Abstract
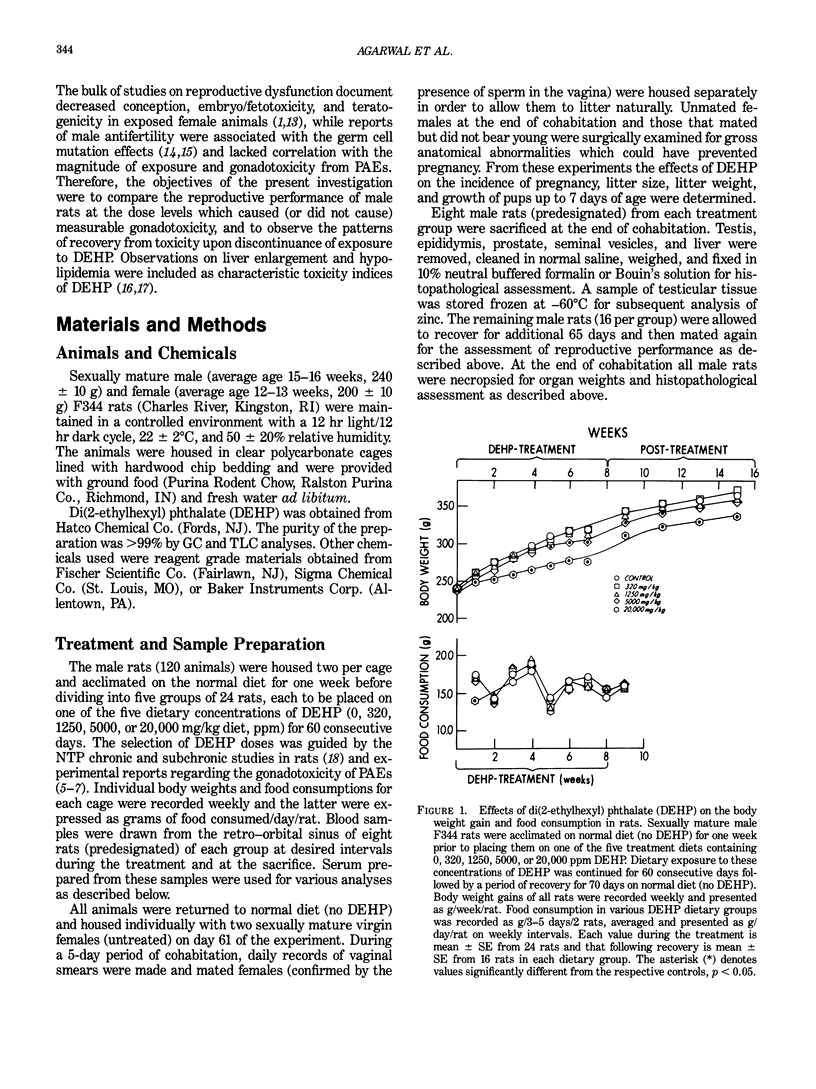
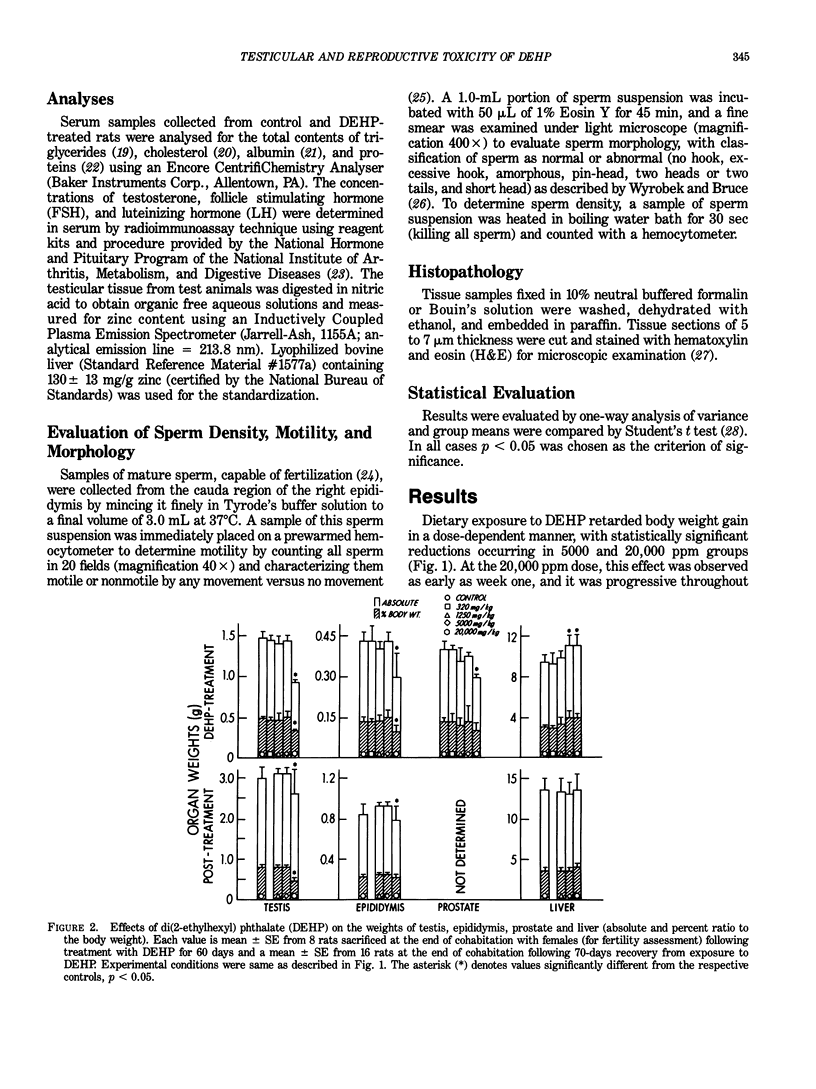
Dietary exposure of adult male F344 rats to 0, 320, 1250, 5000, or 20,000 ppm DEHP for 60 consecutive days resulted in a dose-dependent reduction in total body, testis, epididymis, and prostate weights at 5000 and 20,000 ppm. Degenerative changes were observed in testis, along with decreased testicular zinc content, reduced epididymal sperm density and motility, and increased occurrence of abnormal sperm at 20,000 ppm. There was a trend towards reduced testosterone and increased luteinizing hormone and follicle stimulating hormone in serum at 5000 and 20,000 ppm. The mean percentage of fertile animals was unchanged and reduction in fertility parameters, although not marked in severity, were correlated with gonadal effects. Average litter size was reduced at 20,000 ppm, but initial pup weights and growth were unaffected. There were no grossly observed abnormalities in the offspring and the rate of neonatal deaths was similar in control and DEHP treated groups. Characteristic toxicity manifestations of DEHP included dose-dependent enlargement of liver and reduced sperm triglycerides and cholesterol. Additionally, serum albumin and total proteins were dose dependently increased upon treatment with DEHP. Cessation of exposure to DEHP initiated partial to complete recovery from toxicity in most cases. The magnitude of recovery were variable with that of the gonads being slower than other systems. These data suggest a lack of reproductive dysfunction in F344 male rats at DEHP doses below 20,000 ppm which produced measurable testicular degeneration and afflicted epididymal sperm morphology under the present experimental conditions.
