Abstract
The process of the sexual differentiation of the brain represents a valuable model system for the study of the chemical modification of the mammalian brain. Although there are numerous functional and structural sex differences in the adult brain, these are imposed on an essentially feminine or bipotential brain by testicular hormones during a critical phase of perinatal development in the rat. It is suggested that a relatively marked structural sex difference in the rat brain, the sexually dimorphic nucleus of the preoptic area (SDN-POA), is a morphological signature of the permanent or organizational action of estradiol derived from the aromatization of testicular testosterone. The SDN-POA of the male rat is severalfold larger in volume and is composed of more neurons than that of the female. The observation that the mitotic formation of the neurons of the SDN-POA is specifically prolonged has enabled us to identify the time course and pathway of neuronal migration into the nucleus. Study of the development of the SDN-POA suggests that estradiol in the male increases the number of neurons which survive a phase of neuronal death by exerting a neurite growth promoting action and/or a direct neuronotrophic action. It may not be possible to extrapolate this trophic effect of estradiol to all other structural sex differences since in the anteroventral periventricular nucleus, steroid exposure reduces the number of immunohistochemically defined dopaminergic neurons. Finally, although it is clear that gonadal hormones have dramatic permanent effects on the brain during perinatal development, even after puberty and in adulthood gonadal steroids can alter neuronal structure and, perhaps as a corollary to this, have permanent effects on reproductive function. For example, in the lightly androgenized rat which exhibits the delayed anovulation syndrome, exposure to estrogen prepubertally delays the onset of ovulatory failure, whereas estrogen exposure peri- or post-pubertally has an inhibitory effect on ovulation. Although the brain may be most sensitive to gonadal hormones or exogenous chemical factors during perinatal development, such sensitivity does not appear limited to this period.
Full text
PDF

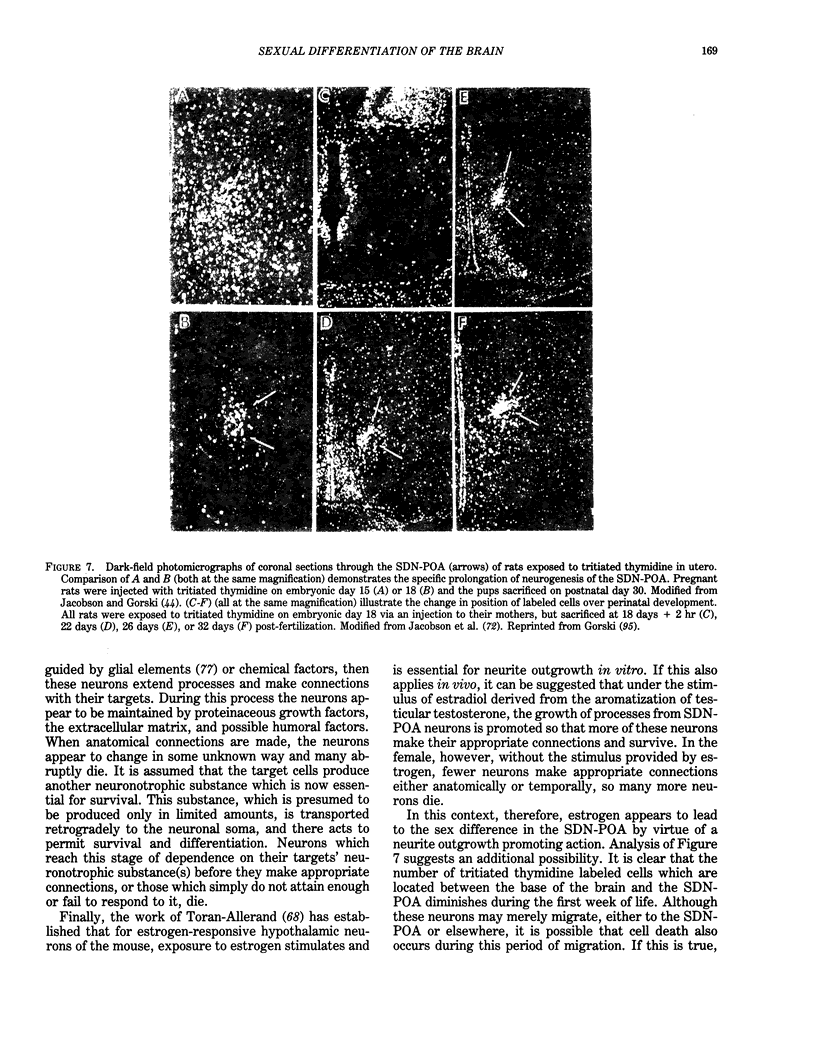
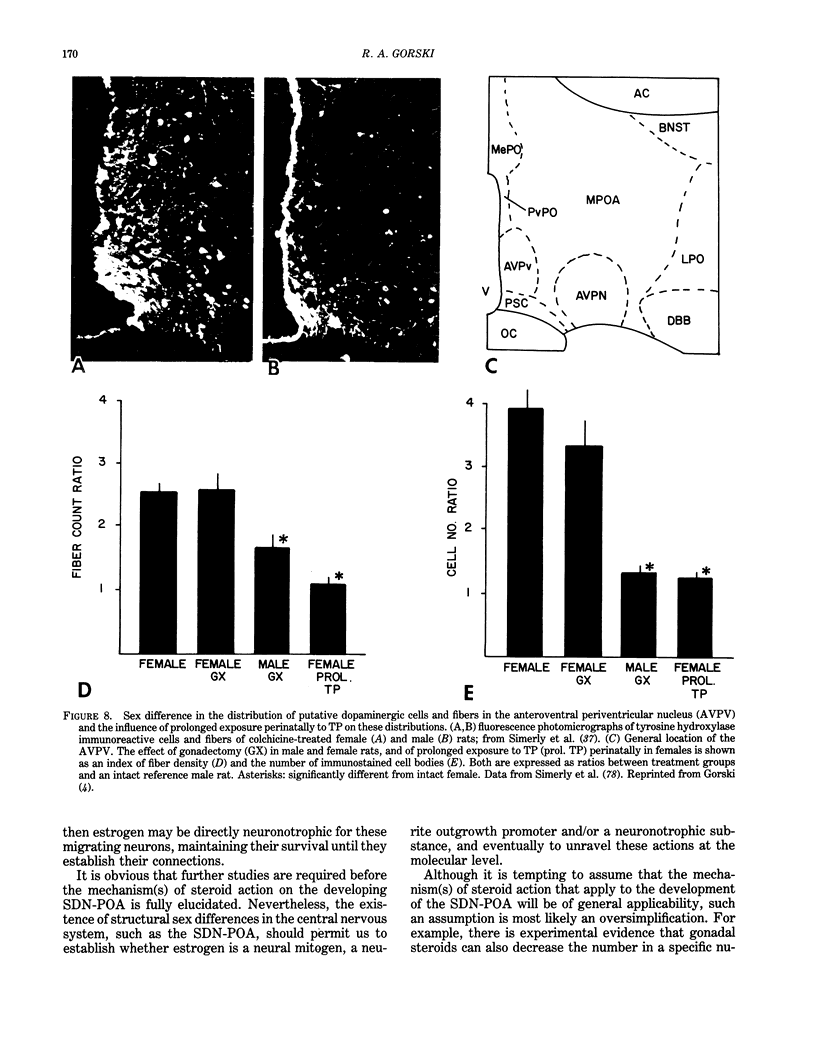
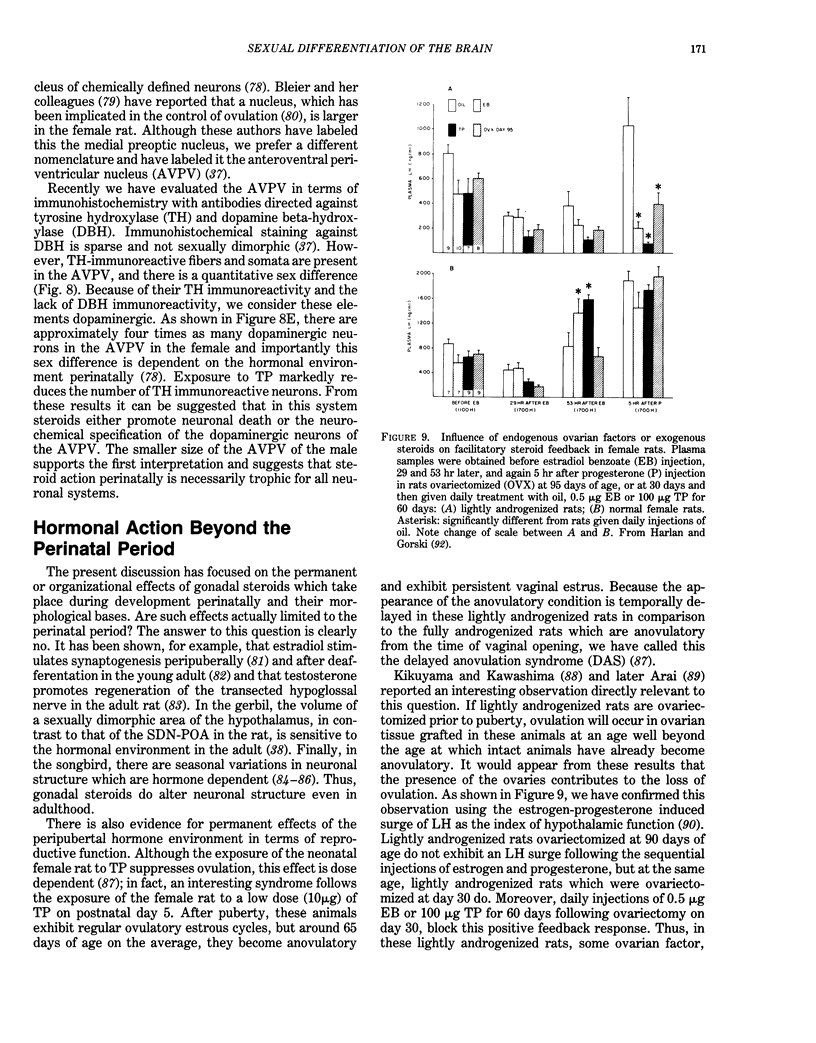




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman J., Bayer S. A. Development of the diencephalon in the rat. I. Autoradiographic study of the time of origin and settling patterns of neurons of the hypothalamus. J Comp Neurol. 1978 Dec 15;182(4 Pt 2):945–971. doi: 10.1002/cne.901820511. [DOI] [PubMed] [Google Scholar]
