Abstract
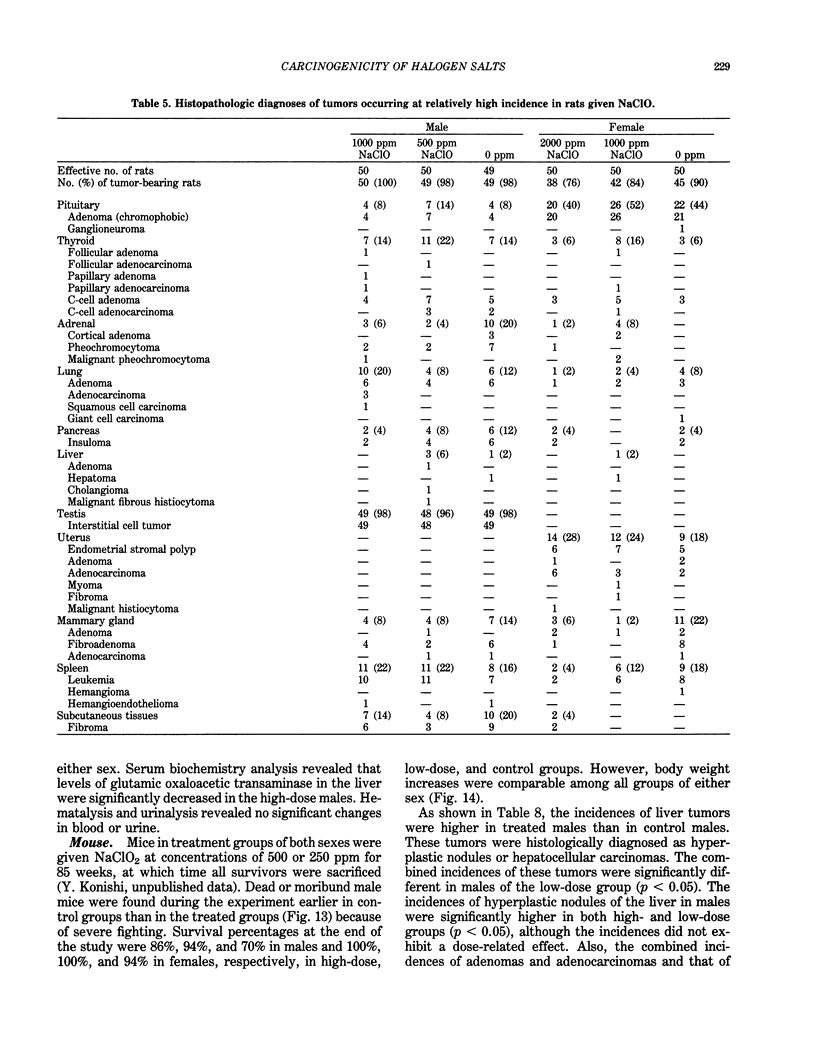
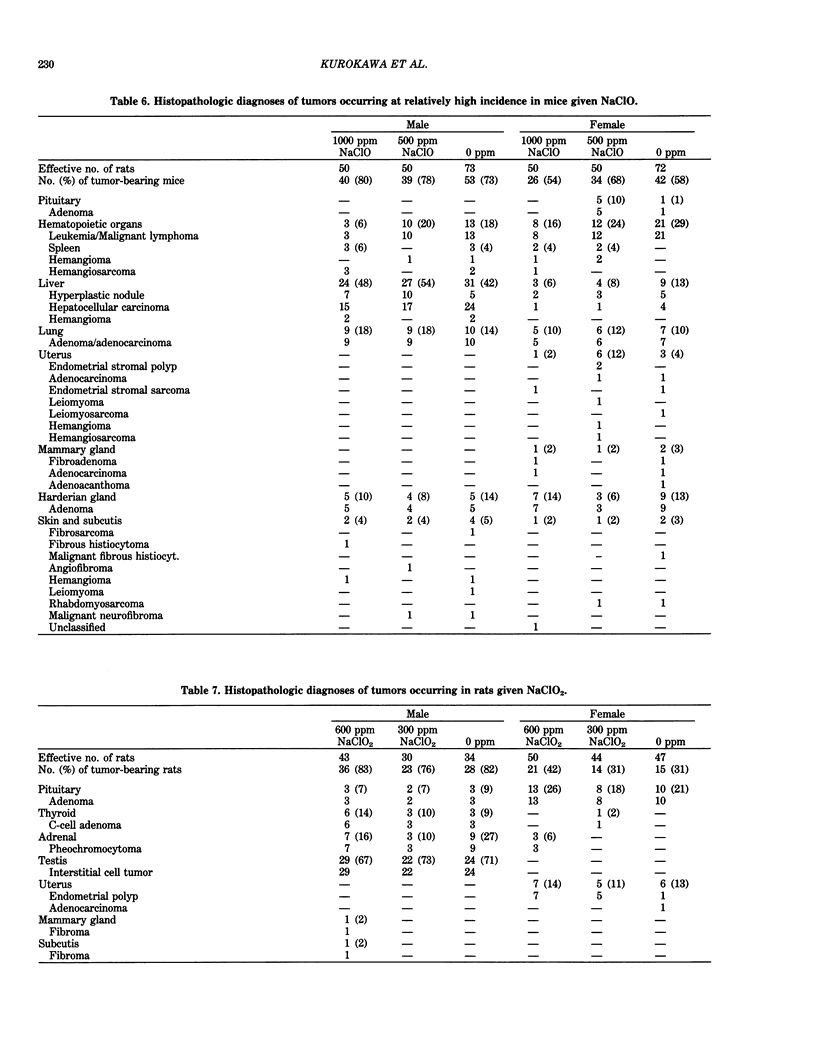
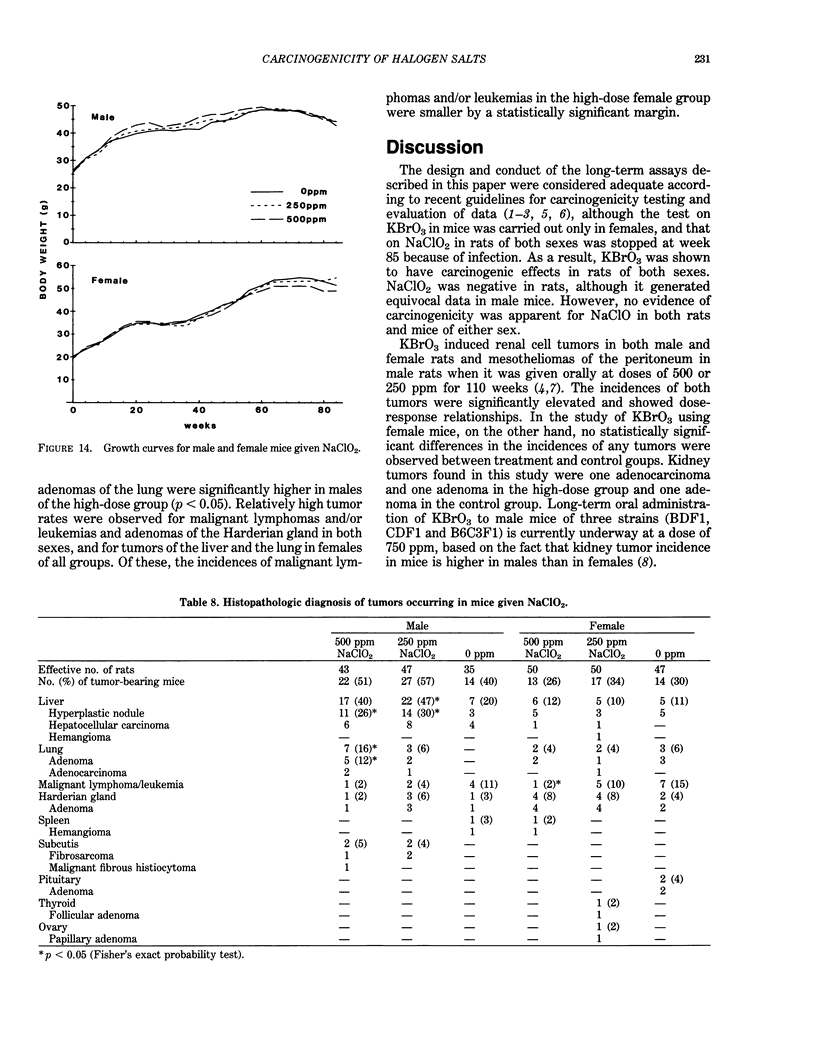
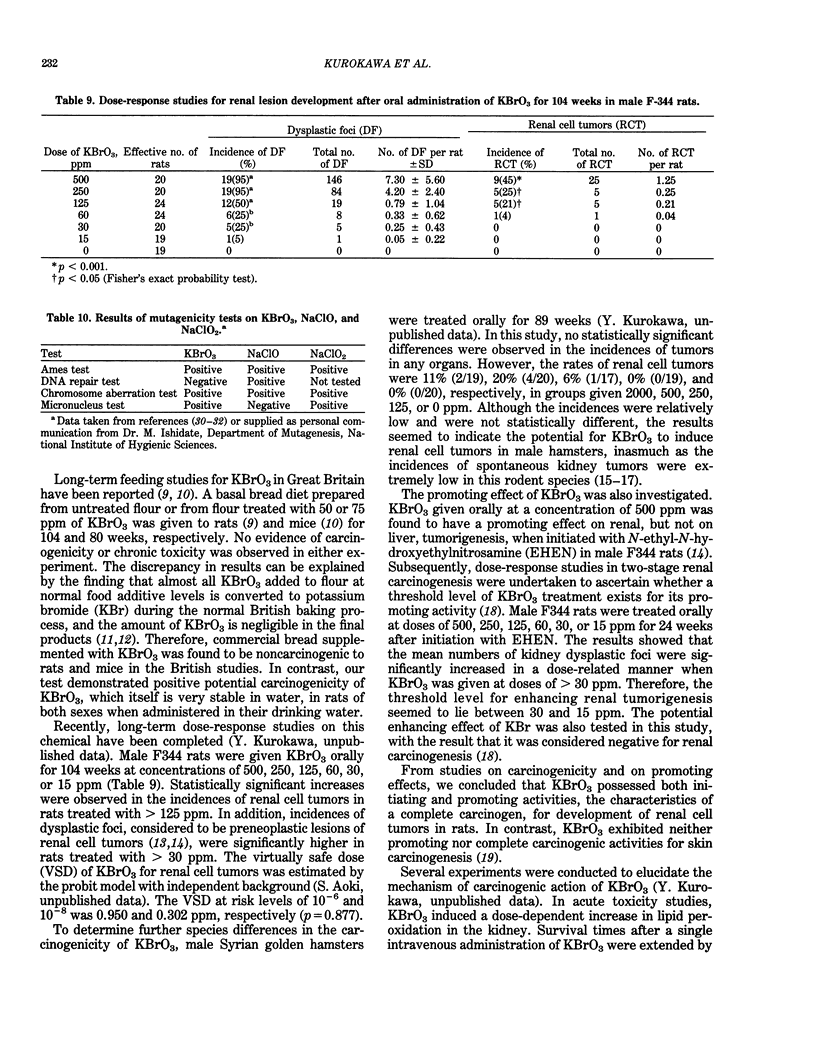
Long-term in vivo carcinogenicity tests of potassium bromate (KBrO3), sodium hypochlorite (NaClO), and sodium chlorite (NaClO2) have been conducted in Japan from 1977 to 1985. In these investigations, groups of approximately 50 male and 50 female F344 rats or B6C3F1 mice were given solutions of the compounds as their drinking water ad libitum at two dose levels determined on the basis of preliminary 13-week tests. Control animals were given distilled water. The carcinogenic potential of KBrO3 was tested by administering doses of 500 or 250 ppm to rats for 110 weeks. Significantly elevated incidences of renal cell tumors in males and females and mesotheliomas of the peritoneum in males as compared to controls were observed. When female mice were given KBrO3 at doses of 1000 or 500 ppm for 78 weeks, no significant differences in tumor incidences between experimental and control groups were apparent. NaClO was administered to male and female rats, respectively, at doses of 1000 or 500 ppm and 2000 or 1000 ppm for 104 weeks. In mice, NaClO was given at doses of 1000 or 500 ppm to either sex for 103 