Full text
PDF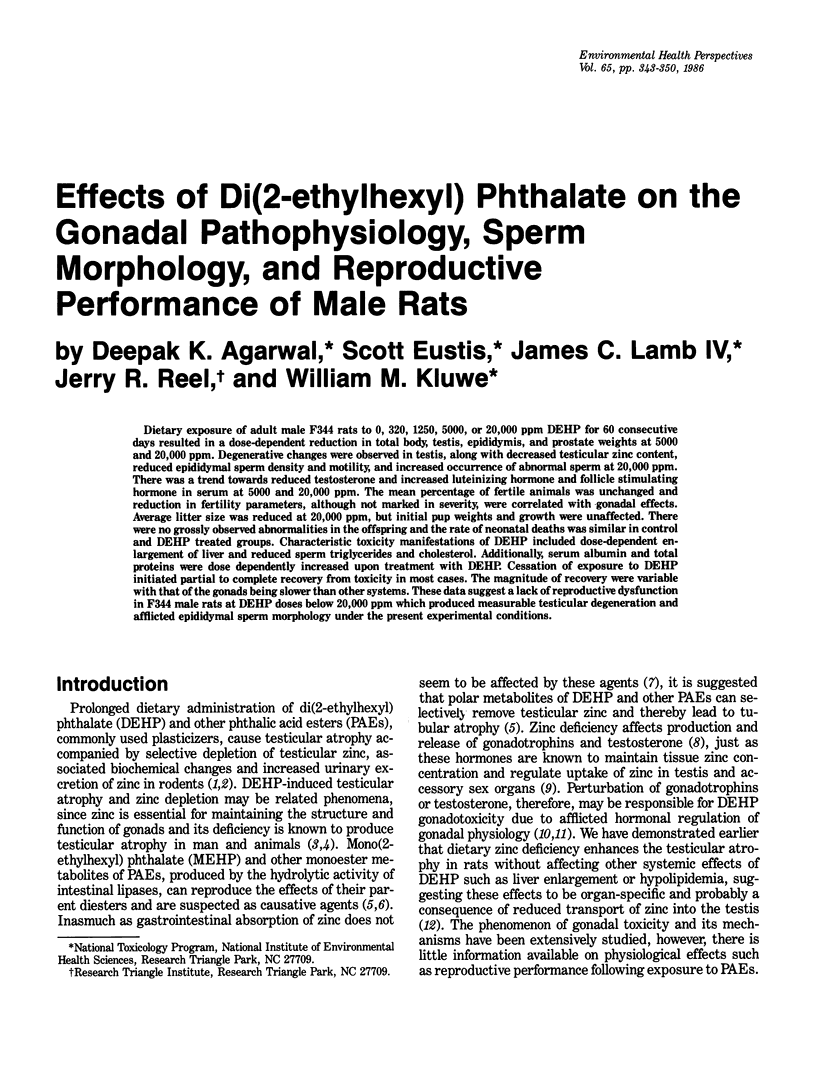
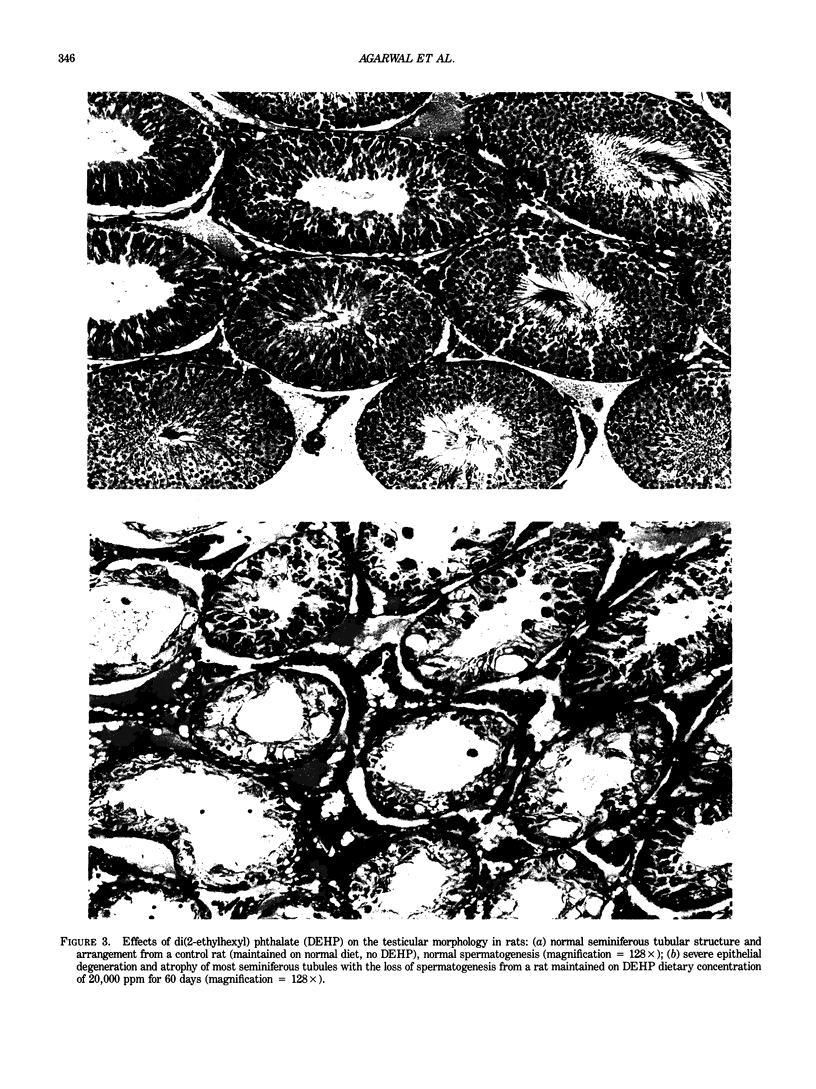
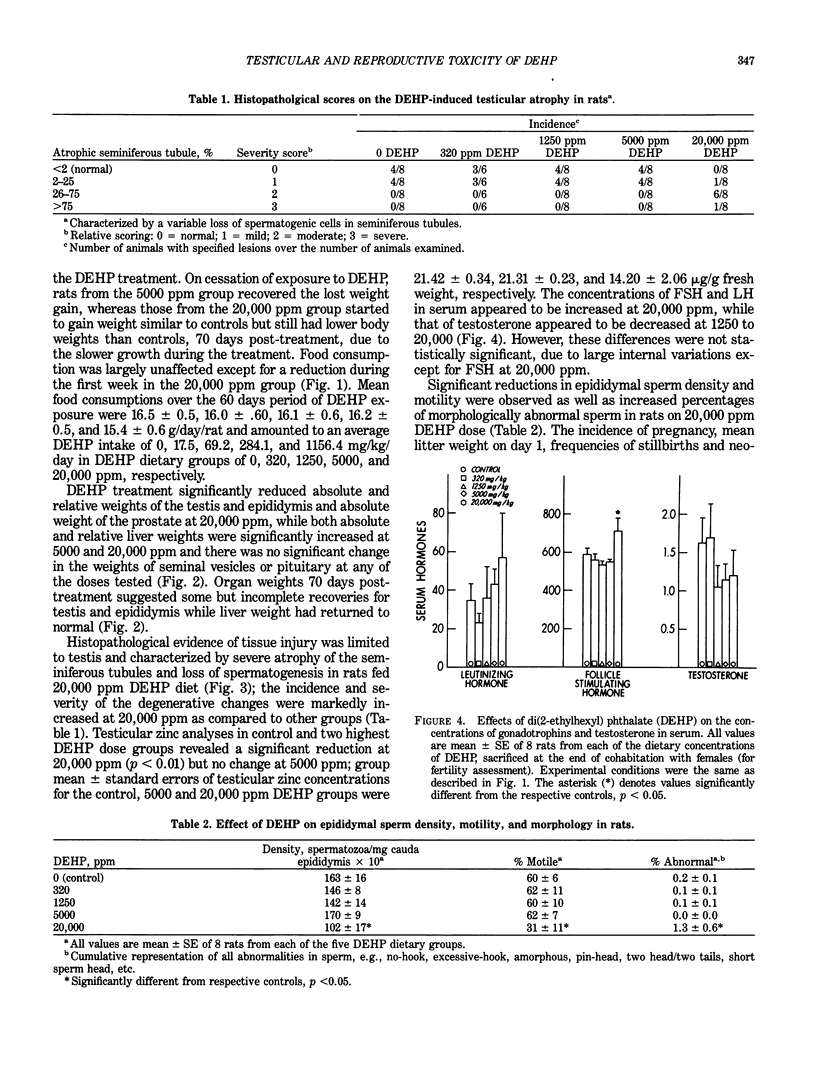
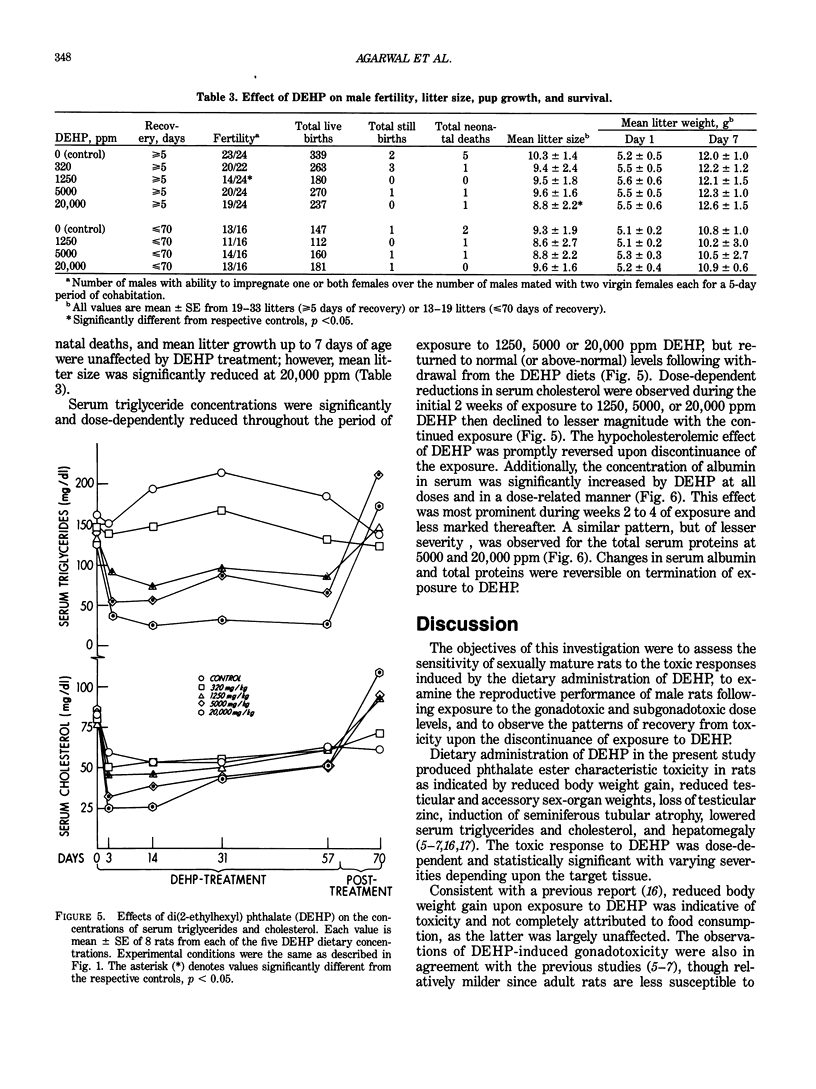
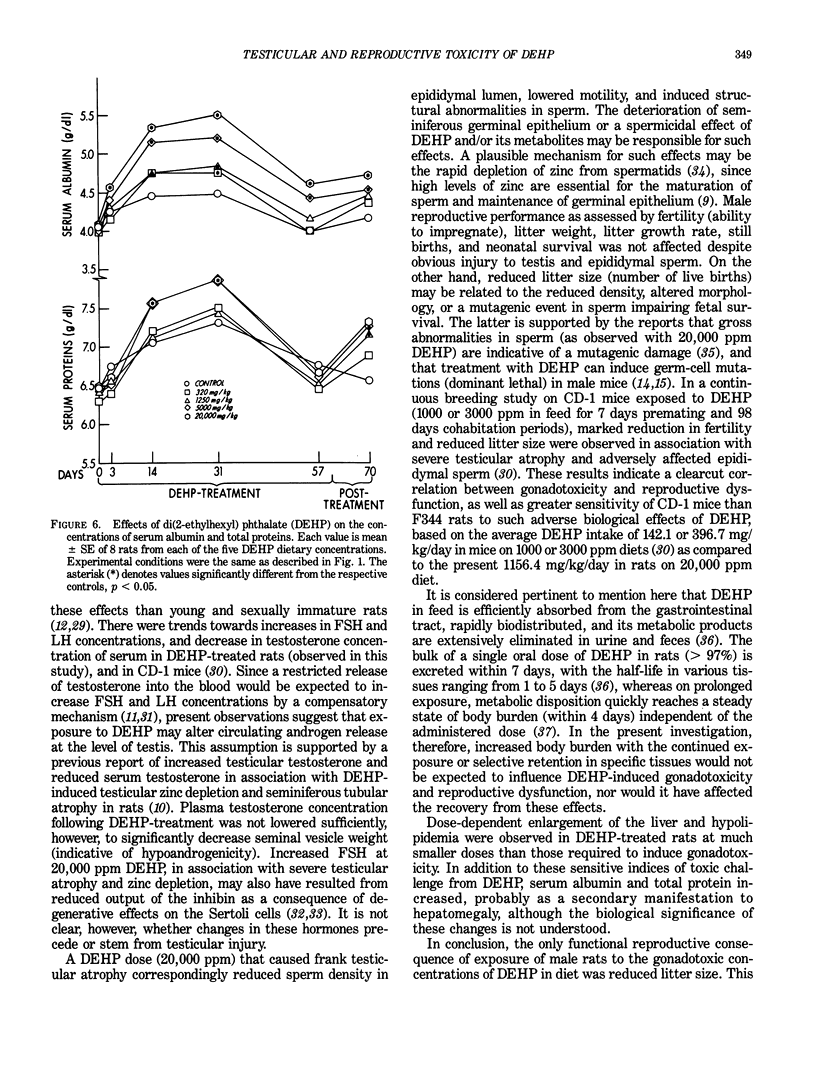

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agarwal D. K., Maronpot R. R., Lamb J. C., 4th, Kluwe W. M. Adverse effects of butyl benzyl phthalate on the reproductive and hematopoietic systems of male rats. Toxicology. 1985 Jun 14;35(3):189–206. doi: 10.1016/0300-483x(85)90015-0. [DOI] [PubMed] [Google Scholar]
- Albro P. W., Corbett J. T., Schroeder J. L., Jordan S., Matthews H. B. Pharmacokinetics, interactions with macromolecules and species differences in metabolism of DEHP. Environ Health Perspect. 1982 Nov;45:19–25. doi: 10.1289/ehp.824519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allain C. C., Poon L. S., Chan C. S., Richmond W., Fu P. C. Enzymatic determination of total serum cholesterol. Clin Chem. 1974 Apr;20(4):470–475. [PubMed] [Google Scholar]
