Abstract
Several lines of evidence suggest that planning eye movements and directing visuospatial attention share overlapping brain mechanisms. This study tested whether spatial attention can be enhanced by altering oculomotor signals within the brain. Monkeys performed a spatial attention task while neurons within the frontal eye field, an oculomotor area within prefrontal cortex, were electrically stimulated below the level at which eye movements are evoked. We found that we could improve the monkey's performance with microstimulation when, but only when, the object to be attended was positioned in the space represented by the cortical stimulation site.
Keywords: frontal eye fields, microstimulation, saccades, target selection
Spatial attention allows us to selectively perceive stimuli at relevant locations at the expense of stimuli at other locations. In the visual system, neural correlates of this process can be observed at many stages (1–3), yet the source of these attentive signals remains unknown. Several lines of evidence suggest that planning an eye movement to a particular location can enhance the processing of visual stimuli at that location, regardless of whether the movement is actually carried out (4–7). If this is true, it should be possible to direct visual attention by modifying oculomotor signals within the brain. We attempted to modify the performance of monkeys trained to detect visual events by injecting subthreshold oculomotor signals into the brain. This was done via electrical stimulation of the frontal eye field (FEF), an area with known oculomotor involvement. We hypothesized that if oculomotor planning gives rise to attentional filtering of visual signals, stimulation of an oculomotor area at levels below the movement threshold should allocate attention to targets positioned in the part of space represented by neurons at the stimulation site. We chose the FEF for this experiment because of its known involvement in the programming of saccadic eye movements and in target selection (8, 9), its coherent map of visual space in oculomotor coordinates (10), and its dense connections with posterior visual areas, including posterior parietal cortex (11, 12).
We trained two monkeys to make manual responses to signal the transient dimming (“blink”) of a peripheral visual target in the presence of flashing distractors (Fig. 1; ref. 13). We then tested the effects of FEF microstimulation on their performance. In each experimental session, we first determined the location in space to which suprathreshold microstimulation shifted the direction of gaze, defined here as the motor field (MF). We then lowered the current below the movement threshold level and tested the effects of subthreshold microstimulation on the monkey's performance when the target was placed inside of the MF. We did this in the following way. During blink trials, a 100-ms subthreshold stimulation train preceded the dimming of the target. During “catch” trials, the stimulation still occurred, but the target did not blink. Randomly interleaved with these trials were control trials during which no stimulation occurred. During both stimulation and control trials we measured psychophysical thresholds, defined as the minimum luminance change in the target stimulus the monkey could detect.
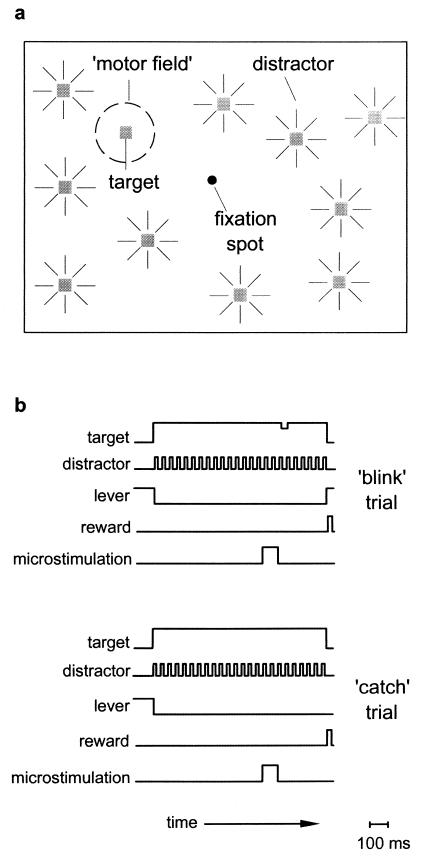
Figure 1.
