Abstract
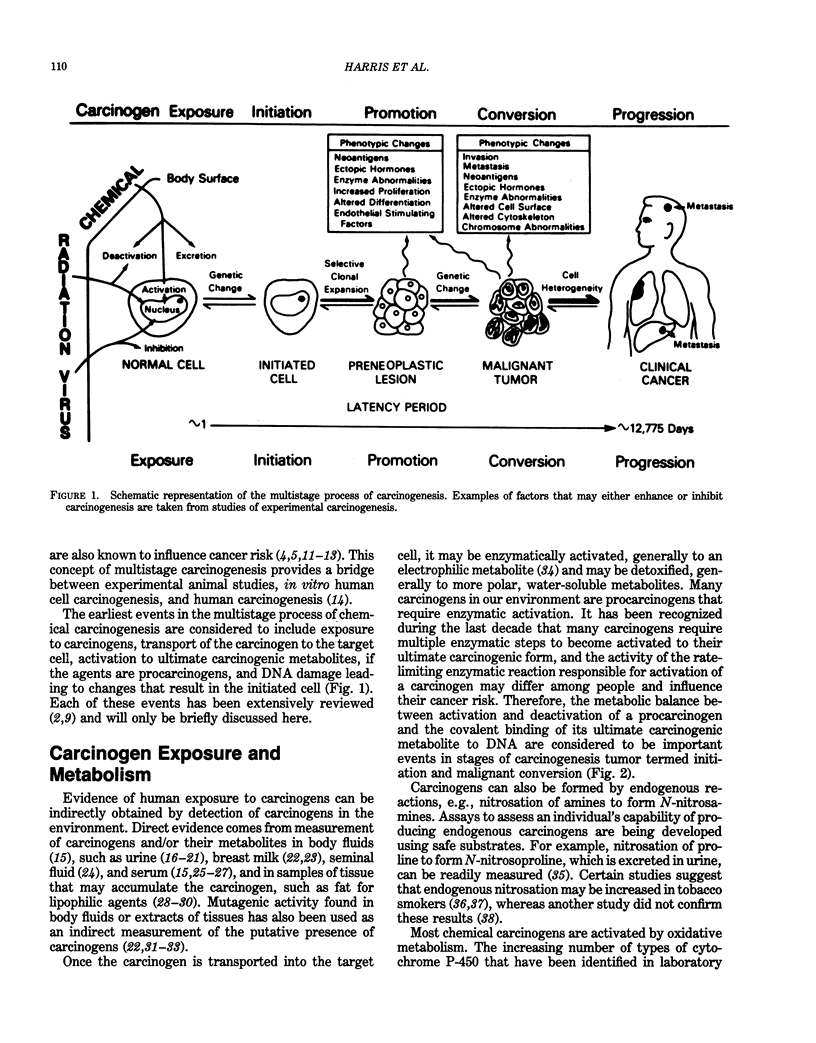
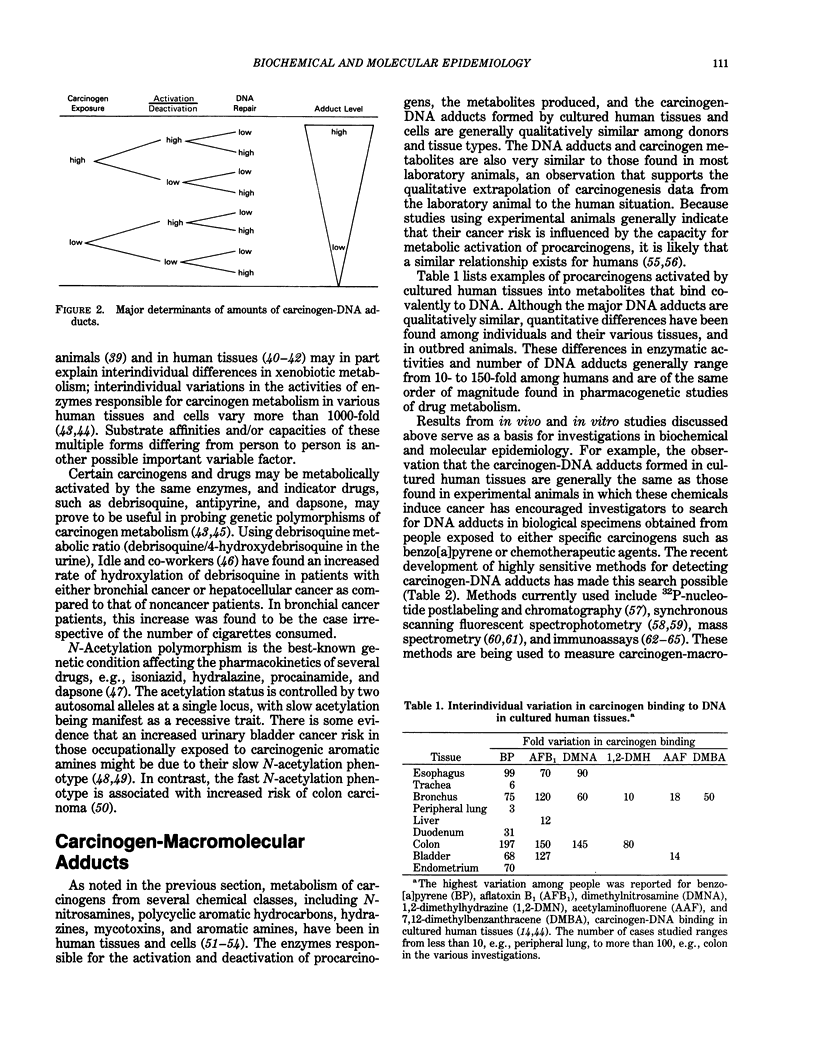
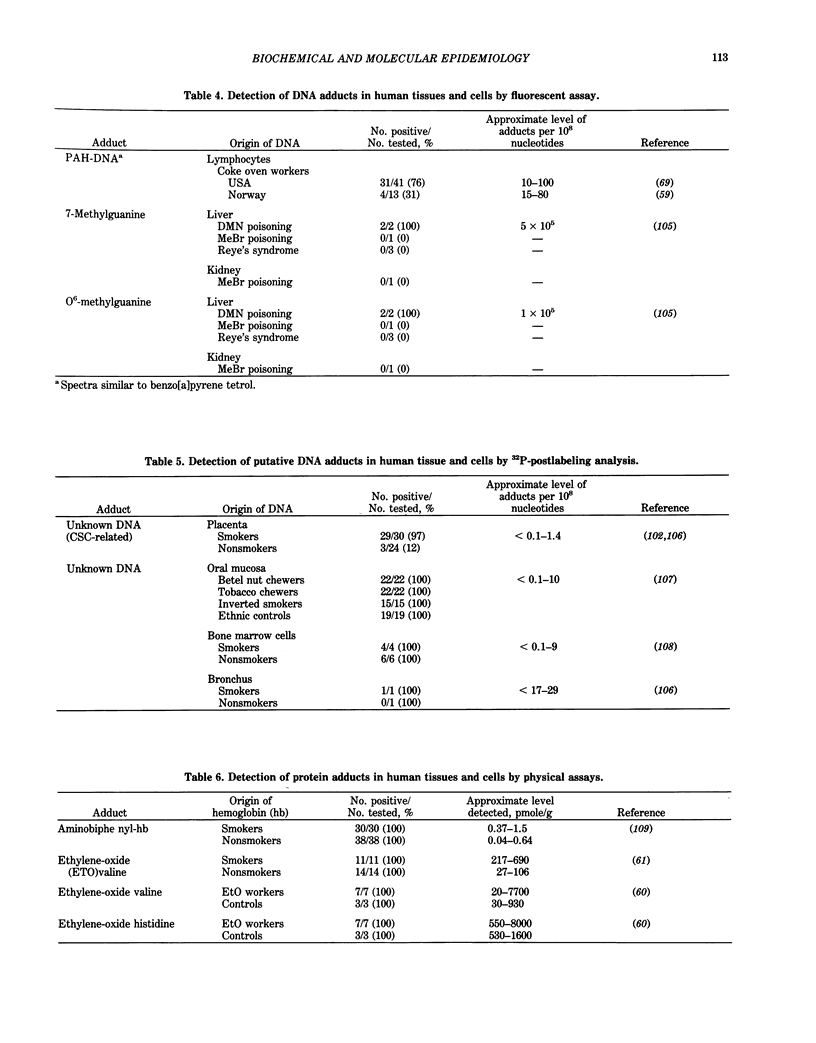
The primary goal of biochemical and molecular epidemiology is to identify individuals at high cancer risk by obtaining evidence of high exposure to carcinogens, leading to pathobiological lesions in target cells, and/or increased oncogenic susceptibility due to either inherited or acquired host factors. This emerging and multidisciplinary area of cancer research combines epidemiological and laboratory approaches. Because DNA is considered to be an important target for modification by mutagens and carcinogens, damage to DNA can be used as an internal, molecular dosimeter of carcinogen exposure. The reactive species of these carcinogens may directly bind to DNA to form adducts and may indirectly cause secondary DNA lesions, e.g., via induction of free radicals and aldehydes. Highly sensitive and specific methods have been developed to measure the minute amounts of DNA lesions and DNA repair products found in biological specimens from humans exposed to carcinogens. For example, DNA adducts have been measured in cells and tissues from people occupationally exposed to carcinogenic polycyclic aromatic hydrocarbons. Antibodies recognizing carcinogen-DNA adducts have also been detected in human sera. Inherited predisposition to cancer has been revealed by recent advances in molecular genetics, including restriction-fragment-length polymorphism. For example, the hypothesis that rare alleles of the Ha-ras proto-oncogene are associated with an increased risk of lung cancer is currently being tested. These approaches afford the potential of biochemical and molecular epidemiology to predict disease risk for individual persons, instead of for populations, and before the onset of clinically evident disease.
