Abstract
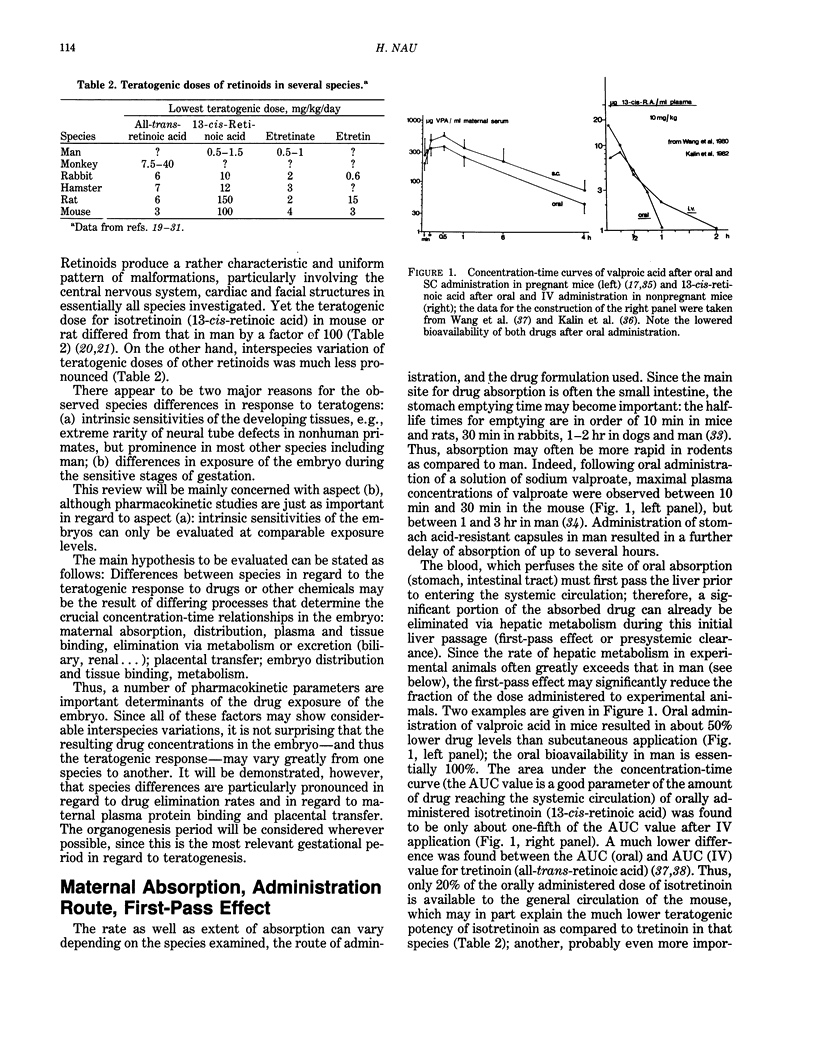
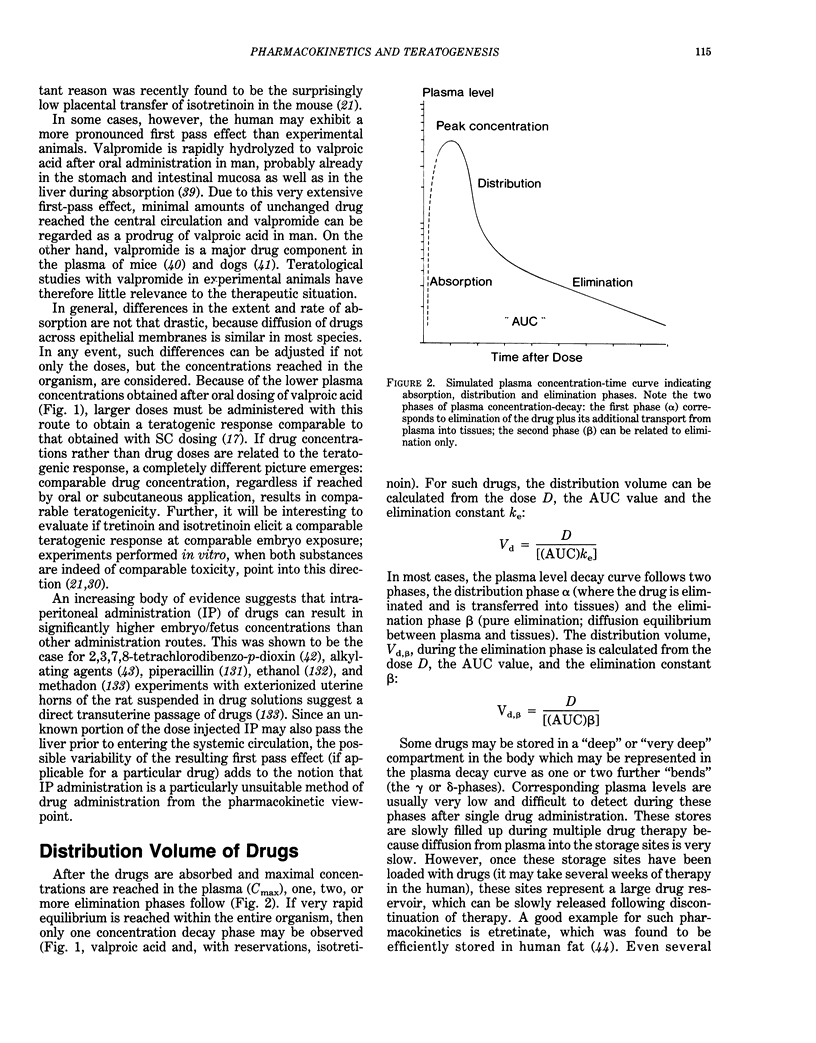
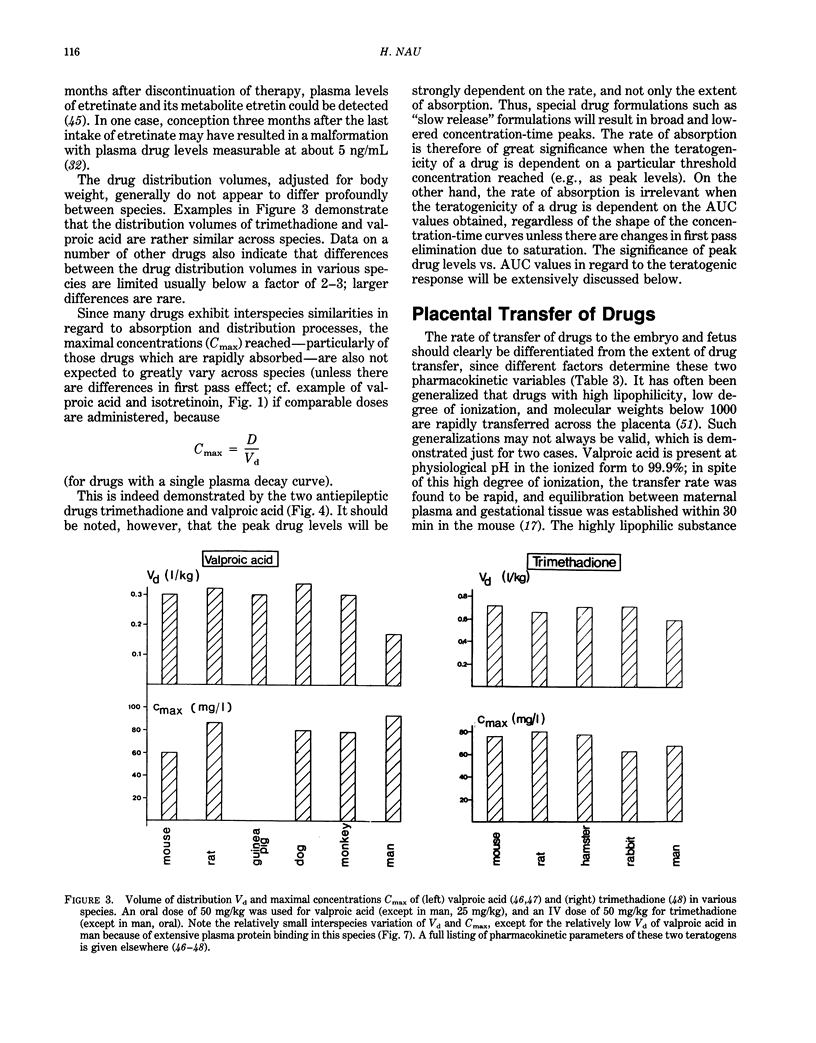
Interspecies differences in regard to the teratogenicity of drugs can be the result of differing pharmacokinetic processes that determine the crucial concentration-time relationships in the embryo. Maternal absorption, as well as distribution, of the drugs does not usually show great species differences. The first-pass effect after oral application is often more pronounced in animals than man (e.g., valproic acid, 13-cis-retinoic acid), although in some cases the reverse was found (e.g., hydrolysis of valpromide). Existing differences can be adjusted by appropriate choice of the administration route and measurements of drug levels. Many variables determine the placental transfer of drugs: developmental stage, type of placenta, properties of the drug. Even closely related drugs (e.g., retinoids) may differ greatly in regard to placental transfer. Maternal protein binding is an important determinant of placental transfer, since only the free concentration in maternal plasma can equilibrate with the embryo during organogenesis; this parameter differs greatly across species (e.g., valproic acid: five times higher free fractions in mouse and hamster than in monkey and man). The metabolic pattern has not yet been demonstrated to be a major cause of species differences, although recent evidence on phenytoin and thalidomide support the hypothesis that some species differences can be the result of differing activation/deactivation pathways. Laboratory animals usually have a much higher rate of drug elimination than man. Drastic drug level fluctuations are therefore present during teratogenicity testing in animals, but not to the same degree in human therapy. It must, therefore, be investigated if peak concentrations (such as for valproic acid and possibly caffeine) or the area under the concentration-time curve (AUC) (such as for cyclophosphamide and possibly retinoids) correlate with the teratogenic response. Only then is a rational and scientific basis for interspecies comparison possible. It is concluded that the prediction of the human response based on animal studies can be improved by consideration of the appropriate pharmacokinetic determinants.
Full text
PDF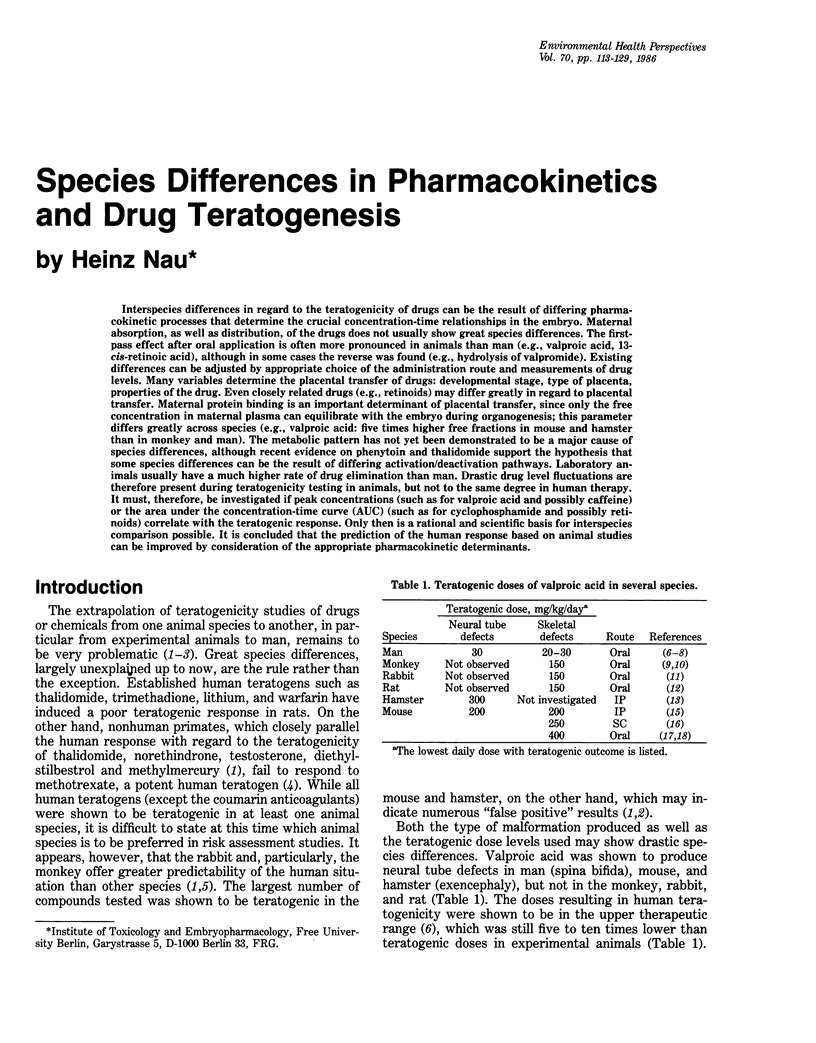
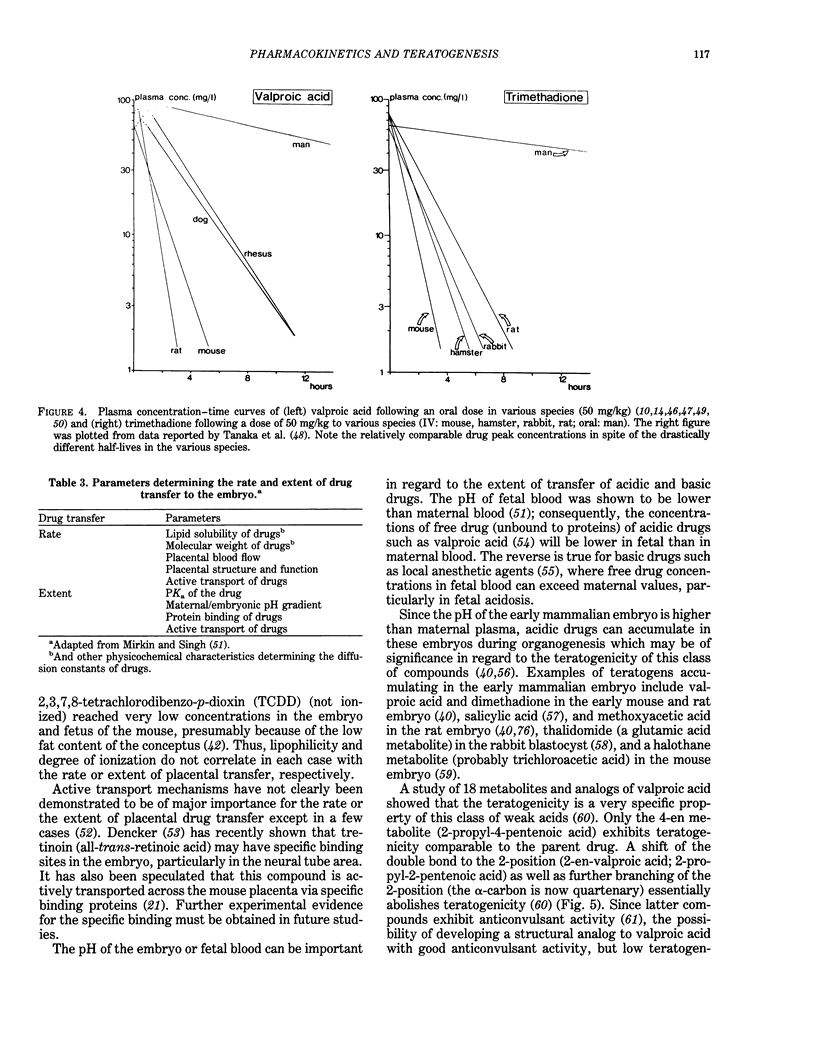
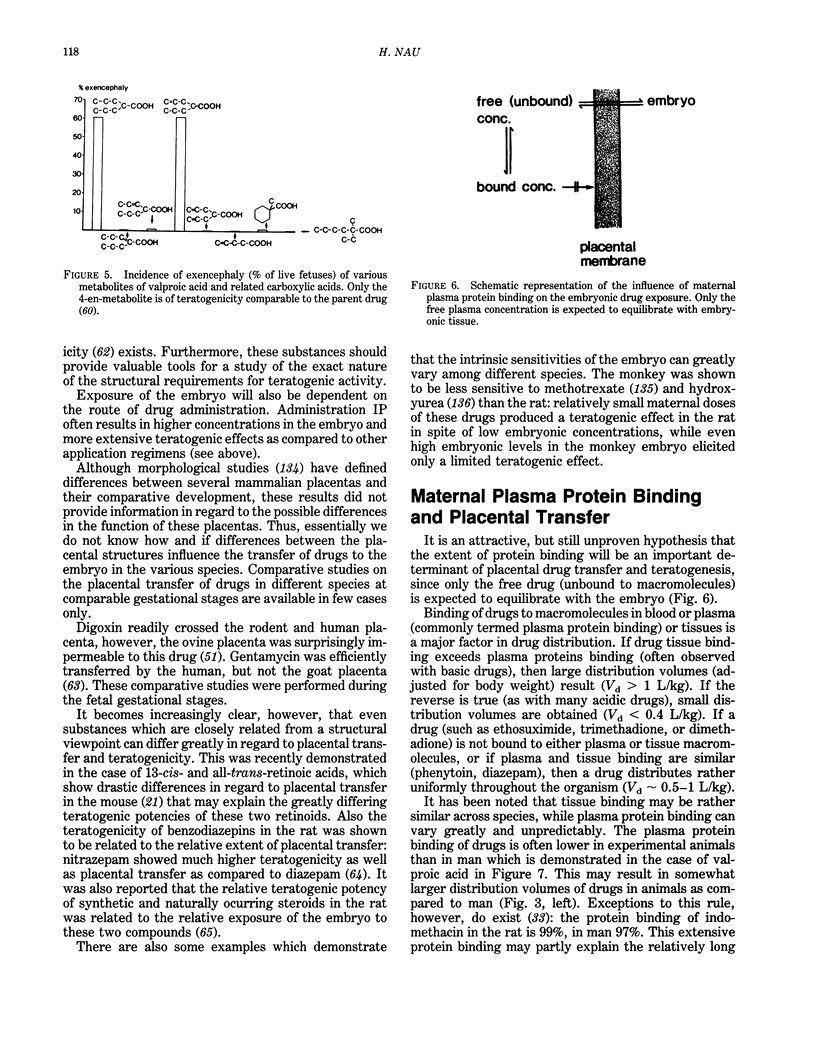
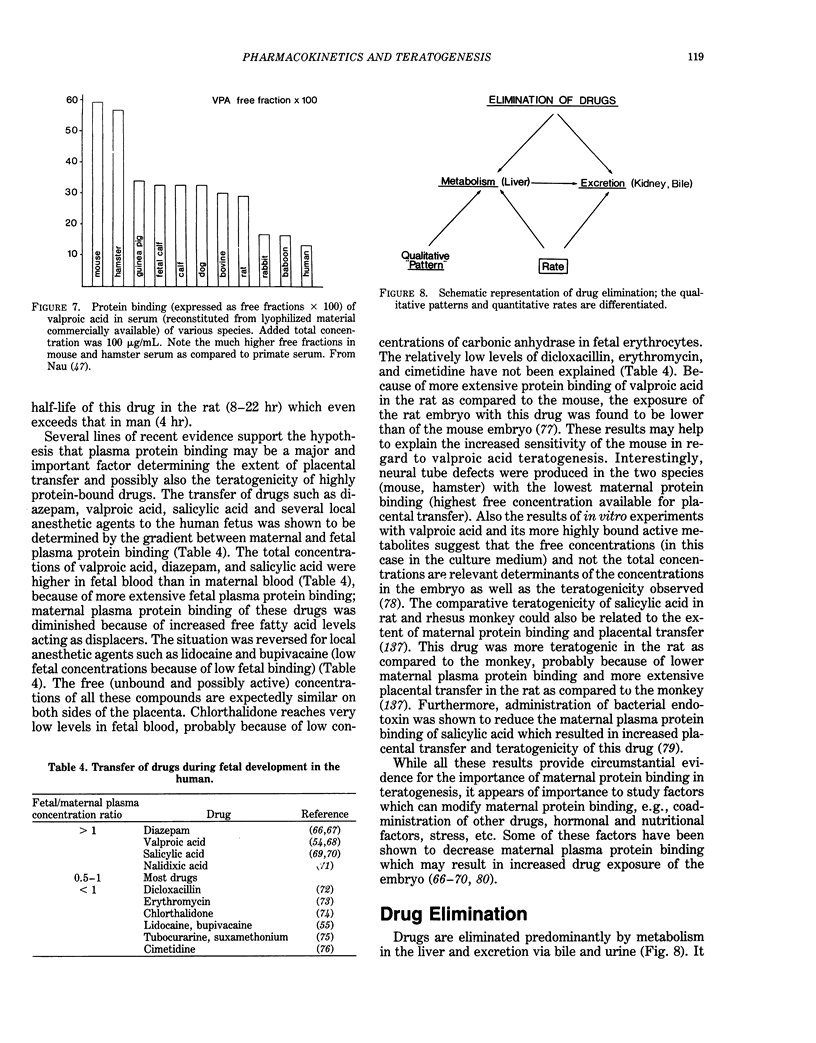


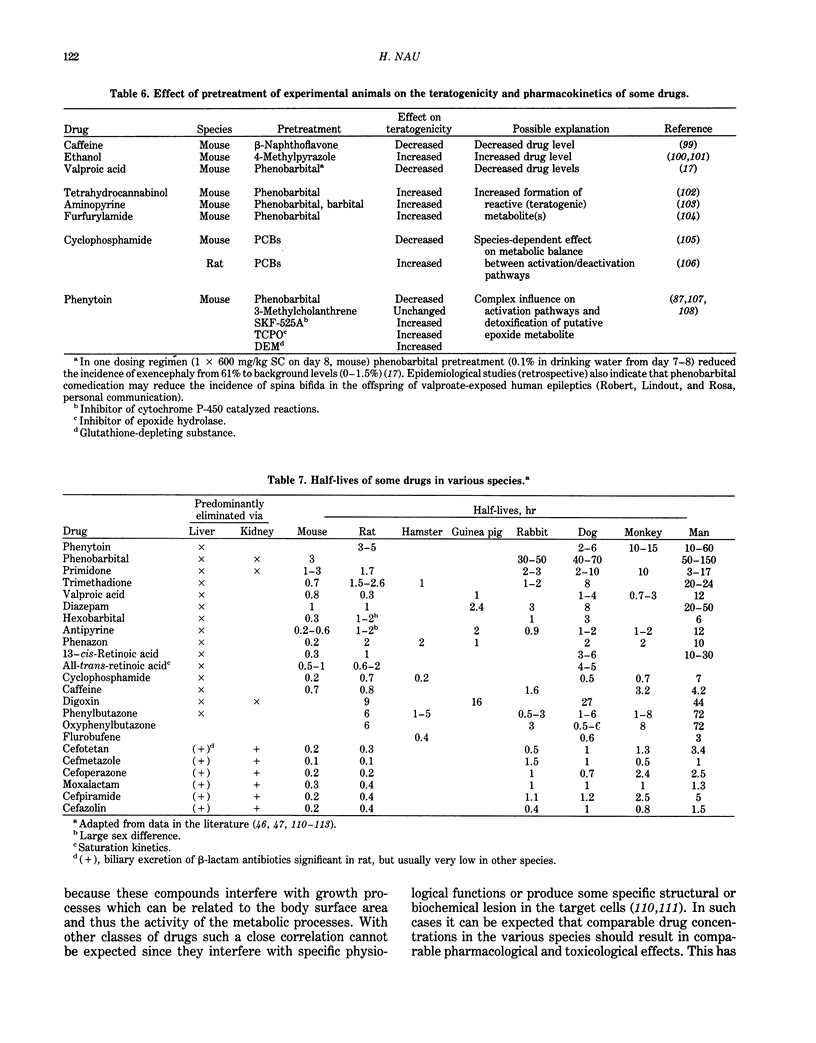





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atlas S. A., Zweier J. L., Nebert D. W. Genetic differences in phenytoin pharmacokinetics. In vivo clearance and in vitro metabolism among inbred strains of mice. Dev Pharmacol Ther. 1980;1(5):281–304. [PubMed] [Google Scholar]
- Batra V., Morrison J., Kaleita E. Comparative pharmacokinetics of 14C-piperacillin following intravenous and intraperitoneal administration in pregnant and non-pregnant rats. Eur J Drug Metab Pharmacokinet. 1984 Jan-Mar;9(1):31–39. doi: 10.1007/BF03189603. [DOI] [PubMed] [Google Scholar]
- Beck F. Comparative placental morphology and function. Environ Health Perspect. 1976 Dec;18:5–12. doi: 10.1289/ehp.76185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialer M., Hussein Z., Dubrovsky J., Raz I., Abramsky O. Pharmacokinetics of valproic acid obtained after administration of three oral formulations to humans. Isr J Med Sci. 1984 Jan;20(1):46–49. [PubMed] [Google Scholar]