- Autian J. Antifertility effects and dominant lethal assays for mutagenic effects of DEHP. Environ Health Perspect. 1982 Nov;45:115–118. doi: 10.1289/ehp.8245115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell F. P. Effects of phthalate esters on lipid metabolism in various tissues, cells and organelles in mammals. Environ Health Perspect. 1982 Nov;45:41–50. doi: 10.1289/ehp.824541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cater B. R., Cook M. W., Gangolli S. D., Grasso P. Studies on dibutyl phthalate-induced testicular atrophy in the rat: effect on zinc metabolism. Toxicol Appl Pharmacol. 1977 Sep;41(3):609–618. doi: 10.1016/s0041-008x(77)80014-8. [DOI] [PubMed] [Google Scholar]
- Creasy D. M., Foster J. R., Foster P. M. The morphological development of di-N-pentyl phthalate induced testicular atrophy in the rat. J Pathol. 1983 Mar;139(3):309–321. doi: 10.1002/path.1711390307. [DOI] [PubMed] [Google Scholar]
- Daniel J. W., Bratt H. The absorption, metabolism and tissue distribution of di(2-ethylhexyl)phthalate in rats. Toxicology. 1974 Mar;2(1):51–65. doi: 10.1016/0300-483x(74)90042-0. [DOI] [PubMed] [Google Scholar]