Schematic depiction of the spatial attention paradigm used to measure the effects of microstimulation. (a) Illustration of the visual display viewed by the monkey. The monkey was trained to fixate on the central fixation spot and attend to a peripheral target that could transiently dim at a random time during the trial. While the monkey awaited the blink event, visual distractors were randomly flashed throughout the display to further require the monkey to attend only to the target stimulus. The dotted circle marked “motor field” signifies the location to which the eyes move during suprathreshold stimulation. (b) The temporal sequence of events during a typical microstimulation trial. The monkey attains fixation, depresses the lever to start the trial, and then releases the lever if and when the target dims. Throughout each trial, a distractor is flashed at random locations at a fixed rate (32 Hz). The top set of traces marked ‘blink’ trial shows the sequence of events in a trial in which the target transiently dims (32–48 ms) at a random time (500–1,300 ms) and the monkey is rewarded for releasing the lever within 600–800 ms. Note than in this trial a 100-ms (subthreshold) stimulation train immediately precedes the blink event. The interval between the onset of the 100-ms stimulation train and the onset of the blink event, which varied from 50 to 175 ms, was defined as the stimulation onset asynchrony. In the bottom set of traces, the target does not dim (‘catch’ trial), the stimulation still occurs, and the monkey is rewarded for holding the lever for the duration of the trial (2,100 ms). Not shown are control trials (blink and catch), which are identical, except that no stimulation was administered.
Methods
Behavioral Task.
In each trial, the monkey first fixated on the central fixation point and depressed a lever, at which time a stable, visual target appeared at a single location. Next, at an unpredictable time, the target dimmed transiently (blink trials), and the monkey was rewarded for releasing the lever within a specified time window (600–800 ms). During one-third of the trials, however, the target did not dim and instead the monkey was rewarded for holding down the lever throughout the duration of the trial (catch trials). Catch trials were used to prevent the monkey from simply releasing on every trial. During all trials, distractor stimuli were continually flashed throughout the display while the monkey waited for the target luminance to change. The distractor stimulus, which had a similar shape and brightness to the target stimulus, was intended to draw attention away from the target stimulus (14), thus making the detection of the target luminance change more difficult. Throughout each trial, the monkey was required to maintain fixation within a 3° fixation window.
Visual Stimuli.
The target stimulus was a white square (0.25–1.25 deg2) with an initial luminance of 26 cd/m2 (background, 1 cd/m2) displayed on a video monitor (30 cm vertical × 40 cm horizontal, 60 Hz) positioned ≈57 cm in front of the monkey. The distractor stimulus was also a white square (0.1–1 deg2) displayed at the same luminance as the target. A distractor was flashed for 16 ms and replotted every 32 ms at random locations excluding a 2–5° circular region surrounding the target stimulus. Initial behavioral tests with and without the distractor stimuli showed that performance was always hindered when the distractors were present. In other words, the minimum target dim amount detectable by the monkey was always higher in the presence of distractors. Control of the display, stimulation, and data storage was maintained by way of a cortex data acquisition system (http://cog.nimh.nih.gov/CORTEX/). Throughout all behavioral testing, eye position was monitored and stored at 200 Hz by the scleral search coil method.
Electrical Stimulation and MF Mapping.
Electrical stimulation consisted of a 100-ms train of biphasic current pulses (0.2 ms, 200 Hz) delivered with a Grass stimulator (S88) and two Grass stimulation isolation units (PSIU-6). Current amplitude was measured via the voltage drop across a 1-kΩ resistor in series with the return lead of the current source. All stimulation was delivered via Pt/Ir electrodes (0.1–1.0 MΩ impedance, at 1 kHz). In each monkey, the FEF was first localized on the basis of its surrounding physiological and anatomical landmarks and our being able to evoke fixed-vector, saccadic eye movements with stimulation at currents of less than 150 μA (typically around 50 μA). The slopes of the duration–amplitude tradeoff functions (“main sequence”) of stimulation evoked saccadic eye movements in the two monkeys, 1.3 ms/deg and 2.2 ms/deg eccentricity, are within the range of visually evoked saccades described in published data (15).
