Abstract
The roles of different forms of cytochrome P-450 in activation and deactivation of toxic chemicals, synthesis and breakdown of steroid hormones, and other functions, indicate the significance of these enzymes. Monooxygenase systems have been studied in species from several phyla of aquatic organisms. However, cytochrome P-450, the dominant catalyst in xenobiotic monooxygenase activity, is best studied in fish. Forms of cytochrome P-450 have been purified from several teleost species, including scup (Stenotomus chrysops), rainbow trout (Salmo gairdneri), and cod (Gadus morhua). Cytochrome P-450E from scup, cytochrome P-450 LM4b from trout, and cytochrome P-450c from cod have properties similar to each other and appear to be homologous hydrocarbon or BNF-inducible isozymes. Partially purified cytochrome DBA-P-450-I from little skate, Raja erinacea, is possibly an elasmobranch counterpart of these teleost forms. Cytochrome P-450E from scup is immunochemically related to the major BNF-inducible isozyme (cytochrome P-450c or BNF-B) in rats, indicating homology between the fish and mammalian BNF-inducible isozymes. Several other cytochrome P-450 forms with interesting or unusual properties have been purified from aquatic species. Mammalian homologs are not yet known for these isozymes. Further studies of cytochrome P-450 forms in aquatic species should establish additional homologies and the regulation of these forms by chemical and biological variables, possibly providing fundamental insights into the function and evolution of these proteins.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Binder R. L., Stegeman J. J. Basal levels and induction of hepatic aryl hydrocarbon hydroxylase activity during the embryonic period of development in brook trout. Biochem Pharmacol. 1983 Apr 1;32(7):1324–1327. doi: 10.1016/0006-2952(83)90292-7. [DOI] [PubMed] [Google Scholar]
- Binder R. L., Stegeman J. J., Lech J. J. Induction of cytochrome P-450-dependent monooxygenase systems in embryos and eleutheroembryos of the killfish Fundulus heteroclitus. Chem Biol Interact. 1985 Oct;55(1-2):185–202. doi: 10.1016/s0009-2797(85)80127-7. [DOI] [PubMed] [Google Scholar]
- Binder R. L., Stegeman J. J. Microsomal electron transport and xenobiotic monooxygenase activities during the embryonic period of development in the killifish, Fundulus heteroclitus. Toxicol Appl Pharmacol. 1984 May;73(3):432–443. doi: 10.1016/0041-008x(84)90096-6. [DOI] [PubMed] [Google Scholar]
- Conney A. H. Pharmacological implications of microsomal enzyme induction. Pharmacol Rev. 1967 Sep;19(3):317–366. [PubMed] [Google Scholar]
- Fitch W. M. Distinguishing homologous from analogous proteins. Syst Zool. 1970 Jun;19(2):99–113. [PubMed] [Google Scholar]
- Goksøyr A. Purification of hepatic microsomal cytochromes P-450 from beta-naphthoflavone-treated Atlantic cod (Gadus morhua), a marine teleost fish. Biochim Biophys Acta. 1985 Jul 5;840(3):409–417. [PubMed] [Google Scholar]
- Gorsky L. D., Coon M. J. Effects of conditions for reconstitution with cytochrome b5 on the formation of products in cytochrome P-450-catalyzed reactions. Drug Metab Dispos. 1986 Jan-Feb;14(1):89–96. [PubMed] [Google Scholar]
