Abstract
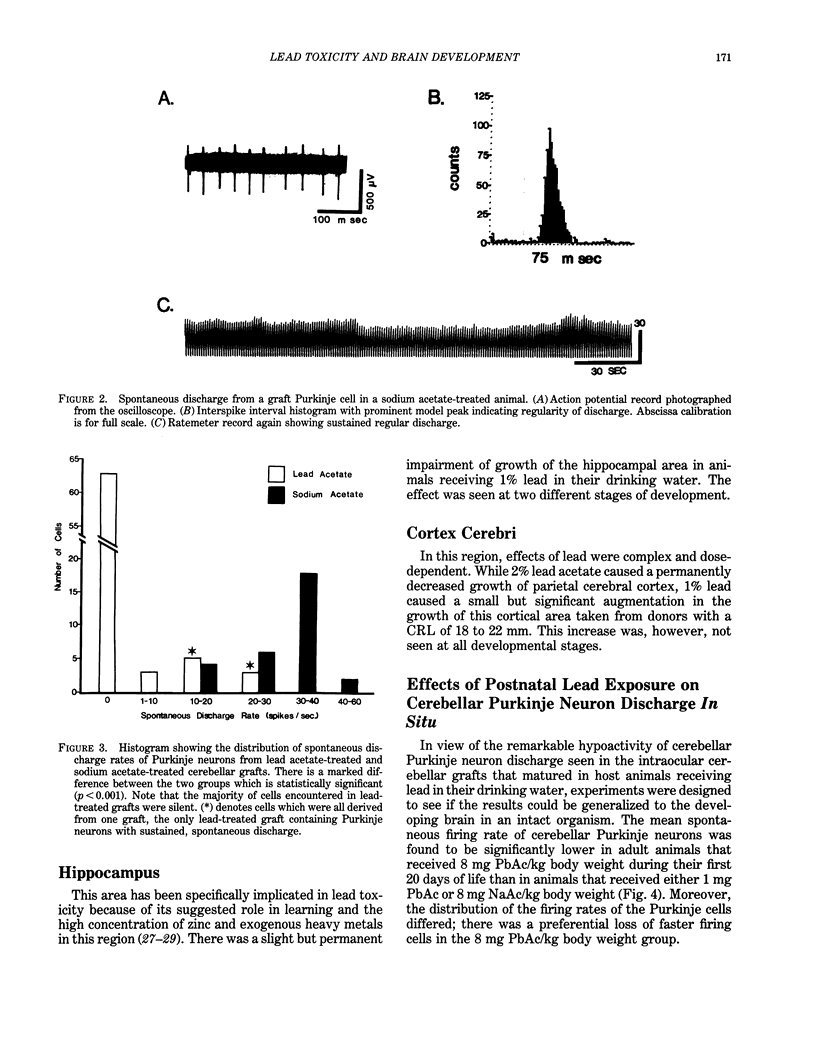
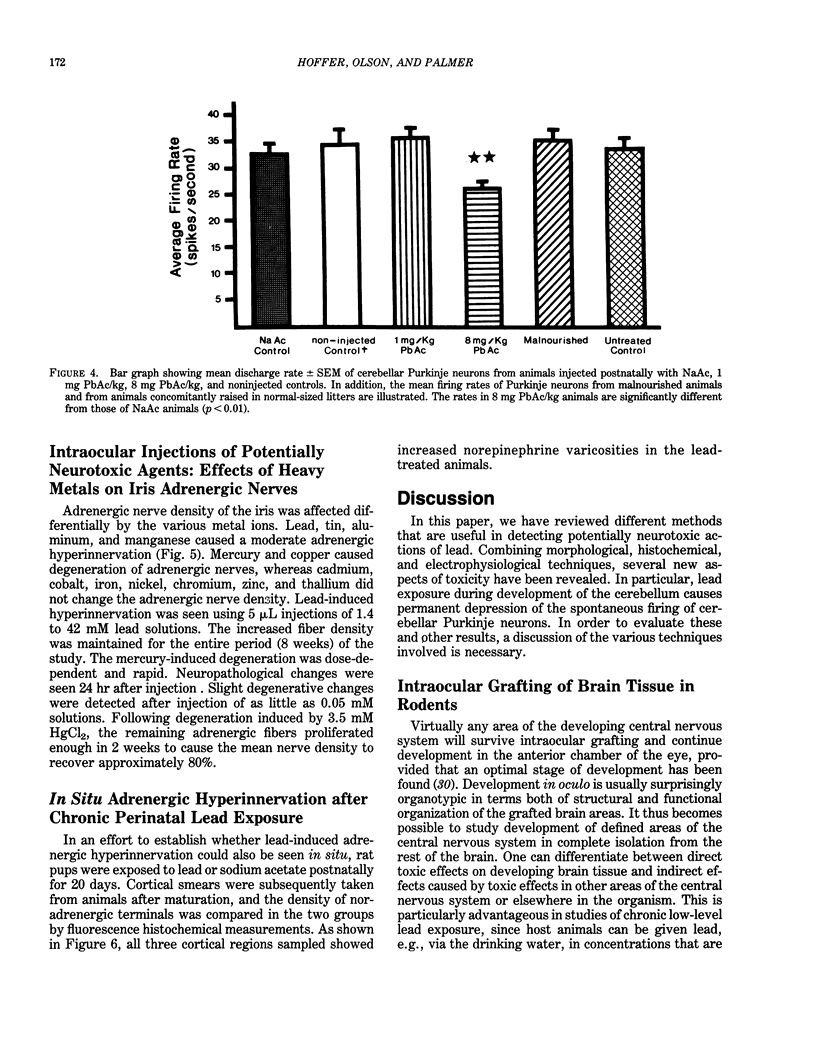
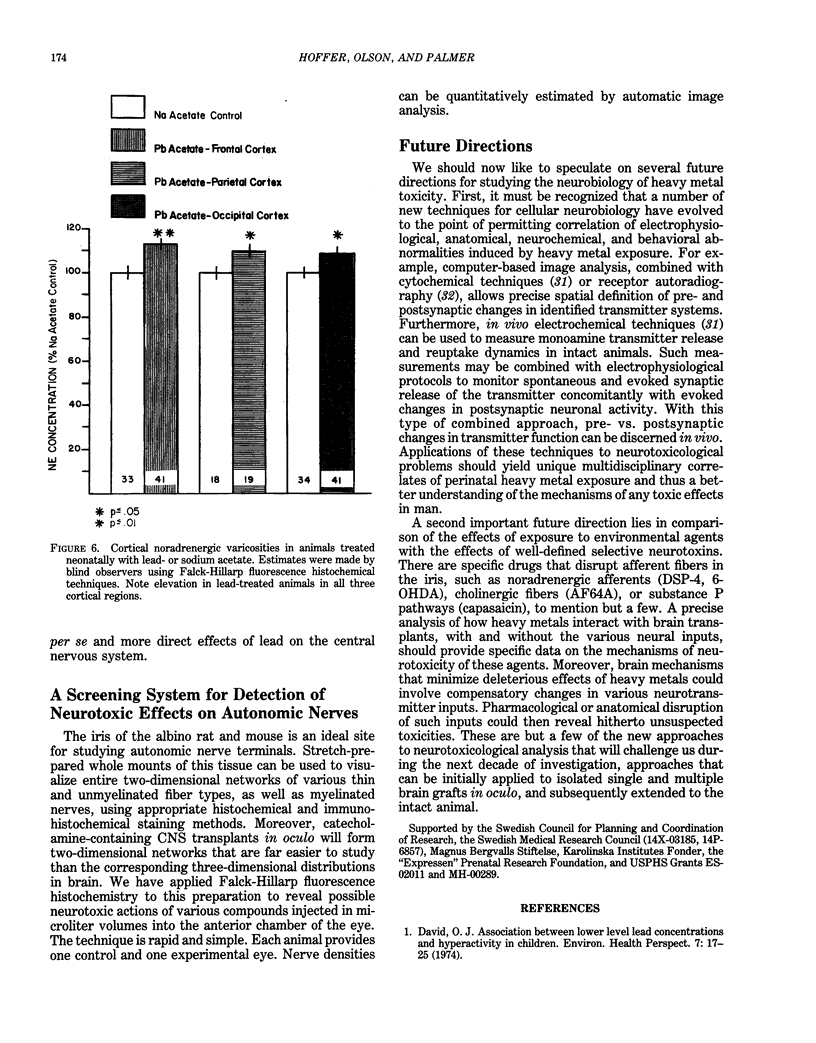
Modern man is chronically exposed to lead in the biosphere at levels several orders of magnitude higher than the natural level that once existed. There is much concern about the possible adverse effects of this population-wide, low-level lead exposure, particularly on the developing organism, wherein the central nervous system may be one primary target. We have developed in oculo test systems, which permit temporal and spatial discrimination of possible effects of lead and other potential neurotoxic agents in the environment on the developing central nervous system as well as on different types of peripheral nerves in the adult. In one experimental protocol, defined areas of the fetal rat brain are grafted to the anterior chamber of the eye of adult rat recipients that are exposed to lead. Such grafts will become vascularized from the host iris and continue developing in oculo. Thus, grafted brain tissue and host brain will share circulation and therefore be exposed to similar amounts of lead. Studies of cerebellar grafts revealed that, although there was a normal gross cytological development in the presence of lead, there was a marked and permanent impairment of spontaneous discharge rates of the grafted Purkinje neurons as observed with electrophysiological techniques long after cessation of lead treatment. The host Purkinje neurons were not affected. A similar, although less dramatic, impairment of cerebellar function could be subsequently demonstrated in intact animals when newborn rats were given lead during the first 20 days of life and studied as adults.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander F. W., Clayton B. E., Delves H. T. Mineral and trace-metal balances in children receiving normal and synthetic diets. Q J Med. 1974 Jan;43(169):89–111. [PubMed] [Google Scholar]