Full text
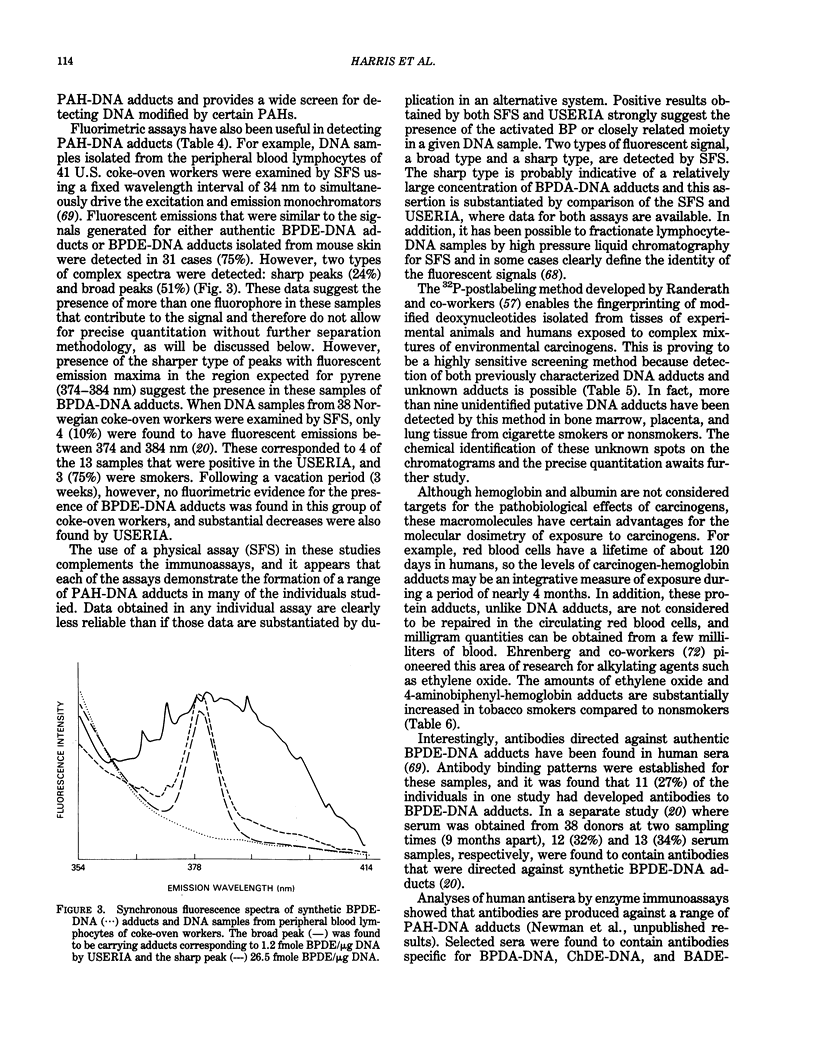
PDF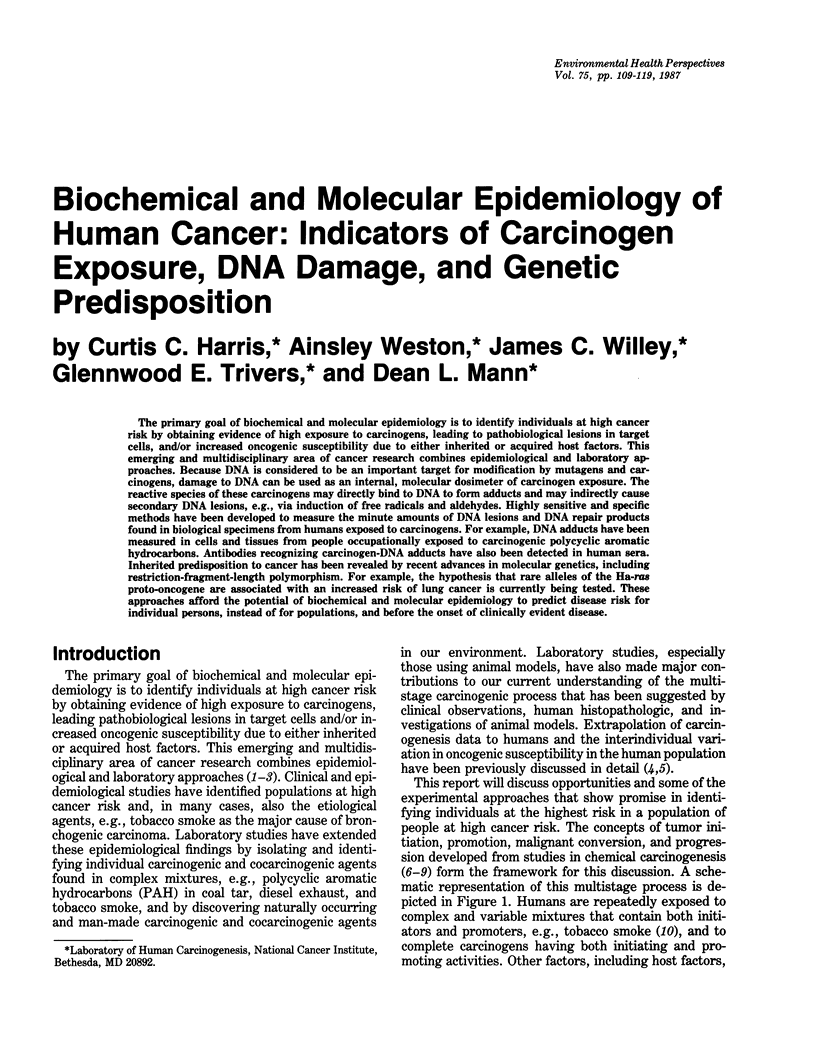






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames B. N. Identifying environmental chemicals causing mutations and cancer. Science. 1979 May 11;204(4393):587–593. doi: 10.1126/science.373122. [DOI] [PubMed] [Google Scholar]
- Ayesh R., Idle J. R., Ritchie J. C., Crothers M. J., Hetzel M. R. Metabolic oxidation phenotypes as markers for susceptibility to lung cancer. Nature. 1984 Nov 8;312(5990):169–170. doi: 10.1038/312169a0. [DOI] [PubMed] [Google Scholar]
- Cairns J. The origin of human cancers. Nature. 1981 Jan 29;289(5796):353–357. doi: 10.1038/289353a0. [DOI] [PubMed] [Google Scholar]
- Campbell T. C., Caedo J. P., Jr, Bulatao-Jayme J., Salamat L., Engel R. W. Aflatoxin M1 in human urine. Nature. 1970 Jul 25;227(5256):403–404. doi: 10.1038/227403a0. [DOI] [PubMed] [Google Scholar]
- Cartwright R. A., Glashan R. W., Rogers H. J., Ahmad R. A., Barham-Hall D., Higgins E., Kahn M. A. Role of N-acetyltransferase phenotypes in bladder carcinogenesis: a pharmacogenetic epidemiological approach to bladder cancer. Lancet. 1982 Oct 16;2(8303):842–845. doi: 10.1016/s0140-6736(82)90810-8. [DOI] [PubMed] [Google Scholar]
- Cerutti P. A. Prooxidant states and tumor promotion. Science. 1985 Jan 25;227(4685):375–381. doi: 10.1126/science.2981433. [DOI] [PubMed] [Google Scholar]
- Chu G., Vollrath D., Davis R. W. Separation of large DNA molecules by contour-clamped homogeneous electric fields. Science. 1986 Dec 19;234(4783):1582–1585. doi: 10.1126/science.3538420. [DOI] [PubMed] [Google Scholar]
- Conney A. H. Induction of microsomal enzymes by foreign chemicals and carcinogenesis by polycyclic aromatic hydrocarbons: G. H. A. Clowes Memorial Lecture. Cancer Res. 1982 Dec;42(12):4875–4917. [PubMed] [Google Scholar]
- Doll R. An epidemiological perspective of the biology of cancer. Cancer Res. 1978 Nov;38(11 Pt 1):3573–3583. [PubMed] [Google Scholar]
- Dunn B. P., Stich H. F. 32P-postlabelling analysis of aromatic DNA adducts in human oral mucosal cells. Carcinogenesis. 1986 Jul;7(7):1115–1120. doi: 10.1093/carcin/7.7.1115. [DOI] [PubMed] [Google Scholar]
- EVANS D. A., WHITE T. A. HUMAN ACETYLATION POLYMORPHISM. J Lab Clin Med. 1964 Mar;63:394–403. [PubMed] [Google Scholar]
- Ekström G., von Bahr C., Glaumann H., Ingelman-Sundberg M. Interindividual variation in benzo(a)pyrene metabolism and composition of isoenzymes of cytochrome P-450 as revealed by SDS-gel electrophoresis of human liver microsomal fractions. Acta Pharmacol Toxicol (Copenh) 1982 Apr;50(4):251–260. doi: 10.1111/j.1600-0773.1982.tb00971.x. [DOI] [PubMed] [Google Scholar]
- Everson R. B., Randerath E., Santella R. M., Cefalo R. C., Avitts T. A., Randerath K. Detection of smoking-related covalent DNA adducts in human placenta. Science. 1986 Jan 3;231(4733):54–57. doi: 10.1126/science.3941892. [DOI] [PubMed] [Google Scholar]
- Farber E. Chemical carcinogenesis: a current biological perspective. Carcinogenesis. 1984 Jan;5(1):1–5. doi: 10.1093/carcin/5.1.1. [DOI] [PubMed] [Google Scholar]