- Bialer M., Rubinstein A. A comparative study on the pharmacokinetics of valpramide after intravenous administration in dogs. J Pharm Pharmacol. 1983 Sep;35(9):607–609. doi: 10.1111/j.2042-7158.1983.tb04347.x. [DOI] [PubMed] [Google Scholar]
- Blakley P. M., Scott W. J., Jr Determination of the proximate teratogen of the mouse fetal alcohol syndrome. 1. Teratogenicity of ethanol and acetaldehyde. Toxicol Appl Pharmacol. 1984 Feb;72(2):355–363. doi: 10.1016/0041-008x(84)90320-x. [DOI] [PubMed] [Google Scholar]
- Blakley P. M., Scott W. J., Jr Determination of the proximate teratogen of the mouse fetal alcohol syndrome. 2. Pharmacokinetics of the placental transfer of ethanol and acetaldehyde. Toxicol Appl Pharmacol. 1984 Feb;72(2):364–371. doi: 10.1016/0041-008x(84)90321-1. [DOI] [PubMed] [Google Scholar]
- Bonati M., Latini R., Tognoni G., Young J. F., Garattini S. Interspecies comparison of in vivo caffeine pharmacokinetics in man, monkey, rabbit, rat, and mouse. Drug Metab Rev. 1984;15(7):1355–1383. doi: 10.3109/03602538409029964. [DOI] [PubMed] [Google Scholar]
- Brodie B. B., Reid W. D. Some pharmacological consequences of species variation in rates of metabolism. Fed Proc. 1967 Jul-Aug;26(4):1062–1070. [PubMed] [Google Scholar]
- Brown N. A., Fabro S. The value of animal teratogenicity testing for predicting human risk. Clin Obstet Gynecol. 1983 Jun;26(2):467–477. doi: 10.1097/00003081-198306000-00028. [DOI] [PubMed] [Google Scholar]
- Caldwell J. The current status of attempts to predict species differences in drug metabolism. Drug Metab Rev. 1981;12(2):221–237. doi: 10.3109/03602538108994030. [DOI] [PubMed] [Google Scholar]
- Clark B., Smith D. A. Pharmacokinetics and toxicity testing. Crit Rev Toxicol. 1984;12(4):343–385. doi: 10.3109/10408448409044214. [DOI] [PubMed] [Google Scholar]
- Clarke D. W., Steenaart N. A., Breedon T. H., Brien J. F. Differential pharmacokinetics for oral and intraperitoneal administration of ethanol to the pregnant guinea pig. Can J Physiol Pharmacol. 1985 Feb;63(2):169–172. doi: 10.1139/y85-033. [DOI] [PubMed] [Google Scholar]
- Cress C. R., Peters M. A. Transuterine passage of drugs in the rat. Proc West Pharmacol Soc. 1984;27:81–84. [PubMed] [Google Scholar]
- Danielsson B. R., Ghantous H., Dencker L. Accumulation in murine amniotic fluid of halothane and its metabolites. Acta Pharmacol Toxicol (Copenh) 1984 Nov;55(5):410–417. doi: 10.1111/j.1600-0773.1984.tb02003.x. [DOI] [PubMed] [Google Scholar]
- Dayton P. G., Sanders J. E. Dose-dependent pharmacokinetics: emphasis on phase I metabolism. Drug Metab Rev. 1983;14(3):347–405. doi: 10.3109/03602538308991394. [DOI] [PubMed] [Google Scholar]
- Depp R., Kind A. C., Kirby W. M., Johnson W. L. Transplacental passage of methicillin and dicloxacillin into the fetus and amniotic fluid. Am J Obstet Gynecol. 1970 Aug 1;107(7):1054–1057. doi: 10.1016/0002-9378(70)90628-9. [DOI] [PubMed] [Google Scholar]
- Doherty P. A., Ferm V. H., Smith R. P. Congenital malformations induced by infusion of sodium cyanide in the golden hamster. Toxicol Appl Pharmacol. 1982 Jul;64(3):456–464. doi: 10.1016/0041-008x(82)90242-3. [DOI] [PubMed] [Google Scholar]
