mercer WE, Calabretta B, Baserga R. Inhibition of cellular proliferation by antisense oligodeoxynucleotides to PCNA cyclin. Science. 1988;240:1544–1546. doi: 10.1126/science.2897717. [DOI] [PubMed] [Google Scholar]
- Johnson SE, Allen RE. Proliferating cell nuclear antigen (PCNA) is expressed in activated rat skeletal muscle satellite cells. J Cell Physiol. 1993;154:39–43. doi: 10.1002/jcp.1041540106. [DOI] [PubMed] [Google Scholar]
- Krakower GR, James RG, Arnaud C, Etienne J, Keller RH, Kissebah AH. Regional adipocyte precursors in the female rat. Influence of ovarian factors. J Clin Invest. 1988;81:641–648. doi: 10.1172/JCI113367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruszynska YT, Mukherjee R, Jow L, Dana S, Paterniti JR, Olefsky JM. Skeletal muscle peroxisome proliferator-activated receptor-gamma expression in obesity and non-insulin-dependent diabetes mellitus. J Clin Invest. 1998;101:543–548. doi: 10.1172/JCI1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapsys NM, Kriketos AD, Lim-Fraser M, Poynten AM, Lowy A, Furler SM, et al. Expression of genes involved in lipid metabolism correlate with peroxisome proliferator-activated receptor gamma expression in human skeletal muscle. J Clin Endocrinol Metab. 2000;85:4293–4297. doi: 10.1210/jcem.85.11.6973. [DOI] [PubMed] [Google Scholar]
- Llado I, Pico C, Palou A, Pons A. Protein and amino acid intake in cafeteria fed obese rats. Physiol Behav. 1995;58:513–519. doi: 10.1016/0031-9384(95)00081-s. [DOI] [PubMed] [Google Scholar]
- Lobstein T, Frelut ML. Prevalence of overweight among children in Europe. Obes Rev. 2003;4:195–200. doi: 10.1046/j.1467-789x.2003.00116.x. [DOI] [PubMed] [Google Scholar]
- Martin JF, Johnston CS, Han CT, Benyshek DC. Nutritional origins of insulin resistance: a rat model for diabetes-prone human populations. J Nutr. 2000;130:741–744. doi: 10.1093/jn/130.4.741. [DOI] [PubMed] [Google Scholar]
- Marx J. Unraveling the causes of diabetes. Science. 2002;296:686–689. doi: 10.1126/science.296.5568.686. [DOI] [PubMed] [Google Scholar]
- McCroskery S, Thomas M, Maxwell L, Sharma M, Kambadur R. Myostatin negatively regulates satellite cell activation and self-renewal. J Cell Biol. 2003;162:1135–1147. doi: 10.1083/jcb.200207056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minokoshi Y, Kahn CR, Kahn BB. Tissue-specific ablation of the GLUT4 glucose transporter or the insulin receptor challenges assumptions about insulin action and glucose homeostasis. J Biol Chem. 2003;278:33609–33612. doi: 10.1074/jbc.R300019200. [DOI] [PubMed] [Google Scholar]
- Moitra J, Mason MM, Olive M, Krylov D, Gavrilova O, Marcus-Samuels B, et al. Life without white fat: a transgenic mouse. Genes Dev. 1998;12:3168–3181. doi: 10.1101/gad.12.20.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossberg HO. 40-year follow-up of overweight children. Lancet. 1989;2:491–493. doi: 10.1016/s0140-6736(89)92098-9. [DOI] [PubMed] [Google Scholar]
- Must A, Jacques PF, Dallal GE, Bajema CJ, Dietz WH. Long-term morbidity and mortality of overweight adolescents. A follow-up of the Harvard Growth Study of 1922–35. N Engl J Med. 1992;327:1350–1355. doi: 10.1056/NEJM199211053271904. [DOI] [PubMed] [Google Scholar]
- Norris AW, Chen L, Fisher SJ, Szanto I, Ristow M, Jozsi AC, et al. Muscle-specific PPARgamma-deficient mice develop increased adiposity and insulin resistance but respond to thiazolidinediones. J Clin Invest. 2003;112:608–618. doi: 10.1172/JCI17305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden CL, Flegal KM, Carroll MD, Johnson CL. Prevalence and trends in overweight among US children and adolescents, 1999–2000. JAMA. 2002;288:1728–1732. doi: 10.1001/jama.288.14.1728. [DOI] [PubMed] [Google Scholar]
- Owino V, Yang SY, Goldspink G. Age-related loss of skeletal muscle function and the inability to express the autocrine form of insulin-like growth factor-1 (MGF) in response to mechanical overload. FEBS Lett. 2001;505:259–263. doi: 10.1016/s0014-5793(01)02825-3. [DOI] [PubMed] [Google Scholar]
- Prakash YS, Fournier M, Sieck GC. Effects of prenatal undernutrition on developing rat diaphragm. J Appl Physiol. 1993;75:1044–1052. doi: 10.1152/jappl.1993.75.3.1044. [DOI] [PubMed] [Google Scholar]
- Rayne J, Crawford GN. Increase in fibre numbers of the rat pterygoid muscles during postnatal growth. J Anat. 1975;119:347–357. [PMC free article] [PubMed] [Google Scholar]
- Rieusset J, Andreelli F, Auboeuf D, Roques M, Vallier P, Riou JP, et al. Insulin acutely regulates the expression of the peroxisome proliferator-activated receptor-gamma in human adipocytes. Diabetes. 1999;48:699–705. doi: 10.2337/diabetes.48.4.699. [DOI] [PubMed] [Google Scholar]
- Rothwell NJ, Stock MJ. A role for brown adipose tissue in diet-induced thermogenesis. Nature. 1979;281:31–35. doi: 10.1038/281031a0. [DOI] [PubMed] [Google Scholar]
- Roy RR, Powell PL, Kanim P, Simpson DR. Architectural and histochemical analysis of the semitendinosus muscle in mice, rats, guinea pigs, and rabbits. J Morph. 1984;181:155–160. doi: 10.1002/jmor.1051810204. [DOI] [PubMed] [Google Scholar]
- Vickers MH, Breier BH, McCarthy D, Gluckman PD. Sedentary behavior during postnatal life is determined by the prenatal environment and exacerbated by postnatal hypercaloric nutrition. Am J Physiol Regul Integr Comp Physiol. 2003;285:R271–R273. doi: 10.1152/ajpregu.00051.2003. [DOI] [PubMed] [Google Scholar]
- Vickers MH, Ikenasio BA, Breier BH. IGF-I treatment reduces hyperphagia, obesity, and hypertension in metabolic disorders induced by fetal programming. Endocrinology. 2001;142:3964–3973. doi: 10.1210/endo.142.9.8390. [DOI] [PubMed] [Google Scholar]
- Wilkison WO, Min HY, Claffey KP, Satterberg BL, Spiegelman BM. Control of the adipsin gene in adipocyte differentiation. Identification of distinct nuclear factors binding to single- and double-stranded DNA. J Biol Chem. 1990;265:477–482. [PubMed] [Google Scholar]