weeks. The incidences of tumors in NaClO-treated and control animals of both sexes were not significantly different in both rat and mouse studies. NaClO2 was given to rats of both sexes at a dose of 600 or 300 ppm for 85 weeks. No statistically significant differences were observed in the incidences of tumor formation between NaClO2-treated and control groups of both sexes. NaClO2 was administered to mice at a concentration of 500 or 250 ppm for 85 weeks. In males, the combined incidences of hyperplastic nodules and hepatocellular carcinomas of the liver in a low-dose group, and adenomas and adenocarcinomas of the lung in a high-dose group, were marginally increased compared to controls (p less than 0.05). However, these incidences in treated males were within the range of values of historical control data in our program. We concluded that KBrO3 was carcinogenic in rats of both sexes. NaClO was not carcinogenic in either rats and or mice under the conditions of the present studies. Although NaClO2 was shown to be noncarcinogenic in rats, the results for mice were evaluated as inconclusive. Also the results of two-stage mouse skin carcinogenesis using KBrO3, NaClO, and NaClO2 are presented. The necessity for further testing of oxidant chemicals to determine potential carcinogenic and/or promoting effects is suggested in view of the recently proposed role of active oxygen species in carcinogenesis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdel-Rahman M. S., Couri D., Bull R. J. Kinetics of Cl02 and effects of Cl02, Cl02-, and Cl03- in drinking water on blood glutathione and hemolysis in rat and chicken. J Environ Pathol Toxicol. 1979 Dec;3(1-2):431–449. [PubMed] [Google Scholar]
- Abdel-Rahman M. S., Suh D. H., Bull R. J. Pharmacodynamics and toxicity of chlorine in drinking water in the rat. J Appl Toxicol. 1984 Apr;4(2):82–86. doi: 10.1002/jat.2550040205. [DOI] [PubMed] [Google Scholar]
- Ames B. N. Dietary carcinogens and anticarcinogens. Oxygen radicals and degenerative diseases. Science. 1983 Sep 23;221(4617):1256–1264. doi: 10.1126/science.6351251. [DOI] [PubMed] [Google Scholar]
- Bercz J. P., Jones L., Garner L., Murray D., Ludwig D. A., Boston J. Subchronic toxicity of chlorine dioxide and related compounds in drinking water in the nonhuman primate. Environ Health Perspect. 1982 Dec;46:47–55. doi: 10.1289/ehp.824647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bus J. S., Gibson J. E. Paraquat: model for oxidant-initiated toxicity. Environ Health Perspect. 1984 Apr;55:37–46. doi: 10.1289/ehp.845537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerutti P. A. Prooxidant states and tumor promotion. Science. 1985 Jan 25;227(4685):375–381. doi: 10.1126/science.2981433. [DOI] [PubMed] [Google Scholar]