During each experimental session, we determined the saccade vector elicited at the cortical site under study with the use of a separate behavioral paradigm. In this paradigm, the monkey was required to fixate on a visual stimulus (0.5°) for 250–500 ms, after which time a stimulation pulse was delivered. For each trial, the visual stimulus was positioned at one of five positions, one at the center of gaze and one 10° from center along each cardinal direction. The endpoints of saccades evoked from the center of gaze were then used to define the MF. The MF position was then used as the position for the target stimulus in the attention task. For experiments in which the target was placed outside of the MF, an attempt was made to place the target at a location 90° θ from the MF (relative to the fixation point), but at the same eccentricity and within the same hemifield. In some cases, the dimensions of the monitor constrained the placement of the target, and thus other locations distant from the MF were chosen. The average absolute distance between the MF and the outside location was 17° (range 7–27°).
Psychophysical Threshold Estimates.
We used a simultaneous staircase procedure to determine the luminance change threshold for the stimulation and control conditions. In this procedure, each block of trials consisted of two blink trials and one catch trial per condition. Blocks in which the monkey correctly responded to at least one of two target blink events (50%) at a given level and performed correctly on the corresponding catch trial (100%) were followed by a decrease in the magnitude of the target luminance change (% Michaelson contrast). Blocks that were unsuccessful beyond the starting level were followed by increases in the blink magnitude. Step sizes were always constant within a set of blocks. Luminance change thresholds were estimated as the least-square fit of 10–25 blocks of staircase data with an asymptotic function: y = ax/(b + x), where a is the threshold (at 66.7% performance) and b is the block number at half threshold.
Results
We tested the effects of stimulation at a total of 51 cortical sites in two monkeys. Our initial experiments with the first monkey consisted of a simple performance (% correct) comparison of stimulation and control trials. At 17 FEF sites, there was a small, but statistically significant, performance improvement during stimulation trials over that of control trials when the target was positioned within the MF (stimulation − control = 3.9%; t test, P < 0.03). In contrast, when the target was positioned outside of the MF, there was no significant effect on performance (stimulation − control = −3.4%; t test, P = 0.2).
During these initial experiments, we set the test current level to arbitrary points below the movement threshold (46–88% of threshold current) and observed that when the current level approached threshold, the monkey would often break fixation and saccade to the target stimulus, aborting the trial. Thus, stimulation with current levels too close to the movement threshold actually made the task more difficult because it impaired the maintenance of central fixation. Consistent with this observation was a significant negative correlation between the current level chosen and the magnitude of the behavioral improvement with stimulation (Kendall Rank correlation, P < 0.04). In subsequent experiments, we therefore used currents that were ≤50% of the movement threshold current. In addition, we changed our dependent variable from a simple performance measure to that of a psychophysical threshold to increase the sensitivity of the task to the effects of stimulation.
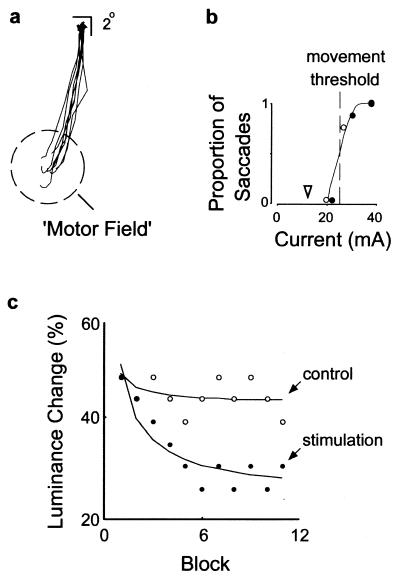
With the use of the lower current levels, we determined the smallest luminance change detectable by the monkey with the same attention task. Psychophysical thresholds were obtained by using a staircase procedure in which the size of the luminance change of the target decreased or increased in steps after each block, depending on the monkey's performance on both blink and catch trials. Fig. 2 illustrates the experimental procedure and an example of the effect of microstimulation in monkey A. In this example, when the FEF site was stimulated with a current of 38 μA, saccadic eye movements were reliably evoked to a location 14° into the lower half-field. With progressively lower currents, fewer saccades were evoked. For this site, the movement threshold, or current at which saccades were evoked half the time, was about 26 μA. During the attention task, microstimulation with a 13-μA (subthreshold) current preceded the blink event on some trials. During trials with no stimulation, the monkey was able to reliably detect a 44% change in target luminance but could not detect smaller changes. In contrast, during stimulation trials the monkey was able to reliably detect a 30% change in target luminance. Thus, in this case, the luminance change required to obtain equal performance between the two experimental conditions differed by 14%, with stimulation trials yielding lower thresholds.
