Abstract
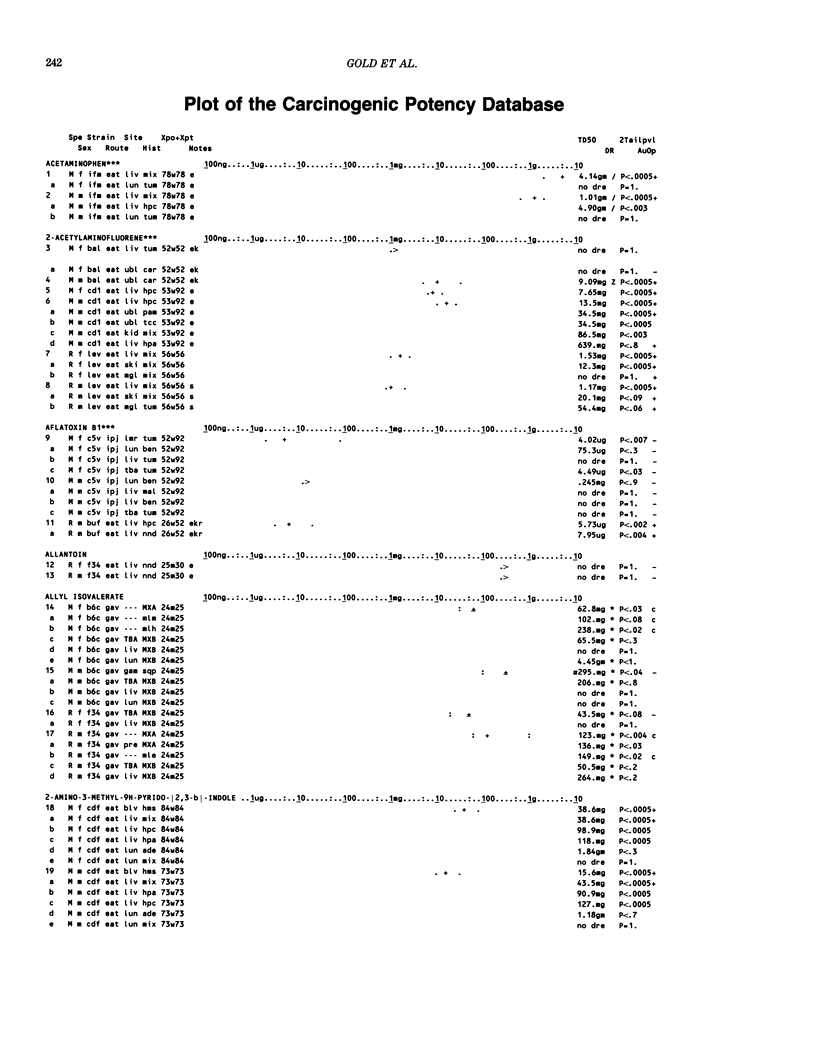
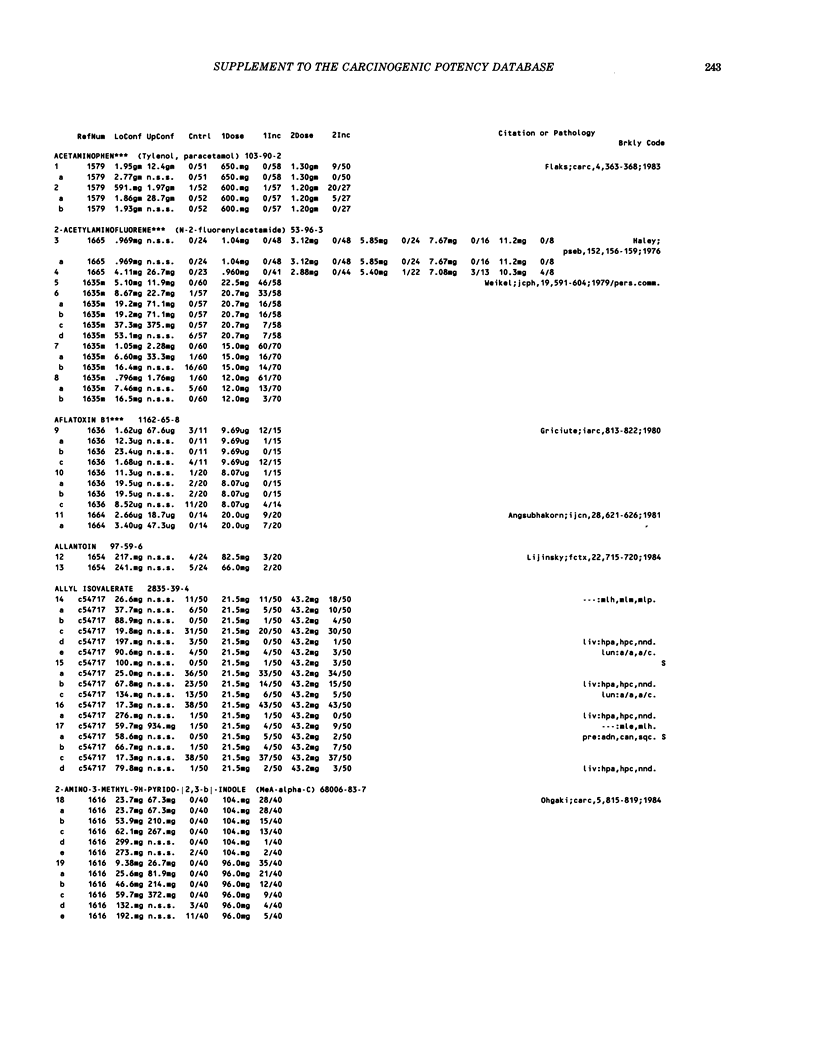
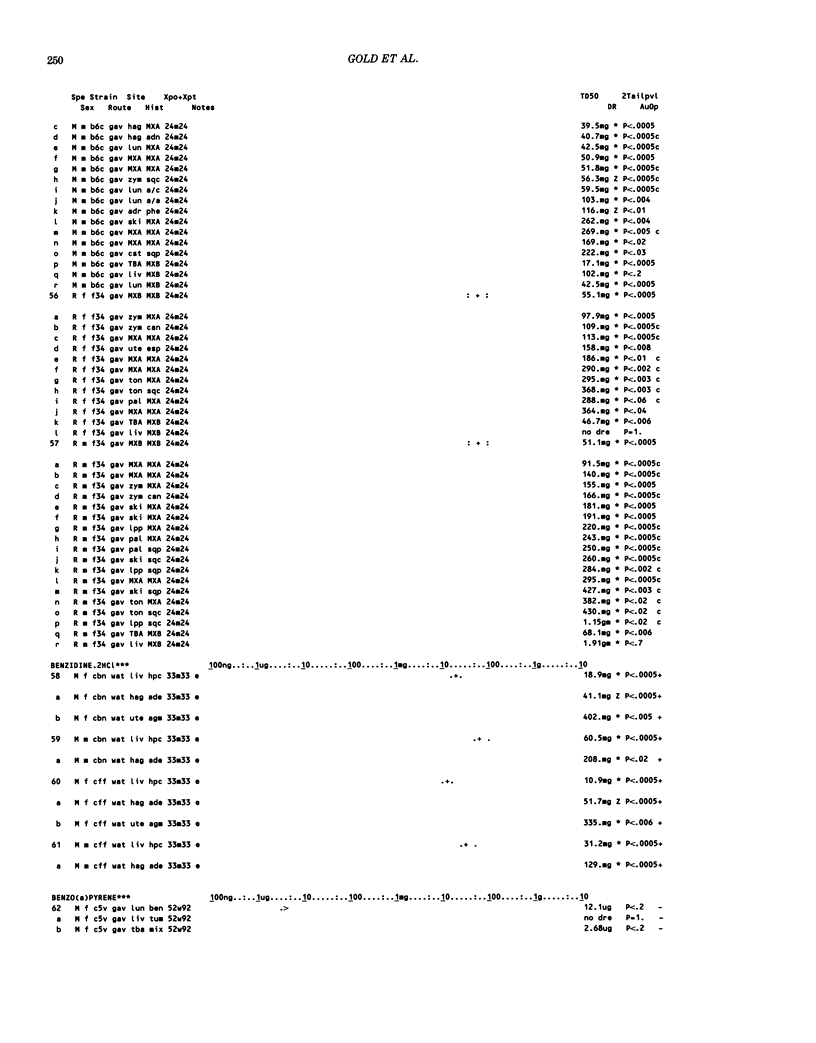
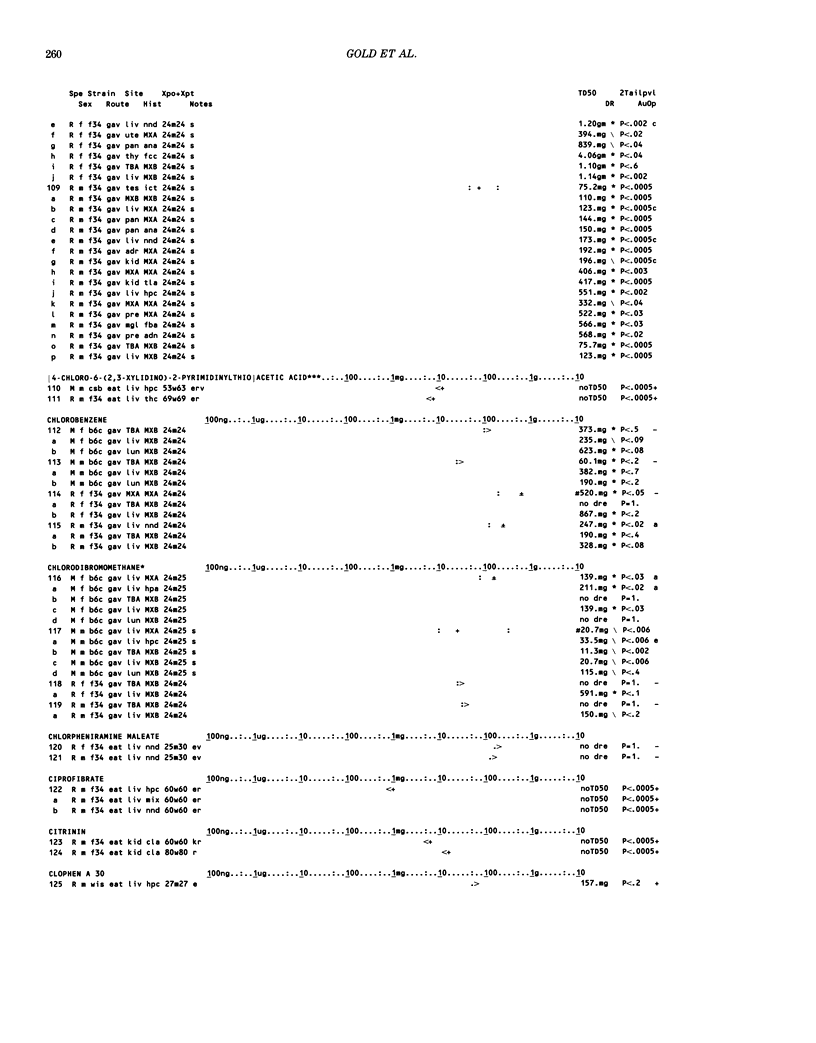
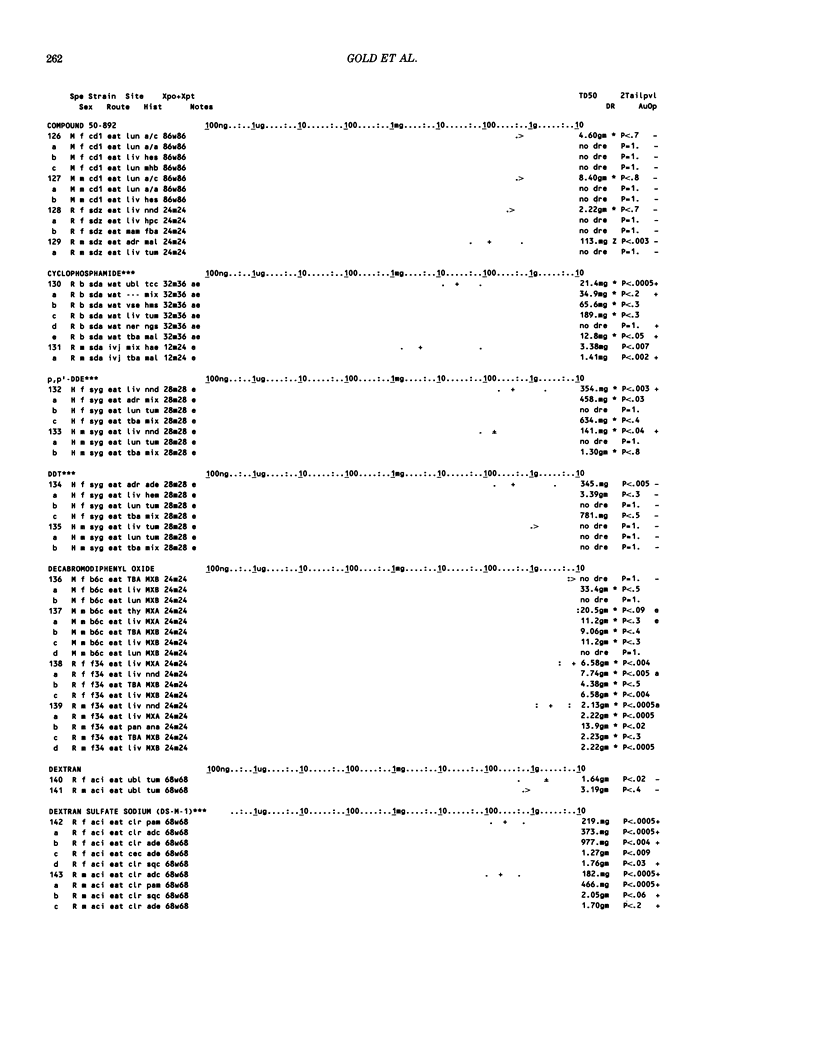
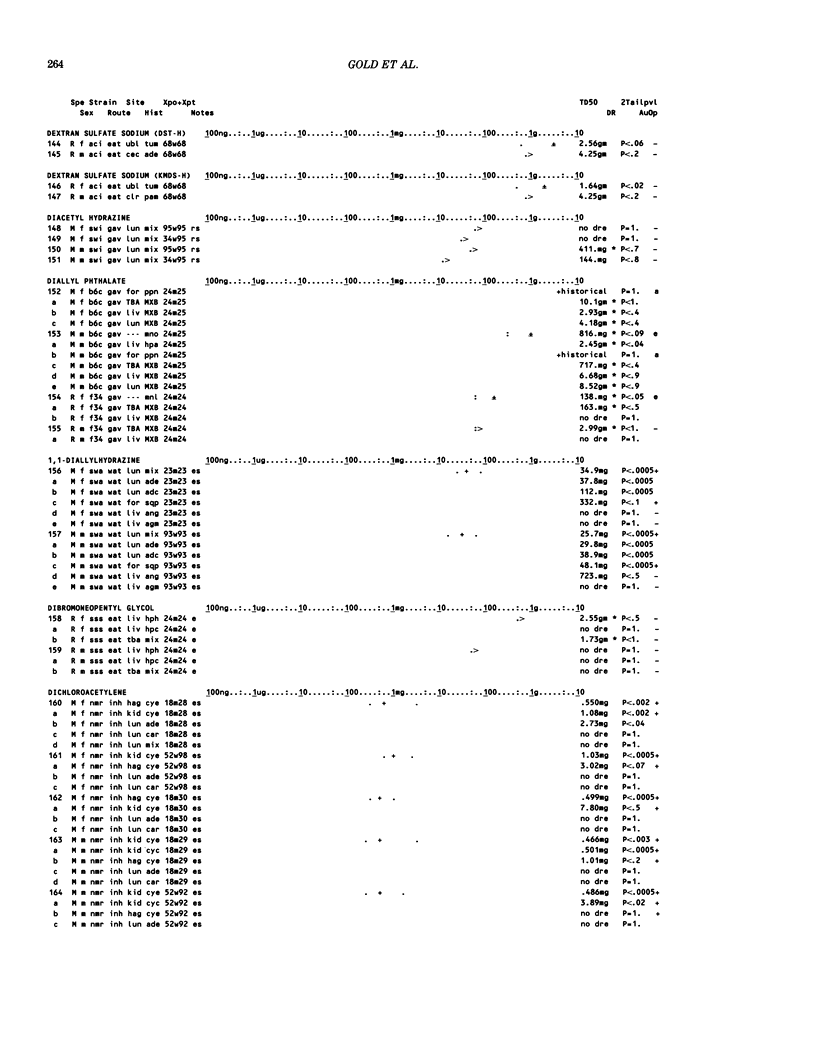
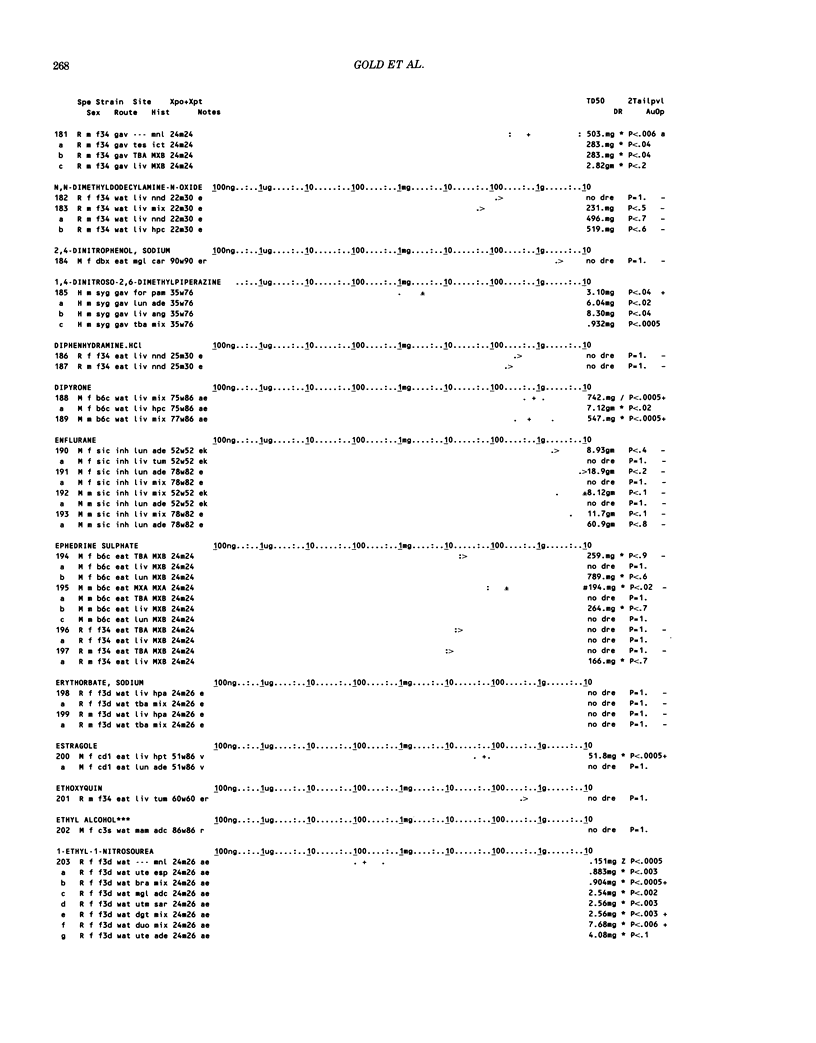
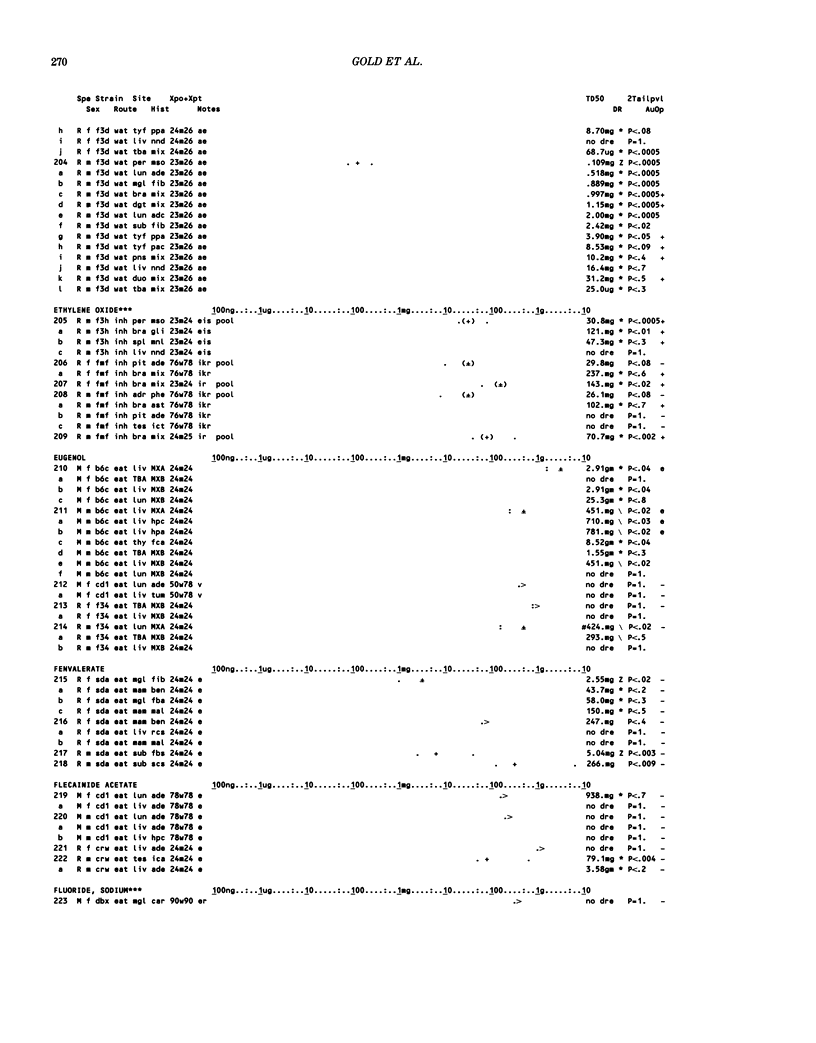
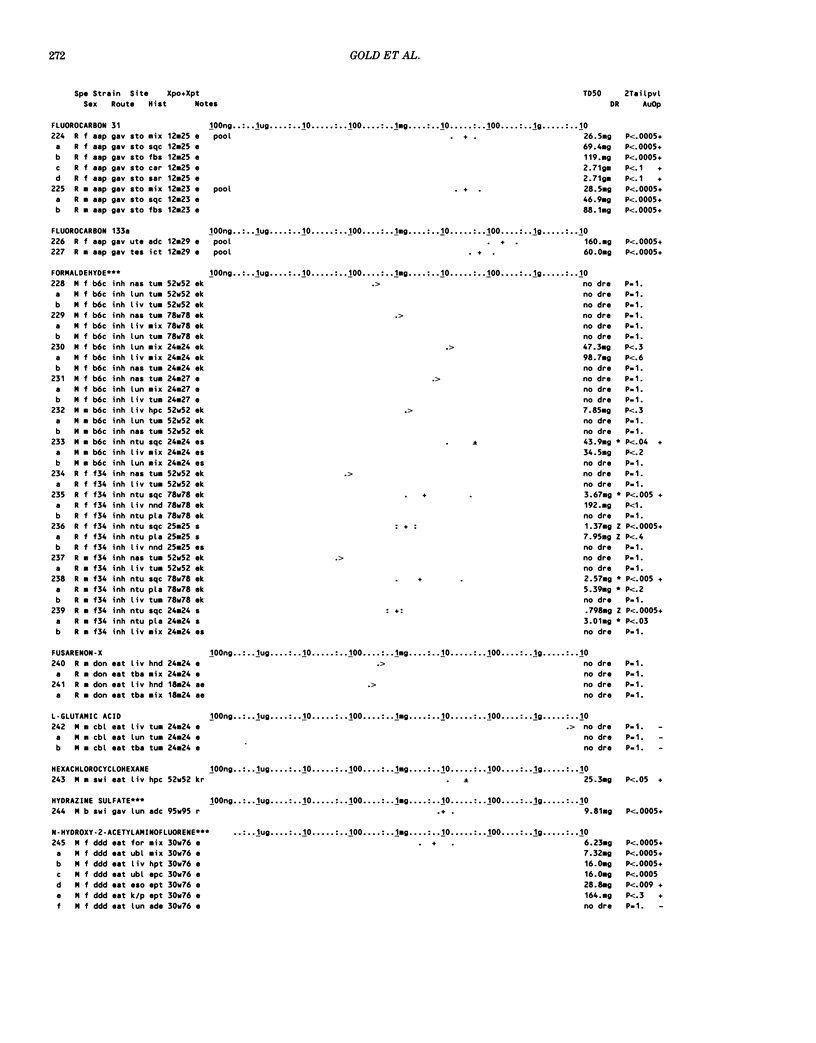
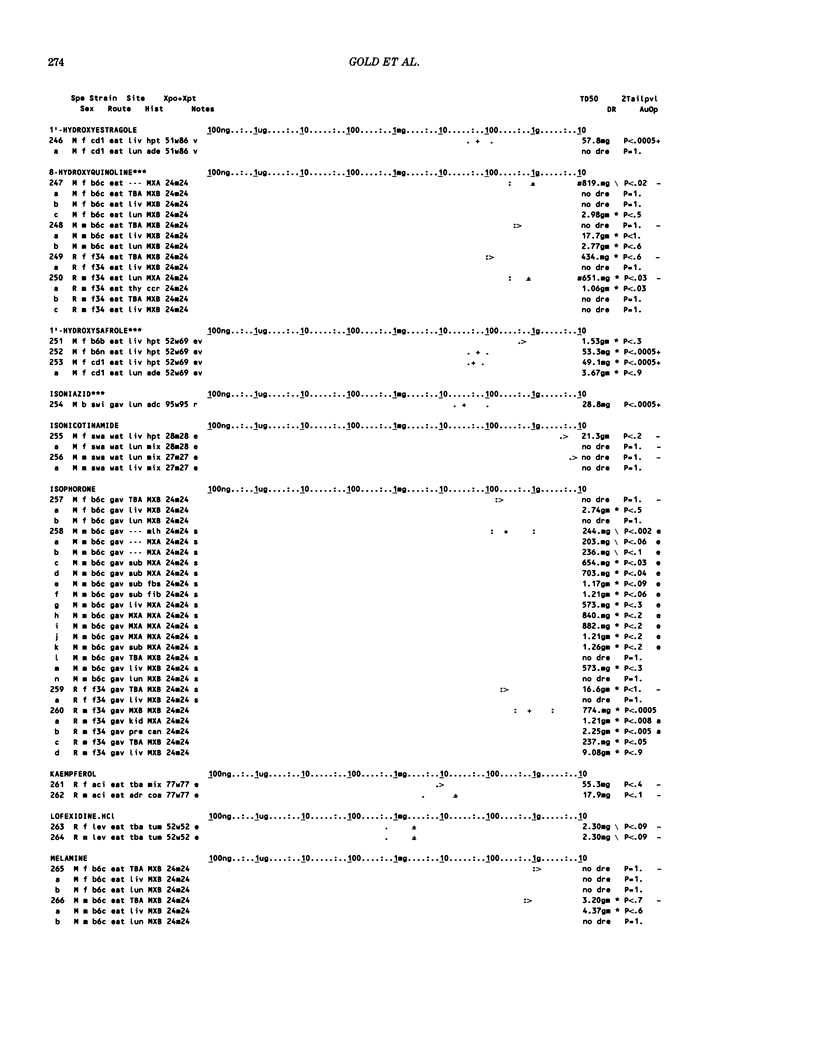
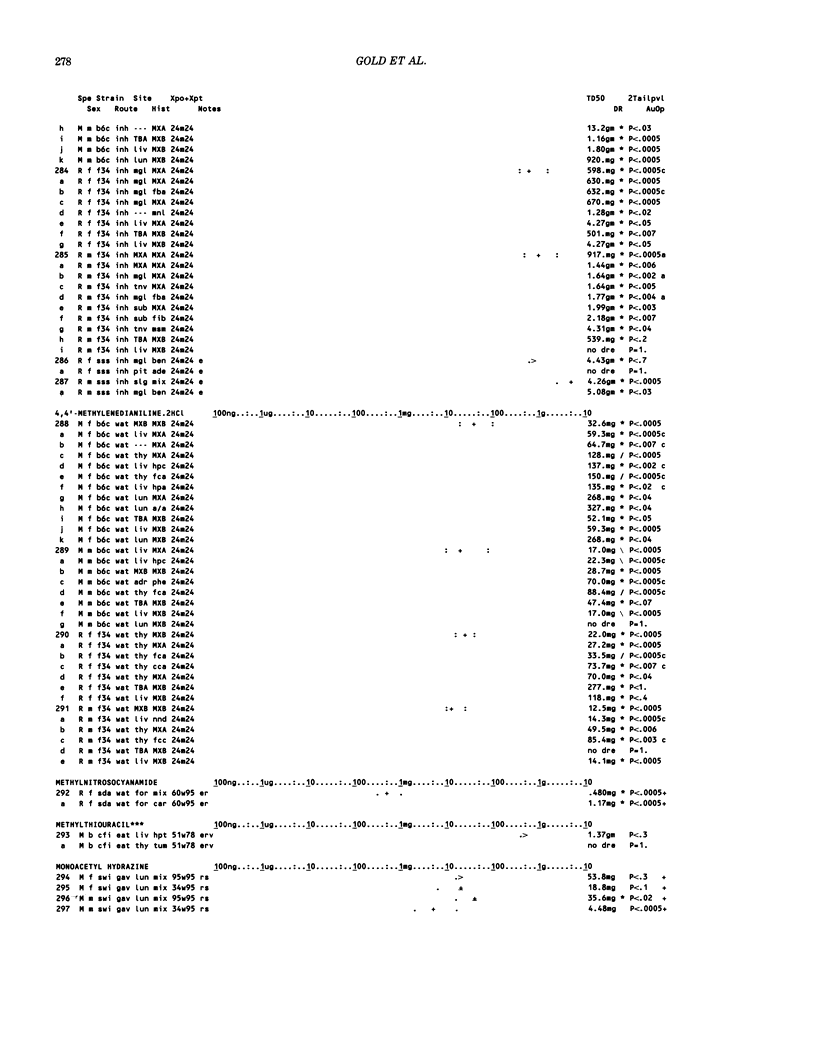
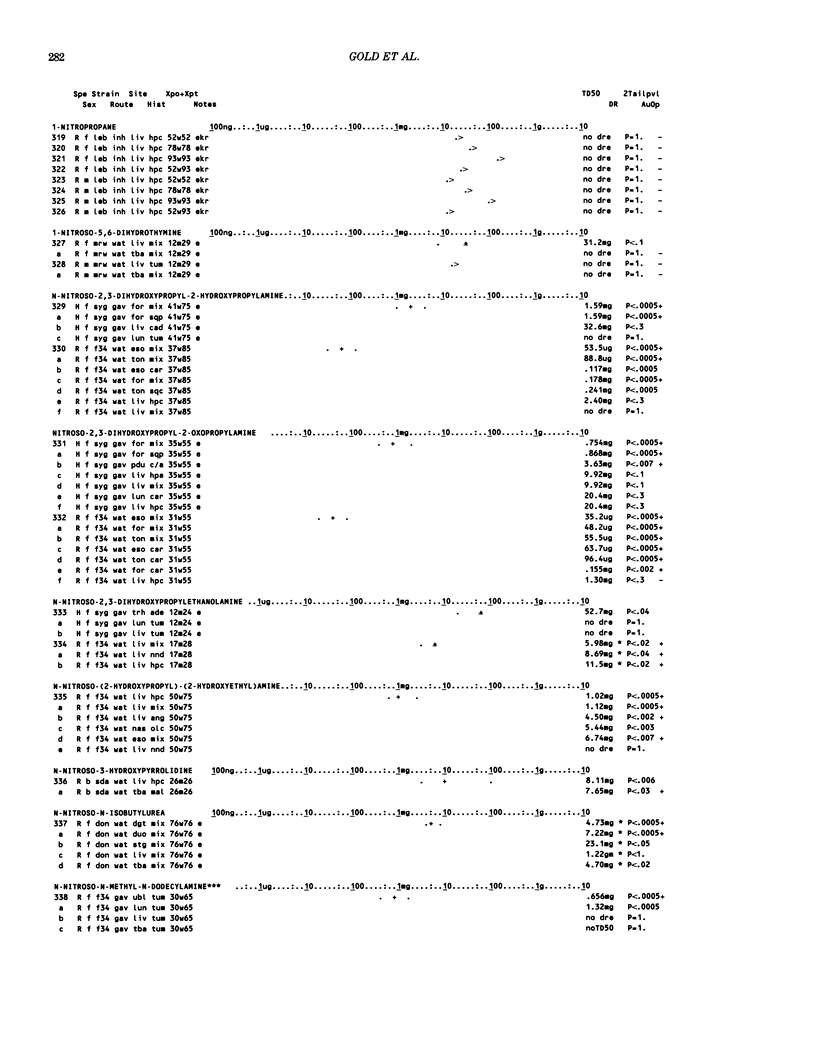
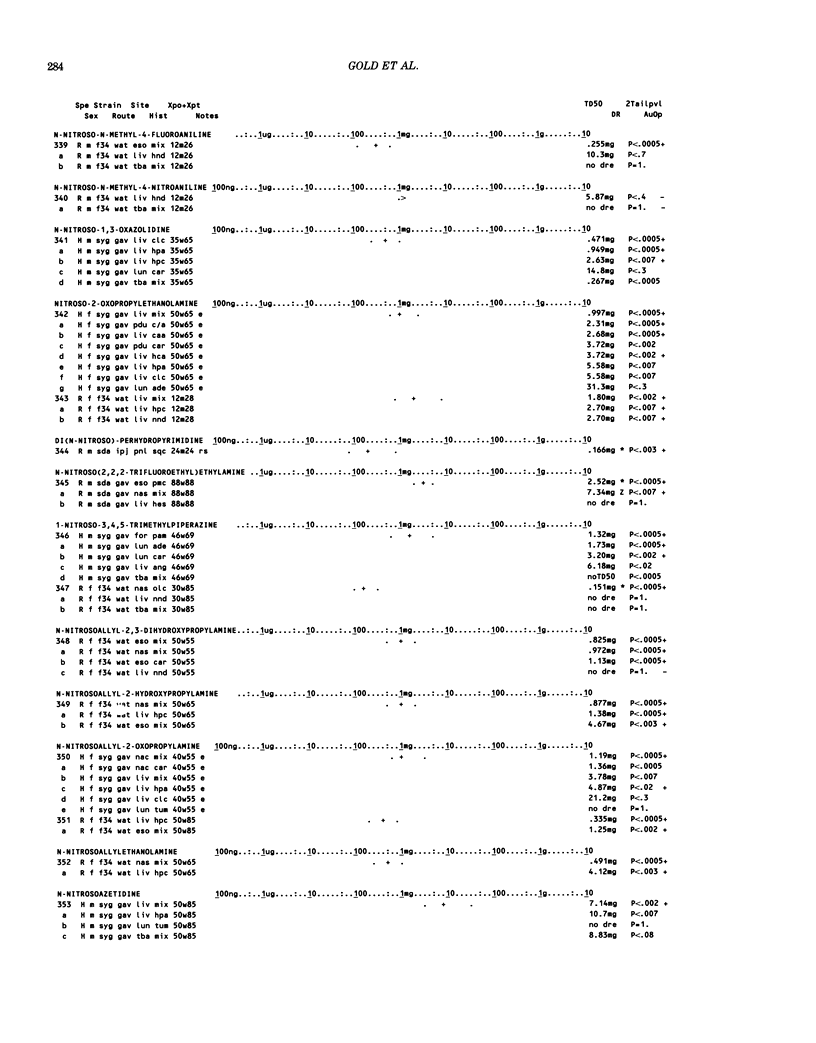
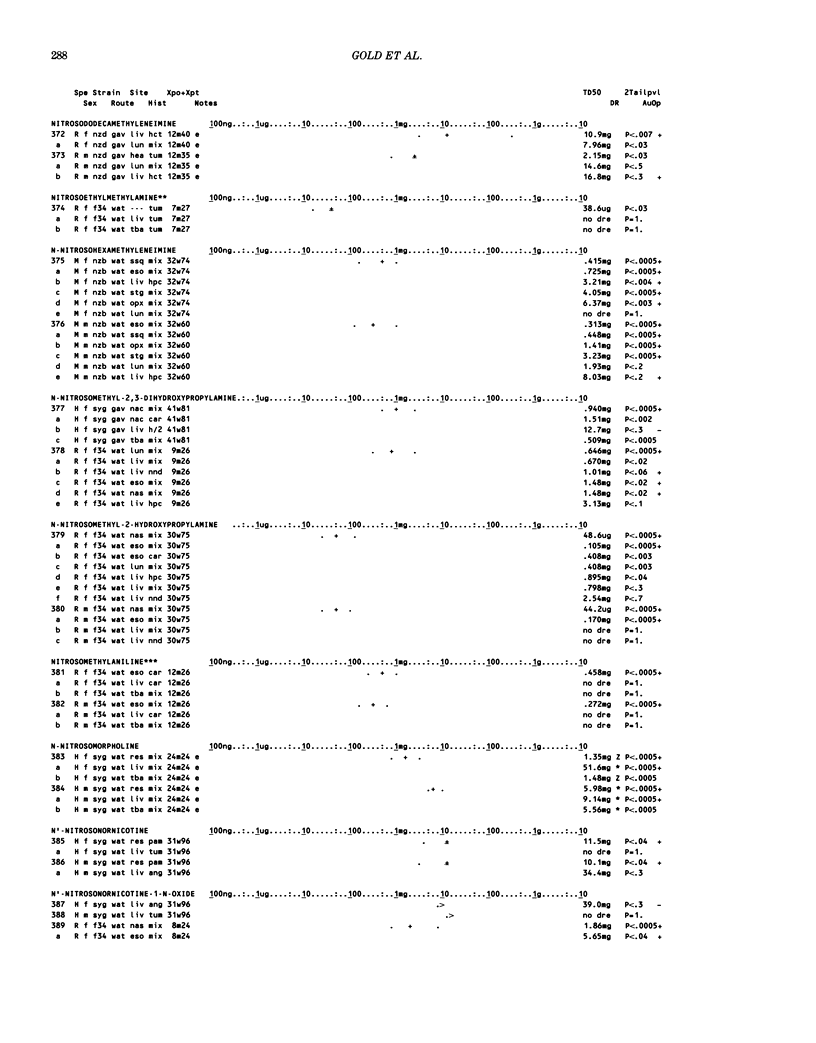