- Copeland E. S. A National Institutes of Health Workshop report. Free radicals in promotion--a chemical pathology study section workshop. Cancer Res. 1983 Nov;43(11):5631–5637. [PubMed] [Google Scholar]
- Couri D., Abdel-Rahman M. S., Bull R. J. Toxicological effects of chlorine dioxide, chlorite and chlorate. Environ Health Perspect. 1982 Dec;46:13–17. doi: 10.1289/ehp.824613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dees J. H., Heatfield B. M., Reuber M. D., Trump B. F. Adenocarcinoma of the kidney. III. Histogenesis of renal adenocarcinomas induced in rats by N-(4'-fluoro-4-biphenylyl)acetamide. J Natl Cancer Inst. 1980 Jun;64(6):1537–1545. doi: 10.1093/jnci/64.6.1537. [DOI] [PubMed] [Google Scholar]
- Demopoulos H. B., Pietronigro D. D., Flamm E. S., Seligman M. L. The possible role of free radical reactions in carcinogenesis. J Environ Pathol Toxicol. 1980 Mar;3(4 Spec No):273–303. [PubMed] [Google Scholar]
- Druckrey H. Chloriertes trikwasser, toxizitäts-Prüfungen an Ratten über sieben Generationen. Food Cosmet Toxicol. 1968 Aug;6(2):147–154. doi: 10.1016/0015-6264(68)90196-x. [DOI] [PubMed] [Google Scholar]