- Farmer P. B., Bailey E., Gorf S. M., Törnqvist M., Osterman-Golkar S., Kautiainen A., Lewis-Enright D. P. Monitoring human exposure to ethylene oxide by the determination of haemoglobin adducts using gas chromatography-mass spectrometry. Carcinogenesis. 1986 Apr;7(4):637–640. doi: 10.1093/carcin/7.4.637. [DOI] [PubMed] [Google Scholar]
- Fearon E. R., Feinberg A. P., Hamilton S. H., Vogelstein B. Loss of genes on the short arm of chromosome 11 in bladder cancer. 1985 Nov 28-Dec 4Nature. 318(6044):377–380. doi: 10.1038/318377a0. [DOI] [PubMed] [Google Scholar]
- Fearon E. R., Vogelstein B., Feinberg A. P. Somatic deletion and duplication of genes on chromosome 11 in Wilms' tumours. Nature. 1984 May 10;309(5964):176–178. doi: 10.1038/309176a0. [DOI] [PubMed] [Google Scholar]
- Francomano C. A., Kazazian H. H., Jr DNA analysis in genetic disorders. Annu Rev Med. 1986;37:377–395. doi: 10.1146/annurev.me.37.020186.002113. [DOI] [PubMed] [Google Scholar]
- Frenkel K., Chrzan K. Hydrogen peroxide formation and DNA base modification by tumor promoter-activated polymorphonuclear leukocytes. Carcinogenesis. 1987 Mar;8(3):455–460. doi: 10.1093/carcin/8.3.455. [DOI] [PubMed] [Google Scholar]
- Friend S. H., Bernards R., Rogelj S., Weinberg R. A., Rapaport J. M., Albert D. M., Dryja T. P. A human DNA segment with properties of the gene that predisposes to retinoblastoma and osteosarcoma. Nature. 1986 Oct 16;323(6089):643–646. doi: 10.1038/323643a0. [DOI] [PubMed] [Google Scholar]
- Fujino T., Park S. S., West D., Gelboin H. V. Phenotyping of cytochromes P-450 in human tissues with monoclonal antibodies. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3682–3686. doi: 10.1073/pnas.79.12.3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland W. A., Kuenzig W., Rubio F., Kornychuk H., Norkus E. P., Conney A. H. Urinary excretion of nitrosodimethylamine and nitrosoproline in humans: interindividual and intraindividual differences and the effect of administered ascorbic acid and alpha-tocopherol. Cancer Res. 1986 Oct;46(10):5392–5400. [PubMed] [Google Scholar]
- Geiser A. G., Der C. J., Marshall C. J., Stanbridge E. J. Suppression of tumorigenicity with continued expression of the c-Ha-ras oncogene in EJ bladder carcinoma-human fibroblast hybrid cells. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5209–5213. doi: 10.1073/pnas.83.14.5209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhard D. S., Dracopoli N. C., Bale S. J., Houghton A. N., Watkins P., Payne C. E., Greene M. H., Housman D. E. Evidence against Ha-ras-1 involvement in sporadic and familial melanoma. Nature. 1987 Jan 1;325(6099):73–75. doi: 10.1038/325073a0. [DOI] [PubMed] [Google Scholar]
- Groopman J. D., Donahue P. R., Zhu J. Q., Chen J. S., Wogan G. N. Aflatoxin metabolism in humans: detection of metabolites and nucleic acid adducts in urine by affinity chromatography. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6492–6496. doi: 10.1073/pnas.82.19.6492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen M. F., Koufos A., Gallie B. L., Phillips R. A., Fodstad O., Brøgger A., Gedde-Dahl T., Cavenee W. K. Osteosarcoma and retinoblastoma: a shared chromosomal mechanism revealing recessive predisposition. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6216–6220. doi: 10.1073/pnas.82.18.6216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris C. C. Human tissues and cells in carcinogenesis research. Cancer Res. 1987 Jan 1;47(1):1–10. [PubMed] [Google Scholar]