- Anderson C. H. Time of neuron origin in the anterior hypothalamus of the rat. Brain Res. 1978 Oct 6;154(1):119–122. doi: 10.1016/0006-8993(78)91056-9. [DOI] [PubMed] [Google Scholar]
- Arai Y. A possible process of the secondary sterilization: delayed anovulation syndrome. Experientia. 1971 Apr 15;27(4):463–464. doi: 10.1007/BF02137314. [DOI] [PubMed] [Google Scholar]
- Arimura A., Schally A. V. Augmentation of pituitary responsiveness to LH-releasing hormone (LH-RH) by estrogen. Proc Soc Exp Biol Med. 1971 Jan;136(1):290–293. doi: 10.3181/00379727-136-35249. [DOI] [PubMed] [Google Scholar]
- Attardi B., Geller L. N., Ohno S. Androgen and estrogen receptors in brain cytosol from male, female, and testicular feminized (tfm/y hermaphrodite) mice. Endocrinology. 1976 Apr;98(4):864–874. doi: 10.1210/endo-98-4-864. [DOI] [PubMed] [Google Scholar]
- Bardin C. W., Bullock L., Schneider G., Allison J. E., Stanley A. J. Pseudohermaphrodite rat: end organ insensitivity to testosterone. Science. 1970 Feb 20;167(3921):1136–1137. doi: 10.1126/science.167.3921.1136. [DOI] [PubMed] [Google Scholar]
- Becker J. B., Ramirez V. D. Sex differences in the amphetamine stimulated release of catecholamines from rat striatal tissue in vitro. Brain Res. 1981 Jan 12;204(2):361–372. doi: 10.1016/0006-8993(81)90595-3. [DOI] [PubMed] [Google Scholar]
- Bleier R., Byne W., Siggelkow I. Cytoarchitectonic sexual dimorphisms of the medial preoptic and anterior hypothalamic areas in guinea pig, rat, hamster, and mouse. J Comp Neurol. 1982 Dec 1;212(2):118–130. doi: 10.1002/cne.902120203. [DOI] [PubMed] [Google Scholar]
- Booth J. E. Sexual behaviour of male rats injected with the anti-oestrogen MER-25 during infancy. Physiol Behav. 1977 Jul;19(1):35–39. doi: 10.1016/0031-9384(77)90155-x. [DOI] [PubMed] [Google Scholar]
- Christensen L. W., Gorski R. A. Independent masculinization of neuroendocrine systems by intracerebral implants of testosterone or estradiol in the neonatal female rat. Brain Res. 1978 May 12;146(2):325–340. doi: 10.1016/0006-8993(78)90977-0. [DOI] [PubMed] [Google Scholar]
- Commins D., Yahr P. Adult testosterone levels influence the morphology of a sexually dimorphic area in the Mongolian gerbil brain. J Comp Neurol. 1984 Mar 20;224(1):132–140. doi: 10.1002/cne.902240112. [DOI] [PubMed] [Google Scholar]
- DeVoogd T., Nottebohm F. Gonadal hormones induce dendritic growth in the adult avian brain. Science. 1981 Oct 9;214(4517):202–204. doi: 10.1126/science.7280692. [DOI] [PubMed] [Google Scholar]
- Doughty C., McDonald P. G. Hormonal control of sexual differentiation of the hypothalamus in the neonatal female rat. Differentiation. 1974 Oct;2(5):275–285. doi: 10.1111/j.1432-0436.1974.tb00362.x. [DOI] [PubMed] [Google Scholar]
