Abstract
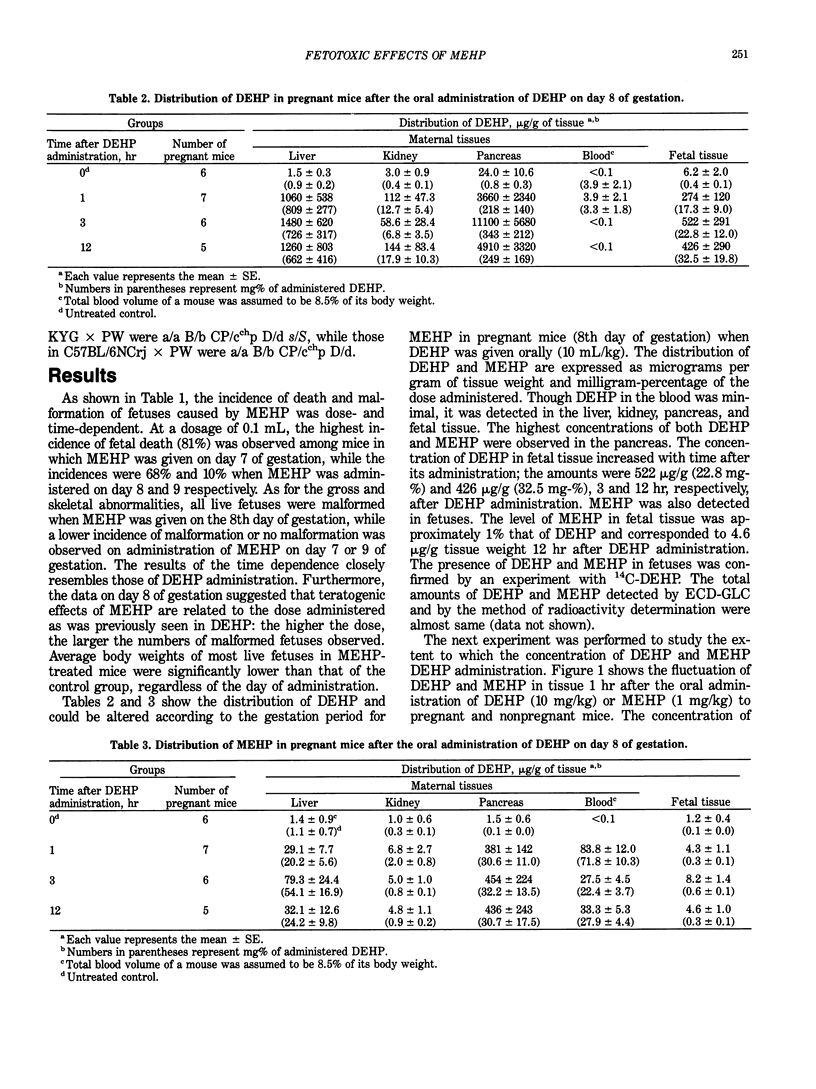
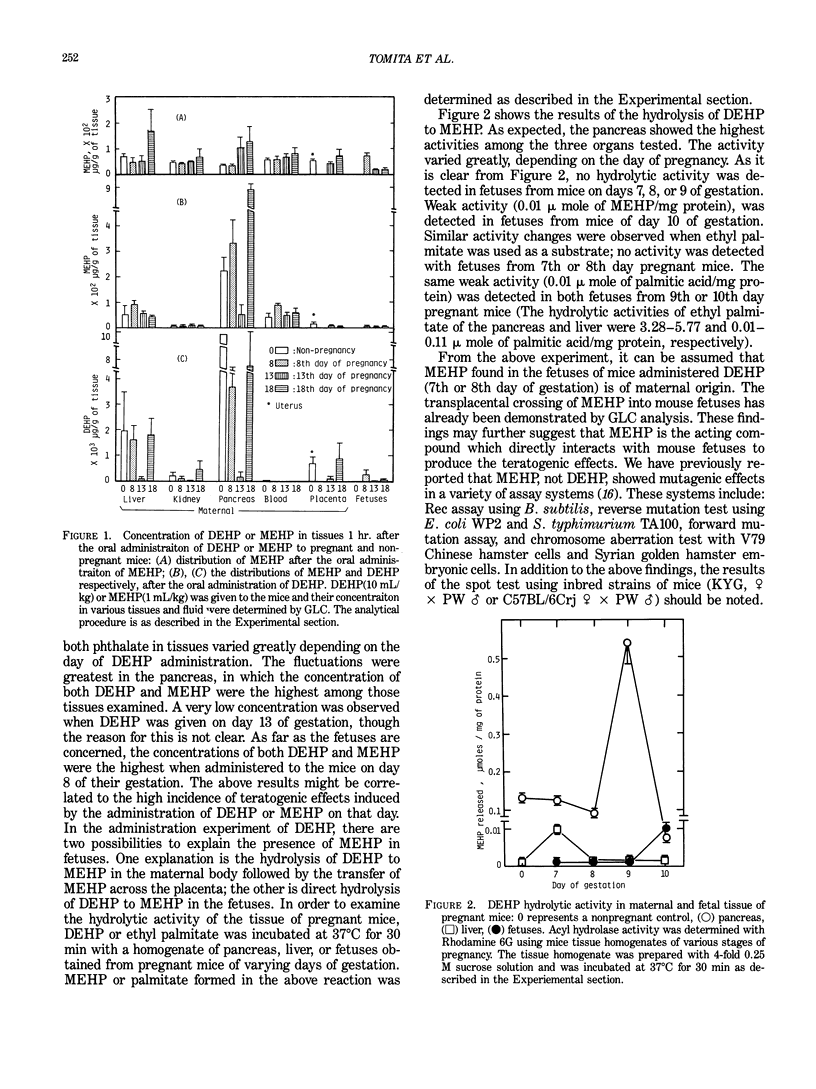
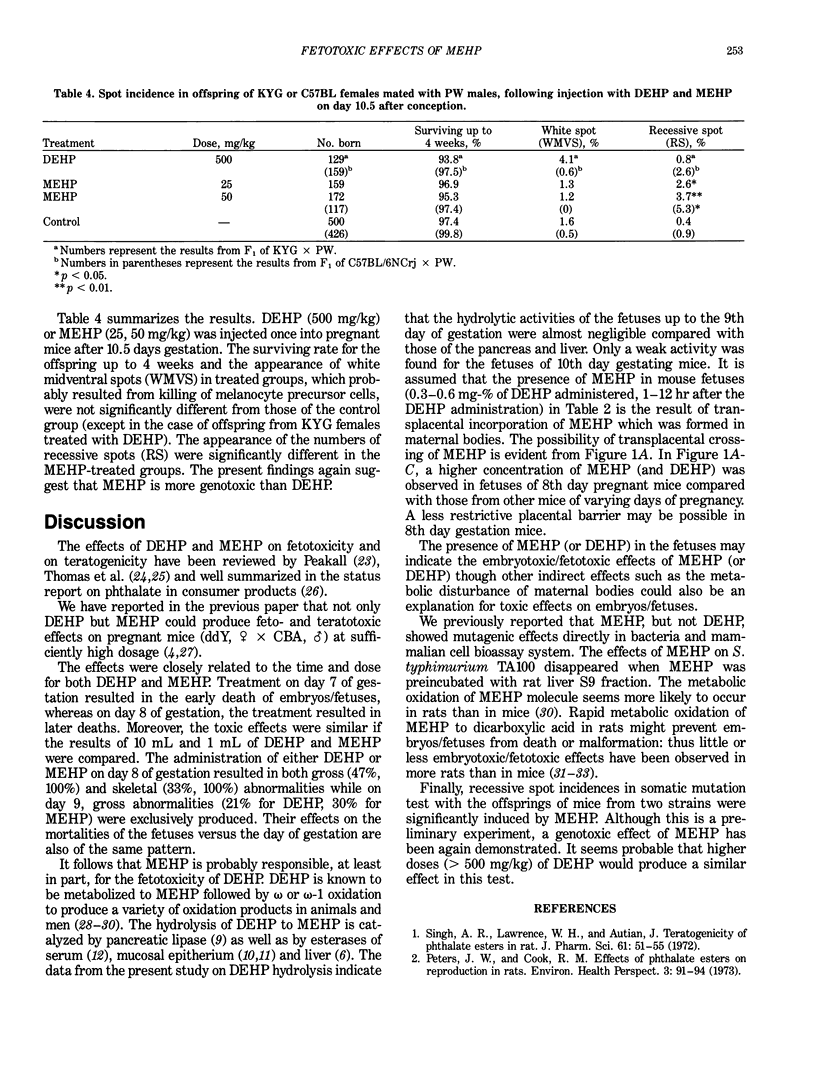
Mono(2-ethylhexyl) phthalate (MEHP), one of the main metabolites of di(2-ethylhexyl) phthalate (DEHP), exerted embryo/fetotoxic effects similar to those of DEHP at lower doses. Oral administration of MEHP (1 mL/kg) to the mice of 8 days gestation resulted in less than 32% of live fetuses, all of which were deformed. When DEHP (10 mL/kg) was given to the pregnant mice of 8 days gestation, approximately 0.03% and 0.003% of the administered dose was found in fetuses as DEHP and MEHP, respectively, after 12 hr. The presence of the MEHP in fetuses is probably due to the transplacental crossing of the MEHP formed in the maternal body, since the fetuses of mice up to day 9 of pregnancy showed no hydrolytic activity of DEHP to MEHP. Crossing of MEHP through the placenta was proven by an experiment in which MEHP was administered in pregnant mice. A single injection of MEHP (25 or 50 mg/kg), but not DEHP (500 mg/kg) into pregnant mice, induced a significantly high incidence of somatic mutations in the coat hair of offspring of mice (KYG, female X PW, male; C57BL/6Crj, female X PW, male). All these data suggest that MEHP could be responsible for the embryotoxic/fetotoxic effects observed with DEHP.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albro P. W., Corbett J. T., Schroeder J. L., Jordan S., Matthews H. B. Pharmacokinetics, interactions with macromolecules and species differences in metabolism of DEHP. Environ Health Perspect. 1982 Nov;45:19–25. doi: 10.1289/ehp.824519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albro P. W., Thomas R. O. Enzymatic hydrolysis of di-(2-ethylhexyl) phthalate by lipases. Biochim Biophys Acta. 1973 Jun 21;306(3):380–390. doi: 10.1016/0005-2760(73)90176-8. [DOI] [PubMed] [Google Scholar]