- Eluma F. O., Sucheston M. E., Hayes T. G., Paulson R. B. Teratogenic effects of dosage levels and time of administration of carbamazepine, sodium valproate, and diphenylhydantoin on craniofacial development in the CD-1 mouse fetus. J Craniofac Genet Dev Biol. 1984;4(3):191–210. [PubMed] [Google Scholar]
- Fantel A. G., Shepard T. H., Newell-Morris L. L., Moffett B. C. Teratogenic effects of retinoic acid in pigtail monkeys (Macaca nemestrina). I. General features. Teratology. 1977 Feb;15(1):65–71. doi: 10.1002/tera.1420150109. [DOI] [PubMed] [Google Scholar]
- Ferm V. H., Hanlon D. P. Constant rate exposure of pregnant hamsters to arsenate during early gestation. Environ Res. 1985 Aug;37(2):425–432. doi: 10.1016/0013-9351(85)90124-0. [DOI] [PubMed] [Google Scholar]
- Gabrielsson J., Paalzow L., Larsson S., Blomquist I. Constant rate of infusion--improvement of tests for teratogenicity and embryotoxicity. Life Sci. 1985 Dec 16;37(24):2275–2282. doi: 10.1016/0024-3205(85)90018-9. [DOI] [PubMed] [Google Scholar]
- Gordon G. B., Spielberg S. P., Blake D. A., Balasubramanian V. Thalidomide teratogenesis: evidence for a toxic arene oxide metabolite. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2545–2548. doi: 10.1073/pnas.78.4.2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales B. F. Modification of the mutagenicity and teratogenicity of cyclophosphamide in rats with inducers of the cytochromes P-450. Teratology. 1981 Aug;24(1):1–11. doi: 10.1002/tera.1420240102. [DOI] [PubMed] [Google Scholar]
- Hansen D. K., Hodes M. E. Metabolism of phenytoin in teratogenesis-susceptible and -resistant strains of mice. Drug Metab Dispos. 1983 Jan-Feb;11(1):21–24. [PubMed] [Google Scholar]
- Happle R., Traupe H., Bounameaux Y., Fisch T. Teratogene Wirkung von Etretinat beim Menschen. Dtsch Med Wochenschr. 1984 Sep 28;109(39):1476–1480. doi: 10.1055/s-2008-1069397. [DOI] [PubMed] [Google Scholar]
- Harbison R. D., Mantilla-Plata B., Lubin D. J. Alteration of delta 9-tetrahydrocannabinol-induced teratogenicity by stimulation and inhibition of its metabolism. J Pharmacol Exp Ther. 1977 Aug;202(2):455–465. [PubMed] [Google Scholar]
- Heinemeyer G., Nau H., Hildebrandt A. G., Roots I. Oxidation and glucuronidation of valproic acid in male rats--influence of phenobarbital, 3-methylcholanthrene, beta-naphthoflavone and clofibrate. Biochem Pharmacol. 1985 Jan 1;34(1):133–139. doi: 10.1016/0006-2952(85)90111-x. [DOI] [PubMed] [Google Scholar]
- Hendrickx A. G., Silverman S., Pellegrini M., Steffek A. J. Teratological and radiocephalometric analysis of craniofacial malformations induced with retinoic acid in rhesus monkeys (Macaca mulatta). Teratology. 1980 Aug;22(1):13–22. doi: 10.1002/tera.1420220104. [DOI] [PubMed] [Google Scholar]
- Henry E. C., Miller R. K. Comparison of the disposition of diethylstilbestrol and estradiol in the fetal rat. Correlation with teratogenic potency. Biochem Pharmacol. 1986 Jun 15;35(12):1993–2001. doi: 10.1016/0006-2952(86)90732-x. [DOI] [PubMed] [Google Scholar]
- Itami T., Kanoh S. Studies on the pharmacological bases of fetal toxicity of drugs (IV). Effect of endotoxin and starvation on serum protein binding of salicylic acid in pregnant rats. Jpn J Pharmacol. 1983 Dec;33(6):1199–1204. doi: 10.1254/jjp.33.1199. [DOI] [PubMed] [Google Scholar]
- Jiritano L., Bortolotti A., Gaspari F., Bonati M. Caffeine disposition after oral administration to pregnant rats. Xenobiotica. 1985 Dec;15(12):1045–1051. doi: 10.3109/00498258509049099. [DOI] [PubMed] [Google Scholar]
