Abstract
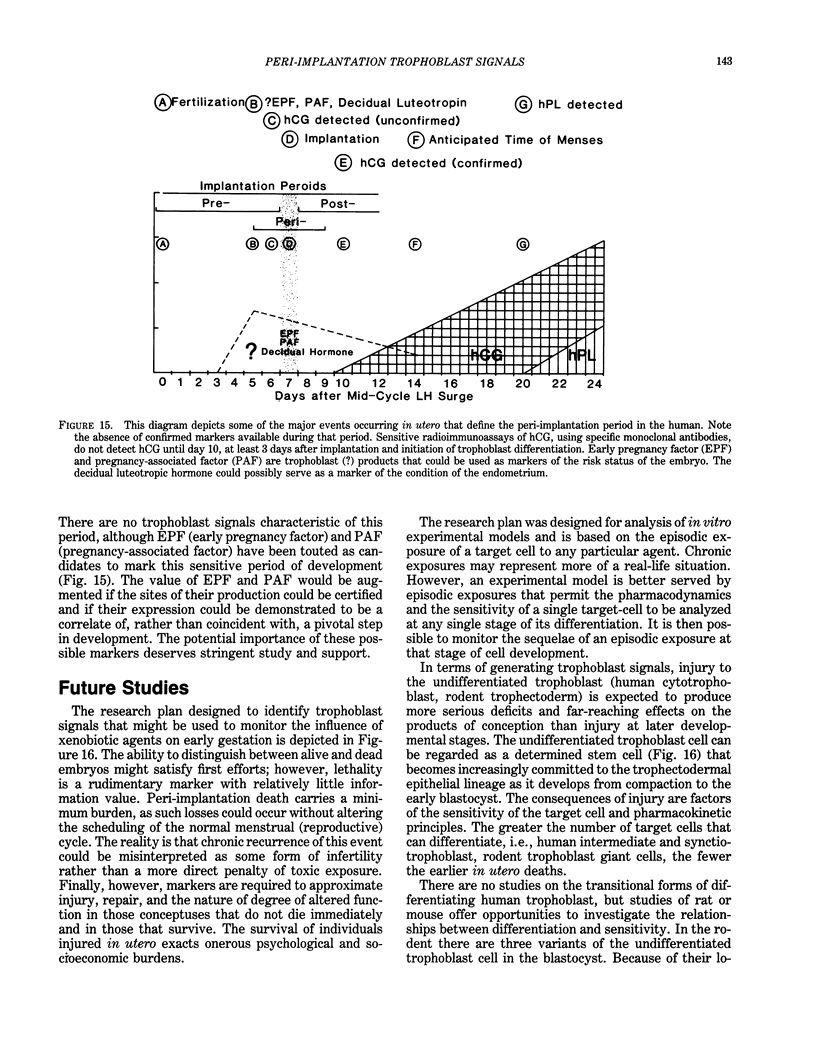
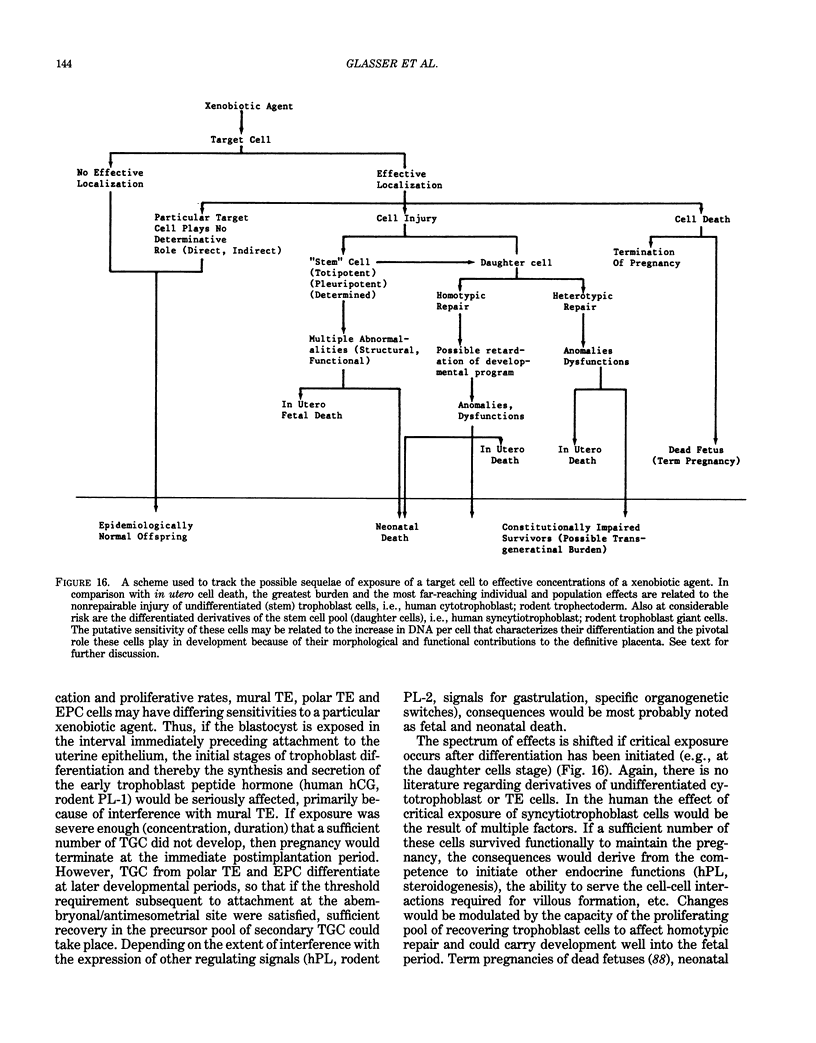
The peri-implantation period extends from the time the blastocyst is free in the uterus, through the processes of recognition and attachment, to the beginning of trophoblast differentiation and the interactions between the embryo and the uterine endometrium which initiate establishment of the hemochorial placenta. It is during the peri-implantation period that the embryo and hormonally regulated endometrial cells appear to be most sensitive to factors which introduce risk into the intrauterine environment. There are no markers which can be used practically to assess pregnancy risk during the peri-implantation period of either human or laboratory rodents. Experimental studies, using in vitro laboratory models of differentiating trophoblast cells, have identified peptide hormone markers of pivotal developmental processes. Exposure of trophoblast during the expression of these processes could have severe and far-reaching effects individually and societally. While these trophoblast signals are limited in their utility with respect to health monitoring extrapolation of these findings to human pregnancy, the signals could serve to identify more practical and sensitive markers to assess risk in early gestation. Human chorionic gonadotropin (hCG) has been used extensively as a marker to assess risk during the early stages of pregnancy. Extrapolation of experimental data indicates how hCG could be used more effectively in analyses of possible cause and effect relationships. The limitations of hCG as a marker for risk during the human peri-implantation period are discussed. Peptide hormones which could serve to assess risk during this critical period of extraordinary sensitivity to toxic factors are introduced.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams S. O., Nissley S. P., Handwerger S., Rechler M. M. Developmental patterns of insulin-like growth factor-I and -II synthesis and regulation in rat fibroblasts. Nature. 1983 Mar 10;302(5904):150–153. doi: 10.1038/302150a0. [DOI] [PubMed] [Google Scholar]
- Barlow P. W., Sherman M. I. The biochemistry of differentiation of mouse trophoblast: studies on polyploidy. J Embryol Exp Morphol. 1972 Apr;27(2):447–465. [PubMed] [Google Scholar]
- Bell S. C. Protein synthesis during deciduoma morphogenesis in the rat. Biol Reprod. 1979 May;20(4):811–821. doi: 10.1095/biolreprod20.4.811. [DOI] [PubMed] [Google Scholar]
- Billington W. D. Biology of the trophoblast. Adv Reprod Physiol. 1971;5:27–66. [PubMed] [Google Scholar]