- Albro P. W., Thomas R., Fishbein L. Metabolism of diethylhexyl phthalate by rats. Isolation and characterization of the urinary metabolites. J Chromatogr. 1973 Feb 28;76(2):321–330. doi: 10.1016/s0021-9673(01)96915-8. [DOI] [PubMed] [Google Scholar]
- Chu I., Villeneuve D. C., Secours V., Franklin C., Rock G., Viau A. Metabolism and tissue distribution of mono-2-ethylhexyl phthalate in the rat. Drug Metab Dispos. 1978 Mar-Apr;6(2):146–149. [PubMed] [Google Scholar]
- Daniel J. W., Bratt H. The absorption, metabolism and tissue distribution of di(2-ethylhexyl)phthalate in rats. Toxicology. 1974 Mar;2(1):51–65. doi: 10.1016/0300-483x(74)90042-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lake B. G., Phillips J. C., Linnell J. C., Gangolli S. D. The in vitro hydrolysis of some phthalate diesters by hepatic and intestinal preparations from various species. Toxicol Appl Pharmacol. 1977 Feb;39(2):239–248. doi: 10.1016/0041-008x(77)90157-0. [DOI] [PubMed] [Google Scholar]
- Lewandowski M., Fernandes J., Chen T. S. Assessment of the teratogenic potential of plasma-soluble extracts of diethylhexyl phthalate plasticized polyvinyl chloride plastics in rats. Toxicol Appl Pharmacol. 1980 Jun 15;54(1):141–147. doi: 10.1016/0041-008x(80)90014-9. [DOI] [PubMed] [Google Scholar]
- Peakall D. B. Phthalate esters: Occurrence and biological effects. Residue Rev. 1975;54:1–41. doi: 10.1007/978-1-4612-9857-1_1. [DOI] [PubMed] [Google Scholar]