- Fisher N., Hutchinson J. B., Berry R., Hardy J., Ginocchio A. V., Waite V. Long-term toxicity and carcinogenicity studies of the bread improver potassium bromate 1. Studies in rats. Food Cosmet Toxicol. 1979 Feb;17(1):33–39. doi: 10.1016/0015-6264(79)90156-1. [DOI] [PubMed] [Google Scholar]
- Furukawa F., Kurata Y., Kokubo T., Takahashi M., Nakadate M. [Oral acute and subchronic toxicity studies for sodium hypochlorite in F-344 rat (author's transl)]. Eisei Shikenjo Hokoku. 1980;98:62–69. [PubMed] [Google Scholar]
- Ginocchio A. V., Waite V., Hardy J., Fisher N., Hutchinson J. B., Berry R. Long-term toxicity and carcinogenicity studies of the bread improver potassium bromate 2. Studies in mice. Food Cosmet Toxicol. 1979 Feb;17(1):41–47. doi: 10.1016/0015-6264(79)90157-3. [DOI] [PubMed] [Google Scholar]
- Griesemer R. A., Cueto C., Jr Toward a classification scheme for degrees of experimental evidence for the carcinogenicity of chemicals for animals. IARC Sci Publ. 1980;(27):259–281. [PubMed] [Google Scholar]
- Haseman J. K., Crawford D. D., Huff J. E., Boorman G. A., McConnell E. E. Results from 86 two-year carcinogenicity studies conducted by the National Toxicology Program. J Toxicol Environ Health. 1984;14(5-6):621–639. doi: 10.1080/15287398409530613. [DOI] [PubMed] [Google Scholar]