- Boime I., Boothby M., Hoshina M., Daniels-McQueen S., Darnell R. Expression and structures of human placental hormone genes as a function of placental development. Biol Reprod. 1982 Feb;26(1):73–91. doi: 10.1095/biolreprod26.1.73. [DOI] [PubMed] [Google Scholar]
- Brinsmead M. W., Bancroft B. J., Thorburn G. D., Waters M. J. Fetal and material ovine placental lactogen during hyperglycaemia, hypoglycaemia and fasting. J Endocrinol. 1981 Sep;90(3):337–343. doi: 10.1677/joe.0.0900337. [DOI] [PubMed] [Google Scholar]
- Canfield R. E., Birken S., Ehrlich P., Armstrong G. Immunochemistry of human chorionic gonadotropin. Adv Exp Med Biol. 1984;176:199–215. doi: 10.1007/978-1-4684-4811-5_12. [DOI] [PubMed] [Google Scholar]
- Copp A. J. Interaction between inner cell mass and trophectoderm of the mouse blastocyst. II. The fate of the polar trophectoderm. J Embryol Exp Morphol. 1979 Jun;51:109–120. [PubMed] [Google Scholar]
- Cunha G. R., Bigsby R. M., Cooke P. S., Sugimura Y. Stromal-epithelial interactions in adult organs. Cell Differ. 1985 Sep;17(3):137–148. doi: 10.1016/0045-6039(85)90481-6. [DOI] [PubMed] [Google Scholar]
- Daly D. C., Maslar I. A., Riddick D. H. Prolactin production during in vitro decidualization of proliferative endometrium. Am J Obstet Gynecol. 1983 Mar 15;145(6):672–678. doi: 10.1016/0002-9378(83)90572-0. [DOI] [PubMed] [Google Scholar]
- Davies J., Glasser S. R. Histological and fine structural observations on the placenta of the rat. Acta Anat (Basel) 1968;69(4):542–608. doi: 10.1159/000143100. [DOI] [PubMed] [Google Scholar]
- Davies J., Glasser S. R. Light and electron microscopic observations on a human placenta 2 weeks after fetal death. Am J Obstet Gynecol. 1967 Aug 15;98(8):1111–1124. doi: 10.1016/0002-9378(67)90037-3. [DOI] [PubMed] [Google Scholar]
- Dickmann Z., Dey S. K. Steroidogenesis in the preimplantation rat embryo and its possible influence on morula-blastocyst transformation and implantation. J Reprod Fertil. 1974 Mar;37(1):91–93. doi: 10.1530/jrf.0.0370091. [DOI] [PubMed] [Google Scholar]
- Dreskin R. B., Spicer S. S., Greene W. B. Ultrastructural localization of chorionic gonadotropin in human term placenta. J Histochem Cytochem. 1970 Dec;18(12):862–874. doi: 10.1177/18.12.862. [DOI] [PubMed] [Google Scholar]
- ENDERS A. C. FORMATION OF SYNCYTIUM FROM CYTOTROPHOBLAST IN THE HUMAN PLACENTA. Obstet Gynecol. 1965 Mar;25:378–386. [PubMed] [Google Scholar]
- Edwards R. G., Steptoe P. C., Purdy J. M. Establishing full-term human pregnancies using cleaving embryos grown in vitro. Br J Obstet Gynaecol. 1980 Sep;87(9):737–756. doi: 10.1111/j.1471-0528.1980.tb04610.x. [DOI] [PubMed] [Google Scholar]
