Abstract
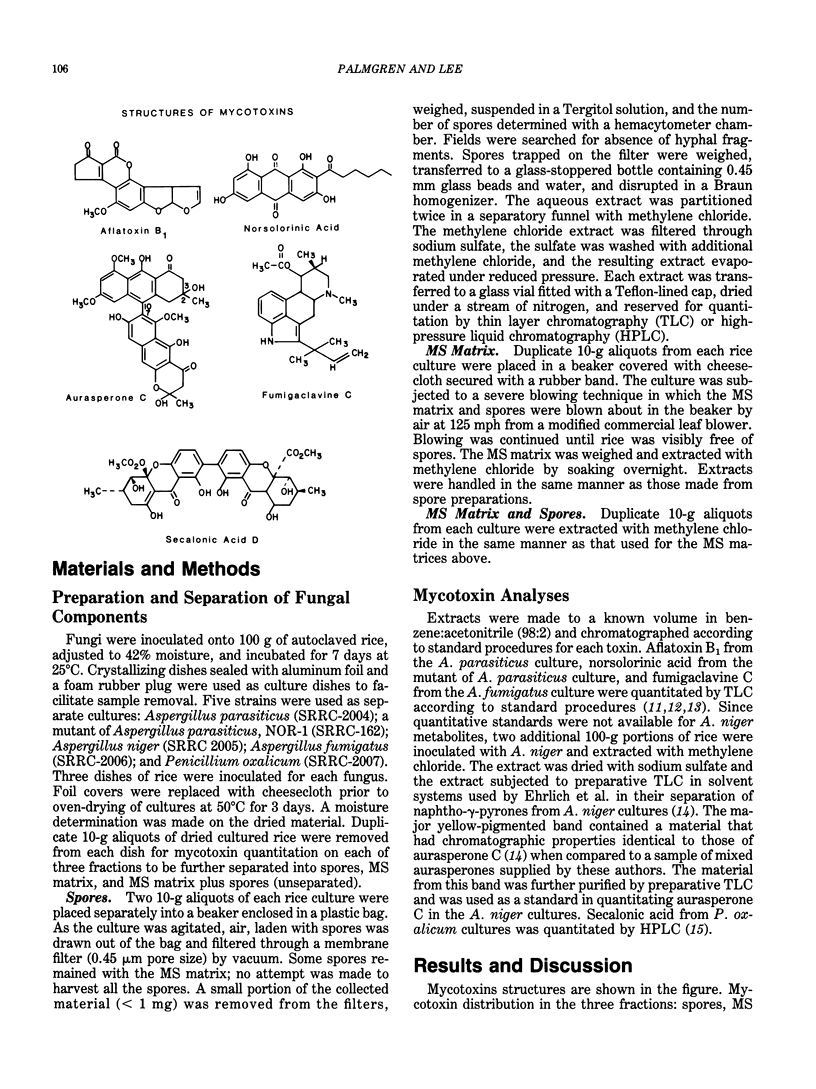
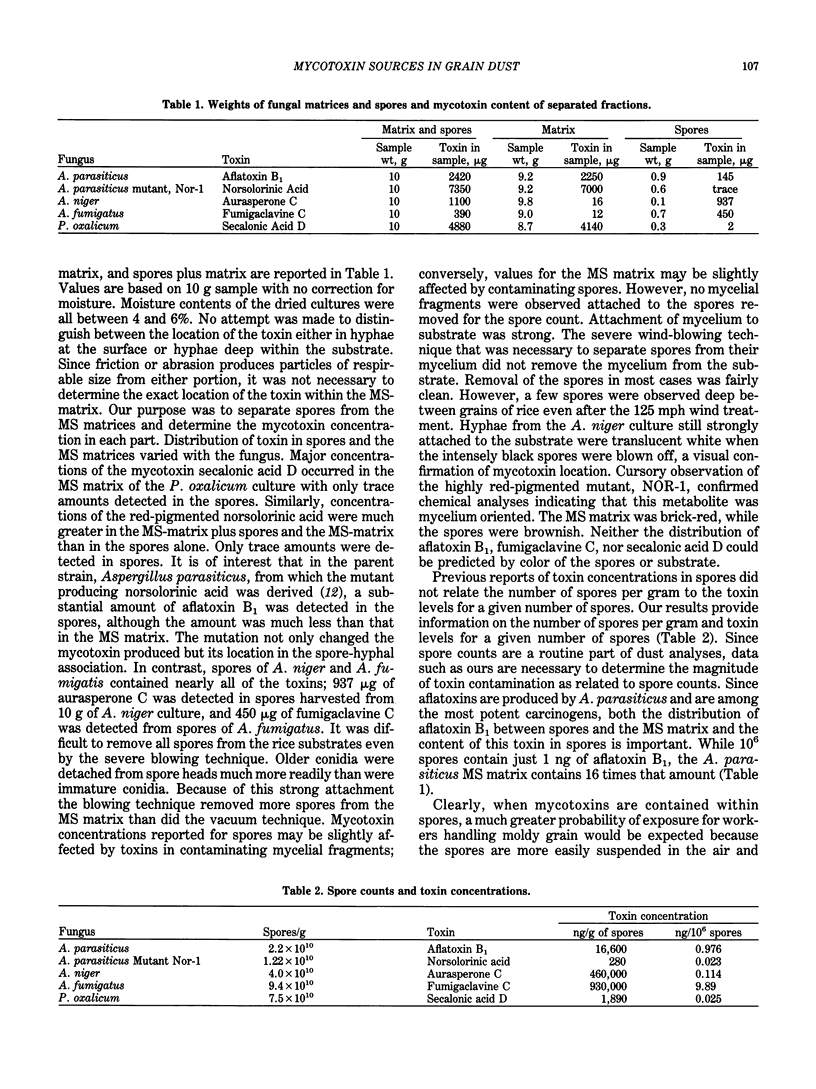
Two distinct reservoirs of mycotoxins exist in fungal-infected cereal grains--the fungal spores and the spore-free mycelium-substrate matrix. Many fungal spores are of respirable size and the mycelium-substrate matrix can be pulverized to form particles of respirable size during routine handling of grain. In order to determine the contribution of each source to the level of mycotoxin contamination of dust, we developed techniques to harvest and separate mycelium-substrate matrices from spores of fungi. Conventional quantitative chromatographic analyses of separated materials indicated that aflatoxin from Aspergillus parasiticus, norsolorinic acid from a mutant of A. parasiticus, and secalonic acid D from Penicillium oxalicum were concentrated in the mycelium-substrate matrices and not in the spores. In contrast, spores of Aspergillus niger and Aspergillus fumigatus contained significant concentrations of aurasperone C and fumigaclavine C, respectively; only negligible amounts of the toxins were detected in the mycelium-substrate matrices of these two fungi.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ciegler A., Hayes A. W., Vesonder R. F. Production and biological activity of secalonic Acid d. Appl Environ Microbiol. 1980 Feb;39(2):285–287. doi: 10.1128/aem.39.2.285-287.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole R. J., Kirksey J. W., Dorner J. W., Wilson D. M., Johnson J. C., Jr, Johnson A. N., Bedell D. M., Springer J. P., Chexal K. K., Clardy J. C. Mycotoxins produced by Aspergillus fumigatus species isolated from molded silage. J Agric Food Chem. 1977 Jul-Aug;25(4):826–830. doi: 10.1021/jf60212a015. [DOI] [PubMed] [Google Scholar]