- Harris C. C., Trump B. F., Grafstrom R., Autrup H. Differences in metabolism of chemical carcinogens in cultured human epithelial tissues and cells. J Cell Biochem. 1982;18(3):285–294. doi: 10.1002/jcb.1982.240180304. [DOI] [PubMed] [Google Scholar]
- Harris C. C., Vahakangas K., Autrup H., Trivers G. E., Shamsuddin A. K., Trump B. F., Boman B. M., Mann D. L. Biochemical and molecular epidemiology of human cancer risk. Monogr Pathol. 1985;(26):140–167. [PubMed] [Google Scholar]
- Harris C. C., Vahakangas K., Newman M. J., Trivers G. E., Shamsuddin A., Sinopoli N., Mann D. L., Wright W. E. Detection of benzo[a]pyrene diol epoxide-DNA adducts in peripheral blood lymphocytes and antibodies to the adducts in serum from coke oven workers. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6672–6676. doi: 10.1073/pnas.82.19.6672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugen A., Becher G., Benestad C., Vahakangas K., Trivers G. E., Newman M. J., Harris C. C. Determination of polycyclic aromatic hydrocarbons in the urine, benzo(a)pyrene diol epoxide-DNA adducts in lymphocyte DNA, and antibodies to the adducts in sera from coke oven workers exposed to measured amounts of polycyclic aromatic hydrocarbons in the work atmosphere. Cancer Res. 1986 Aug;46(8):4178–4183. [PubMed] [Google Scholar]
- Higginson J. Developing concepts on environmental cancer: the role of geographical pathology. Environ Mutagen. 1983;5(6):929–940. doi: 10.1002/em.2860050616. [DOI] [PubMed] [Google Scholar]
- Hoffmann D., Brunnemann K. D. Endogenous formation of N-nitrosoproline in cigarette smokers. Cancer Res. 1983 Nov;43(11):5570–5574. [PubMed] [Google Scholar]
- Hoffmann D., Hecht S. S., Wynder E. L. Tumor promoters and cocarcinogens in tobacco carcinogenesis. Environ Health Perspect. 1983 Apr;50:247–257. doi: 10.1289/ehp.8350247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu I. C., Poirier M. C., Yuspa S. H., Grunberger D., Weinstein I. B., Yolken R. H., Harris C. C. Measurement of benzo(a)pyrene-DNA adducts by enzyme immunoassays and radioimmunoassay. Cancer Res. 1981 Mar;41(3):1091–1095. [PubMed] [Google Scholar]
- Hudec T., Thean J., Kuehl D., Dougherty R. C. Tris(dichloropropyl)phosphate, a mutagenic flame retardant: frequent cocurrence in human seminal plasma. Science. 1981 Feb 27;211(4485):951–952. doi: 10.1126/science.7466368. [DOI] [PubMed] [Google Scholar]
- Kaelbling M., Klinger H. P. Suppression of tumorigenicity in somatic cell hybrids. III. Cosegregation of human chromosome 11 of a normal cell and suppression of tumorigenicity in intraspecies hybrids of normal diploid x malignant cells. Cytogenet Cell Genet. 1986;41(2):65–70. doi: 10.1159/000132206. [DOI] [PubMed] [Google Scholar]
- Koufos A., Hansen M. F., Lampkin B. C., Workman M. L., Copeland N. G., Jenkins N. A., Cavenee W. K. Loss of alleles at loci on human chromosome 11 during genesis of Wilms' tumour. Nature. 1984 May 10;309(5964):170–172. doi: 10.1038/309170a0. [DOI] [PubMed] [Google Scholar]
