Abstract
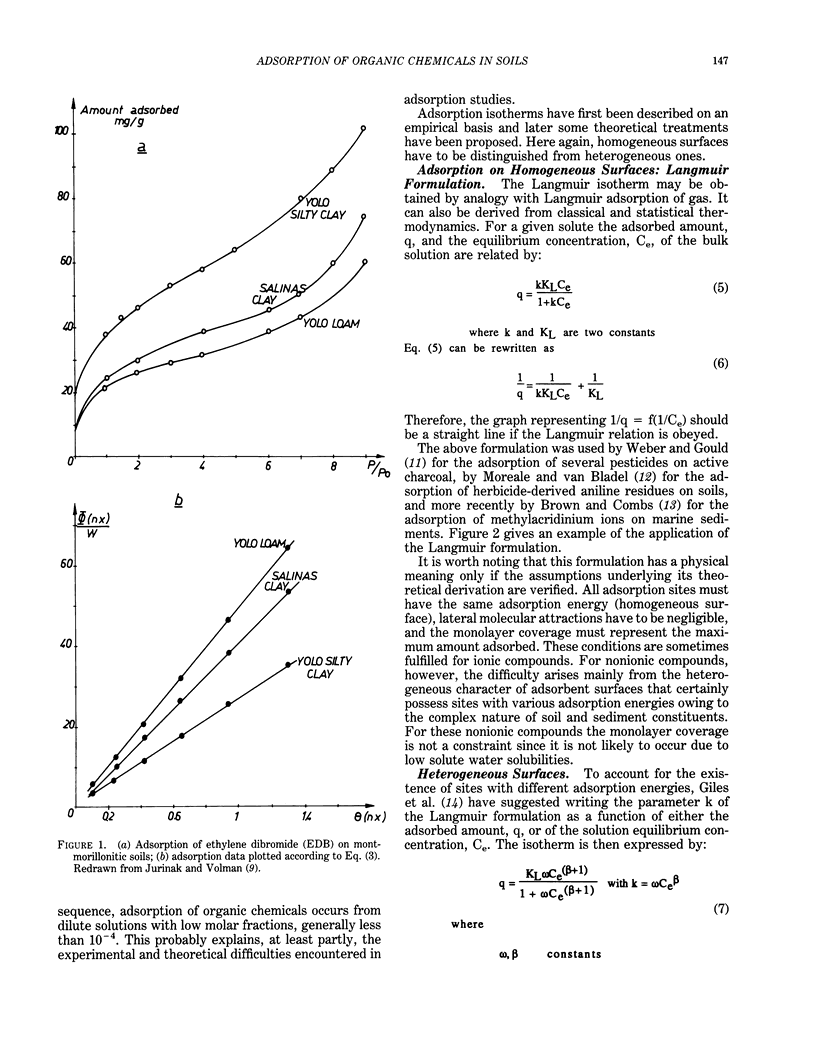
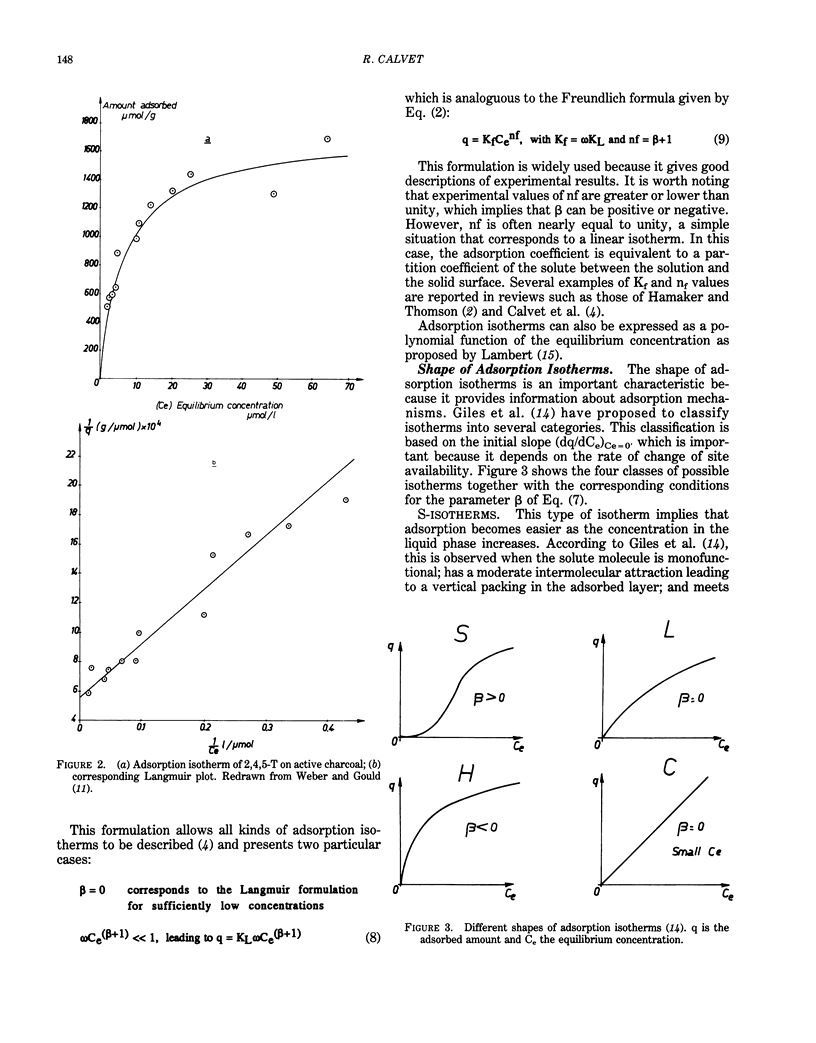
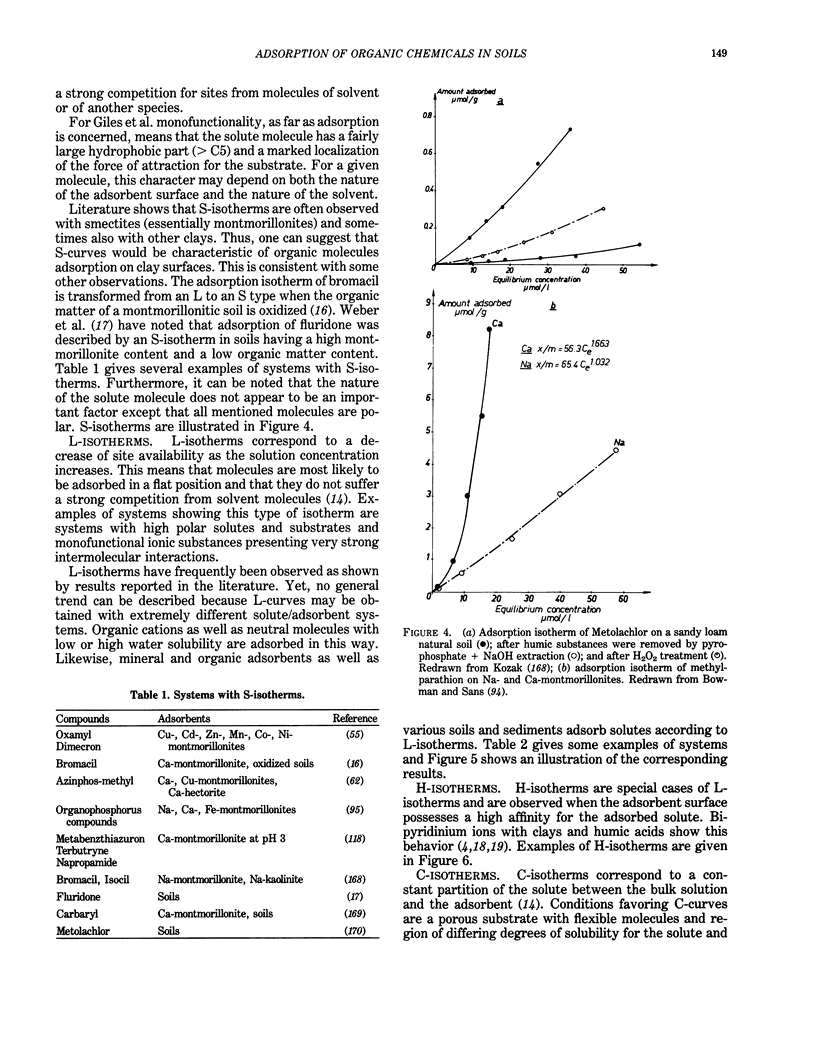
This paper presents a review on adsorption of organic chemicals on soils sediments and their constituents. The first part of this review deals with adsorption from gas and liquid phases and gives a discussion on the physical meaning of the shape of adsorption isotherms. Results show that no general rules can be proposed to describe univocally the relation between the shape of isotherms and the nature of adsorbate-adsorbent system. Kinetics of adsorption is discussed through the description of various models. Theoretical developments exist both for the thermodynamics and the kinetics of adsorption, but there is a strong need for experimental results. Possible adsorption mechanisms are ion exchange, interaction with metallic cations, hydrogen bonds, charge transfers, and London-van der Waals dispersion forces/hydrophobic effect. However, direct proofs of a given mechanism are rare. Several factors influence adsorption behavior. Electronic structure of adsorbed molecules, properties of adsorbents, and characteristics of the liquid phase are discussed in relation to adsorption. Such properties as water solubility, organic carbon content of adsorbing materials, and the composition of the liquid phase are particularly important. Evaluation of adsorption can be obtained through either laboratory measurements or use of several correlations. Adsorption measurements must be interpreted, taking into account treatment of adsorbent materials, experimental conditions, and secondary phenomena such as degradations. Correlations between adsorption coefficients and water-octanol partition coefficient or water solubility are numerous. They may be useful tools for prediction purposes. Relations with transport, bioavailability, and degradation are described.