- Juchau M. R., Bark D. H., Shewey L. M., Greenaway J. C. Generation of reactive dysmorphogenic intermediates by rat embryos in culture: effects of cytochrome P-450 inducers. Toxicol Appl Pharmacol. 1985 Dec;81(3 Pt 1):533–544. doi: 10.1016/0041-008x(85)90424-7. [DOI] [PubMed] [Google Scholar]
- Jäger-Roman E., Deichl A., Jakob S., Hartmann A. M., Koch S., Rating D., Steldinger R., Nau H., Helge H. Fetal growth, major malformations, and minor anomalies in infants born to women receiving valproic acid. J Pediatr. 1986 Jun;108(6):997–1004. doi: 10.1016/s0022-3476(86)80949-0. [DOI] [PubMed] [Google Scholar]
- KEBERLE H., LOUSTALOT P., MALLER R. K., FAIGLE J. W., SCHMID K. BIOCHEMICAL EFFECTS OF DRUGS ON THE MAMMALIAN CONCEPTUS. Ann N Y Acad Sci. 1965 Mar 12;123:252–262. doi: 10.1111/j.1749-6632.1965.tb12264.x. [DOI] [PubMed] [Google Scholar]
- Kalin J. R., Starling M. E., Hill D. L. Disposition of all-trans-retinoic acid in mice following oral doses. Drug Metab Dispos. 1981 May-Jun;9(3):196–201. [PubMed] [Google Scholar]
- Kalin J. R., Wells M. J., Hill D. L. Disposition of 13-cis-retinoic acid and N-(2-hydroxyethyl)retinamide in mice after oral doses. Drug Metab Dispos. 1982 Jul-Aug;10(4):391–398. [PubMed] [Google Scholar]
- Kamm J. J. Toxicology, carcinogenicity, and teratogenicity of some orally administered retinoids. J Am Acad Dermatol. 1982 Apr;6(4 Pt 2 Suppl):652–659. doi: 10.1016/s0190-9622(82)70054-4. [DOI] [PubMed] [Google Scholar]
- Kauffman R. E., Morris J. A., Azarnoff D. L. Placental transfer and fetal urinary excretion of gentamicin during constant rate maternal infusion. Pediatr Res. 1975 Feb;9(2):104–107. doi: 10.1203/00006450-197502000-00009. [DOI] [PubMed] [Google Scholar]
- Kietzmann H., Schwarze I., Grote W., Ravens U., Jänig U., Harms D. Embryonale Fehlbildung bei Etretinat-Therapie der Mutter wegen Morbus Darier. Dtsch Med Wochenschr. 1986 Jan 10;111(2):60–62. doi: 10.1055/s-2008-1068402. [DOI] [PubMed] [Google Scholar]
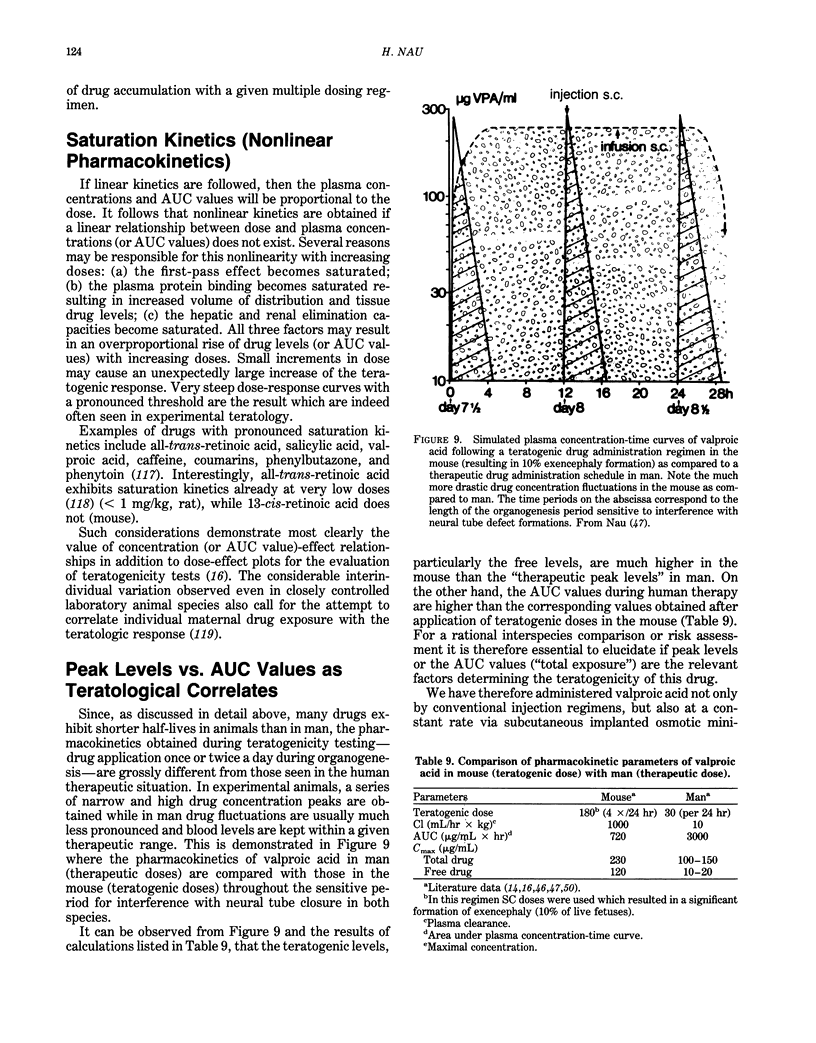
- Kimmel C. A., Young J. F. Correlating pharmacokinetics and teratogenic endpoints. Fundam Appl Toxicol. 1983 Jul-Aug;3(4):250–255. doi: 10.1016/s0272-0590(83)80136-5. [DOI] [PubMed] [Google Scholar]
- Kistler A., Hummler H. Teratogenesis and reproductive safety evaluation of the retinoid etretin (Ro 10-1670). Arch Toxicol. 1985 Oct;58(1):50–56. doi: 10.1007/BF00292617. [DOI] [PubMed] [Google Scholar]
- Koch S., Jäger-Roman E., Rating D., Helge H. Possible teratogenic effect of valproate during pregnancy. J Pediatr. 1983 Dec;103(6):1007–1008. doi: 10.1016/s0022-3476(83)80750-1. [DOI] [PubMed] [Google Scholar]
- Kochhar D. M., Penner J. D., Tellone C. I. Comparative teratogenic activities of two retinoids: effects on palate and limb development. Teratog Carcinog Mutagen. 1984;4(4):377–387. doi: 10.1002/tcm.1770040407. [DOI] [PubMed] [Google Scholar]
- Kuhnz W., Nau H. Differences in in vitro binding of diazepam and N-desmethyldiazepam to maternal and fetal plasma proteins at birth: relation to free fatty acid concentration and other parameters. Clin Pharmacol Ther. 1983 Aug;34(2):220–226. doi: 10.1038/clpt.1983.156. [DOI] [PubMed] [Google Scholar]
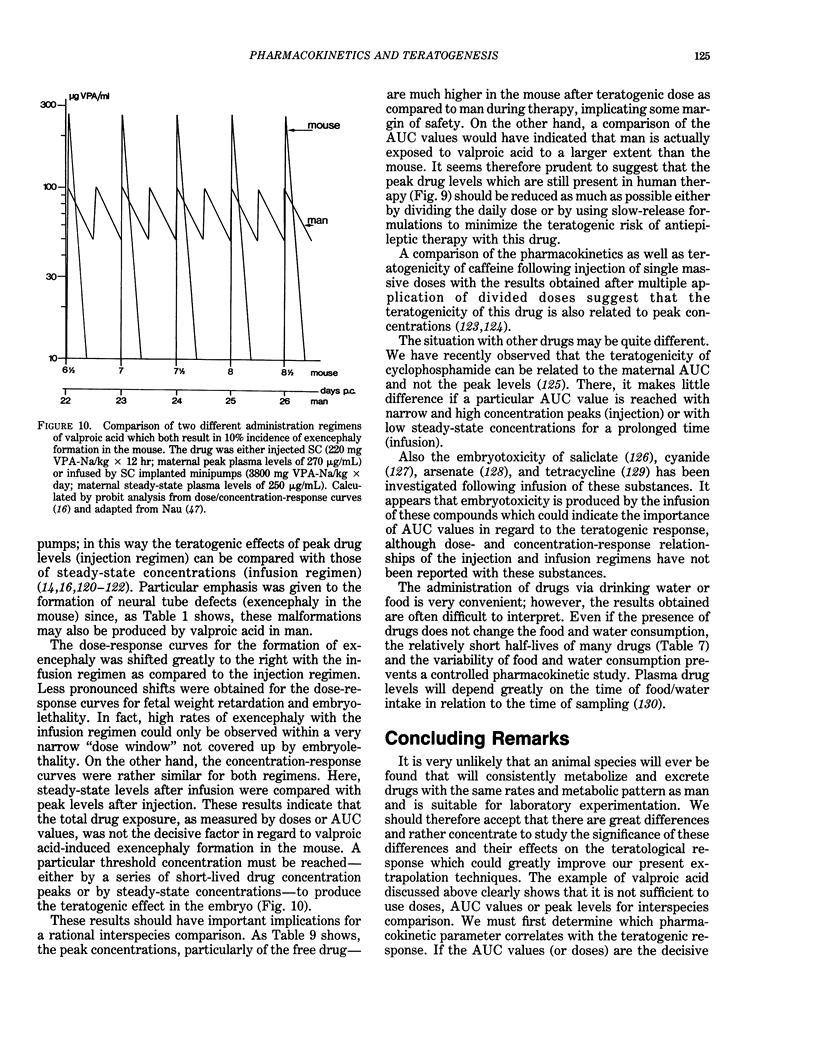