- Kriebel D., Commoner B., Bollinger D., Bronsdon A., Gold J., Henry J. Detection of occupational exposure to genotoxic agents with a urinary mutagen assay. Mutat Res. 1983 Mar;108(1-3):67–79. doi: 10.1016/0027-5107(83)90110-0. [DOI] [PubMed] [Google Scholar]
- Kutz F. W., Strassman S. C., Sperling J. F. Survey of selected organochlorine pesticides in the general population of the United States: fiscal years 1970-1975. Ann N Y Acad Sci. 1979 May 31;320:60–68. doi: 10.1111/j.1749-6632.1979.tb56593.x. [DOI] [PubMed] [Google Scholar]
- Lang N. P., Chu D. Z., Hunter C. F., Kendall D. C., Flammang T. J., Kadlubar F. F. Role of aromatic amine acetyltransferase in human colorectal cancer. Arch Surg. 1986 Nov;121(11):1259–1261. doi: 10.1001/archsurg.121.11.1259. [DOI] [PubMed] [Google Scholar]
- Leadon S. A., Hanawalt P. C. Monoclonal antibody to DNA containing thymine glycol. Mutat Res. 1983 Aug;112(4):191–200. doi: 10.1016/0167-8817(83)90006-8. [DOI] [PubMed] [Google Scholar]
- Lidereau R., Escot C., Theillet C., Champeme M. H., Brunet M., Gest J., Callahan R. High frequency of rare alleles of the human c-Ha-ras-1 proto-oncogene in breast cancer patients. J Natl Cancer Inst. 1986 Sep;77(3):697–701. doi: 10.1093/jnci/77.3.697. [DOI] [PubMed] [Google Scholar]
- Lower G. M., Jr, Nilsson T., Nelson C. E., Wolf H., Gamsky T. E., Bryan G. T. N-acetyltransferase phenotype and risk in urinary bladder cancer: approaches in molecular epidemiology. Preliminary results in Sweden and Denmark. Environ Health Perspect. 1979 Apr;29:71–79. doi: 10.1289/ehp.792971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz W. K. Quantitative evaluation of DNA binding data for risk estimation and for classification of direct and indirect carcinogens. J Cancer Res Clin Oncol. 1986;112(2):85–91. doi: 10.1007/BF00404387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mes J., Campbell D. S., Robinson R. N., Davies D. J. Polychlorinated biphenyl and organochlorine pesticide residues in adipose tissue of Canadians. Bull Environ Contam Toxicol. 1977 Feb;17(2):196–203. doi: 10.1007/BF01685550. [DOI] [PubMed] [Google Scholar]
- Miller E. C., Miller J. A. Mechanisms of chemical carcinogenesis. Cancer. 1981 Mar 1;47(5 Suppl):1055–1064. doi: 10.1002/1097-0142(19810301)47:5+<1055::aid-cncr2820471302>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Minchin R. F., McManus M. E., Boobis A. R., Davies D. S., Thorgeirsson S. S. Polymorphic metabolism of the carcinogen 2-acetylaminofluorene in human liver microsomes. Carcinogenesis. 1985 Dec;6(12):1721–1724. doi: 10.1093/carcin/6.12.1721. [DOI] [PubMed] [Google Scholar]
- Moolgavkar S. H., Knudson A. G., Jr Mutation and cancer: a model for human carcinogenesis. J Natl Cancer Inst. 1981 Jun;66(6):1037–1052. doi: 10.1093/jnci/66.6.1037. [DOI] [PubMed] [Google Scholar]
- Murphree A. L., Benedict W. F. Retinoblastoma: clues to human oncogenesis. Science. 1984 Mar 9;223(4640):1028–1033. doi: 10.1126/science.6320372. [DOI] [PubMed] [Google Scholar]
- Müller R., Rajewsky M. F. Antibodies specific for DNA components structurally modified by chemical carcinogens. J Cancer Res Clin Oncol. 1981;102(2):99–113. doi: 10.1007/BF00410662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebert D. W., Negishi M. Multiple forms of cytochrome P-450 and the importance of molecular biology and evolution. Biochem Pharmacol. 1982 Jul 15;31(14):2311–2317. doi: 10.1016/0006-2952(82)90523-8. [DOI] [PubMed] [Google Scholar]
