Abstract
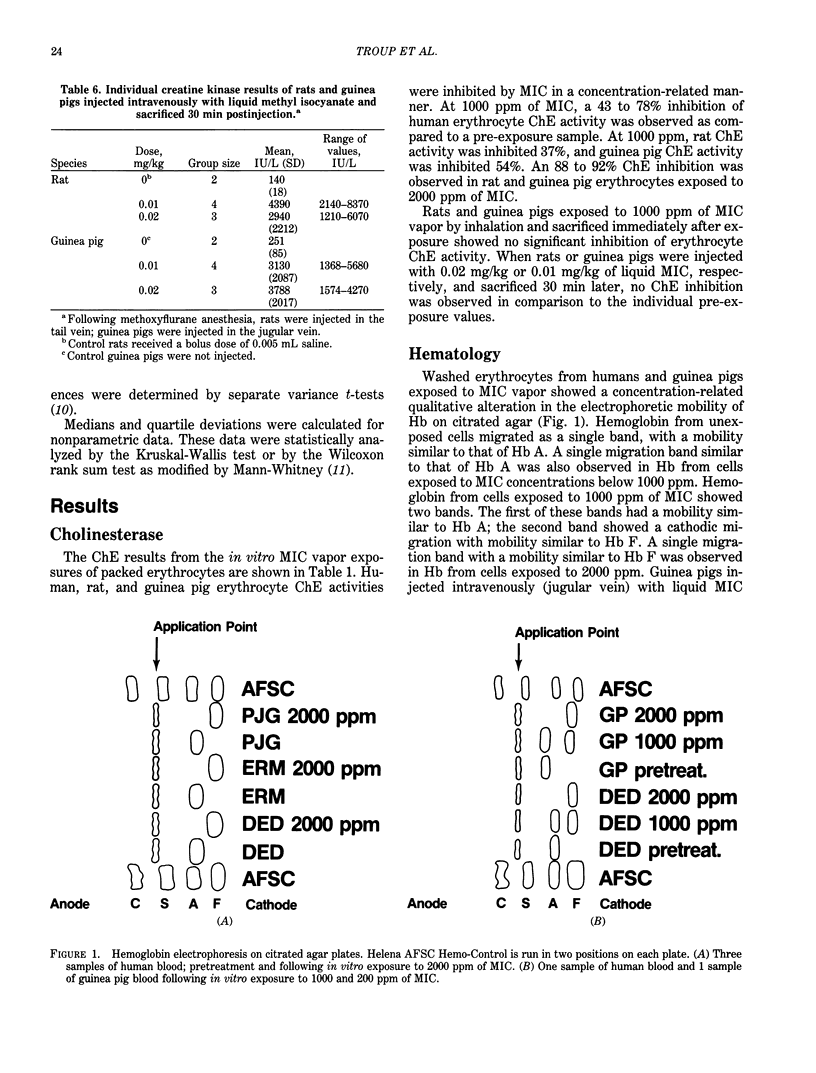
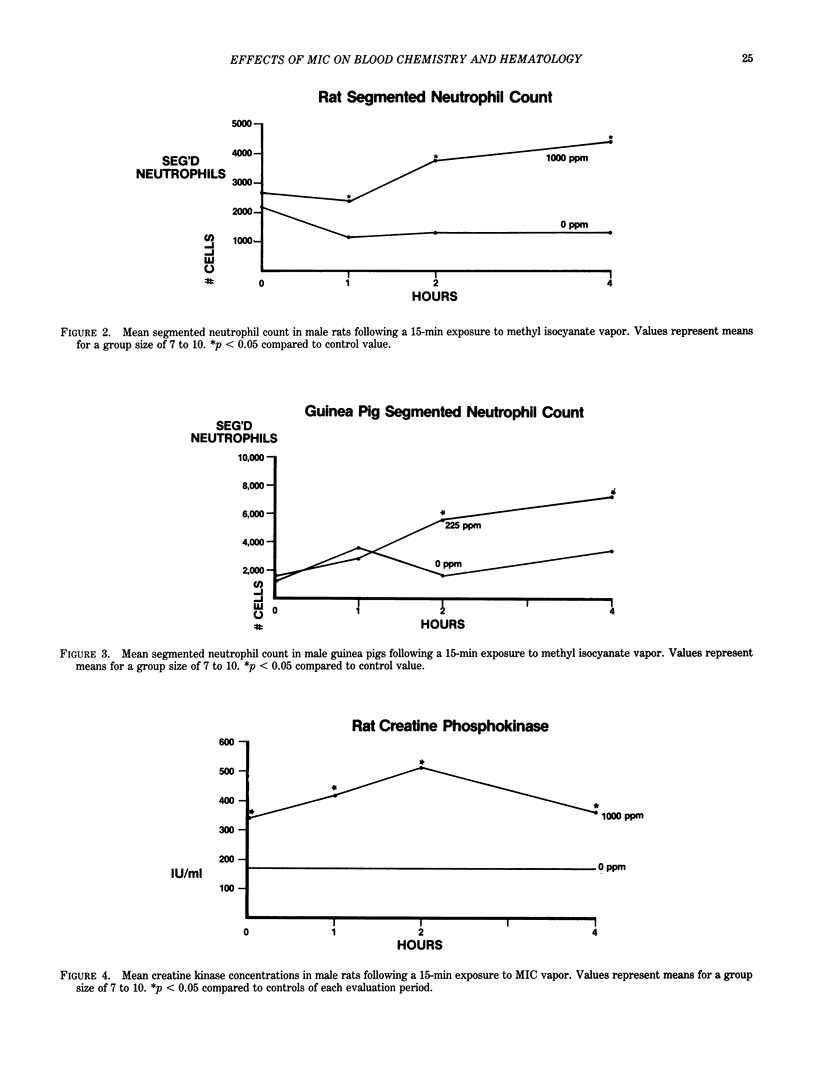
Human, rat, and guinea pig packed erythrocytes exposed to 100, 500, or 1000 ppm of methyl isocyanate (MIC) vapor in vitro showed a concentration-related inhibition of cholinesterase (ChE) activity. Rat and guinea pig packed erythrocytes showed an almost complete inhibition of ChE activity at 2000 ppm. In vitro exposures of human and guinea pig blood to 1000 or 2000 ppm of MIC vapor resulted in qualitative alterations in the electrophoretic mobility of hemoglobin (Hb) as measured by citrated agar electrophoresis. In rats and guinea pigs, neither IV injection of liquid MIC nor in vivo exposure to 1000 ppm of MIC by inhalation resulted in any inhibition of erythrocyte ChE activity or alteration in Hb electrophoretic mobility. As a result of these observations, it was concluded that neither ChE inhibition nor structural alteration of Hb were major contributing factors to death resulting from MIC exposure. Rats and guinea pigs receiving IV injections of liquid MIC showed an increase in creatine kinase (CK) levels. This increase could not be attributed to a specific isoenzyme of CK by ion exchange chromatography. Rats exposed to 100, 600, or 1000 ppm of MIC and guinea pigs exposed to 25, 125, or 225 ppm of MIC and bled immediately following a 15-min exposure or at 1, 2, 4, or 16 hr postexposure had the following alterations in blood parameters: an increase in CK, increases in hemoglobin concentration and hematocrit, reticulocytosis (rats only), neutrophilia, a decrease in blood pH and PO2, and an increase in blood PCO2.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown W. E., Green A. H., Karol M. H., Alarie Y. C. Inhibition of cholinesterase activity by isocyanates. Toxicol Appl Pharmacol. 1982 Mar 30;63(1):45–52. doi: 10.1016/0041-008x(82)90025-4. [DOI] [PubMed] [Google Scholar]
- Dodd D. E., Frank F. R., Fowler E. H., Troup C. M., Milton R. M. Biological effects of short-term, high-concentration exposure to methyl isocyanate. I. Study objectives and inhalation exposure design. Environ Health Perspect. 1987 Jun;72:13–19. doi: 10.1289/ehp.877213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedde M. R., Dodd D. E., Troup C. M., Fowler E. H. Biological effects of short-term, high-concentration exposure to methyl isocyanate. III. Influence on gas exchange in the guinea pig lung. Environ Health Perspect. 