- Haseman J. K., Huff J., Boorman G. A. Use of historical control data in carcinogenicity studies in rodents. Toxicol Pathol. 1984;12(2):126–135. doi: 10.1177/019262338401200203. [DOI] [PubMed] [Google Scholar]
- Hayatsu H., Hoshino H., Kawazoe Y. Potential cocarcinogenicity of sodium hypochlorite. Nature. 1971 Oct 15;233(5320):495–495. doi: 10.1038/233495a0. [DOI] [PubMed] [Google Scholar]
- Hirota N., Yokoyama T. Enhancing effect of hydrogen peroxide upon duodenal and upper jejunal carcinogenesis in rats. Gan. 1981 Oct;72(5):811–812. [PubMed] [Google Scholar]
- Ito A., Watanabe H., Naito M., Naito Y. Induction of duodenal tumors in mice by oral administration of hydrogen peroxide. Gan. 1981 Feb;72(1):174–175. [PubMed] [Google Scholar]
- Ito A., Watanabe H., Naito M., Naito Y., Kawashima K. Correlation between induction of duodenal tumor by hydrogen peroxide and catalase activity in mice. Gan. 1984 Jan;75(1):17–21. [PubMed] [Google Scholar]
- Jones G. R. Skin cancer: risk to individuals using the tumour promoter benzoyl peroxide for acne treatment. Hum Toxicol. 1985 Jan;4(1):75–78. doi: 10.1177/096032718500400110. [DOI] [PubMed] [Google Scholar]