Figure 2.
Illustration of a typical stimulation experiment and the effect of stimulation on psychophysical performance. (a) Individual saccade vectors obtained with suprathreshold stimulation at an FEF site in monkey A, illustrating how the MF was mapped. Vector traces show eight saccades evoked in eight trials at a current of 38 μA. (b) The proportion of evoked saccades was measured at different current levels, both before (●) and after (○) the attention task, to determine the current threshold. The open arrowhead indicates the subthreshold current (13 μA) used during the spatial attention task. (c) Staircase data and luminance change threshold estimates (% contrast) obtained with (●) and without (○) microstimulation (stimulation onset asynchrony = 175 ms). Each staircase plot shows the relative progress made by the monkey and the asymptotic level reached after 11 blocks of testing.
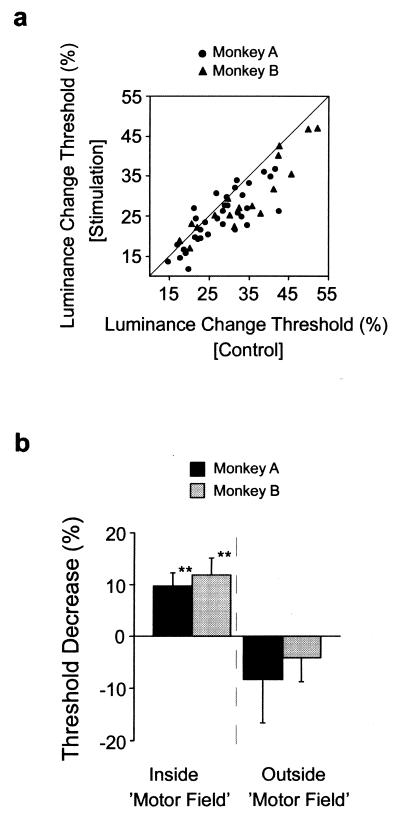
We measured luminance change thresholds during 77 experiments at 34 FEF sites in the two monkeys. MF locations ranged from 6° to 18° in eccentricity in all directions throughout the visual field contralateral to the FEF sites. Comparison of luminance change thresholds measured with and without microstimulation revealed that when the task was performed with the target inside of the MF, both monkeys were able to detect smaller luminance changes when stimulation preceded the blink event. Although psychophysical thresholds varied greatly between experimental sessions, they were consistently lower on stimulation trials than control trials (paired t test: P < 0.0005, monkey A; P < 0.0005, monkey B) (Fig. 3a). Fig. 3b shows that, on average, microstimulation reduced thresholds by about 11% when the task was performed with the target inside of the MF. The effect of stimulation was statistically indistinguishable between the two monkeys (9.7%, monkey A; 11.9%, monkey B; t test, P > 0.6).
Figure 3.
Effect of microstimulation on psychophysical thresholds in two monkeys performing the spatial attention task. (a) Comparison of the distribution of luminance change thresholds obtained with and without stimulation when the attention task was performed inside of the MF. The vast majority of threshold points fall below the line of unity, illustrating the tendency of thresholds to be lower with stimulation. (b) Average decrease in psychophysical thresholds obtained when the attention task was performed inside or outside the MF in each monkey. The reduction in threshold signifies the difference in target luminance change required for equal performance on stimulation and control trials, expressed as a percentage of the control contrast threshold [(1 − [contraststim./contrastcontrol]) × 100]. Positive values denote decreases in threshold with stimulation, whereas negative values denote increases. Targets positioned outside of the MF were on average 17° from the MF, but within the same hemifield. Double asterisks (**) denote a significance of P < 0.001.