- Ehrlich K. C., DeLucca A. J., 2nd, Ciegler A. Naphtho-gamma-pyrone production by Aspergillus niger isolated from stored cottonseed. Appl Environ Microbiol. 1984 Jul;48(1):1–4. doi: 10.1128/aem.48.1.1-4.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosal S., Biswas K., Chakrabarti D. K. Toxic naphtho-gamma-pyrones from Aspergillus niger. J Agric Food Chem. 1979 Nov-Dec;27(6):1347–1351. doi: 10.1021/jf60226a018. [DOI] [PubMed] [Google Scholar]
- Hesseltine C. W., Shotwell O. L., Ellis J. J., Stubblefield R. D. Aflatoxin formation by Aspergillus flavus. Bacteriol Rev. 1966 Dec;30(4):795–805. doi: 10.1128/br.30.4.795-805.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmgren M. S., Lee L. S., Delucca A. J., 2nd, Ciegler A. Preliminary study of mycoflora and mycotoxins in grain dust from New Orleans area grain elevators. Am Ind Hyg Assoc J. 1983 Jul;44(7):485–488. doi: 10.1080/15298668391405184. [DOI] [PubMed] [Google Scholar]
- Reddy C. S., Reddy R. V., Hayes A. W. High-performance liquid chromatographic analysis of the mycotoxin secalonic acid D and its application to biological fluids. J Chromatogr. 1981 Apr 3;208(1):17–26. doi: 10.1016/s0021-9673(00)87954-6. [DOI] [PubMed] [Google Scholar]
- Wicklow D. T., Shotwell O. L. Intrafungal distribution of aflatoxins among conidia and sclerotia of Aspergillus flavus and Aspergillus parasiticus. Can J Microbiol. 1983 Jan;29(1):1–5. doi: 10.1139/m83-001. [DOI] [PubMed] [Google Scholar]