- Klein-Szanto A. J., Slaga T. J. Effects of peroxides on rodent skin: epidermal hyperplasia and tumor promotion. J Invest Dermatol. 1982 Jul;79(1):30–34. doi: 10.1111/1523-1747.ep12510444. [DOI] [PubMed] [Google Scholar]
- Kurokawa Y., Aoki S., Imazawa T., Hayashi Y., Matsushima Y., Takamura N. Dose-related enhancing effect of potassium bromate on renal tumorigenesis in rats initiated with N-ethyl-N-hydroxyethyl-nitrosamine. Jpn J Cancer Res. 1985 Jul;76(7):583–589. [PubMed] [Google Scholar]
- Kurokawa Y., Hayashi Y., Maekawa A., Takahashi M., Kokubo T. Induction of renal cell tumors in F-344 rats by oral administration of potassium bromate, a food additive. Gan. 1982 Apr;73(2):335–338. [PubMed] [Google Scholar]
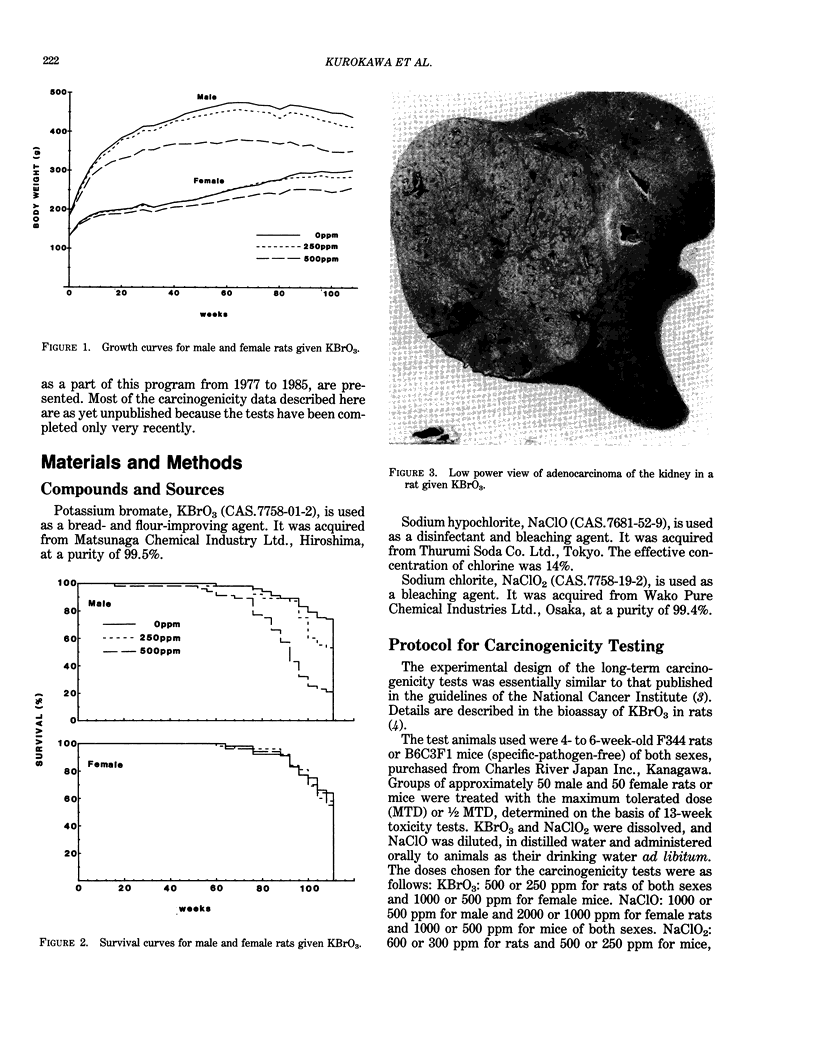
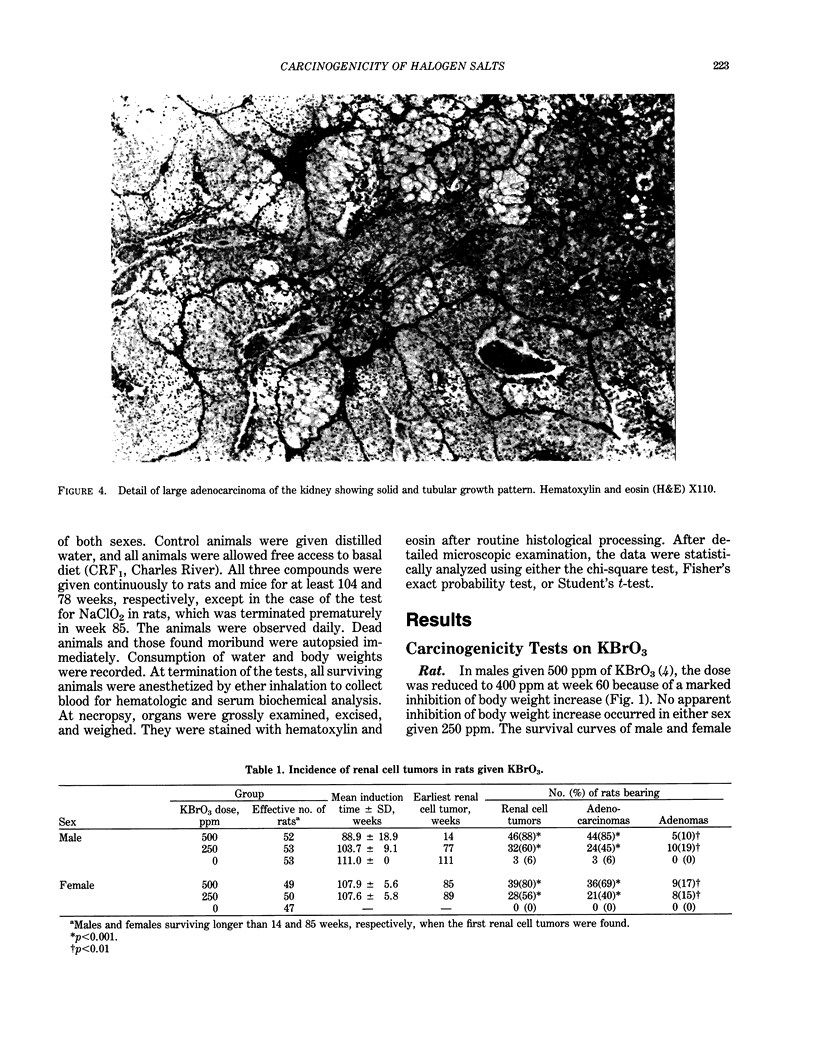
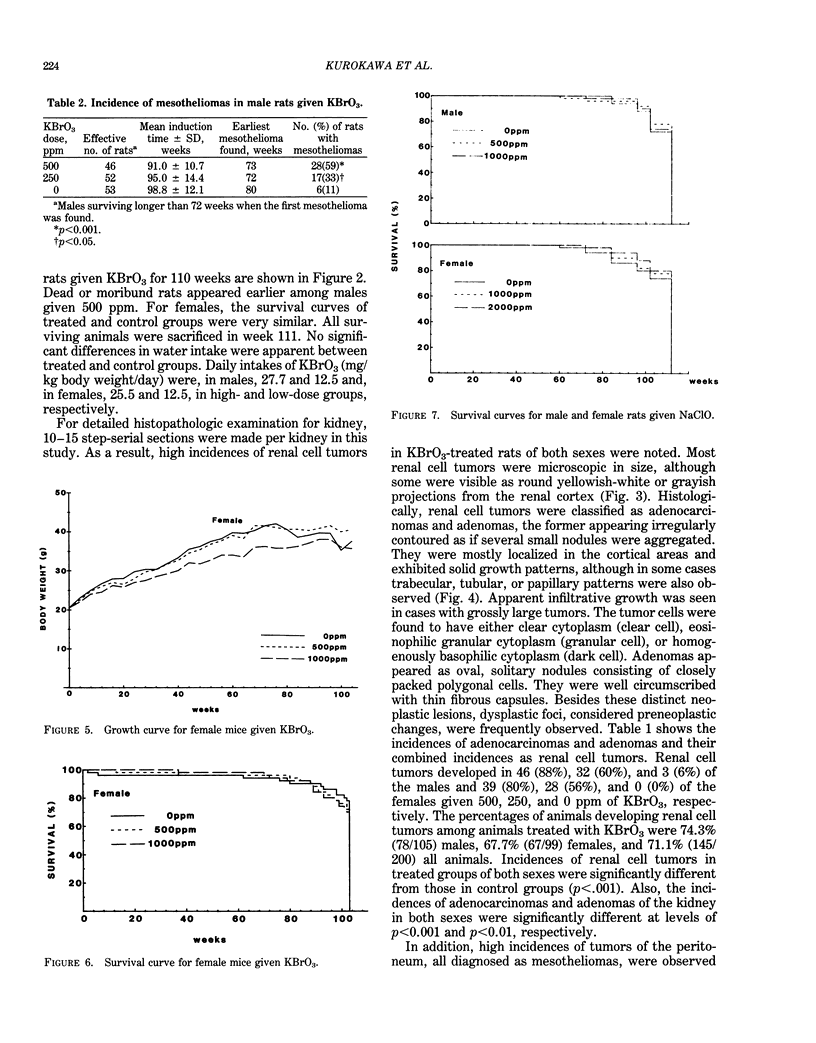
- Kurokawa Y., Hayashi Y., Maekawa A., Takahashi M., Kokubo T., Odashima S. Carcinogenicity of potassium bromate administered orally to F344 rats. J Natl Cancer Inst. 1983 Nov;71(5):965–972. [PubMed] [Google Scholar]
- Kurokawa Y., Takahashi M., Kokubo T., Ohno Y., Hayashi Y. Enhancement by potassium bromate of renal tumorigenesis initiated by N-ethyl-N-hydroxyethylnitrosamine in F-344 rats. Gan. 1983 Oct;74(5):607–610. [PubMed] [Google Scholar]