- Foster P. M., Foster J. R., Cook M. W., Thomas L. V., Gangolli S. D. Changes in ultrastructure and cytochemical localization of zinc in rat testis following the administration of di-n-pentyl phthalate. Toxicol Appl Pharmacol. 1982 Mar 30;63(1):120–132. doi: 10.1016/0041-008x(82)90031-x. [DOI] [PubMed] [Google Scholar]
- Gangolli S. D. Testicular effects of phthalate esters. Environ Health Perspect. 1982 Nov;45:77–84. doi: 10.1289/ehp.824577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray T. J., Butterworth K. R., Gaunt I. F., Grasso G. P., Gangolli S. D. Short-term toxicity study of di-(2-ethylhexyl) phthalate in rats. Food Cosmet Toxicol. 1977 Oct;15(5):389–399. doi: 10.1016/s0015-6264(77)80003-5. [DOI] [PubMed] [Google Scholar]
- Gray T. J., Butterworth K. R. Testicular atrophy produced by phthalate esters. Arch Toxicol Suppl. 1980;4:452–455. doi: 10.1007/978-3-642-67729-8_106. [DOI] [PubMed] [Google Scholar]
- Gray T. J., Gangolli S. D. Aspects of the testicular toxicity of phthalate esters. Environ Health Perspect. 1986 Mar;65:229–235. doi: 10.1289/ehp.8665229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence W. H. Phthalate esters: the question of safety. Clin Toxicol. 1978;13(1):89–139. doi: 10.3109/15563657808988230. [DOI] [PubMed] [Google Scholar]