Full text
PDF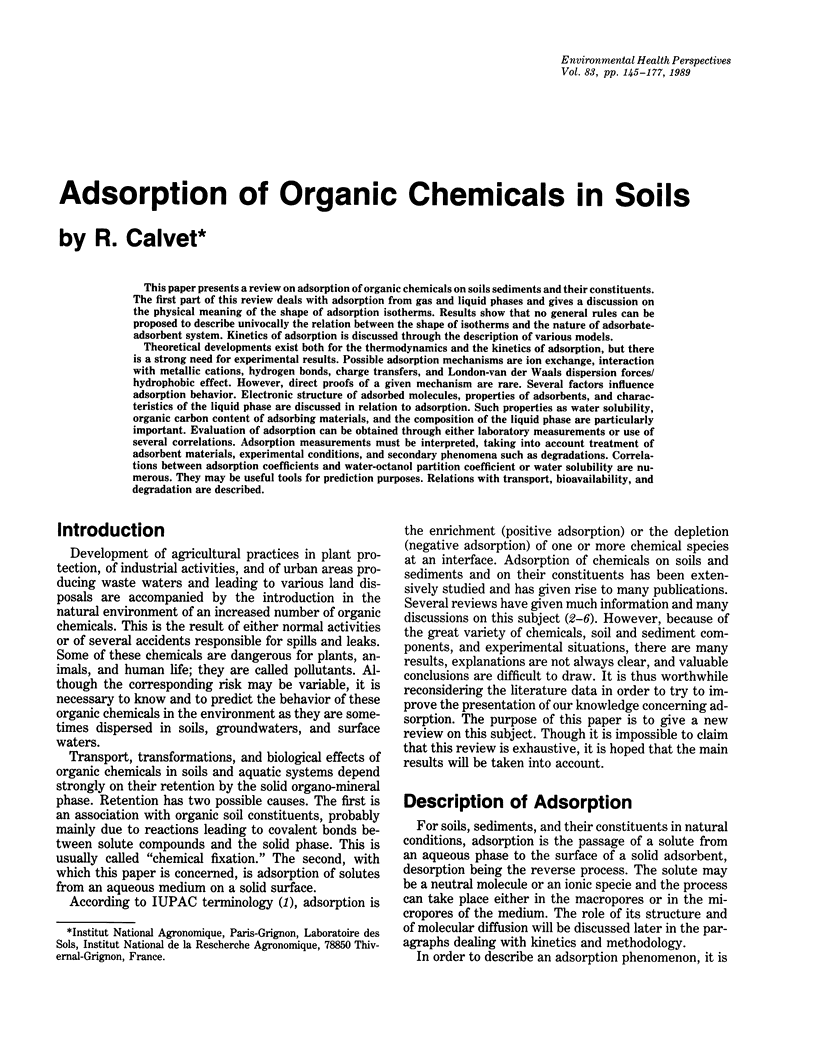














Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aharonson N., Kafkafi U. Adsorption of benzimidazole fungicides on montmorillonite and kaolinite clay surfaces. J Agric Food Chem. 1975 May-Jun;23(3):434–437. doi: 10.1021/jf60199a069. [DOI] [PubMed] [Google Scholar]
- Aharonson N., Kafkafi U. Adsorption, mobility, and persistence of thiabendazole and methyl 2-benzimidazolecarbamate in soils. J Agric Food Chem. 1975 Jul-Aug;23(4):720–724. doi: 10.1021/jf60200a017. [DOI] [PubMed] [Google Scholar]
- Browne J. E., Feldkamp J. R., White J. L., Hem S. L. Potential of organic cation-saturated montmorillonite as treatment for poisoning by weak bases. J Pharm Sci. 1980 Dec;69(12):1393–1395. doi: 10.1002/jps.2600691210. [DOI] [PubMed] [Google Scholar]
- Carringer R. D., Weber J. B., Monaco T. J. Adsorption-desorption of selected pesticides by organic matter and montmorillonite. J Agric Food Chem. 1975 May-Jun;23(3):568–572. doi: 10.1021/jf60199a037. [DOI] [PubMed] [Google Scholar]
- Chiou C. T., Peters L. J., Freed V. H. A physical concept of soil-water equilibria for nonionic organic compounds. Science. 1979 Nov 16;206(4420):831–832. doi: 10.1126/science.206.4420.831. [DOI] [PubMed] [Google Scholar]
- Dieguez-Carbonell D., Rodriguez Pascual C. Adsorption of the herbicide "2,4-D" by montmorillonite. Environ Qual Saf Suppl. 1975;3:237–242. [PubMed] [Google Scholar]
- Haque R., Sexton R. Kinetic and equilibrium study of the adsorption of 2,4-dichlorophenoxy acetic acid on some surfaces. J Colloid Interface Sci. 1968 Aug;27(4):818–827. doi: 10.1016/0021-9797(68)90115-x. [DOI] [PubMed] [Google Scholar]
- Hayes M. H. Adsorption of triazine herbicides on soil organic matter, including a short review on soil organic matter chemistry. Residue Rev. 1970;32:131–174. doi: 10.1007/978-1-4615-8464-3_6. [DOI] [PubMed] [Google Scholar]
- Israelachvili J., Pashley R. The hydrophobic interaction is long range, decaying exponentially with distance. Nature. 1982 Nov 25;300(5890):341–342. doi: 10.1038/300341a0. [DOI] [PubMed] [Google Scholar]