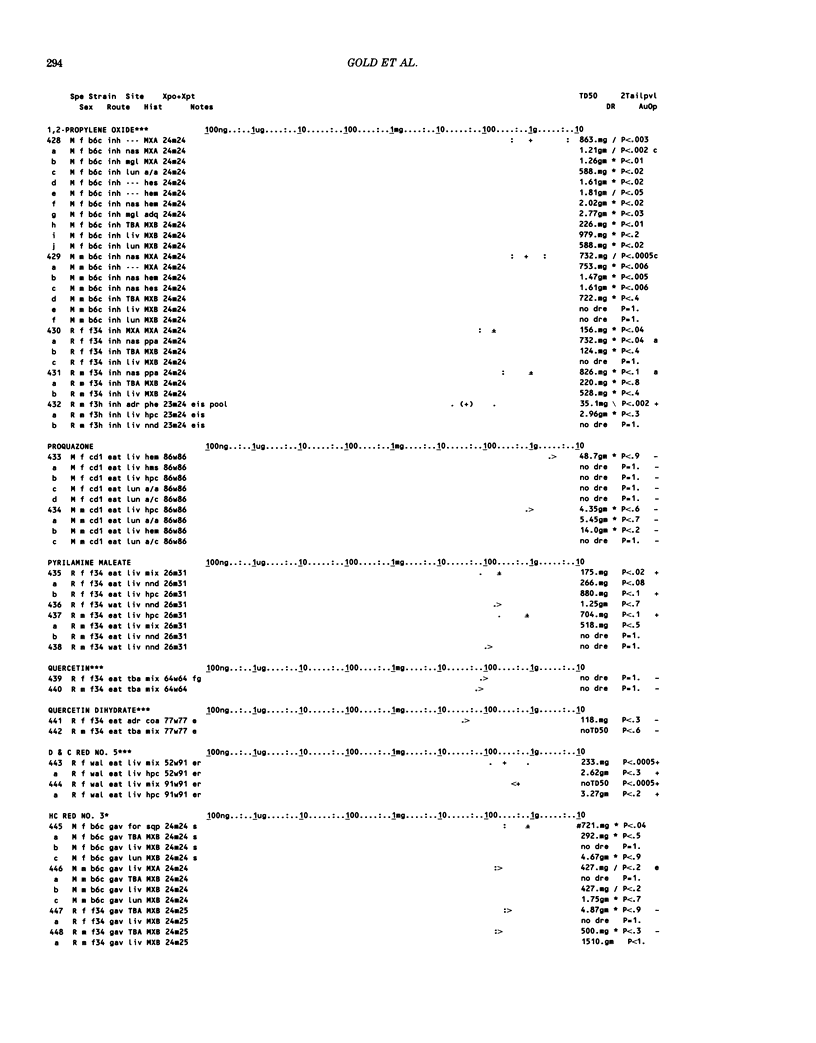
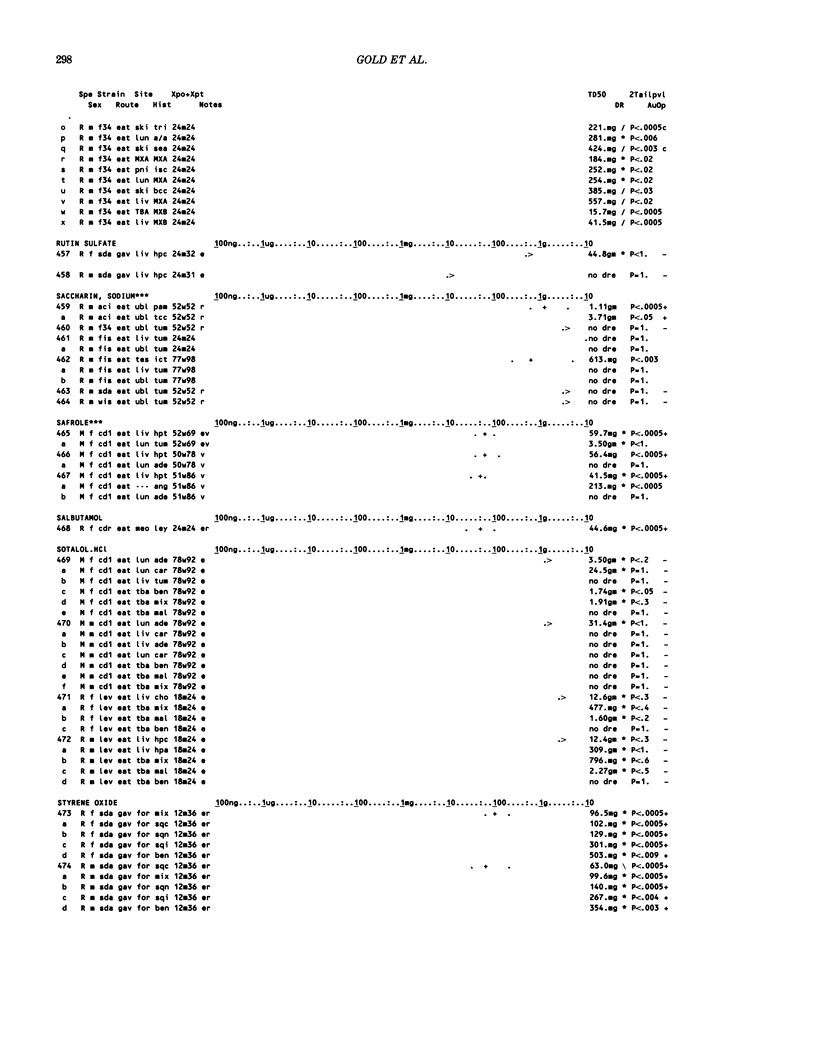
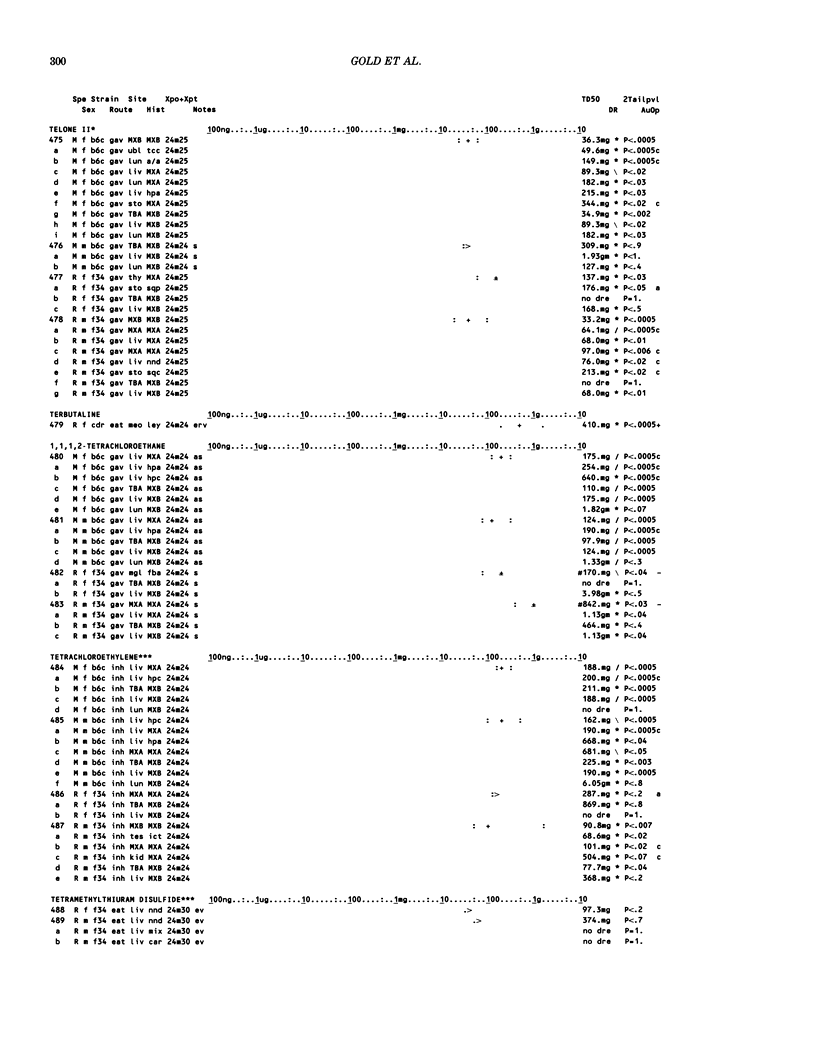
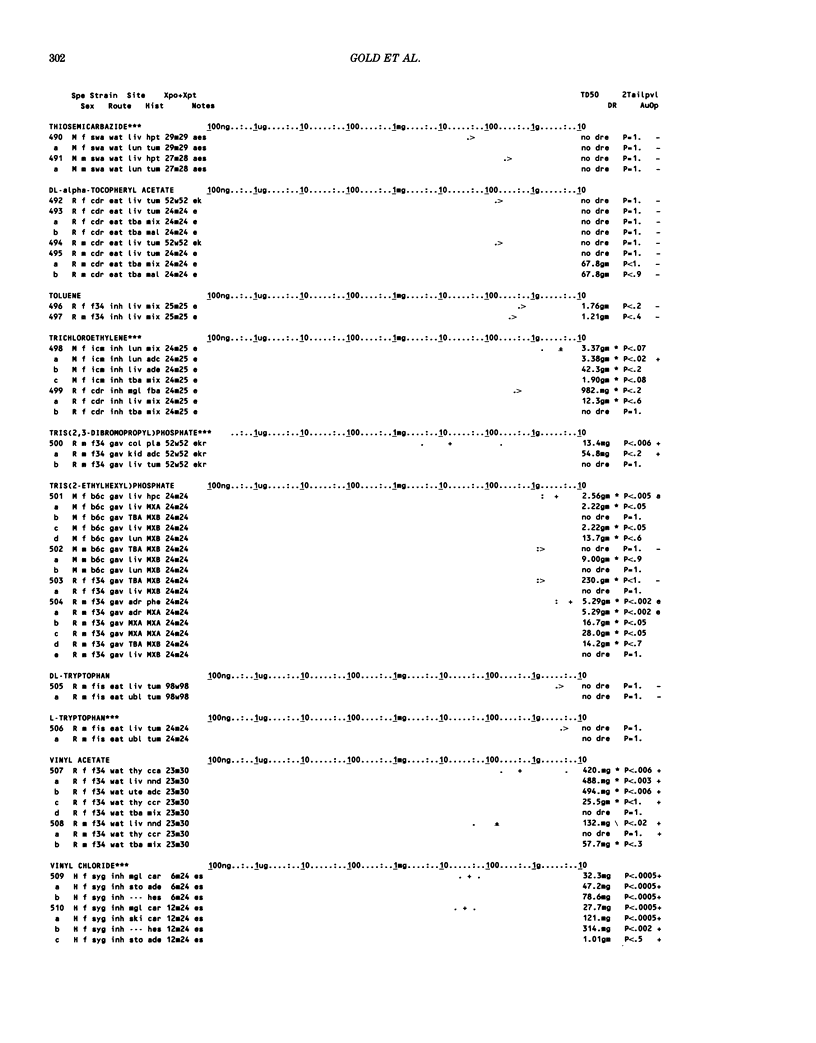
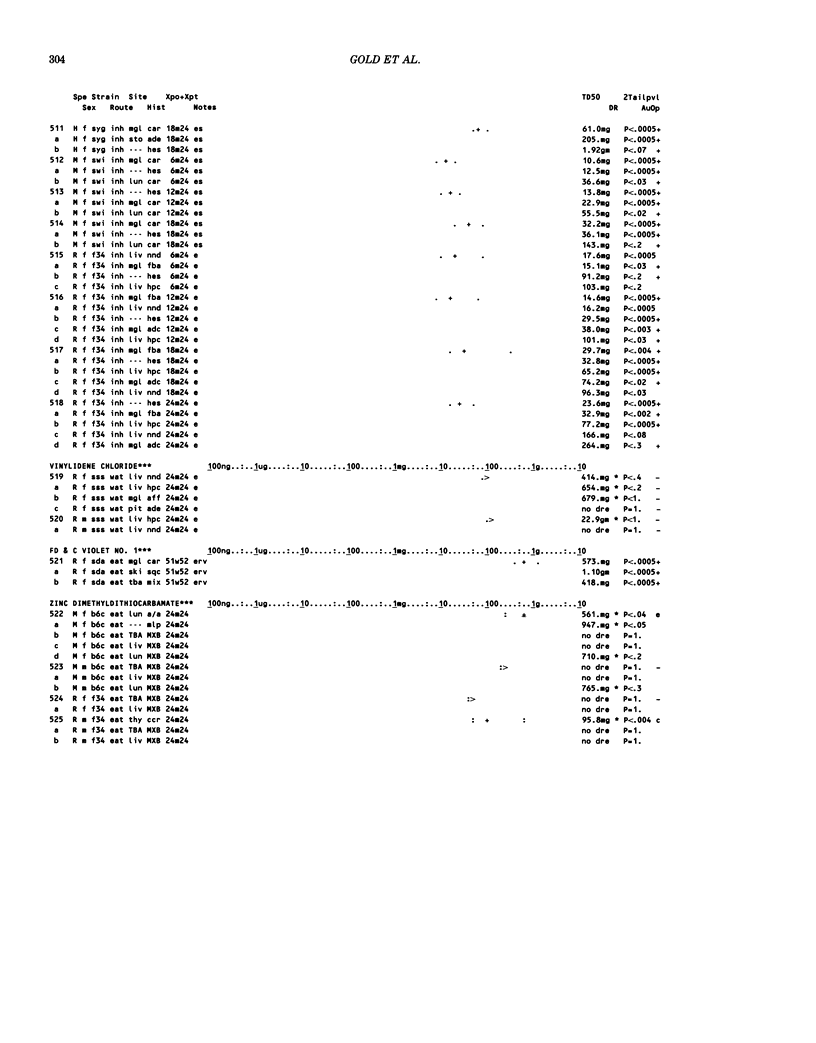
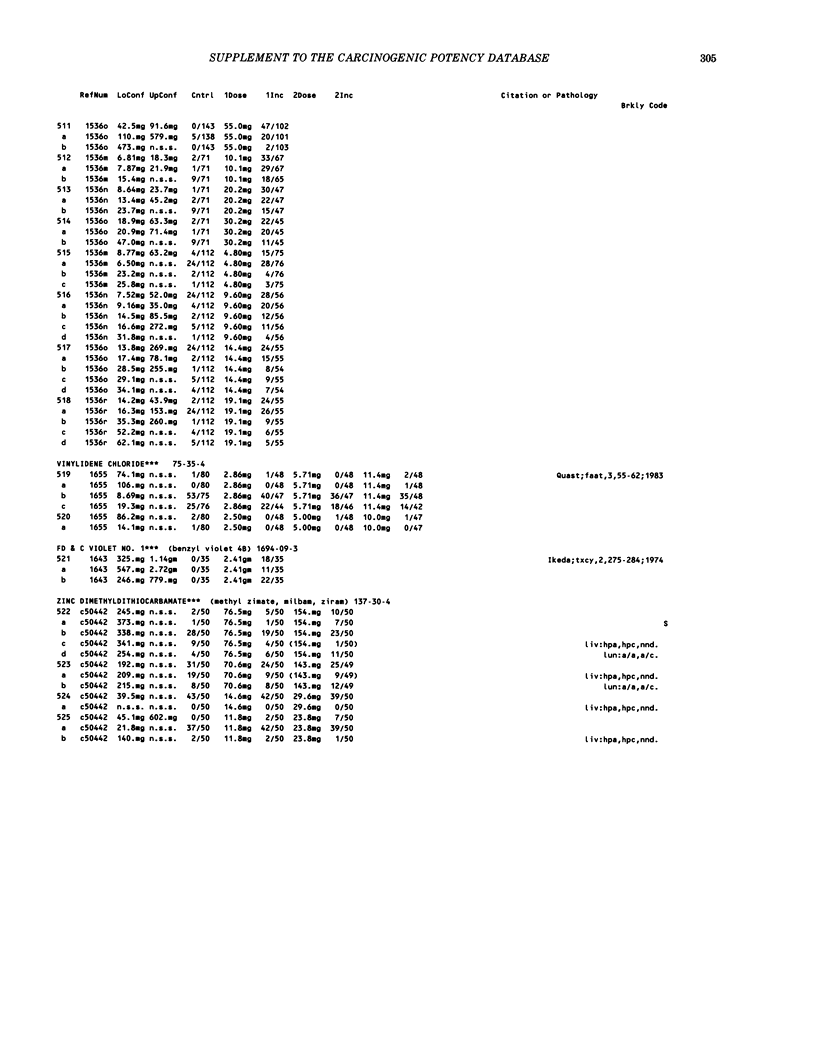
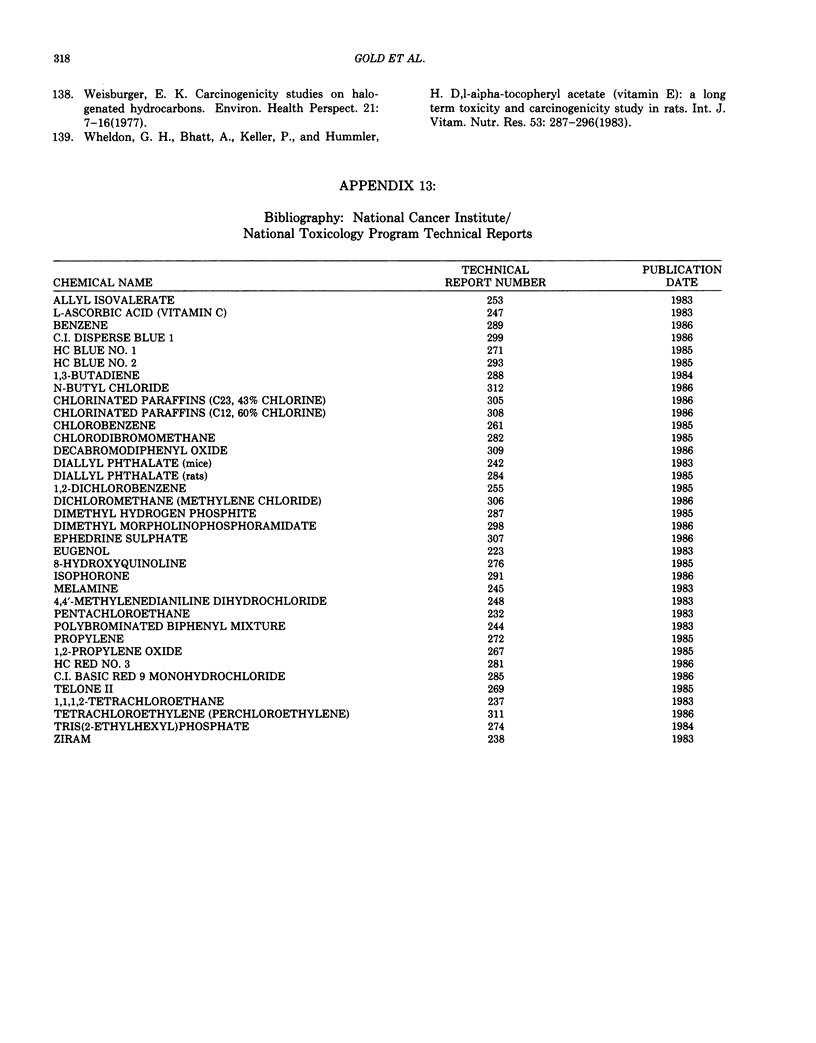
This paper is the second chronological supplement to the Carcinogenic Potency Database, published earlier in this journal (1,2,4). We report here results of carcinogenesis bioassays published in the general literature between January 1983 and December 1984, and