- Lammer E. J., Chen D. T., Hoar R. M., Agnish N. D., Benke P. J., Braun J. T., Curry C. J., Fernhoff P. M., Grix A. W., Jr, Lott I. T. Retinoic acid embryopathy. N Engl J Med. 1985 Oct 3;313(14):837–841. doi: 10.1056/NEJM198510033131401. [DOI] [PubMed] [Google Scholar]
- Legraverend C., Guenthner T. M., Nebert D. W. Importance of the route of administration for genetic differences in benzo[a]pyrene-induced in utero toxicity and teratogenicity. Teratology. 1984 Feb;29(1):35–47. doi: 10.1002/tera.1420290106. [DOI] [PubMed] [Google Scholar]
- Levy G., Procknal J. A., Garrettson L. K. Distribution of salicylate between neonatal and maternal serum at diffusion equilibrium. Clin Pharmacol Ther. 1975 Aug;18(2):210–214. doi: 10.1002/cpt1975182210. [DOI] [PubMed] [Google Scholar]
- Lewandowski C., Klug S., Nau H., Neubert D. Pharmacokinetic aspects of drug effects in vitro: effects of serum protein binding on concentration and teratogenicity of valproic acid and 2-en-valproic acid in whole embryos in culture. Arch Toxicol. 1986 Apr;58(4):239–242. doi: 10.1007/BF00297113. [DOI] [PubMed] [Google Scholar]
- Lorenz J., Glatt H. R., Fleischmann R., Ferlinz R., Oesch F. Drug metabolism in man and its relationship to that in three rodent species: monooxygenase, epoxide hydrolase, and glutathione S-transferase activities in subcellular fractions of lung and liver. Biochem Med. 1984 Aug;32(1):43–56. doi: 10.1016/0006-2944(84)90007-3. [DOI] [PubMed] [Google Scholar]
- Löscher W., Nau H. Pharmacological evaluation of various metabolites and analogues of valproic acid. Anticonvulsant and toxic potencies in mice. Neuropharmacology. 1985 May;24(5):427–435. doi: 10.1016/0028-3908(85)90028-0. [DOI] [PubMed] [Google Scholar]
- Löscher W., Nau H. Valproic acid: metabolite concentrations in plasma and brain, anticonvulsant activity, and effects on GABA metabolism during subacute treatment in mice. Arch Int Pharmacodyn Ther. 1982 May;257(1):20–31. [PubMed] [Google Scholar]
- Manchester D., Jacoby E. Decreased placental monooxygenase activities associated with birth defects. Teratology. 1984 Aug;30(1):31–37. doi: 10.1002/tera.1420300105. [DOI] [PubMed] [Google Scholar]
- Martz F., Failinger C., 3rd, Blake D. A. Phenytoin teratogenesis: correlation between embryopathic effect and covalent binding of putative arene oxide metabolite in gestational tissue. J Pharmacol Exp Ther. 1977 Oct;203(1):231–239. [PubMed] [Google Scholar]
- Massarella J., Vane F., Buggé C., Rodriguez L., Cunningham W. J., Franz T., Colburn W. Etretinate kinetics during chronic dosing in severe psoriasis. Clin Pharmacol Ther. 1985 Apr;37(4):439–446. doi: 10.1038/clpt.1985.68. [DOI] [PubMed] [Google Scholar]
- Mast T. J., Cukierski M. A., Nau H., Hendrickx A. G. Predicting the human teratogenic potential of the anticonvulsant, valproic acid, from a non-human primate model. Toxicology. 1986 May;39(2):111–119. doi: 10.1016/0300-483x(86)90129-0. [DOI] [PubMed] [Google Scholar]
- Mellett L. B. Comparative drug metabolism. Prog Drug Res. 1969;13:136–169. doi: 10.1007/978-3-0348-7068-9_3. [DOI] [PubMed] [Google Scholar]
- Mirkes P. E. Cyclophosphamide teratogenesis: a review. Teratog Carcinog Mutagen. 1985;5(2):75–88. doi: 10.1002/tcm.1770050202. [DOI] [PubMed] [Google Scholar]
- Mulley B. A., Parr G. D., Pau W. K., Rye R. M., Mould J. J., Siddle N. C. Placental transfer of chlorthalidone and its elimination in maternal milk. Eur J Clin Pharmacol. 1978 May 17;13(2):129–131. doi: 10.1007/BF00609757. [DOI] [PubMed] [Google Scholar]