- Peters J. W., Cook R. M. Effect of phthalate esters on reproduction in rats. Environ Health Perspect. 1973 Jan;3:91–94. doi: 10.1289/ehp.730391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock G., Secours V. E., Franklin C. A., Chu I., Villeneuve D. C. The accumulation of mono-2-ethylhexylphthalate (MEHP) during storage of whole blood and plasma. Transfusion. 1978 Sep-Oct;18(5):553–558. doi: 10.1046/j.1537-2995.1978.18579036383.x. [DOI] [PubMed] [Google Scholar]
- Rowland I. R., Cottrell R. C., Phillips J. C. Hydrolysis of phthalate esters by the gastro-intestinal contents of the rat. Food Cosmet Toxicol. 1977 Feb;15(1):17–21. doi: 10.1016/s0015-6264(77)80257-5. [DOI] [PubMed] [Google Scholar]
- Ruddick J. A., Villeneuve D. C., Chu I., Nestmann E., Miles D. An assessment of the teratogenicity in the rat and mutagenicity in Salmonella of mono-2-ethylhexyl phthalate. Bull Environ Contam Toxicol. 1981 Aug;27(2):181–186. doi: 10.1007/BF01611005. [DOI] [PubMed] [Google Scholar]
- Russell L. B. Validation of the in vivo somatic mutation method in the mouse as a prescreen for germinal point mutations. Arch Toxicol. 1977 Sep 21;38(1-2):75–85. doi: 10.1007/BF00293665. [DOI] [PubMed] [Google Scholar]
- Shiota K., Chou M. J., Nishimura H. Embryotoxic effects of di-2-ethylhexyl phthalate (DEHP) and di-n-buty phthalate (DBP) in mice. Environ Res. 1980 Jun;22(1):245–253. doi: 10.1016/0013-9351(80)90136-x. [DOI] [PubMed] [Google Scholar]
- Singh A. R., Lawrence W. H., Autian J. Maternal-fetal transfer of 14C-di-2-ethylhexyl phthalate and 14C-diethyl phthalate in rats. J Pharm Sci. 1975 Aug;64(8):1347–1350. doi: 10.1002/jps.2600640819. [DOI] [PubMed] [Google Scholar]