- Guengerich F. P., Dannan G. A., Wright S. T., Martin M. V., Kaminsky L. S. Purification and characterization of liver microsomal cytochromes p-450: electrophoretic, spectral, catalytic, and immunochemical properties and inducibility of eight isozymes isolated from rats treated with phenobarbital or beta-naphthoflavone. Biochemistry. 1982 Nov 9;21(23):6019–6030. doi: 10.1021/bi00266a045. [DOI] [PubMed] [Google Scholar]
- Hansson T., Gustafsson J. A. In-vitro metabolism of 4-androstene-3,17-dione by hepatic microsomes from the rainbow trout (Salmo gairdnerii): effects of hypophysectomy and oestradiol-17 beta. J Endocrinol. 1981 Jul;90(1):103–112. doi: 10.1677/joe.0.0900103. [DOI] [PubMed] [Google Scholar]
- Kleinow K. M., Melancon M. J., Lech J. J. Biotransformation and induction: implications for toxicity, bioaccumulation and monitoring of environmental xenobiotics in fish. Environ Health Perspect. 1987 Apr;71:105–119. doi: 10.1289/ehp.8771105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotz A. V., Stegeman J. J., Walsh C. An aryl hydrocarbon hydroxylating hepatic cytochrome P-450 from the marine fish Stenotomus chrysops. Arch Biochem Biophys. 1983 Oct 15;226(2):578–592. doi: 10.1016/0003-9861(83)90327-2. [DOI] [PubMed] [Google Scholar]
- Klotz A. V., Stegeman J. J., Woodin B. R., Snowberger E. A., Thomas P. E., Walsh C. Cytochrome P-450 isozymes from the marine teleost Stenotomus chrysops: their roles in steroid hydroxylation and the influence of cytochrome b5. Arch Biochem Biophys. 1986 Sep;249(2):326–338. doi: 10.1016/0003-9861(86)90009-3. [DOI] [PubMed] [Google Scholar]
- Lu A. Y., West S. B. Multiplicity of mammalian microsomal cytochromes P-45. Pharmacol Rev. 1979 Dec;31(4):277–295. [PubMed] [Google Scholar]
- Nebert D. W., Eisen H. J., Negishi M., Lang M. A., Hjelmeland L. M., Okey A. B. Genetic mechanisms controlling the induction of polysubstrate monooxygenase (P-450) activities. Annu Rev Pharmacol Toxicol. 1981;21:431–462. doi: 10.1146/annurev.pa.21.040181.002243. [DOI] [PubMed] [Google Scholar]
- Nebert D. W., Jensen N. M. The Ah locus: genetic regulation of the metabolism of carcinogens, drugs, and other environmental chemicals by cytochrome P-450-mediated monooxygenases. CRC Crit Rev Biochem. 1979;6(4):401–437. doi: 10.3109/10409237909105427. [DOI] [PubMed] [Google Scholar]
- Okino S. T., Quattrochi L. C., Barnes H. J., Osanto S., Griffin K. J., Johnson E. F., Tukey R. H. Cloning and characterization of cDNAs encoding 2,3,7,8-tetrachlorodibenzo-p-dioxin-inducible rabbit mRNAs for cytochrome P-450 isozymes 4 and 6. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5310–5314. doi: 10.1073/pnas.82.16.5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
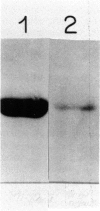
- Park S. S., Miller H., Klotz A. V., Kloepper-Sams P. J., Stegeman J. J., Gelboin H. V. Monoclonal antibodies to liver microsomal cytochrome P-450E of the marine fish Stenotomus chrysops (scup): cross reactivity with 3-methylcholanthrene induced rat cytochrome P-450. Arch Biochem Biophys. 1986 Sep;249(2):339–350. doi: 10.1016/0003-9861(86)90010-x. [DOI] [PubMed] [Google Scholar]
- Reik L. M., Levin W., Ryan D. E., Thomas P. E. Immunochemical relatedness of rat hepatic microsomal cytochromes P-450c and P-450d. J Biol Chem. 1982 Apr 10;257(7):3950–3957. [PubMed] [Google Scholar]
- Ryan D. E., Iida S., Wood A. W., Thomas P. E., Lieber C. S., Levin W. Characterization of three highly purified cytochromes P-450 from hepatic microsomes of adult male rats. J Biol Chem. 1984 Jan 25;259(2):1239–1250. [PubMed] [Google Scholar]