- Freemark M., Handwerger S. Ovine placental lactogen stimulates amino acid transport in rat diaphragm. Endocrinology. 1982 Jun;110(6):2201–2203. doi: 10.1210/endo-110-6-2201. [DOI] [PubMed] [Google Scholar]
- Friedman S. J., Skehan P. Morphological differentiation of human choriocarcinoma cells induced by methotrexate. Cancer Res. 1979 Jun;39(6 Pt 1):1960–1967. [PubMed] [Google Scholar]
- Glasser S. R., Julian J. Intermediate filaments of the midgestation rat trophoblast giant cell. Dev Biol. 1986 Feb;113(2):356–363. doi: 10.1016/0012-1606(86)90170-3. [DOI] [PubMed] [Google Scholar]
- Hall J., Talamantes F. Immunocytochemical localization of mouse placental lactogen in the mouse placenta. J Histochem Cytochem. 1984 Apr;32(4):379–382. doi: 10.1177/32.4.6368678. [DOI] [PubMed] [Google Scholar]
- Hilf G., Merz W. E. Influence of cyclic nucleotides on receptor binding, immunological activity, and microheterogeneity of human choriogonadotropin synthesized in placental tissue culture. Mol Cell Endocrinol. 1985 Feb;39(2):151–159. doi: 10.1016/0303-7207(85)90133-9. [DOI] [PubMed] [Google Scholar]
- Hoshina M., Boothby M., Hussa R., Pattillo R., Camel H. M., Boime I. Linkage of human chorionic gonadotrophin and placental lactogen biosynthesis to trophoblast differentiation and tumorigenesis. Placenta. 1985 Mar-Apr;6(2):163–172. doi: 10.1016/s0143-4004(85)80066-7. [DOI] [PubMed] [Google Scholar]
- Hurley T. W., Kuhn C. M., Schanberg S. M., Handwerger S. Differential effects of placental lactogen, growth hormone and prolactin on rat liver ornithine decarboxylase activity in the perinatal period. Life Sci. 1980 Dec 8;27(23):2269–2275. doi: 10.1016/0024-3205(80)90394-x. [DOI] [PubMed] [Google Scholar]
- Ilgren E. B. Review article: control of trophoblastic growth. Placenta. 1983 Jul-Sep;4(3):307–328. doi: 10.1016/s0143-4004(83)80010-1. [DOI] [PubMed] [Google Scholar]
- Jayatilak P. G., Glaser L. A., Warshaw M. L., Herz Z., Gruber J. R., Gibori G. Relationship between luteinizing hormone and decidual luteotropin in the maintenance of luteal steroidogenesis. Biol Reprod. 1984 Oct;31(3):556–564. doi: 10.1095/biolreprod31.3.556. [DOI] [PubMed] [Google Scholar]
- Johnson M. H., Rossant J. Molecular studies on cells of the trophectodermal lineage of the postimplantation mouse embryo. J Embryol Exp Morphol. 1981 Feb;61:103–116. [PubMed] [Google Scholar]