Subthreshold stimulation of the FEF appeared to facilitate attention to the target stimulus positioned in the MF. However, we considered the possibility that stimulation might merely affect the monkey's arousal, thus preparing it to respond to the blink event, should it occur. If so, one would expect stimulation to lower thresholds throughout the visual field. Thus, the effect of stimulation should also be obtained when the target is placed at a location outside of the MF. However, we did not observe this. Instead, we found that when the task was performed with the target outside of the MF, but within the same hemifield, psychophysical thresholds on stimulation trials were slightly higher than on control trials, although this effect was not significant (paired t test: P = 0.19, monkey A; P = 0.15, monkey B) (Fig. 3b). Thus, the effect of stimulation appears to operate via a spatially restricted facilitation of the salience of objects inside of the MF.
Discussion
Our results show that cortical sites from which saccadic eye movements can be evoked with microstimulation coincide with sites from which enhancement in attentional performance can also be obtained. This evidence suggests that increased activity among neurons representing specific locations within oculomotor space also initiates increased signaling of visual targets within that space, even when a movement is not made. This interpretation is consistent with anatomical evidence revealing widespread and topographically segregated “feedback” projections from the FEF to posterior visual areas (11, 12). These pathways may provide one mechanism by which the multitude of attentional modulations observed within visual cortex (1–3, 16–18) is brought about.
It is important, however, to consider the degree to which the stimulation effects were due to direct activation of FEF neurons, as opposed to other brain structures. Electrical stimulation of neural tissue activates all neural elements in the vicinity of the electrode tip (19). Our effects could thus have resulted from direct antidromic stimulation of neurons forming connections with the FEF, rather than from activation of neurons within the FEF, and the subsequent activation of structures to which the FEF projects. Although antidromic activation typically requires current levels greater than that used here (median = 10 μA) (20, 21), we cannot rule out the contribution such activation may have made to our results. Moreover, the FEF is heavily interconnected with other cortical and subcortical structures, such as the superior colliculus and the lateral intraparietal area (LTP) (22), both of which are known to be involved in oculomotor behavior and spatial attention (13, 23–24). Thus, FEF stimulation may have orthodromically recruited these structures, which in turn produced the attentional modulation. Evidence of deficits in attention has, in fact, been observed in monkeys with lesions of the FEF, LIP, or the superior colliculus (25–27). Regardless of the site of activation, the present results provide evidence of a direct effect of oculomotor signaling on the allocation of spatial attention. Moreover, the present study provides a basis for studies of the links between motor planning and visual processing, a necessary step in our understanding of sensorimotor integration.
Acknowledgments
We thank C. G. Gross, M. S. A. Graziano, and E. J. Tehovnik for providing valuable comments on earlier versions of the manuscript. We also thank J. Y. Lee and M. W. Wagers for their help. This work was supported by National Institutes of Health Grants NEI-11347 and MH-12336.
Abbreviations
- FEF
frontal eye field
- MF
motor field
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.021549498.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.021549498