- Lei K. Y., Abbasi A., Prasad A. S. Function of pituitary-gonadal axis in zinc-deficient rats. Am J Physiol. 1976 Jun;230(6):1730–1732. doi: 10.1152/ajplegacy.1976.230.6.1730. [DOI] [PubMed] [Google Scholar]
- MILLAR M. J., FISCHER M. I., ELCOATE P. V., MAWSON C. A. The effects of dietary zinc deficiency on the reproductive system of male rats. Can J Biochem Physiol. 1958 Jun;36(6):557–569. [PubMed] [Google Scholar]
- Megraw R. E., Dunn D. E., Biggs H. G. Manual and continuous-flow colorimetry of triacylglycerols by a fully enzymic method. Clin Chem. 1979 Feb;25(2):273–278. [PubMed] [Google Scholar]
- OAKBERG E. F. Duration of spermatogenesis in the mouse and timing of stages of the cycle of the seminiferous epithelium. Am J Anat. 1956 Nov;99(3):507–516. doi: 10.1002/aja.1000990307. [DOI] [PubMed] [Google Scholar]
- Oishi S., Hiraga K. Testicular atrophy induced by di-2-ethylhexyl phthalate: effect of zinc supplement. Toxicol Appl Pharmacol. 1983 Aug;70(1):43–48. doi: 10.1016/0041-008x(83)90177-1. [DOI] [PubMed] [Google Scholar]