- Omenn G. S. Predictive identification of hypersusceptible individuals. J Occup Med. 1982 May;24(5):369–374. doi: 10.1097/00043764-198205000-00007. [DOI] [PubMed] [Google Scholar]
- Orkin S. H., Goldman D. S., Sallan S. E. Development of homozygosity for chromosome 11p markers in Wilms' tumour. Nature. 1984 May 10;309(5964):172–174. doi: 10.1038/309172a0. [DOI] [PubMed] [Google Scholar]
- Pelkonen O., Nebert D. W. Metabolism of polycyclic aromatic hydrocarbons: etiologic role in carcinogenesis. Pharmacol Rev. 1982 Jun;34(2):189–222. [PubMed] [Google Scholar]
- Perera F. P., Poirier M. C., Yuspa S. H., Nakayama J., Jaretzki A., Curnen M. M., Knowles D. M., Weinstein I. B. A pilot project in molecular cancer epidemiology: determination of benzo[a]pyrene--DNA adducts in animal and human tissues by immunoassays. Carcinogenesis. 1982;3(12):1405–1410. doi: 10.1093/carcin/3.12.1405. [DOI] [PubMed] [Google Scholar]
- Perera F. P., Weinstein I. B. Molecular epidemiology and carcinogen-DNA adduct detection: new approaches to studies of human cancer causation. J Chronic Dis. 1982;35(7):581–600. doi: 10.1016/0021-9681(82)90078-9. [DOI] [PubMed] [Google Scholar]
- Phillips D. H., Hewer A., Grover P. L. Aromatic DNA adducts in human bone marrow and peripheral blood leukocytes. Carcinogenesis. 1986 Dec;7(12):2071–2075. doi: 10.1093/carcin/7.12.2071. [DOI] [PubMed] [Google Scholar]
- Poirier M. C. Antibodies to carcinogen-DNA adducts. J Natl Cancer Inst. 1981 Sep;67(3):515–519. [PubMed] [Google Scholar]
- Poirier M. C., Reed E., Zwelling L. A., Ozols R. F., Litterst C. L., Yuspa S. H. Polyclonal antibodies to quantitate cis-diamminedichloroplatinum(II)--DNA adducts in cancer patients and animal models. Environ Health Perspect. 1985 Oct;62:89–94. doi: 10.1289/ehp.856289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier M. C., Santella R., Weinstein I. B., Grunberger D., Yuspa S. H. Quantitation of benzo(a)pyrene-deoxyguanosine adducts by radioimmunoassay. Cancer Res. 1980 Feb;40(2):412–416. [PubMed] [Google Scholar]
- Rahn R. O., Chang S. S., Holland J. M., Shugart L. R. A fluorometric-HPLC assay for quantitating the binding of benzo[a]pyrene metabolites to DNA. Biochem Biophys Res Commun. 1982 Nov 16;109(1):262–268. doi: 10.1016/0006-291x(82)91594-7. [DOI] [PubMed] [Google Scholar]
- Randerath E., Avitts T. A., Reddy M. V., Miller R. H., Everson R. B., Randerath K. Comparative 32P-analysis of cigarette smoke-induced DNA damage in human tissues and mouse skin. Cancer Res. 1986 Nov;46(11):5869–5877. [PubMed] [Google Scholar]
- Randerath K., Randerath E., Agrawal H. P., Gupta R. C., Schurdak M. E., Reddy M. V. Postlabeling methods for carcinogen-DNA adduct analysis. Environ Health Perspect. 1985 Oct;62:57–65. doi: 10.1289/ehp.856257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve A. E., Housiaux P. J., Gardner R. J., Chewings W. E., Grindley R. M., Millow L. J. Loss of a Harvey ras allele in sporadic Wilms' tumour. Nature. 1984 May 10;309(5964):174–176. doi: 10.1038/309174a0. [DOI] [PubMed] [Google Scholar]
