Abstract
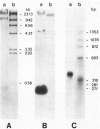
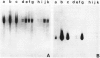
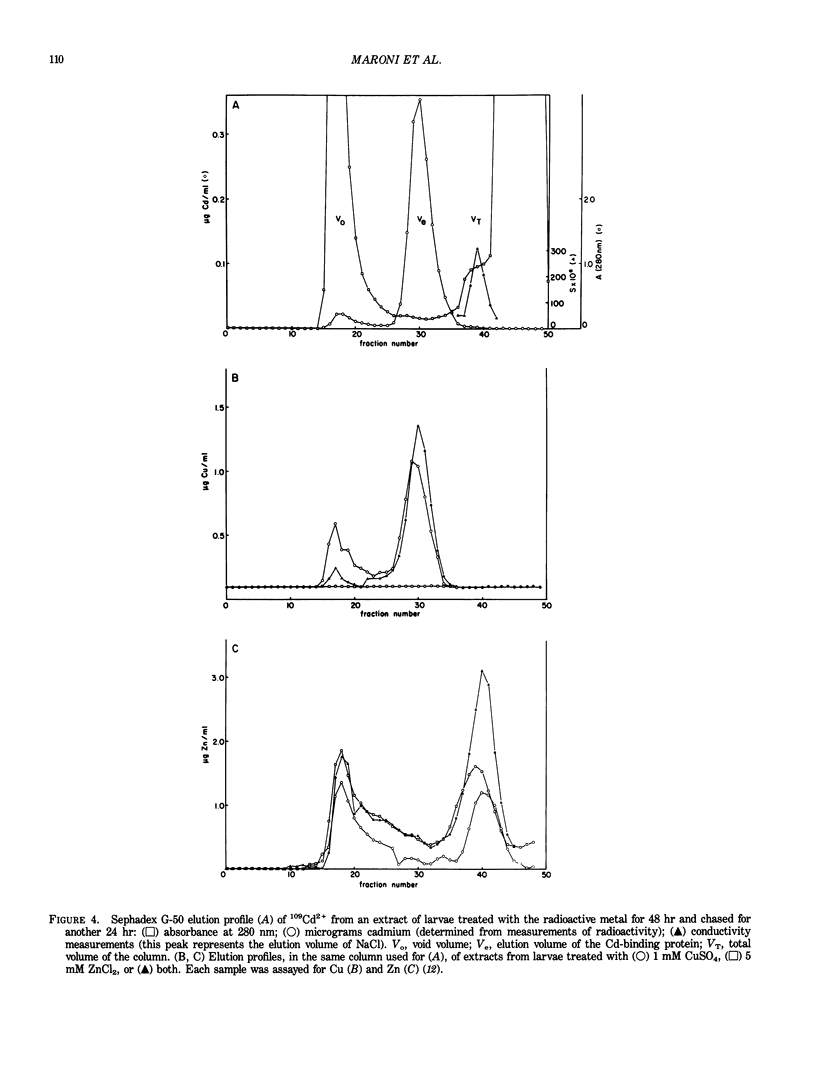
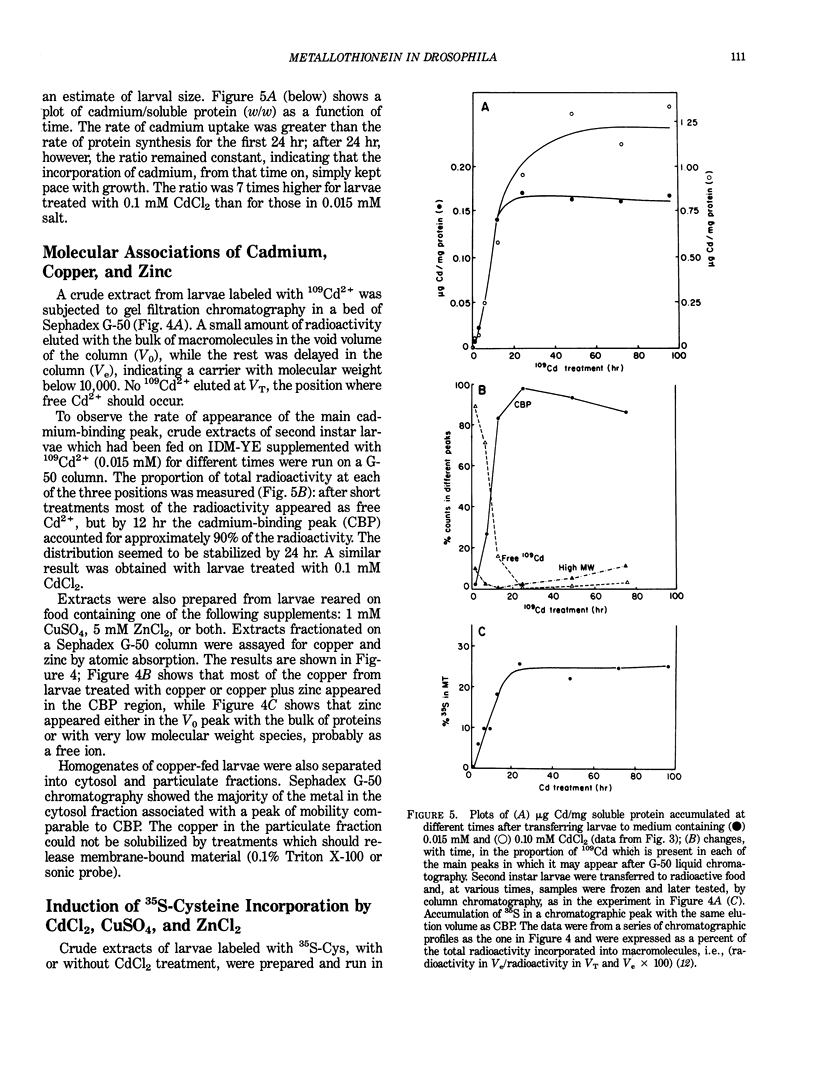
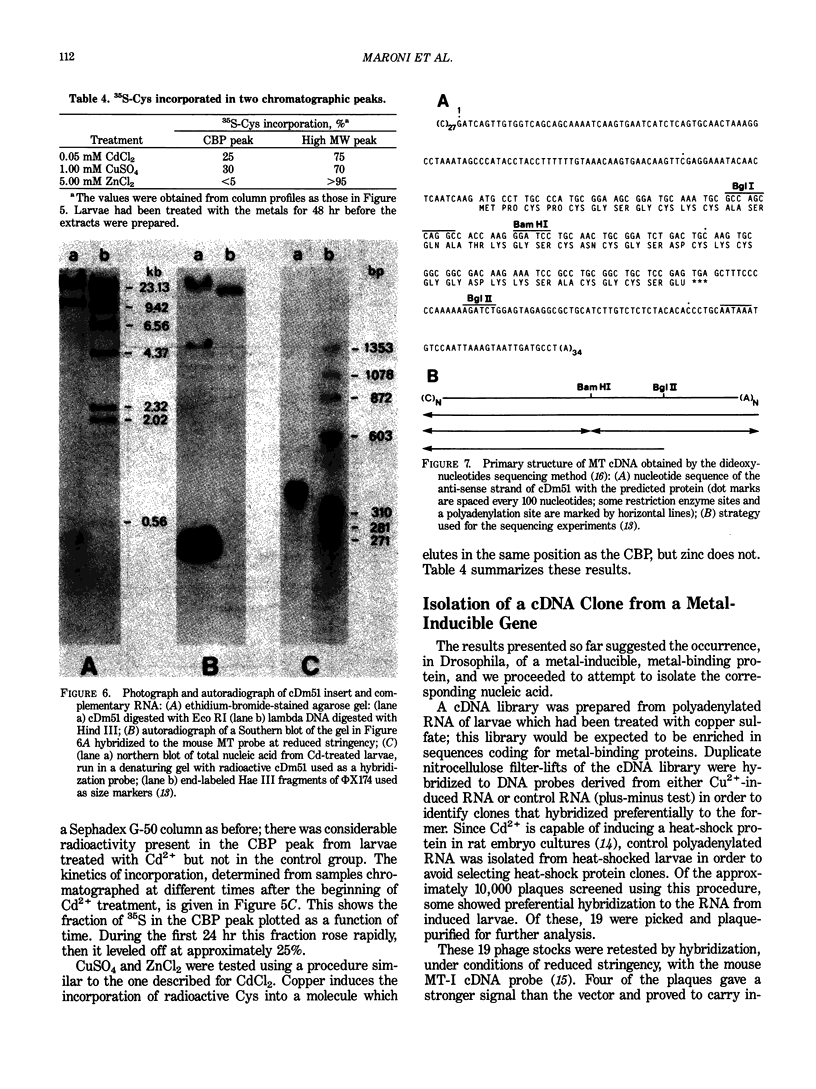
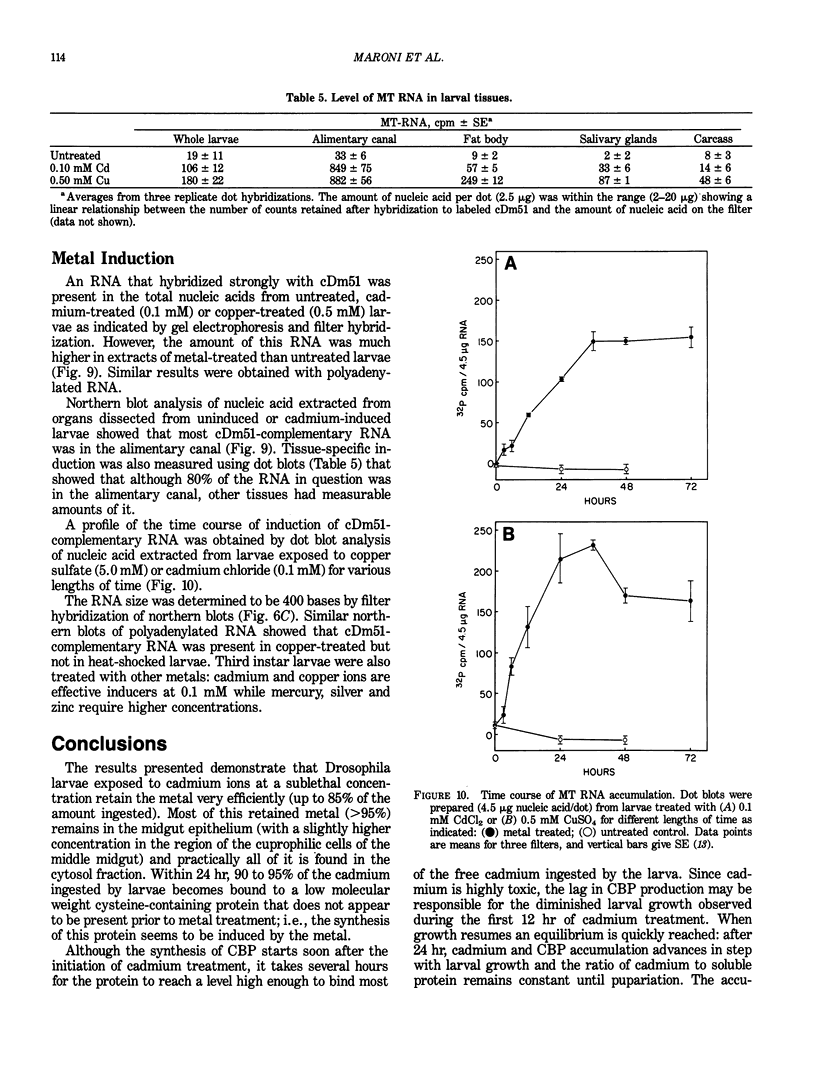
Drosophila melanogaster larvae reared on food containing radioactive cadmium retained over 80% of it, mostly in the intestinal epithelium. The majority of this radioactivity was associated with a soluble protein of less than 10,000 molecular weight. Synthesis of this cadmium-binding protein was induced by the metal as demonstrated by incorporation of radioactive cysteine. Most copper ingested by larvae was also found to associate with a low molecular weight, inducible protein, but some of it was found in an insoluble fraction. Zinc was unable to, or very inefficient at, binding or inducing the synthesis of a similar protein. A D. melanogaster cDNA clone was isolated based on its more intense hybridization to copies of RNA sequences from copper-fed larvae than from control larvae. This clone showed strong hybridization to mouse metallothionein-I cDNA at