- Kelly P. A., Shiu R. P., Robertson M. C., Friesen H. G. Characterization of rat chorionic mammotropin. Endocrinology. 1975 May;96(5):1187–1195. doi: 10.1210/endo-96-5-1187. [DOI] [PubMed] [Google Scholar]
- Kliman H. J., Nestler J. E., Sermasi E., Sanger J. M., Strauss J. F., 3rd Purification, characterization, and in vitro differentiation of cytotrophoblasts from human term placentae. Endocrinology. 1986 Apr;118(4):1567–1582. doi: 10.1210/endo-118-4-1567. [DOI] [PubMed] [Google Scholar]
- Lenton E. A., Neal L. M., Sulaiman R. Plasma concentrations of human chorionic gonadotropin from the time of implantation until the second week of pregnancy. Fertil Steril. 1982 Jun;37(6):773–778. doi: 10.1016/s0015-0282(16)46337-5. [DOI] [PubMed] [Google Scholar]
- Marcal J. M., Chew N. J., Salomon D. S., Sherman M. I. Delta5,3beta-hydroxysteroid dehydrogenase activities in rat trophoblast and ovary during pregnancy. Endocrinology. 1975 May;96(5):1270–1279. doi: 10.1210/endo-96-5-1270. [DOI] [PubMed] [Google Scholar]
- Martal J., Djiane J., Dubois M. P. Immunofluorescent localization of ovine placental lactogen. Cell Tissue Res. 1977 Nov 23;184(4):427–433. doi: 10.1007/BF00220966. [DOI] [PubMed] [Google Scholar]
- Matthies D. L. Studies of the luteotropic and mammotropic factor found in trophoblast and maternal peripheral blood of the rat at mid-pregnancy. Anat Rec. 1967 Sep;159(1):55–67. doi: 10.1002/ar.1091590109. [DOI] [PubMed] [Google Scholar]
- McCormack S. A., Glasser S. R. Differential response of individual uterine cell types from immature rats treated with estradiol. Endocrinology. 1980 May;106(5):1634–1649. doi: 10.1210/endo-106-5-1634. [DOI] [PubMed] [Google Scholar]
- McCormack S. A., Glasser S. R. Ontogeny and regulation of rat placental estrogen receptor. Endocrinology. 1978 Jan;102(1):273–280. doi: 10.1210/endo-102-1-273. [DOI] [PubMed] [Google Scholar]
- Pijnenborg R., Robertson W. B., Brosens I. Morphological aspects of placental ontogeny and phylogeny. Placenta. 1985 Mar-Apr;6(2):155–162. doi: 10.1016/s0143-4004(85)80065-5. [DOI] [PubMed] [Google Scholar]
- Randerath K., Randerath E., Agrawal H. P., Gupta R. C., Schurdak M. E., Reddy M. V. Postlabeling methods for carcinogen-DNA adduct analysis. Environ Health Perspect. 1985 Oct;62:57–65. doi: 10.1289/ehp.856257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson M. C., Gillespie B., Friesen H. G. Characterization of the two forms of rat placental lactogen (rPL): rPL-I and rPL-II. Endocrinology. 1982 Dec;111(6):1862–1866. doi: 10.1210/endo-111-6-1862. [DOI] [PubMed] [Google Scholar]