- Sager R. Genetic suppression of tumor formation. Adv Cancer Res. 1985;44:43–68. doi: 10.1016/s0065-230x(08)60025-1. [DOI] [PubMed] [Google Scholar]
- Schneider N. R., Williams W. R., Chaganti R. S. Genetic epidemiology of familial aggregation of cancer. Adv Cancer Res. 1986;47:1–36. doi: 10.1016/s0065-230x(08)60196-7. [DOI] [PubMed] [Google Scholar]
- Seizinger B. R., Martuza R. L., Gusella J. F. Loss of genes on chromosome 22 in tumorigenesis of human acoustic neuroma. Nature. 1986 Aug 14;322(6080):644–647. doi: 10.1038/322644a0. [DOI] [PubMed] [Google Scholar]
- Shamsuddin A. K., Sinopoli N. T., Hemminki K., Boesch R. R., Harris C. C. Detection of benzo(a)pyrene:DNA adducts in human white blood cells. Cancer Res. 1985 Jan;45(1):66–68. [PubMed] [Google Scholar]
- Shireman R. B., Schneider M. Association of aflatoxin B1 with plasma components in vitro. Toxicol Lett. 1982 Dec;14(3-4):213–220. doi: 10.1016/0378-4274(82)90054-6. [DOI] [PubMed] [Google Scholar]
- Stanbridge E. J., Der C. J., Doersen C. J., Nishimi R. Y., Peehl D. M., Weissman B. E., Wilkinson J. E. Human cell hybrids: analysis of transformation and tumorigenicity. Science. 1982 Jan 15;215(4530):252–259. doi: 10.1126/science.7053574. [DOI] [PubMed] [Google Scholar]
- Tsuboi S., Nakagawa T., Tomita M., Seo T., Ono H., Kawamura K., Iwamura N. Detection of aflatoxin B1 in serum samples of male Japanese subjects by radioimmunoassay and high-performance liquid chromatography. Cancer Res. 1984 Mar;44(3):1231–1234. [PubMed] [Google Scholar]
- Törnqvist M., Osterman-Golkar S., Kautiainen A., Jensen S., Farmer P. B., Ehrenberg L. Tissue doses of ethylene oxide in cigarette smokers determined from adduct levels in hemoglobin. Carcinogenesis. 1986 Sep;7(9):1519–1521. doi: 10.1093/carcin/7.9.1519. [DOI] [PubMed] [Google Scholar]
- Umbenhauer D., Wild C. P., Montesano R., Saffhill R., Boyle J. M., Huh N., Kirstein U., Thomale J., Rajewsky M. F., Lu S. H. O(6)-methyldeoxyguanosine in oesophageal DNA among individuals at high risk of oesophageal cancer. Int J Cancer. 1985 Dec 15;36(6):661–665. doi: 10.1002/ijc.2910360607. [DOI] [PubMed] [Google Scholar]
- Vahakangas K., Haugen A., Harris C. C. An applied synchronous fluorescence spectrophotometric assay to study benzo[a]pyrene-diolepoxide-DNA adducts. Carcinogenesis. 1985 Aug;6(8):1109–1115. doi: 10.1093/carcin/6.8.1109. [DOI] [PubMed] [Google Scholar]
- Wassermann M., Wassermann D., Cucos S., Miller H. J. World PCBs map: storage and effects in man and his biologic environment in the 1970s. Ann N Y Acad Sci. 1979 May 31;320:69–124. doi: 10.1111/j.1749-6632.1979.tb13137.x. [DOI] [PubMed] [Google Scholar]
- Weston A., Trivers G., Vahakangas K., Newman M., Rowe M., Mann D., Harris C. C. Detection of carcinogen-DNA adducts in human cells and antibodies to these adducts in human sera. Prog Exp Tumor Res. 1987;31:76–85. doi: 10.1159/000413905. [DOI] [PubMed] [Google Scholar]
- Wogan G. N., Gorelick N. J. Chemical and biochemical dosimetry of exposure to genotoxic chemicals. Environ Health Perspect. 1985 Oct;62:5–18. doi: 10.1289/ehp.85625. [DOI] [PMC free article] [PubMed] [Google Scholar]