- Nau H., Bass R. Transfer of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) to the mouse embryo and fetus. Toxicology. 1981;20(4):299–308. doi: 10.1016/0300-483x(81)90037-8. [DOI] [PubMed] [Google Scholar]
- Nau H. Clinical pharmacokinetics in pregnancy and perinatology. I. Placental transfer and fetal side effects of local anaesthetic agents. Dev Pharmacol Ther. 1985;8(3):149–181. doi: 10.1159/000457034. [DOI] [PubMed] [Google Scholar]
- Nau H., Helge H., Luck W. Valproic acid in the perinatal period: decreased maternal serum protein binding results in fetal accumulation and neonatal displacement of the drug and some metabolites. J Pediatr. 1984 Apr;104(4):627–634. doi: 10.1016/s0022-3476(84)80567-3. [DOI] [PubMed] [Google Scholar]
- Nau H., Krauer B. Serum protein binding of valproic acid in fetus-mother pairs throughout pregnancy: correlation with oxytocin administration and albumin and free fatty acid concentrations. J Clin Pharmacol. 1986 Mar;26(3):215–221. doi: 10.1002/j.1552-4604.1986.tb02937.x. [DOI] [PubMed] [Google Scholar]
- Nau H., Kuhnz W., Egger H. J., Rating D., Helge H. Anticonvulsants during pregnancy and lactation. Transplacental, maternal and neonatal pharmacokinetics. Clin Pharmacokinet. 1982 Nov-Dec;7(6):508–543. doi: 10.2165/00003088-198207060-00003. [DOI] [PubMed] [Google Scholar]
- Nau H., Luck W., Kuhnz W., Wegener S. Serum protein binding of diazepam, desmethyldiazepam, furosemide, indomethacin, warfarin, and phenobarbital in human fetus, mother, and newborn infant. Pediatr Pharmacol (New York) 1983;3(3-4):219–227. [PubMed] [Google Scholar]
- Nau H., Löscher W. Pharmacologic evaluation of various metabolites and analogs of valproic acid: teratogenic potencies in mice. Fundam Appl Toxicol. 1986 May;6(4):669–676. doi: 10.1016/0272-0590(86)90180-6. [DOI] [PubMed] [Google Scholar]
- Nau H., Löscher W., Schäfer H. Anticonvulsant activity and embryotoxicity of valproic acid. Neurology. 1984 Mar;34(3):400–401. doi: 10.1212/wnl.34.3.400-b. [DOI] [PubMed] [Google Scholar]
- Nau H., Löscher W. Valproic acid and metabolites: pharmacological and toxicological studies. Epilepsia. 1984;25 (Suppl 1):S14–S22. doi: 10.1111/j.1528-1157.1984.tb05632.x. [DOI] [PubMed] [Google Scholar]
- Nau H., Scott W. J., Jr Weak acids may act as teratogens by accumulating in the basic milieu of the early mammalian embryo. Nature. 1986 Sep 18;323(6085):276–278. doi: 10.1038/323276a0. [DOI] [PubMed] [Google Scholar]
- Nau H., Spielmann H., Lo Turco Mortler C. M., Winckler K., Riedel L., Obe G. Mutagenic, teratogenic and pharmacokinetic properties of cyclophosphamide and some of its deuterated derivatives. Mutat Res. 1982 Aug;95(2-3):105–118. doi: 10.1016/0027-5107(82)90250-0. [DOI] [PubMed] [Google Scholar]
- Nau H. Teratogenic valproic acid concentrations: infusion by implanted minipumps vs conventional injection regimen in the mouse. Toxicol Appl Pharmacol. 1985 Sep 15;80(2):243–250. doi: 10.1016/0041-008x(85)90081-x. [DOI] [PubMed] [Google Scholar]
- Nau H. Transfer of valproic acid and its main active unsaturated metabolite to the gestational tissue: correlation with neural tube defect formation in the mouse. Teratology. 1986 Feb;33(1):21–27. doi: 10.1002/tera.1420330105. [DOI] [PubMed] [Google Scholar]