reduced stringency. Its nucleotide sequence includes an open-reading segment which codes for a 40-amino acid protein; this protein was identified as metallothionein based on its similarity to the amino-terminal portion of mammalian and crab metalloproteins. The ten cysteine residues present occur in five pairs of near-vicinal cysteines (Cys-X-Cys). This cDNA sequence hybridized to a 400-nucleotide polyadenylated RNA whose presence in the cells of the alimentary canal of larvae was stimulated by ingestion of cadmium or copper; in other tissues this RNA was present at much lower levels. Mercury, silver, and zinc induced metallothionein to a lesser extent. Whether (any of) the protein(s) discussed above correspond(s) to that coded by this RNA sequence has not yet been determined.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benoist C., O'Hare K., Breathnach R., Chambon P. The ovalbumin gene-sequence of putative control regions. Nucleic Acids Res. 1980 Jan 11;8(1):127–142. doi: 10.1093/nar/8.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cass A. E., Hill H. A. Copper proteins and copper enzymes. Ciba Found Symp. 1980;79:71–91. doi: 10.1002/9780470720622.ch5. [DOI] [PubMed] [Google Scholar]
- Durnam D. M., Perrin F., Gannon F., Palmiter R. D. Isolation and characterization of the mouse metallothionein-I gene. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6511–6515. doi: 10.1073/pnas.77.11.6511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hightower L. E., White F. P. Cellular responses to stress: comparison of a family of 71--73-kilodalton proteins rapidly synthesized in rat tissue slices and canavanine-treated cells in culture. J Cell Physiol. 1981 Aug;108(2):261–275. doi: 10.1002/jcp.1041080216. [DOI] [PubMed] [Google Scholar]
- Karin M., Richards R. I. Human metallothionein genes: molecular cloning and sequence analysis of the mRNA. Nucleic Acids Res. 1982 May 25;10(10):3165–3173. doi: 10.1093/nar/10.10.3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
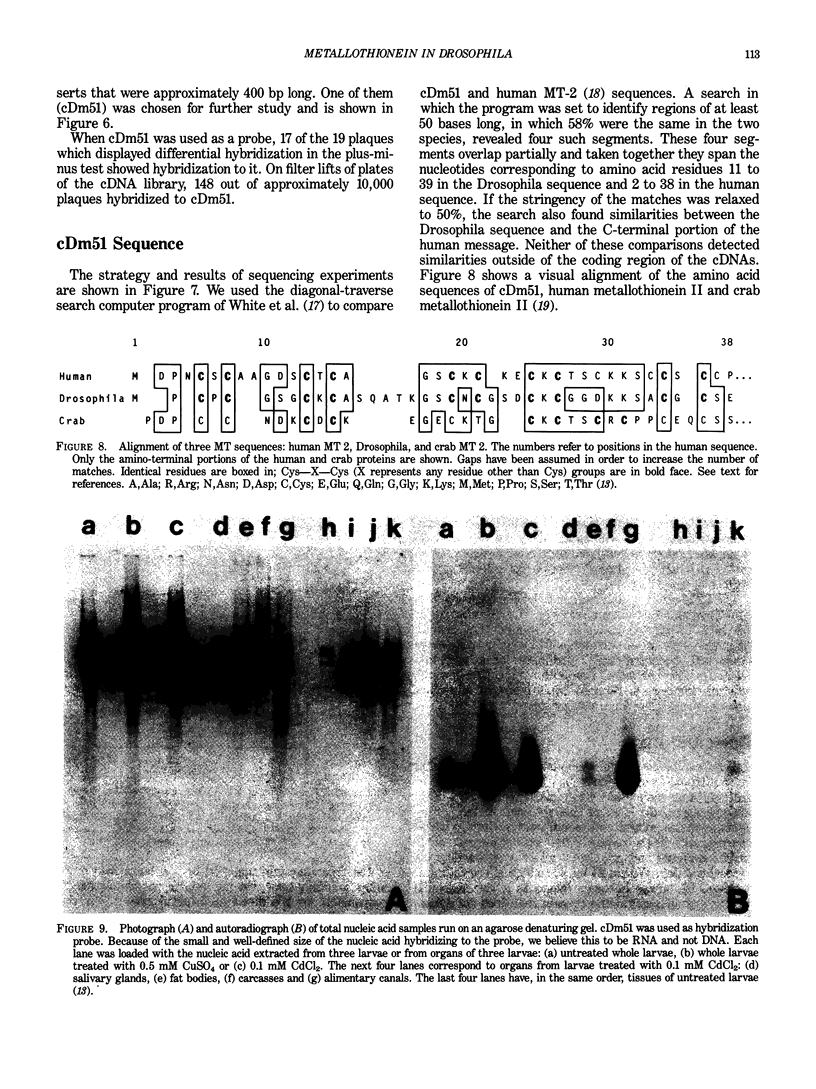
- Lastowski-Perry D., Otto E., Maroni G. Nucleotide sequence and expression of a Drosophila metallothionein. J Biol Chem. 1985 Feb 10;260(3):1527–1530. [PubMed] [Google Scholar]
