Abstract
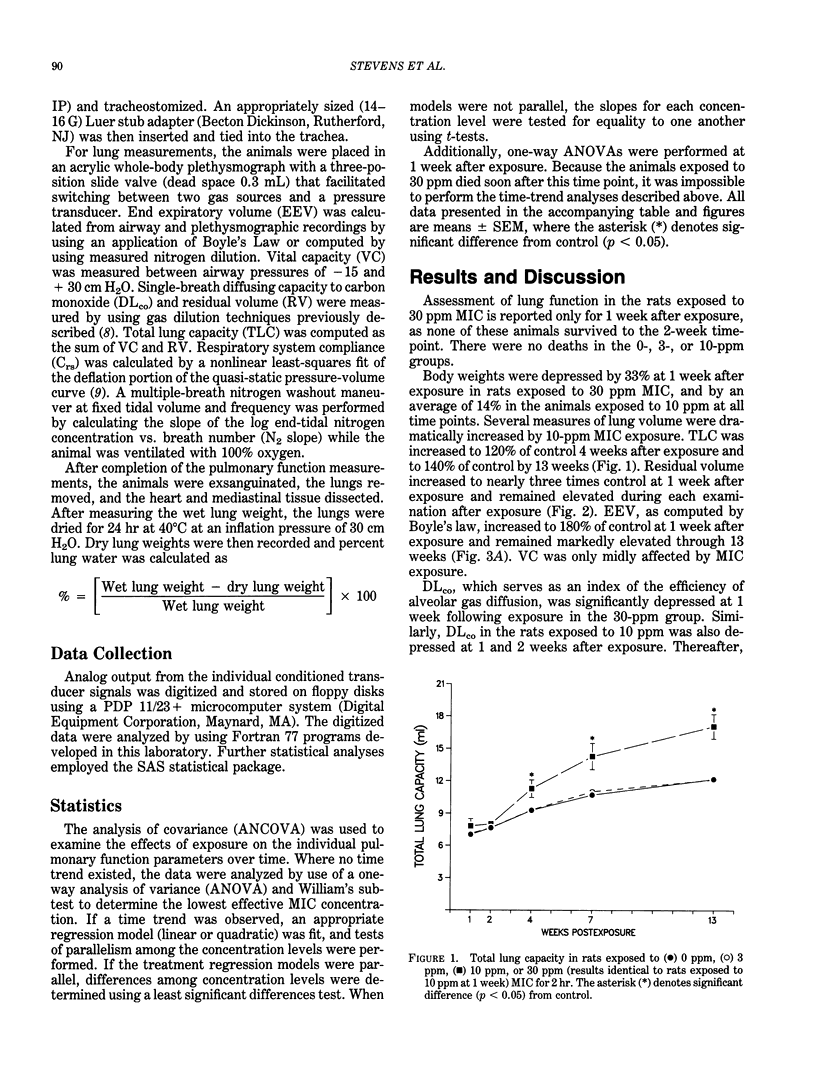
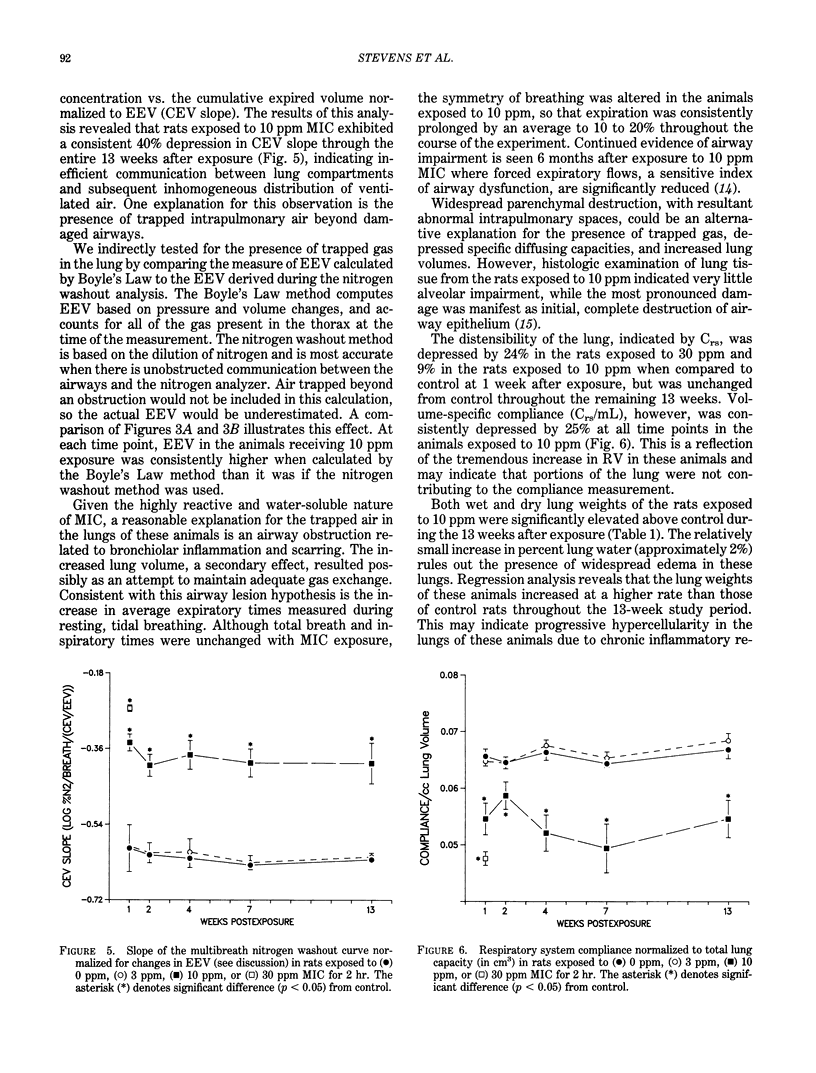
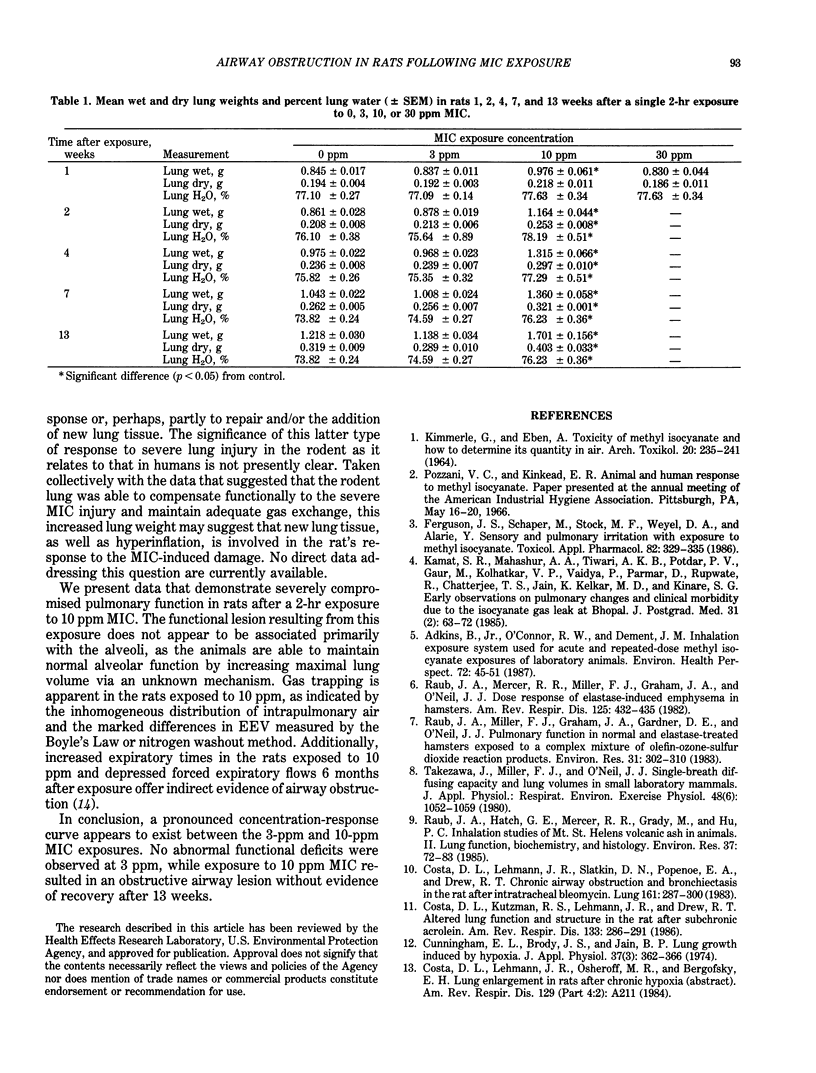
Pulmonary function was assessed in male, F344 rats 1,2,4,7, and 13 weeks after a single 2-hr exposure to 0, 3, 10, or 30 ppm methyl isocyanate. No significant changes were observed in the rats exposed to 3 ppm through 13 weeks. Diffusing capacity (DLco), quasistatic lung compliance, and homogeneity of ventilation, as determined by multibreath nitrogen washout, were depressed in the rats exposed to 10 and 30 ppm by 1 week after exposure. None of the rats exposed to 30 ppm survived beyond 1 week. By 13 weeks, dramatic increases in lung volumes were observed in the rats exposed to 10 ppm, while DLco and lung compliance were only mildly affected. However, volume-specific DLco and compliance were depressed in the rats exposed to 10 ppm, suggesting that lung hyperinflation or other compensatory means of increasing lung size occurred in response to the methyl isocyanate-induced lung lesion. This group also exhibited increased expiratory times during tidal breathing and severely impaired distribution of ventilated air. Collectively, these results suggest the development and likely progression of a severe, obstructive airway lesion with associated gas trapping, and the existence of a pronounced concentration-response relationship between 3 and 10 ppm methyl isocyanate exposures.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adkins B., Jr, O'Connor R. W., Dement J. M. Inhalation exposure system used for acute and repeated-dose methyl isocyanate exposures of laboratory animals. Environ Health Perspect. 