- Nau H. Valproic acid teratogenicity in mice after various administration and phenobarbital-pretreatment regimens: the parent drug and not one of the metabolites assayed is implicated as teratogen. Fundam Appl Toxicol. 1986 May;6(4):662–668. doi: 10.1016/0272-0590(86)90179-x. [DOI] [PubMed] [Google Scholar]
- Nau H., Zierer R., Spielmann H., Neubert D., Gansau C. A new model for embryotoxicity testing: teratogenicity and pharmacokinetics of valproic acid following constant-rate administration in the mouse using human therapeutic drug and metabolite concentrations. Life Sci. 1981 Dec 28;29(26):2803–2814. doi: 10.1016/0024-3205(81)90541-5. [DOI] [PubMed] [Google Scholar]
- Neubert D., Chahoud I. Significance of species and strain differences in pre- and perinatal toxicology. Acta Histochem Suppl. 1985;31:23–35. [PubMed] [Google Scholar]
- Nomura T., Isa Y., Kurokawa N., Kanzaki T., Tanaka H., Tada E., Sakamoto Y. Enhancement effects of barbital on the teratogenicity of aminopyrine. Toxicology. 1984 Feb;29(4):281–291. doi: 10.1016/0300-483x(84)90160-4. [DOI] [PubMed] [Google Scholar]
- Nomura T., Kondo S. The enhancement effect of phenobarbital on toxicity of furylfuramide in mouse embryo. Mutat Res. 1976 Apr;35(1):167–172. doi: 10.1016/0027-5107(76)90179-2. [DOI] [PubMed] [Google Scholar]
- Olanoff L. S., Anderson J. M. Controlled release of tetracycline--III: A physiological pharmacokinetic model of the pregnant rat. J Pharmacokinet Biopharm. 1980 Dec;8(6):599–620. doi: 10.1007/BF01060056. [DOI] [PubMed] [Google Scholar]
- Ong L. L., Schardein J. L., Petrere J. A., Sakowski R., Jordan H., Humphrey R. R., Fitzgerald J. E., de la Iglesia F. A. Teratogenesis of calcium valproate in rats. Fundam Appl Toxicol. 1983 Mar-Apr;3(2):121–126. doi: 10.1016/s0272-0590(83)80067-0. [DOI] [PubMed] [Google Scholar]
- Peiker G., Traeger A. Die Plazentapassage von Nalidixinsäure (Negram) und pharmakokinetische Untersuchungen bei Neugeborenen. Pharmazie. 1983 Sep;38(9):613–615. [PubMed] [Google Scholar]
- Pelkonen O. Biotransformation of xenobiotics in the fetus. Pharmacol Ther. 1980;10(2):261–281. doi: 10.1016/0163-7258(80)90083-2. [DOI] [PubMed] [Google Scholar]
- Philipson A., Sabath L. D., Charles D. Transplacental passage of erythromycin and clindamycin. N Engl J Med. 1973 Jun 7;288(23):1219–1221. doi: 10.1056/NEJM197306072882307. [DOI] [PubMed] [Google Scholar]
- Platzek T., Bochert G., Rahm U. Embryotoxicity induced by alkylating agents. Teratogenicity of acetoxymethyl-methylnitrosamine: dose-response relationship, application route dependency and phase specificity. Arch Toxicol. 1983 Jan;52(1):45–69. doi: 10.1007/BF00317981. [DOI] [PubMed] [Google Scholar]
- Pruitt A. W., McNay J. L., Dayton P. G. Transfer characteristics of triamterene and its analogs. Central nervous system, placenta, and kidney. Drug Metab Dispos. 1975 Jan-Feb;3(1):30–41. [PubMed] [Google Scholar]
- Rowland J. M., Althaus Z. R., Slikker W., Jr, Hendrickx A. G. Comparative distribution and metabolism of triamcinolone acetonide and cortisol in the rat embryomaternal unit. Teratology. 1983 Jun;27(3):333–341. doi: 10.1002/tera.1420270307. [DOI] [PubMed] [Google Scholar]
- Saito H., Kobayashi H., Takeno S., Sakai T. Fetal toxicity of benzodiazepines in rats. Res Commun Chem Pathol Pharmacol. 1984 Dec;46(3):437–447. [PubMed] [Google Scholar]