in Technical Reports of the National Cancer Institute/National Toxicology Program between January 1983 and May 1986. This supplement includes results of 525 long-term, chronic experiments of 199 test compounds, and reports the same information about each experiment in the same plot format as the earlier papers: e.g., the species and strain of test animal, the route and duration of compound administration, dose level and other aspects of experimental protocol, histopathology and tumor incidence, TD50 (carcinogenic potency) and its statistical significance, dose response, author's opinion about carcinogenicity, and literature citation. We refer the reader to the 1984 publications for a description of the numerical index of carcinogenic potency (TD50), a guide to the plot of the database, and a discussion of the sources of data, the rationale for the inclusion of particular experiments and particular target sites, and the conventions adopted in summarizing the literature. The three plots of the database are to be used together, since results of experiments published in earlier plots are not repeated. Taken together, the three plots include results for more than 3500 experiments on 975 chemicals. Appendix 14 is an index to all chemicals in the database and indicates which plot(s) each chemical appears in.
Full text
PDF







































































Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames B. N., Magaw R., Gold L. S. Ranking possible carcinogenic hazards. Science. 1987 Apr 17;236(4799):271–280. doi: 10.1126/science.3563506. [DOI] [PubMed] [Google Scholar]
- Bernstein L., Gold L. S., Ames B. N., Pike M. C., Hoel D. G. Some tautologous aspects of the comparison of carcinogenic potency in rats and mice. Fundam Appl Toxicol. 1985 Feb;5(1):79–86. doi: 10.1016/0272-0590(85)90051-x. [DOI] [PubMed] [Google Scholar]
- Gold L. S., Bernstein L., Kaldor J., Backman G., Hoel D. An empirical comparison of methods used to estimate carcinogenic potency in long-term animal bioassays: lifetable vs summary incidence data. Fundam Appl Toxicol. 1986 Feb;6(2):263–269. doi: 10.1016/0272-0590(86)90239-3. [DOI] [PubMed] [Google Scholar]