- Döhler K. D., Coquelin A., Davis F., Hines M., Shryne J. E., Gorski R. A. Differentiation of the sexually dimorphic nucleus in the preoptic area of the rat brain is determined by the perinatal hormone environment. Neurosci Lett. 1982 Dec 13;33(3):295–298. doi: 10.1016/0304-3940(82)90388-3. [DOI] [PubMed] [Google Scholar]
- Döhler K. D., Srivastava S. S., Shryne J. E., Jarzab B., Sipos A., Gorski R. A. Differentiation of the sexually dimorphic nucleus in the preoptic area of the rat brain is inhibited by postnatal treatment with an estrogen antagonist. Neuroendocrinology. 1984 Apr;38(4):297–301. doi: 10.1159/000123907. [DOI] [PubMed] [Google Scholar]
- Fink G., Jamieson M. G. Immunoreactive luteinizing hormone releasing factor in rat pituitary stalk blood: effects of electrical stimulation of the medial preoptic area. J Endocrinol. 1976 Jan;68(1):71–87. doi: 10.1677/joe.0.0680071. [DOI] [PubMed] [Google Scholar]
- Gorski R. A., Gordon J. H., Shryne J. E., Southam A. M. Evidence for a morphological sex difference within the medial preoptic area of the rat brain. Brain Res. 1978 Jun 16;148(2):333–346. doi: 10.1016/0006-8993(78)90723-0. [DOI] [PubMed] [Google Scholar]
- Gorski R. A., Harlan R. E., Jacobson C. D., Shryne J. E., Southam A. M. Evidence for the existence of a sexually dimorphic nucleus in the preoptic area of the rat. J Comp Neurol. 1980 Sep 15;193(2):529–539. doi: 10.1002/cne.901930214. [DOI] [PubMed] [Google Scholar]
- Gorski R. A. Influence of age on the response to paranatal administration of a low dose of androgen. Endocrinology. 1968 May;82(5):1001–1004. doi: 10.1210/endo-82-5-1001. [DOI] [PubMed] [Google Scholar]
- Gorski R. A. Sexual dimorphisms of the brain. J Anim Sci. 1985;61 (Suppl 3):38–61. doi: 10.1093/ansci/61.supplement_3.38. [DOI] [PubMed] [Google Scholar]
- Gorski R. A. The 13th J. A. F. Stevenson memorial lecture. Sexual differentiation of the brain: possible mechanisms and implications. Can J Physiol Pharmacol. 1985 Jun;63(6):577–594. doi: 10.1139/y85-098. [DOI] [PubMed] [Google Scholar]
- Greenough W. T., Carter C. S., Steerman C., DeVoogd T. J. Sex differences in dentritic patterns in hamster preoptic area. Brain Res. 1977 Apr 22;126(1):63–72. doi: 10.1016/0006-8993(77)90215-3. [DOI] [PubMed] [Google Scholar]
- Güldner F. H. Sexual dimorphisms of axo-spine synapses and postsynaptic density material in the suprachiasmatic nucleus of the rat. Neurosci Lett. 1982 Feb 12;28(2):145–150. doi: 10.1016/0304-3940(82)90143-4. [DOI] [PubMed] [Google Scholar]