1987 Jun;72:29–33. doi: 10.1289/ehp.877229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler E. H., Dodd D. E., Troup C. M. Biological effects of short-term, high-concentration exposure to methyl isocyanate. V. Morphologic evaluation of rat and guinea pig lungs. Environ Health Perspect. 1987 Jun;72:39–44. doi: 10.1289/ehp.877239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry P. D., Bloor C. M., Sobel B. E. Increased serum creatine phosphokinase activity in experimental pulmonary embolism. Am J Cardiol. 1970 Aug;26(2):151–155. doi: 10.1016/0002-9149(70)90773-3. [DOI] [PubMed] [Google Scholar]
- Humiston C. G., Wright G. J. An automated method for the determination of cholinesterase activity. Toxicol Appl Pharmacol. 1967 May;10(3):467–480. doi: 10.1016/0041-008x(67)90087-7. [DOI] [PubMed] [Google Scholar]
- Kolb W. P., Savary J. R., Troup C. M., Dodd D. E., Tamerius J. D. Biological effects of short-term, high-concentration exposure to methyl isocyanate. VI. In vitro and in vivo complement activation studies. Environ Health Perspect. 1987 Jun;72:189–195. doi: 10.1289/ehp.8772189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. K. Methylisocyanate as an antisickling agent and its reaction with hemoglobin S. J Biol Chem. 1976 Oct 25;251(20):6226–6231. [PubMed] [Google Scholar]
- Maginniss L. A., Szewczak J. M., Troup C. M. Biological effects of short-term, high-concentration exposure to methyl isocyanate. IV. Influence on the oxygen-binding properties of guinea pig blood. Environ Health Perspect. 1987 Jun;72:35–38. doi: 10.1289/ehp.877235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemery B., Dinsdale D., Sparrow S., Ray D. E. Effects of methyl isocyanate on the respiratory tract of rats. Br J Ind Med. 1985 Dec;42(12):799–805. doi: 10.1136/oem.42.12.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Till G. O., Ward P. A. Systemic complement activation and acute lung injury. Fed Proc. 1986 Jan;45(1):13–18. [PubMed] [Google Scholar]
- Wilson E., Winter W. P. Use of citrate agar electrophoresis in evaluation of antisickling agents. J Natl Med Assoc. 1981 Feb;73(2):111–116. [PMC free article] [PubMed] [Google Scholar]