- Kurokawa Y., Takamura N., Matsushima Y., Imazawa T., Hayashi Y. Studies on the promoting and complete carcinogenic activities of some oxidizing chemicals in skin carcinogenesis. Cancer Lett. 1984 Oct;24(3):299–304. doi: 10.1016/0304-3835(84)90026-0. [DOI] [PubMed] [Google Scholar]
- Menzel D. B. Ozone: an overview of its toxicity in man and animals. J Toxicol Environ Health. 1984;13(2-3):183–204. [PubMed] [Google Scholar]
- Morrow P. E. Toxicological data on NOx: an overview. J Toxicol Environ Health. 1984;13(2-3):205–227. doi: 10.1080/15287398409530494. [DOI] [PubMed] [Google Scholar]
- Pour P., Mohr U., Althoff J., Cardesa A., Kmoch N. Spontaneous tumors and common diseases in two colonies of Syrian hamsters. III. Urogenital system and endocrine glands. J Natl Cancer Inst. 1976 May;56(5):949–961. doi: 10.1093/jnci/56.5.949. [DOI] [PubMed] [Google Scholar]
- Slaga T. J., Klein-Szanto A. J., Triplett L. L., Yotti L. P., Trosko K. E. Skin tumor-promoting activity of benzoyl peroxide, a widely used free radical-generating compound. Science. 1981 Aug 28;213(4511):1023–1025. doi: 10.1126/science.6791284. [DOI] [PubMed] [Google Scholar]
- Thewlis B. H. The fate of potassium bromate when used as a breadmaking improver. J Sci Food Agric. 1974 Dec;25(12):1471–1475. doi: 10.1002/jsfa.2740251207. [DOI] [PubMed] [Google Scholar]