- Oishi S., Hiraga K. Testicular atrophy induced by phthalic acid esters: effect on testosterone and zinc concentrations. Toxicol Appl Pharmacol. 1980 Mar 30;53(1):35–41. doi: 10.1016/0041-008x(80)90378-6. [DOI] [PubMed] [Google Scholar]
- Oishi S., Hiraga K. Testicular atrophy induced by phthalic acid monoesters: effects of zinc and testosterone concentrations. Toxicology. 1980;15(3):197–202. doi: 10.1016/0300-483x(80)90053-0. [DOI] [PubMed] [Google Scholar]
- Soares E. R., Sheridan W., Haseman J. K., Segall M. Increased frequencies of aberrant sperm as indicators of mutagenic damage in mice. Mutat Res. 1979 Feb;64(1):27–35. doi: 10.1016/0165-1161(79)90133-x. [DOI] [PubMed] [Google Scholar]
- Thomas J. A., Thomas M. J. Biological effects of di-(2-ethylhexyl) phthalate and other phthalic acid esters. Crit Rev Toxicol. 1984;13(4):283–317. doi: 10.3109/10408448409023761. [DOI] [PubMed] [Google Scholar]
- Wyrobek A. J., Bruce W. R. Chemical induction of sperm abnormalities in mice. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4425–4429. doi: 10.1073/pnas.72.11.4425. [DOI] [PMC free article] [PubMed] [Google Scholar]