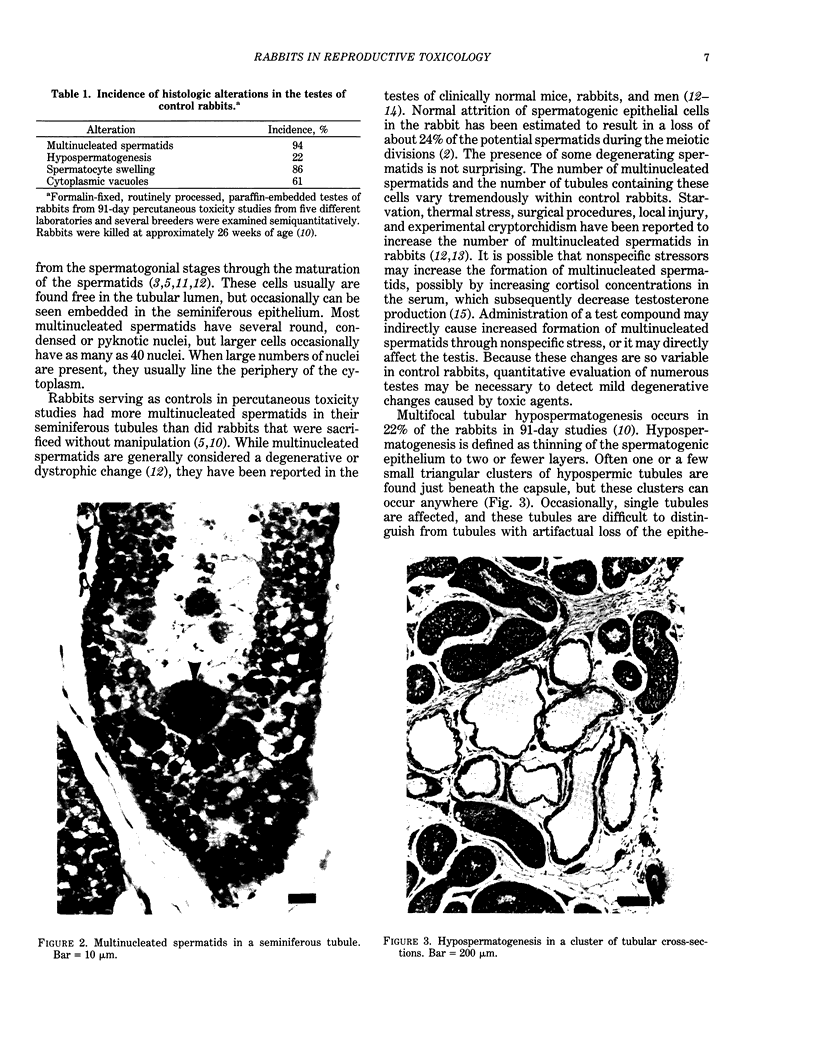
Abstract
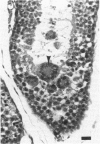
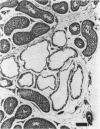
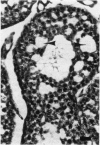
The rabbit is the smallest and least expensive laboratory animal in which serial semen samples can be obtained for morphologic, biochemical, and fertility evaluation. The female rabbit has a predictable reproductive cycle and can be artificially inseminated with a known amount of sperm during fertility testing. These advantages make the rabbit an extremely valuable model for studying the effects of chemicals or other stimuli on the male reproductive system. Quantitative evaluation of the testis, semen, and accessory reproductive organs is important in order to detect subtle effects of a chemical on reproductive capacity. Evaluation of testis size, serum hormone concentrations, and the number, morphology, motility, and fertility of sperm in the ejaculate can be performed serially in the live rabbit. Weights of testes and accessory reproductive organs, estimates of daily sperm production, and histomorphometric data on the seminiferous epithelium can be obtained after sacrifice. Multinucleated spermatids, focal tubular hypospermatogenesis, swelling of spermatocytes, and cytoplasmic vacuoles in Sertoli's cells occur commonly in testes of control rabbits. These changes may be confused with toxic lesions. The incidence of multinucleated spermatids may be increased by stress associated with handling or the environment. Histomorphometric evaluation may be required to prove that a test compound has an adverse effect on the male reproductive system.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amann R. P. Detection of alterations in testicular and epididymal function in laboratory animals. Environ Health Perspect. 1986 Dec;70:149–158. doi: 10.1289/ehp.8670149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann R. P. The male rabbit. IV. Quantitative testicular histology and comparisons between daily sperm production as determined histologically and daily sperm output. Fertil Steril. 1970 Sep;21(9):662–672. [PubMed] [Google Scholar]
- Amann R. P. Use of animal models for detecting specific alterations in reproduction. Fundam Appl Toxicol. 1982 Jan-Feb;2(1):13–26. doi: 10.1016/s0272-0590(82)80059-6. [DOI] [PubMed] [Google Scholar]
- Benitz K. F., Dambach G. The toxicological significance of multinucleated giant cells in dystrophic testes of laboratory mammals and man. Arzneimittelforschung. 1965 Apr;15(4):391–404. [PubMed] [Google Scholar]