References
- 1.Luck S J, Chelazzi L, Hillyard S A, Desimone R. J Neurophysiol. 1997;77:24–42. doi: 10.1152/jn.1997.77.1.24. [DOI] [PubMed] [Google Scholar]
- 2.Treue S, Maunsell J H. Nature (London) 1996;382:539–541. doi: 10.1038/382539a0. [DOI] [PubMed] [Google Scholar]
- 3.Corbetta M, Akbudak E, Conturo T E, Snyder A Z, Ollinger J M, Drury H A, Linenweber M R, Petersen S E, Raichle M E, Van Essen D C, et al. Neuron. 1998;21:761–773. doi: 10.1016/s0896-6273(00)80593-0. [DOI] [PubMed] [Google Scholar]
- 4.Rizzolatti G. In: Advances in Vertebrate Neuroethology. Ewert J P, Capranica R R, Ingle D J, editors. New York: Plenum; 1983. pp. 261–297. [Google Scholar]
- 5.Shepherd M, Findlay J M, Hockey R J. Q J Exp Psychol A. 1986;38:475–491. doi: 10.1080/14640748608401609. [DOI] [PubMed] [Google Scholar]
- 6.Sheliga B M, Riggio L, Rizzolatti G. Exp Brain Res. 1994;98:507–522. doi: 10.1007/BF00233988. [DOI] [PubMed] [Google Scholar]
- 7.Kowler E, Anderson E, Dosher B, Blaser E. Vision Res. 1995;35:1897–1916. doi: 10.1016/0042-6989(94)00279-u. [DOI] [PubMed] [Google Scholar]
- 8.Robinson D A, Fuchs A F. J Neurophysiol. 1969;32:637–648. doi: 10.1152/jn.1969.32.5.637. [DOI] [PubMed] [Google Scholar]
- 9.Schall J D, Hanes D P, Thompson K G, King D J. J Neurosci. 1995;15:6905–6918. doi: 10.1523/JNEUROSCI.15-10-06905.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bruce C J, Goldberg M E, Bushnell M C, Stanton G B. J Neurophysiol. 1985;54:714–734. doi: 10.1152/jn.1985.54.3.714. [DOI] [PubMed] [Google Scholar]
- 11.Stanton G B, Bruce C J, Goldberg M E. J Comp Neurol. 1995;353:291–305. doi: 10.1002/cne.903530210. [DOI] [PubMed] [Google Scholar]
- 12.Schall J D, Morel A, King D J, Bullier J. J Neurosci. 1995;15:4464–4487. doi: 10.1523/JNEUROSCI.15-06-04464.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wurtz R H, Mohler C W. J Neurophysiol. 1976;39:766–772. doi: 10.1152/jn.1976.39.4.766. [DOI] [PubMed] [Google Scholar]
- 14.Remington R W, Johnston J C, Yantis S. Percept Psychophys. 1992;51:279–290. doi: 10.3758/bf03212254. [DOI] [PubMed] [Google Scholar]
- 15.Becker W. In: Neurobiology of Saccadic Eye Movements. Wurtz R H, Goldberg M E, editors. New York: Elsevier; 1989. pp. 13–67. [Google Scholar]
- 16.Desimone R, Duncan J. Annu Rev Neurosci. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- 17.Connor C E, Preddie D C, Gallant J L, Van Essen D C. J Neurosci. 1997;17:3201–3214. doi: 10.1523/JNEUROSCI.17-09-03201.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reynolds J H, Pasternak T, Desimone R. Neuron. 2000;26:703–714. doi: 10.1016/s0896-6273(00)81206-4. [DOI] [PubMed] [Google Scholar]
- 19.Tehovnik E J. J Neurosci Methods. 1996;65:1–17. doi: 10.1016/0165-0270(95)00131-x. [DOI] [PubMed] [Google Scholar]
- 20.Sommer M A, Wurtz R H. J Neurophysiol. 2000;83:1979–2001. doi: 10.1152/jn.2000.83.4.1979. [DOI] [PubMed] [Google Scholar]
- 21.Pare M, Wurtz R H. J Neurophysiol. 1997;78:3493–3497. doi: 10.1152/jn.1997.78.6.3493. [DOI] [PubMed] [Google Scholar]
- 22.Schall J D. In: Cerebral Cortex. Rockland K S, Kaas J H, Peters A, editors. Vol. 12. New York: Plenum; 1997. pp. 527–638. [Google Scholar]
- 23.Colby C L, Duhamel J R, Goldberg M E. J Neurophysiol. 1996;76:2841–2852. doi: 10.1152/jn.1996.76.5.2841. [DOI] [PubMed] [Google Scholar]
- 24.Kustov A A, Robinson D L. Nature (London) 1996;384:74–77. doi: 10.1038/384074a0. [DOI] [PubMed] [Google Scholar]
- 25.Welch K, Stuteville P. Brain. 1958;81:341–347. doi: 10.1093/brain/81.3.341. [DOI] [PubMed] [Google Scholar]
- 26.Schiller P H, True S D, Conway J L. Science. 1979;206:590–592. doi: 10.1126/science.115091. [DOI] [PubMed] [Google Scholar]
- 27.Lynch J C, McLaren J W. J Neurophysiol. 1989;61:74–90. doi: 10.1152/jn.1989.61.1.74. [DOI] [PubMed] [Google Scholar]