- Robertson M. C., Owens R. E., Klindt J., Friesen H. G. Ovariectomy leads to a rapid increase in rat placental lactogen secretion. Endocrinology. 1984 May;114(5):1805–1811. doi: 10.1210/endo-114-5-1805. [DOI] [PubMed] [Google Scholar]
- Rossant J., Ofer L. Properties of extra-embryonic ectoderm isolated from postimplantation mouse embryos. J Embryol Exp Morphol. 1977 Jun;39:183–194. [PubMed] [Google Scholar]
- Rossant J., Tamura-Lis W. Effect of culture conditions on diploid to giant-cell transformation in postimplantation mouse trophoblast. J Embryol Exp Morphol. 1981 Apr;62:217–227. [PubMed] [Google Scholar]
- Saxena B. B., Hasan S. H., Haour F., Schmidt-Gollwitzer M. Radioreceptor assay of human chorionic gonadotropin: detection of early pregnancy. Science. 1974 May 17;184(4138):793–795. doi: 10.1126/science.184.4138.793. [DOI] [PubMed] [Google Scholar]
- Schlafke S., Enders A. C. Cellular basis of interaction between trophoblast and uterus at implantation. Biol Reprod. 1975 Feb;12(1):41–65. doi: 10.1095/biolreprod12.1.41. [DOI] [PubMed] [Google Scholar]
- Sherman M. I., McLaren A., Walker P. M. Mechanism of accumulation of DNA in giant cells of mouse trophoblast. Nat New Biol. 1972 Aug 9;238(84):175–176. doi: 10.1038/newbio238175a0. [DOI] [PubMed] [Google Scholar]
- Shiu R. P., Kelly P. A., Friesen H. G. Radioreceptor assay for prolactin and other lactogenic hormones. Science. 1973 Jun 1;180(4089):968–971. doi: 10.1126/science.180.4089.968. [DOI] [PubMed] [Google Scholar]
- Smith D. M., Smith A. E. Uptake and incorporation of amino acids by cultured mouse embryos: estrogen stimulation. Biol Reprod. 1971 Feb;4(1):66–73. doi: 10.1093/biolreprod/4.1.66. [DOI] [PubMed] [Google Scholar]
- Soares M. J., Bartke A., Colosi P., Talamantes F. Identification of a placental lactogen in pregnant Snell and Ames dwarf mice. Proc Soc Exp Biol Med. 1984 Jan;175(1):106–108. doi: 10.3181/00379727-175-41775. [DOI] [PubMed] [Google Scholar]
- Soares M. J., Colosi P., Talamantes F. The development and characterization of a homologous radioimmunoassay for mouse placental lactogen. Endocrinology. 1982 Feb;110(2):668–670. doi: 10.1210/endo-110-2-668. [DOI] [PubMed] [Google Scholar]
- Soares M. J., Julian J. A., Glasser S. R. Trophoblast giant cell release of placental lactogens: temporal and regional characteristics. Dev Biol. 1985 Feb;107(2):520–526. doi: 10.1016/0012-1606(85)90332-x. [DOI] [PubMed] [Google Scholar]
- Soares M. J., Talamantes F. Placental lactogen secretion in the mouse: in vitro responses and ovarian and hormonal influences. J Exp Zool. 1985 Apr;234(1):97–104. doi: 10.1002/jez.1402340112. [DOI] [PubMed] [Google Scholar]