- Chapin R. E., Ross M. D., Lamb J. C. Immersion fixation methods for glycol methacrylate-embedded testes. Toxicol Pathol. 1984;12(3):221–227. doi: 10.1177/019262338401200303. [DOI] [PubMed] [Google Scholar]
- Dym M., Fawcett D. W. Further observations on the numbers of spermatogonia, spermatocytes, and spermatids connected by intercellular bridges in the mammalian testis. Biol Reprod. 1971 Apr;4(2):195–215. doi: 10.1093/biolreprod/4.2.195. [DOI] [PubMed] [Google Scholar]
- Ford L. Comparison of testicular maturation in two inbred rabbitlines. Am J Vet Res. 1970 May;31(5):941–945. [PubMed] [Google Scholar]
- Gondos B., Renston R. H., Conner L. A. Ultrastructure of germ cells and Sertoli cells in the postnatal rabbit testis. Am J Anat. 1973 Apr;136(4):427–439. doi: 10.1002/aja.1001360404. [DOI] [PubMed] [Google Scholar]
- Holstein A. F., Eckmann C. Multinucleated spermatocytes and spermatids in human seminiferous tubules. Andrologia. 1986 Jan-Feb;18(1):5–16. doi: 10.1111/j.1439-0272.1986.tb01729.x. [DOI] [PubMed] [Google Scholar]
- McGrady A. V. Effects of psychological stress on male reproduction: a review. Arch Androl. 1984;13(1):1–7. doi: 10.3109/01485018408987495. [DOI] [PubMed] [Google Scholar]
- Morton D., Weisbrode S. E., Wyder W. E., Maurer J. K., Capen C. C. Histologic alterations in the testes of laboratory rabbits. Vet Pathol. 1986 Mar;23(2):214–217. doi: 10.1177/030098588602300221. [DOI] [PubMed] [Google Scholar]
- Morton D., Weisbrode S. E., Wyder W. E., Maurer J. K., Capen C. C. Spermatid giant cells, tubular hypospermatogenesis, spermatogonial swelling, cytoplasmic vacuoles, and tubular dilatation in the testes of normal rabbits. Vet Pathol. 1986 Mar;23(2):176–183. doi: 10.1177/030098588602300211. [DOI] [PubMed] [Google Scholar]
- Plöen L. An electron microscope study of the delayed effects on rabbit spermateleosis following experimental cryptorchidism for twenty-four hours. Virchows Arch B Cell Pathol. 1973 Nov 28;14(2):159–184. doi: 10.1007/BF02889185. [DOI] [PubMed] [Google Scholar]
- SWIERSTRA E. E., FOOTE R. H. Cytology and kinetics of spermatogenesis in the rabbit. J Reprod Fertil. 1963 Jun;5:309–322. doi: 10.1530/jrf.0.0050309. [DOI] [PubMed] [Google Scholar]
- Sun E. L., Gondos B. Formation of the blood-testis barrier in the rabbit. Cell Tissue Res. 1986;243(3):575–578. doi: 10.1007/BF00218064. [DOI] [PubMed] [Google Scholar]