- Singh A. R., Lawrence W. H., Autian J. Teratogenicity of phthalate esters in rats. J Pharm Sci. 1972 Jan;61(1):51–55. doi: 10.1002/jps.2600610107. [DOI] [PubMed] [Google Scholar]
- Thomas J. A., Darby T. D., Wallin R. F., Garvin P. J., Martis L. A review of the biological effects of di-(2-ethylhexyl) phthalate. Toxicol Appl Pharmacol. 1978 Jul;45(1):1–27. doi: 10.1016/0041-008x(78)90024-8. [DOI] [PubMed] [Google Scholar]
- Thomas J. A., Northup S. J. Toxicity and metabolism of monoethylhexyl phthalate and diethylhexyl phthalate: a survey of recent literature. J Toxicol Environ Health. 1982 Jan;9(1):141–152. doi: 10.1080/15287398209530149. [DOI] [PubMed] [Google Scholar]
- Tomita I., Nakamura Y., Aoki N., Inui N. Mutagenic/carcinogenic potential of DEHP and MEHP. Environ Health Perspect. 1982 Nov;45:119–125. doi: 10.1289/ehp.8245119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita I., Nakamura Y., Yagi Y. Phthalic acid esters in various foodstuffs and biological materials. Ecotoxicol Environ Saf. 1977 Sep;1(2):275–287. doi: 10.1016/0147-6513(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Tomita I., Nakamura Y., Yagi Y., Tutikawa K. Teratogenicity/fetotoxicity of DEHP in mice. Environ Health Perspect. 1982 Nov;45:71–75. doi: 10.1289/ehp.824571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. T., Blanchfield B. J. Retention, excretion and metabolism of di-(2-ethylhexyl) phthalate administered orally to the rat. Bull Environ Contam Toxicol. 1974 Apr;11(4):371–378. doi: 10.1007/BF01684945. [DOI] [PubMed] [Google Scholar]
- Yagi Y., Nakamura Y., Tomita I., Tsuchikawa K., Shimoi N. Teratogenic potential of di- and mono-(2-ethylhexyl)phthalate in mice. J Environ Pathol Toxicol. 1980 Sep;4(2-3):533–544. [PubMed] [Google Scholar]