- Handa R. J., Gorski R. A. Alterations in the onset of ovulatory failure and gonadotropin secretion following steroid administration to lightly androgenized female rats. Biol Reprod. 1985 Mar;32(2):248–256. doi: 10.1095/biolreprod32.2.248. [DOI] [PubMed] [Google Scholar]
- Harlan R. E., Gorski R. A. Effects of postpubertal ovarian steroids on reproductive function and sexual differentiation of lightly androgenized rats. Endocrinology. 1978 Jun;102(6):1716–1724. doi: 10.1210/endo-102-6-1716. [DOI] [PubMed] [Google Scholar]
- Hines M., Davis F. C., Coquelin A., Goy R. W., Gorski R. A. Sexually dimorphic regions in the medial preoptic area and the bed nucleus of the stria terminalis of the guinea pig brain: a description and an investigation of their relationship to gonadal steroids in adulthood. J Neurosci. 1985 Jan;5(1):40–47. doi: 10.1523/JNEUROSCI.05-01-00040.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ifft J. D. An autoradiographic study of the time of final division of neurons in rat hypothalamic nuclei. J Comp Neurol. 1972 Feb;144(2):193–204. doi: 10.1002/cne.901440204. [DOI] [PubMed] [Google Scholar]
- Jacobson C. D., Csernus V. J., Shryne J. E., Gorski R. A. The influence of gonadectomy, androgen exposure, or a gonadal graft in the neonatal rat on the volume of the sexually dimorphic nucleus of the preoptic area. J Neurosci. 1981 Oct;1(10):1142–1147. doi: 10.1523/JNEUROSCI.01-10-01142.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson C. D., Davis F. C., Gorski R. A. Formation of the sexually dimorphic nucleus of the preoptic area: neuronal growth, migration and changes in cell number. Brain Res. 1985 Jul;353(1):7–18. doi: 10.1016/0165-3806(85)90019-7. [DOI] [PubMed] [Google Scholar]
- Jacobson C. D., Gorski R. A. Neurogenesis of the sexually dimorphic nucleus of the preoptic area in the rat. J Comp Neurol. 1981 Mar 1;196(3):519–529. doi: 10.1002/cne.901960313. [DOI] [PubMed] [Google Scholar]
- Jacobson C. D., Shryne J. E., Shapiro F., Gorski R. A. Ontogeny of the sexually dimorphic nucleus of the preoptic area. J Comp Neurol. 1980 Sep 15;193(2):541–548. doi: 10.1002/cne.901930215. [DOI] [PubMed] [Google Scholar]
- Kalra S. P., Kalra P. S. Neural regulation of luteinizing hormone secretion in the rat. Endocr Rev. 1983 Fall;4(4):311–351. doi: 10.1210/edrv-4-4-311. [DOI] [PubMed] [Google Scholar]
- Knobil E. The neuroendocrine control of the menstrual cycle. Recent Prog Horm Res. 1980;36:53–88. doi: 10.1016/b978-0-12-571136-4.50008-5. [DOI] [PubMed] [Google Scholar]
- Korenbrot C. C., Paup D. C., Gorski R. A. Effects of testosterone propionate or dihydrotestosterone propionate on plasma FSH and LH levels in neonatal rats and on sexual differentiation of the brain. Endocrinology. 1975 Sep;97(3):709–717. doi: 10.1210/endo-97-3-709. [DOI] [PubMed] [Google Scholar]