- Alpern H. P., Greer C. A. A dopaminergic basis for the effects of amphetamine on a mouse "preadolescent hyperkinetic" model. Life Sci. 1977 Jul 1;21(1):93–98. doi: 10.1016/0024-3205(77)90428-3. [DOI] [PubMed] [Google Scholar]
- Björklund H., Palmer M. R., Lind B., Hoffer B. J., Olson L. Postnatal lead exposure alters spontaneous cerebellar Purkinje neuron discharge. Environ Res. 1983 Aug;31(2):448–459. doi: 10.1016/0013-9351(83)90023-3. [DOI] [PubMed] [Google Scholar]
- Blanksma L. A., Sachs H. K., Murray E. F., O'Connell M. J. Incidence of high blood lead levels in Chicago children. Pediatrics. 1969 Nov;44(5):661–667. [PubMed] [Google Scholar]
- Brown D. R. Neonatal lead exposure in the rat: decreased learning as a function of age and blood lead concentrations. Toxicol Appl Pharmacol. 1975 Jun;32(3):628–637. doi: 10.1016/0041-008x(75)90126-x. [DOI] [PubMed] [Google Scholar]
- David O. J. Association between lower level lead concentrations and hyperactivity in children. Environ Health Perspect. 1974 May;7:17–25. doi: 10.1289/ehp.74717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes G. B., Reina J. C. Effect of age on gastrointestinal absorption (Fe, Sr, Pb) in the rat. J Nutr. 1972 May;102(5):647–652. doi: 10.1093/jn/102.5.647. [DOI] [PubMed] [Google Scholar]
- Fox D. A., Lewkowski J. P., Cooper G. P. Acute and chronic effects of neonatal lead exposure on development of the visual evoked responses in rats. Toxicol Appl Pharmacol. 1977 Jun;40(3):449–461. doi: 10.1016/0041-008x(77)90072-2. [DOI] [PubMed] [Google Scholar]
- Freed W. J., Ko G. N., Niehoff D. L., Kuhar M. J., Hoffer B. J., Olson L., Cannon-Spoor H. E., Morihisa J. M., Wyatt R. J. Normalization of spiroperidol binding in the denervated rat striatum by homologous grafts of substantia nigra. Science. 1983 Nov 25;222(4626):937–939. doi: 10.1126/science.6635666. [DOI] [PubMed] [Google Scholar]