1987 Jun;72:45–51. doi: 10.1289/ehp.877245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucher J. R., Boorman G. A., Gupta B. N., Uraih L. C., Hall L. B., Stefanski S. A. Two-hour methyl isocyanate inhalation exposure and 91-day recovery: a preliminary description of pathologic changes in F344 rats. Environ Health Perspect. 1987 Jun;72:71–75. doi: 10.1289/ehp.877271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa D. L., Kutzman R. S., Lehmann J. R., Drew R. T. Altered lung function and structure in the rat after subchronic exposure to acrolein. Am Rev Respir Dis. 1986 Feb;133(2):286–291. doi: 10.1164/arrd.1986.133.2.286. [DOI] [PubMed] [Google Scholar]
- Costa D. L., Lehmann J. R., Slatkin D. N., Popenoe E. A., Drew R. T. Chronic airway obstruction and bronchiectasis in the rat after intratracheal bleomycin. Lung. 1983;161(5):287–300. doi: 10.1007/BF02713874. [DOI] [PubMed] [Google Scholar]
- Cunningham E. L., Brody J. S., Jain B. P. Lung growth induced by hypoxia. J Appl Physiol. 1974 Sep;37(3):362–366. doi: 10.1152/jappl.1974.37.3.362. [DOI] [PubMed] [Google Scholar]
- Ferguson J. S., Schaper M., Stock M. F., Weyel D. A., Alarie Y. Sensory and pulmonary irritation with exposure to methyl isocyanate. Toxicol Appl Pharmacol. 1986 Feb;82(2):329–335. doi: 10.1016/0041-008x(86)90209-7. [DOI] [PubMed] [Google Scholar]
- KIMMERLE G., EBEN A. ZUR TOXICITAET VON METHYLISOCYANAT UND DESSEN QUANTITATIVER BESTIMMUNG IN DER LUFT. Arch Toxikol. 1964 May 27;20:235–241. [PubMed] [Google Scholar]