- Ladosky W., Gaziri L. C. Brain serotonin and sexual differentiation of the nervous system. Neuroendocrinology. 1970;6(3):168–174. doi: 10.1159/000121920. [DOI] [PubMed] [Google Scholar]
- Martin J. E., Tyrey L., Everett J. W., Fellows R. E. Variation in responsiveness to synthetic LH-releasing factor (LRF) in proestrous and diestrous-3 rats. Endocrinology. 1974 Feb;94(2):556–562. doi: 10.1210/endo-94-2-556. [DOI] [PubMed] [Google Scholar]
- Matsumoto A., Arai Y. Sex difference in volume of the ventromedial nucleus of the hypothalamus in the rat. Endocrinol Jpn. 1983 Jun;30(3):277–280. doi: 10.1507/endocrj1954.30.277. [DOI] [PubMed] [Google Scholar]
- McEwen B. S., Lieberburg I., Chaptal C., Krey L. C. Aromatization: important for sexual differentiation of the neonatal rat brain. Horm Behav. 1977 Dec;9(3):249–263. doi: 10.1016/0018-506x(77)90060-5. [DOI] [PubMed] [Google Scholar]
- McEwen B. S., Lieberburg I., Maclusky N., Plapinger L. Do estrogen receptors play a role in the sexual differentiation of the rat brain? J Steroid Biochem. 1977 May;8(5):593–598. doi: 10.1016/0022-4731(77)90267-9. [DOI] [PubMed] [Google Scholar]
- Naess O., Haug E., Attramadal A., Aakvaag A., Hansson V., French F. Androgen receptors in the anterior pituitary and central nervous system of the androgen "insensitive" (Tfm) rat: correlation between receptor binding and effects of androgens on gonadotropin secretion. Endocrinology. 1976 Nov;99(5):1295–1303. doi: 10.1210/endo-99-5-1295. [DOI] [PubMed] [Google Scholar]
- Naftolin F., Ryan K. J., Davies I. J., Reddy V. V., Flores F., Petro Z., Kuhn M., White R. J., Takaoka Y., Wolin L. The formation of estrogens by central neuroendocrine tissues. Recent Prog Horm Res. 1975;31:295–319. doi: 10.1016/b978-0-12-571131-9.50012-8. [DOI] [PubMed] [Google Scholar]
- Nishizuka M., Arai Y. Organizational action of estrogen on synaptic pattern in the amygdala: implications for sexual differentiation of the brain. Brain Res. 1981 Jun 1;213(2):422–426. doi: 10.1016/0006-8993(81)90247-x. [DOI] [PubMed] [Google Scholar]
- Nordeen E. J., Nordeen K. W., Sengelaub D. R., Arnold A. P. Androgens prevent normally occurring cell death in a sexually dimorphic spinal nucleus. Science. 1985 Aug 16;229(4714):671–673. doi: 10.1126/science.4023706. [DOI] [PubMed] [Google Scholar]
- Nottebohm F. A brain for all seasons: cyclical anatomical changes in song control nuclei of the canary brain. Science. 1981 Dec 18;214(4527):1368–1370. doi: 10.1126/science.7313697. [DOI] [PubMed] [Google Scholar]