- Gerhardt G., Rose G., Strömberg I., Conboy G., Olson L., Jonsson G., Hoffer B. Dopaminergic neurotoxicity of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) in the mouse: an in vivo electrochemical study. J Pharmacol Exp Ther. 1985 Oct;235(1):259–265. [PubMed] [Google Scholar]
- Haar G. T., Chadzynski L. An investigation of elevated blood lead levels in Detroit children. Arch Environ Health. 1979 May-Jun;34(3):145–150. doi: 10.1080/00039896.1979.10667386. [DOI] [PubMed] [Google Scholar]
- Kello D., Kostial K. The effect of milk diet on lead metabolism in rats. Environ Res. 1973 Sep;6(3):355–360. doi: 10.1016/0013-9351(73)90048-0. [DOI] [PubMed] [Google Scholar]
- Lorenzo A. V., Gewirtz M., Averill D. CNS lead toxicity in rabbit offspring. Environ Res. 1978 Aug;17(1):131–150. doi: 10.1016/0013-9351(78)90067-1. [DOI] [PubMed] [Google Scholar]
- Louis-Ferdinand R. T., Brown D. R., Fiddler S. F., Daughtrey W. C., Klein A. W. Morphometric and enzymatic effects of neonatal lead exposure in the rat brain. Toxicol Appl Pharmacol. 1978 Feb;43(2):351–360. doi: 10.1016/0041-008x(78)90014-5. [DOI] [PubMed] [Google Scholar]
- MONCRIEFF A. A., KOUMIDES O. P., CLAYTON B. E., PATRICK A. D., RENWICK A. G., ROBERTS G. E. LEAD POISONING IN CHILDREN. Arch Dis Child. 1964 Feb;39:1–13. doi: 10.1136/adc.39.203.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolin D. I. The hyperkinetic child syndrome and brain monoamines: pharmacology and therapeutic implications. J Clin Psychiatry. 1978 Feb;39(2):120-3, 7-30. [PubMed] [Google Scholar]
- Mykkanen H. M., Dickerson J. W., Lancaster M. C. Effect of age on the tissue distribution of lead in the rat. Toxicol Appl Pharmacol. 1979 Dec;51(3):447–454. doi: 10.1016/0041-008x(79)90369-7. [DOI] [PubMed] [Google Scholar]