- Soares M. J., Talamantes F. Pre-parturitional changes in serum prolactin, placental lactogen, growth hormone, progesterone, and corticosterone in the C3H/HeN mouse. J Dev Physiol. 1984 Oct;6(5):423–429. [PubMed] [Google Scholar]
- Surani M. A. Hormonal regulation of proteins in the uterine secretion of ovariectomized rats and the implications for implantation and embryonic diapause. J Reprod Fertil. 1975 Jun;43(3):411–417. doi: 10.1530/jrf.0.0430411. [DOI] [PubMed] [Google Scholar]
- Talamantes F., Ogren L., Markoff E., Woodard S., Madrid J. Phylogenetic distribution, regulation of secretion, and prolactin-like effects of placental lactogens. Fed Proc. 1980 Jun;39(8):2582–2587. [PubMed] [Google Scholar]
- Tonkowicz P. A., Voogt J. L. Ovarian and fetal control of rat placental lactogen and prolactin secretion at midpregnancy. Endocrinology. 1984 Jan;114(1):254–259. doi: 10.1210/endo-114-1-254. [DOI] [PubMed] [Google Scholar]
- Venetianer A., Schiller D. L., Magin T., Franke W. W. Cessation of cytokeratin expression in a rat hepatoma cell line lacking differentiated functions. Nature. 1983 Oct 20;305(5936):730–733. doi: 10.1038/305730a0. [DOI] [PubMed] [Google Scholar]
- Vessey M., Doll R., Peto R., Johnson B., Wiggins P. A long-term follow-up study of women using different methods of contraception--an interim report. J Biosoc Sci. 1976 Oct;8(4):373–427. doi: 10.1017/s0021932000010890. [DOI] [PubMed] [Google Scholar]
- Watkins W. B., Reddy S. Ovine placental lactogen in the cotyledonary and intercotyledonary placenta of the ewe. J Reprod Fertil. 1980 Mar;58(2):411–414. doi: 10.1530/jrf.0.0580411. [DOI] [PubMed] [Google Scholar]
- Watkins W. B. Use of immunocytochemical techniques for the localization of human placental lactogen. J Histochem Cytochem. 1978 Apr;26(4):288–292. doi: 10.1177/26.4.96175. [DOI] [PubMed] [Google Scholar]
- Wooding F. B. Localization of ovine placental lactogen in sheep placentomes by electron microscope immunocytochemistry. J Reprod Fertil. 1981 May;62(1):15–19. doi: 10.1530/jrf.0.0620015. [DOI] [PubMed] [Google Scholar]
- Wooding F. B. The role of the binucleate cell in ruminant placental structure. J Reprod Fertil Suppl. 1982 Nov;31:31–39. [PubMed] [Google Scholar]
- de Ikonicoff L. K., Cedard L. Localization of human chorionic gonadotropic and somatomammotropic hormones by the peroxidase immunohistoenzymologic method in villi and amniotic epithelium of human placentas (from six weeks to term). Am J Obstet Gynecol. 1973 Aug 15;116(8):1124–1132. doi: 10.1016/0002-9378(73)90948-4. [DOI] [PubMed] [Google Scholar]