- Nottebohm F., Arnold A. P. Sexual dimorphism in vocal control areas of the songbird brain. Science. 1976 Oct 8;194(4261):211–213. doi: 10.1126/science.959852. [DOI] [PubMed] [Google Scholar]
- Nunez E., Savu L., Engelmann F., Benassayag C., Crépy O., Jayle M. F. Origine embryonnaire de la protéine sérique fixant l'oestrone et l'oestradiol chez la ratte impubère. C R Acad Sci Hebd Seances Acad Sci D. 1971 Jul 12;273(2):242–245. [PubMed] [Google Scholar]
- Ojeda S. R., Kalra P. S., McCann S. M. Further studies on the maturation of the estrogen negative feedback on gonadotropin release in the female rat. Neuroendocrinology. 1975;18(3):242–255. doi: 10.1159/000122403. [DOI] [PubMed] [Google Scholar]
- Olsen K. L., Whalen R. E. Sexual differentiation of the brain: effects on mating behavior and [3H]-estradiol binding by hypothalamic chromatin in rats. Biol Reprod. 1980 Jun;22(5):1068–1072. doi: 10.1095/biolreprod22.5.1068. [DOI] [PubMed] [Google Scholar]
- PHOENIX C. H., GOY R. W., GERALL A. A., YOUNG W. C. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology. 1959 Sep;65:369–382. doi: 10.1210/endo-65-3-369. [DOI] [PubMed] [Google Scholar]
- Plapinger L., McEwen B. S., Clemens L. E. Ontogeny of estradiol-binding sites in rat brain. II. Characteristics of a neonatal binding macromolecule. Endocrinology. 1973 Nov;93(5):1129–1139. doi: 10.1210/endo-93-5-1129. [DOI] [PubMed] [Google Scholar]
- Porter J. C., Eskay R. L., Oliver C., Ben-Jonathan N., Warberg J., Parker R., Jr, Barnea A. Release of hypothalamic hormones under in vivo and in vitro conditions. Adv Exp Med Biol. 1977;87:181–201. doi: 10.1007/978-1-4615-8849-8_9. [DOI] [PubMed] [Google Scholar]
- RAMIREZ V. D., SAWYER C. H. ADVANCEMENT OF PUBERTY IN THE FEMALE RAT BY ESTROGEN. Endocrinology. 1965 Jun;76:1158–1168. doi: 10.1210/endo-76-6-1158. [DOI] [PubMed] [Google Scholar]
- Rainbow T. C., Parsons B., McEwen B. S. Sex differences in rat brain oestrogen and progestin receptors. Nature. 1982 Dec 16;300(5893):648–649. doi: 10.1038/300648a0. [DOI] [PubMed] [Google Scholar]
- Raisman G., Field P. M. Sexual dimorphism in the neuropil of the preoptic area of the rat and its dependence on neonatal androgen. Brain Res. 1973 May 17;54:1–29. doi: 10.1016/0006-8993(73)90030-9. [DOI] [PubMed] [Google Scholar]
- Raynaud J. P., Mercier-Bodard C., Baulieu E. E. Rat estradiol binding plasma protein (EBP). Steroids. 1971 Dec;18(6):767–788. doi: 10.1016/0039-128x(71)90035-3. [DOI] [PubMed] [Google Scholar]
- Schmechel D. E., Rakic P. Arrested proliferation of radial glial cells during midgestation in rhesus monkey. Nature. 1979 Jan 25;277(5694):303–305. doi: 10.1038/277303a0. [DOI] [PubMed] [Google Scholar]