- Ryan D. E., Ramanathan L., Iida S., Thomas P. E., Haniu M., Shively J. E., Lieber C. S., Levin W. Characterization of a major form of rat hepatic microsomal cytochrome P-450 induced by isoniazid. J Biol Chem. 1985 May 25;260(10):6385–6393. [PubMed] [Google Scholar]
- Ryan D. E., Thomas P. E., Korzeniowski D., Levin W. Separation and characterization of highly purified forms of liver microsomal cytochrome P-450 from rats treated with polychlorinated biphenyls, phenobarbital, and 3-methylcholanthrene. J Biol Chem. 1979 Feb 25;254(4):1365–1374. [PubMed] [Google Scholar]
- Ryan D. E., Thomas P. E., Levin W. Hepatic microsomal cytochrome P-450 from rats treated with isosafrole. Purification and characterization of four enzymic forms. J Biol Chem. 1980 Aug 25;255(16):7941–7955. [PubMed] [Google Scholar]
- Sakaki T., Soga A., Yabusaki Y., Ohkawa H. Characterization of three forms of cytochrome P-450 isolated from liver microsomes of rats treated with 3-methylcholanthrene. J Biochem. 1984 Jul;96(1):117–126. doi: 10.1093/oxfordjournals.jbchem.a134803. [DOI] [PubMed] [Google Scholar]
- Shimada T., Sawabe Y. Activation of 3,4,3',4'-tetrachlorobiphenyl to protein-bound metabolites by rat liver microsomal cytochrome P-448-containing monooxygenase system. Toxicol Appl Pharmacol. 1983 Sep 30;70(3):486–493. doi: 10.1016/0041-008x(83)90166-7. [DOI] [PubMed] [Google Scholar]
- Stegeman J. J., Kloepper-Sams P. J., Farrington J. W. Monooxygenase Induction and Chlorobiphenyls in the Deep-Sea Fish Coryphaenoides armatus. Science. 1986 Mar 14;231(4743):1287–1289. doi: 10.1126/science.231.4743.1287. [DOI] [PubMed] [Google Scholar]
- Stegeman J. J., Pajor A. M., Thomas P. Influence of estradiol and testosterone on cytochrome P-450 and monooxygenase activity in immature brook trout, Salvelinus fontinalis. Biochem Pharmacol. 1982 Dec 15;31(24):3979–3989. doi: 10.1016/0006-2952(82)90644-x. [DOI] [PubMed] [Google Scholar]
- Thomas P. E., Reik L. M., Ryan D. E., Levin W. Regulation of three forms of cytochrome P-450 and epoxide hydrolase in rat liver microsomes. Effects of age, sex, and induction. J Biol Chem. 1981 Jan 25;256(2):1044–1052. [PubMed] [Google Scholar]
- Waxman D. J., Dannan G. A., Guengerich F. P. Regulation of rat hepatic cytochrome P-450: age-dependent expression, hormonal imprinting, and xenobiotic inducibility of sex-specific isoenzymes. Biochemistry. 1985 Jul 30;24(16):4409–4417. doi: 10.1021/bi00337a023. [DOI] [PubMed] [Google Scholar]
- Williams D. E., Buhler D. R. Purified form of cytochrome P-450 from rainbow trout with high activity toward conversion of aflatoxin B1 to aflatoxin B1-2,3-epoxide. Cancer Res. 1983 Oct;43(10):4752–4756. [PubMed] [Google Scholar]
- Williams D. E., Masters B. S., Lech J. J., Buhler D. R. Sex differences in cytochrome P-450 isozyme composition and activity in kidney microsomes of mature rainbow trout. Biochem Pharmacol. 1986 Jun 15;35(12):2017–2023. doi: 10.1016/0006-2952(86)90735-5. [DOI] [PubMed] [Google Scholar]
- Williams D. E., Okita R. T., Buhler D. R., Masters B. S. Regiospecific hydroxylation of lauric acid at the (omega-1) position by hepatic and kidney microsomal cytochromes P-450 from rainbow trout. Arch Biochem Biophys. 1984 Jun;231(2):503–510. doi: 10.1016/0003-9861(84)90414-4. [DOI] [PubMed] [Google Scholar]
- Williams R. T. Comparative patterns of drug metabolism. Fed Proc. 1967 Jul-Aug;26(4):1029–1039. [PubMed] [Google Scholar]