- Needleman H. L., Gunnoe C., Leviton A., Reed R., Peresie H., Maher C., Barrett P. Deficits in psychologic and classroom performance of children with elevated dentine lead levels. N Engl J Med. 1979 Mar 29;300(13):689–695. doi: 10.1056/NEJM197903293001301. [DOI] [PubMed] [Google Scholar]
- Palmer M. R., Björklund H., Freedman R., Taylor D. A., Marwaha J., Olson L., Seiger A., Hoffer B. J. Permanent impairment of spontaneous Purkinje cell discharge in cerebellar grafts caused by chronic lead exposure. Toxicol Appl Pharmacol. 1981 Sep 30;60(3):431–440. doi: 10.1016/0041-008x(81)90328-8. [DOI] [PubMed] [Google Scholar]
- Press M. F. Lead encephalopathy in neonatal Long-Evans rats: morphologic studies. J Neuropathol Exp Neurol. 1977 Jan;36(1):169–193. doi: 10.1097/00005072-197701000-00014. [DOI] [PubMed] [Google Scholar]
- Sauerhoff M. W., Michaelson I. A. Hyperactivity and brain catecholamines in lead-exposed developing rats. Science. 1973 Dec 7;182(4116):1022–1024. doi: 10.1126/science.182.4116.1022. [DOI] [PubMed] [Google Scholar]
- Shaywitz B. A., Cohen D. J., Bowers M. B., Jr CSF monoamine metabolites in children with minimal brain dysfunction: evidence for alteration of brain dopamine. A preliminary report. J Pediatr. 1977 Jan;90(1):67–71. doi: 10.1016/s0022-3476(77)80766-x. [DOI] [PubMed] [Google Scholar]
- Shetty T., Chase T. N. Central monoamines and hyperkinase of childhood. Neurology. 1976 Oct;26(10):1000–1002. doi: 10.1212/wnl.26.10.1000. [DOI] [PubMed] [Google Scholar]
- Silbergeld E. K., Goldberg A. M. Pharmacological and neurochemical investigations of lead-induced hyperactivity. Neuropharmacology. 1975 May-Jun;14(5-6):431–444. doi: 10.1016/0028-3908(75)90026-x. [DOI] [PubMed] [Google Scholar]
- Ziegler E. E., Edwards B. B., Jensen R. L., Mahaffey K. R., Fomon S. J. Absorption and retention of lead by infants. Pediatr Res. 1978 Jan;12(1):29–34. doi: 10.1203/00006450-197801000-00008. [DOI] [PubMed] [Google Scholar]