- Simerly R. B., Swanson L. W., Gorski R. A. Demonstration of a sexual dimorphism in the distribution of serotonin-immunoreactive fibers in the medial preoptic nucleus of the rat. J Comp Neurol. 1984 May 10;225(2):151–166. doi: 10.1002/cne.902250202. [DOI] [PubMed] [Google Scholar]
- Simerly R. B., Swanson L. W., Gorski R. A. The distribution of monoaminergic cells and fibers in a periventricular preoptic nucleus involved in the control of gonadotropin release: immunohistochemical evidence for a dopaminergic sexual dimorphism. Brain Res. 1985 Mar 18;330(1):55–64. doi: 10.1016/0006-8993(85)90007-1. [DOI] [PubMed] [Google Scholar]
- Simerly R. B., Swanson L. W., Handa R. J., Gorski R. A. Influence of perinatal androgen on the sexually dimorphic distribution of tyrosine hydroxylase-immunoreactive cells and fibers in the anteroventral periventricular nucleus of the rat. Neuroendocrinology. 1985 Jun;40(6):501–510. doi: 10.1159/000124122. [DOI] [PubMed] [Google Scholar]
- Swaab D. F., Fliers E. A sexually dimorphic nucleus in the human brain. Science. 1985 May 31;228(4703):1112–1115. doi: 10.1126/science.3992248. [DOI] [PubMed] [Google Scholar]
- Toran-Allerand C. D. On the genesis of sexual differentiation of the general nervous system: morphogenetic consequences of steroidal exposure and possible role of alpha-fetoprotein. Prog Brain Res. 1984;61:63–98. doi: 10.1016/s0079-6123(08)64429-5. [DOI] [PubMed] [Google Scholar]
- Weisz J., Ward I. L. Plasma testosterone and progesterone titers of pregnant rats, their male and female fetuses, and neonatal offspring. Endocrinology. 1980 Jan;106(1):306–316. doi: 10.1210/endo-106-1-306. [DOI] [PubMed] [Google Scholar]
- Whalen R. E., Rezek D. L. Inhibition of lordosis in female rats by subcutaneous implants of testosterone, androstenedione or dihydrotestosterone in infancy. Horm Behav. 1974 Jun;5(2):125–128. doi: 10.1016/0018-506x(74)90035-x. [DOI] [PubMed] [Google Scholar]
- Wiegand S. J., Terasawa E. Discrete lesions reveal functional heterogeneity of suprachiasmatic structures in regulation of gonadotropin secretion in the female rat. Neuroendocrinology. 1982 Jun;34(6):395–404. doi: 10.1159/000123335. [DOI] [PubMed] [Google Scholar]
- Wilson W. E., Agrawal A. K. Brain regional levels of neurotransmitter amines as neurochemical correlates of sex-specific ontogenesis in the rat. Dev Neurosci. 1979;2(4):195–200. doi: 10.1159/000112454. [DOI] [PubMed] [Google Scholar]
- Yu W. H., Yu M. C. Acceleration of the regeneration of the crushed hypoglossal nerve by testosterone. Exp Neurol. 1983 May;80(2):349–360. doi: 10.1016/0014-4886(83)90288-1. [DOI] [PubMed] [Google Scholar]