- Sawada Y., Hanano M., Sugiyama Y., Iga T. Prediction of the disposition of beta-lactam antibiotics in humans from pharmacokinetic parameters in animals. J Pharmacokinet Biopharm. 1984 Jun;12(3):241–261. doi: 10.1007/BF01061720. [DOI] [PubMed] [Google Scholar]
- Schardein J. L., Schwetz B. A., Kenel M. F. Species sensitivities and prediction of teratogenic potential. Environ Health Perspect. 1985 Sep;61:55–67. doi: 10.1289/ehp.856155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shum S., Jensen N. M., Nebert D. W. The murine Ah locus: in utero toxicity and teratogenesis associated with genetic differences in benzo[a]pyrene metabolism. Teratology. 1979 Dec;20(3):365–376. doi: 10.1002/tera.1420200307. [DOI] [PubMed] [Google Scholar]
- Swanson B. N., Frolik C. A., Zaharevitz D. W., Roller P. P., Sporn M. B. Dose-dependent kinetics of all-trans-retinoic acid in rats. Plasma levels and excretion into bile, urine, and faeces. Biochem Pharmacol. 1981 Jan 15;30(2):107–113. doi: 10.1016/0006-2952(81)90180-5. [DOI] [PubMed] [Google Scholar]
- Tanaka E., Kinoshita H., Yoshida T., Kuroiwa Y. Relationship between trimethadione metabolism and hepatic drug-oxidizing enzyme activities in different animal species. J Pharmacobiodyn. 1983 Jul;6(7):481–486. doi: 10.1248/bpb1978.6.481. [DOI] [PubMed] [Google Scholar]
- Walker C. H. Species differences in microsomal monooxygenase activity and their relationship to biological half-lives. Drug Metab Rev. 1978;7(2):295–323. doi: 10.3109/03602537808993770. [DOI] [PubMed] [Google Scholar]
- Welsch F. Effects of acute or chronic polychlorinated biphenyl ingestion on maternal metabolic homeostasis and on the manifestations of embryotoxicity caused by cyclophosphamide in mice. Arch Toxicol. 1985 Jun;57(2):104–113. doi: 10.1007/BF00343119. [DOI] [PubMed] [Google Scholar]
- Willhite C. C., Shealy Y. F. Amelioration of embryotoxicity by structural modification of the terminal group of cancer chemopreventive retinoids. J Natl Cancer Inst. 1984 Mar;72(3):689–695. [PubMed] [Google Scholar]
- Williams K. J., Ferm V. H., Willhite C. C. Teratogenic dose-response relationships of etretinate in the golden hamster. Fundam Appl Toxicol. 1984 Dec;4(6):977–982. doi: 10.1016/0272-0590(84)90236-7. [DOI] [PubMed] [Google Scholar]
- Wilson J. G. Abnormalities of intrauterine development in non-human primates. Acta Endocrinol Suppl (Copenh) 1972;166:261–292. doi: 10.1530/acta.0.071s261. [DOI] [PubMed] [Google Scholar]
- Wilson J. G., Ritter E. J., Scott W. J., Fradkin R. Comparative distribution and embryotoxicity of acetylsalicylic acid in pregnant rats and rhesus monkeys. Toxicol Appl Pharmacol. 1977 Jul;41(1):67–78. doi: 10.1016/0041-008x(77)90054-0. [DOI] [PubMed] [Google Scholar]
- Wilson J. G., Scott W. J., Ritter E. J., Fradkin R. Comparative distribution and embryotoxicity of hydroxyurea in pregnant rats and rhesus monkeys. Teratology. 1975 Apr;11(2):169–178. doi: 10.1002/tera.1420110205. [DOI] [PubMed] [Google Scholar]
- Wilson J. G., Scott W. J., Ritter E. J., Fradkin R. Comparative distribution and embryotoxicity of methotrexate in pregnant rats and rhesus monkeys. Teratology. 1979 Feb;19(1):71–79. doi: 10.1002/tera.1420190111. [DOI] [PubMed] [Google Scholar]
- York R. G., Randall J. L., Scott W. J., Jr Reduction of caffeine teratogenicity in mice by inducing maternal drug metabolism with beta-naphthoflavone. Teratology. 1985 Apr;31(2):217–225. doi: 10.1002/tera.1420310206. [DOI] [PubMed] [Google Scholar]