- Gold L. S., Sawyer C. B., Magaw R., Backman G. M., de Veciana M., Levinson R., Hooper N. K., Havender W. R., Bernstein L., Peto R. A carcinogenic potency database of the standardized results of animal bioassays. Environ Health Perspect. 1984 Dec;58:9–319. doi: 10.1289/ehp.84589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold L. S., Ward J. M., Bernstein L., Stern B. Association between carcinogenic potency and tumor pathology in rodent carcinogenesis bioassays. Fundam Appl Toxicol. 1986 May;6(4):677–690. doi: 10.1016/0272-0590(86)90181-8. [DOI] [PubMed] [Google Scholar]
- Gold L. S., Wright C., Bernstein L., deVeciana M. Reproducibility of results in "near-replicate" carcinogenesis bioassays. J Natl Cancer Inst. 1987 Jun;78(6):1149–1158. [PubMed] [Google Scholar]
- Gold L. S., de Veciana M., Backman G. M., Magaw R., Lopipero P., Smith M., Blumenthal M., Levinson R., Bernstein L., Ames B. N. Chronological supplement to the Carcinogenic Potency Database: standardized results of animal bioassays published through December 1982. Environ Health Perspect. 1986 Aug;67:161–200. doi: 10.1289/ehp.8667161. [DOI] [PMC free article] [PubMed] [Google Scholar]