- Lerch K., Ammer D., Olafson R. W. Crab metallothionein. Primary structures of metallothioneins 1 and 2. J Biol Chem. 1982 Mar 10;257(5):2420–2426. [PubMed] [Google Scholar]
- Maroni G., Laurie-Ahlberg C. C., Adams D. A., Wilton A. N. Genetic variation in the expression of ADH in Drosophila melanogaster. Genetics. 1982 Jul-Aug;101(3-4):431–446. doi: 10.1093/genetics/101.3-4.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulson D. F., Bowen V. T., Hilse R. M., Rubinson A. C. The Copper Metabolism of Drosophila. Proc Natl Acad Sci U S A. 1952 Oct;38(10):912–921. doi: 10.1073/pnas.38.10.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudd C. J., Herschman H. R. Metallothionein in a human cell line: the response of HeLa cells to cadmium and zinc. Toxicol Appl Pharmacol. 1979 Feb;47(2):273–278. doi: 10.1016/0041-008x(79)90321-1. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro S. G., Squibb K. S., Markowitz L. A., Cousins R. J. Cell-free synthesis of metallothionein directed by rat liver polyadenylated messenger ribonucleic acid. Biochem J. 1978 Dec 1;175(3):833–840. doi: 10.1042/bj1750833. [DOI] [PMC free article] [PubMed] [Google Scholar]
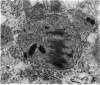
- Sohal R. S., Peters P. D., Hall T. A. Origin, structure, composition and age-dependence of mineralized dense bodies (concretions) in the midgut epithelium of the adult housefly, Musca domestica. Tissue Cell. 1977;9(1):87–102. doi: 10.1016/0040-8166(77)90051-9. [DOI] [PubMed] [Google Scholar]
- Tapp R. L., Hockaday A. Combined histochemical and x-ray microanalytical studies on the copper-accumulating granules in the mid-gut of larval Drosophila. J Cell Sci. 1977 Aug;26:201–215. doi: 10.1242/jcs.26.1.201. [DOI] [PubMed] [Google Scholar]
- Tapp R. L. X-ray microanalysis of the mid-gut epithelium of the fruitfly Drosophila melanogaster. J Cell Sci. 1975 Mar;17(3):449–459. doi: 10.1242/jcs.17.3.449. [DOI] [PubMed] [Google Scholar]
- White C. T., Hardies S. C., Hutchison C. A., 3rd, Edgell M. H. The diagonal-traverse homology search algorithm for locating similarities between two sequences. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):751–766. doi: 10.1093/nar/12.1part2.751. [DOI] [PMC free article] [PubMed] [Google Scholar]