- Kamat S. R., Mahashur A. A., Tiwari A. K., Potdar P. V., Gaur M., Kolhatkar V. P., Vaidya P., Parmar D., Rupwate R., Chatterjee T. S. Early observations on pulmonary changes and clinical morbidity due to the isocyanate gas leak at Bhopal. J Postgrad Med. 1985 Apr;31(2):63–72. [PubMed] [Google Scholar]
- Raub J. A., Hatch G. E., Mercer R. R., Grady M., Hu P. C. Inhalation studies of Mt. St. Helens volcanic ash in animals. II. Lung function, biochemistry, and histology. Environ Res. 1985 Jun;37(1):72–83. doi: 10.1016/0013-9351(85)90050-7. [DOI] [PubMed] [Google Scholar]
- Raub J. A., Mercer R. R., Miller F. J., Graham J. A., O'Neil J. J. Dose response of elastase-induced emphysema in hamsters. Am Rev Respir Dis. 1982 Apr;125(4):432–435. doi: 10.1164/arrd.1982.125.4.432. [DOI] [PubMed] [Google Scholar]
- Raub J. A., Miller F. J., Graham J. A., Gardner D. E., O'Neil J. J. Pulmonary function in normal and elastase-treated hamsters exposed to a complex mixture of olefin-ozone-sulfur dioxide reaction products. Environ Res. 1983 Aug;31(2):302–310. doi: 10.1016/0013-9351(83)90008-7. [DOI] [PubMed] [Google Scholar]
- Takezawa J., Miller F. J., O'Neil J. J. Single-breath diffusing capacity and lung volumes in small laboratory mammals. J Appl Physiol Respir Environ Exerc Physiol. 1980 Jun;48(6):1052–1059. doi: 10.1152/jappl.1980.48.6.1052. [DOI] [PubMed] [Google Scholar]
- Tepper J. S., Wiester M. J., Costa D. L., Watkinson W. P., Weber M. F. Cardiopulmonary effects in awake rats four and six months after exposure to methyl isocyanate. Environ Health Perspect. 1987 Jun;72:95–103. doi: 10.1289/ehp.877295. [DOI] [PMC free article] [PubMed] [Google Scholar]



