Abstract
In the isolated rat mesenteric bed, the 1 min perfusion with 100 nm anandamide, a concentration that did not evoke vasorelaxation, elicited an acute release of 165.1 ± 9.2 pmol nitric oxide (NO) that was paralleled by a 2-fold increase in cGMP tissue levels. The rise in NO released was mimicked by either (R)-(+)-methanandamide or the vanilloid receptor agonists resiniferatoxin and (E)-capsaicin but not by its inactive cis-isomer (Z)-capsaicin. The NO release elicited by either anandamide or capsaicin was reduced by the TRPV1 receptor antagonists 5′-iodoresiniferatoxin, SB 366791 and capsazepine as well as by the cannabinoid CB1 receptor antagonists SR 141716A or AM251. The outflow of NO elicited by anandamide and capsaicin was also reduced by endothelium removal or NO synthase inhibition, suggesting the specific participation of endothelial TRPV1 receptors, rather than the novel endothelial TRPV4 receptors. Consistently, RT-PCR showed the expression of the mRNA coding for the rat TRPV1 receptor in the endothelial cell layer, in addition to its expression in sensory nerves. The participation of sensory nerves on the release of NO was precluded on the basis that neonatal denervation of the myenteric plexus sensory nerves did not modify the pattern of NO release induced by anandamide and capsaicin. We propose that low concentrations of anandamide, devoid of vasorelaxing effects, elicit an acute release of NO mediated predominantly by the activation of endothelial TRPV1 receptors whose physiological significance remains elusive.
Anandamide was formerly recognized as the first endo-genous cannabinoid with preferential affinity for the CB1 cannabinoid receptor (Devane et al. 1992; for a review see Howlett et al. 2002). It was further proposed that anandamide may also act as an endovanilloid, through the activation of the transient receptor potential vanilloid type 1 receptors (TRPV1; Ross, 2003; Van Der Stelt & Di Marzo, 2004). Among other physiological effects, vasodilatation, such as described in the rat mesenteric bed, is partially due to the activation of TRPV1 receptors found in perivascular sensory nerves. In this preparation, the relaxant effects of capsaicin as well as of anandamide are coupled to the release of calcitonin gene-related peptide (CGRP) (Zygmunt et al. 1999). Although controversial, the participation of sensory nerves in anandamide-induced vasodilatation in the rat mesenteric bed might depend on the presence of a functional NO system. For instance, Harris et al. (2002) showed that neuronal NO may be involved in anandamide vasodilatation through sensory nerves whereas Ralevic (2002) reported that the endo-thelial tonic release of NO may have an inhibitory modulator effect of sensory neurotransmission in the rat mesenteric bed. Moreover, the physiological implications of the interaction of anandamide with vanilloid TRPV1 receptors is a matter of controversy, due to the observation that the high concentrations of anandamide, within the micromolar range, required to stimulate vanilloid receptors (Van Der Stelt & Di Marzo, 2004) are unlikely to occur in mammalian tissues. In fact, the actual levels of anandamide in various rat and human tissues are within the nanomolar range. Hence, it is unclear whether the release of endogenous anandamide under physiological conditions may result in concentrations high enough as to stimulate TRPV1 receptors
The aim of the present work was to study whether nanomolar concentrations of anandamide, which do not elicit vasorelaxation, could be somehow linked to the release of NO. In addition, the nature and the location of the receptors involved in the putative release of NO induced by anandamide were also assessed. The present results allowed us to conclude that nanomolar anandamide nevertheless elicits a rapid and transient release of NO mediated through the activation of the endothelial vanilloid TRPV1 receptor channels. Although the presence of endothelial TRPV4 receptors, which are indirectly activated by anandamide, has been reported for the vasculature (Watanabe et al. 2002b, 2003), their participation in the anandamide-induced NO release is likely to be precluded by the present experiments. The role of the TRPV1 receptors in endothelial signalling is discussed.
Methods
Animal treatment and vascular reactivity assays
Adult male Sprague-Dawley rats (230–270 g), bred at the Animal Reproduction Laboratories of the Faculty of Biological Sciences of the P. Catholic University of Chile, were anaesthetized with ketamine: xylazine (25: 2.5 mg kg−1, i.p.). The abdominal cavity was excised at the midline and the rat was injected e.v. with a bolus of KCl to ensure a fast kill. Animal handling followed and conformed to the animal welfare guidelines according to NIH (USA) standards. The Faculty ethical committee for the use of animals in biological research approved the protocols and supervised our strict adherence to the subscribed guidelines.
The superior mesenteric artery was cannulated with polyethylene tubing and perfused with Krebs-Ringer buffer bubbled with 95% O2–5% CO2 at 37°C. The composition of the Krebs-Ringer buffer was as follows (mm): NaCl 118, KCl 5.4, CaCl2 2.5, KH2PO4 1.2, MgSO4 1.2, NaHCO3 23.8, and glucose 11.1. A peristaltic pump (2 ml min−1) was used to perfuse the arterial mesenteric bed; the mesenteric bed was next excised from the intestines and placed in a dish specially designed to collect the perfusate (Boric et al. 1999). A pressure transducer was placed close to the entrance of the main mesenteric artery and connected to a Grass polygraph recorder. Fluctuations of the perfusion pressure were interpreted as changes in the resistance of the arterial mesenteric bed. Mesenteries were perfused for 20 min prior to drug applications. Vasodilatations were quantified in mesenteries precontracted with 10–20 μm phenylephrine following the procedure of Buvinic et al. (2002). Once the perfusion pressure reached a plateau, varying concentrations of either anandamide or capsaicin were perfused for 4 min.
NO release measurements
The overflow of NO released to the mesenteric bed was assessed in non-precontracted tissues perfused for 1 min with either anandamide or (E)-capsaicin or structurally related analogues, such as (R)-(+)-methanandamide (Z)-capsaicin, N-arachidonylglycine, olvanil, N-arachidonyldopamine and resiniferatoxin. One minute samples of the perfusate were collected before, during and up to 5 min after agonist application to quantify the content of NO in the perfusates. Luminal NO, above baseline level, was measured by chemiluminescence using a Sievers 280 NOA analyser (GE Analytical Instruments, Boulder, CO, USA). To reduce the nitrites in the bioassay samples, the instrument reaction chamber was filled with 8 ml of glacial acetic acid containing 100 mg of potassium iodide. A 50 μl sample of the perfusion medium was injected into the chamber and a stream of N2 carried the resulting NO to the cell where the specific chemiluminescence generated by the NO–ozone reaction was quantified (Figueroa et al. 2001). Calibration of the equipment was routinely performed using standards up to 10 μm sodium nitrite. The equipment allows the detection of 0.5–1 pmol NO (10–20 pmol ml−1). Background buffer readings as well as baseline levels were subtracted to determine the net NO release. Results are expressed as the integrated area of the NO peak produced by the agonists above basal values (ΔNO, pmol). A single rat mesentery was used for every agonist concentration. Hence, the concentrations of each agonist causing half maximal NO release (EC50 determinations) have a substantial error, since all values derive from individual rats.
Source of luminal NO and the use of selective antagonists to assess the involvement of TRPV1 or CB1 receptors
To investigate the source of the luminal NO, the endothelial cell layer was removed by perfusing 0.1% saponin for 55 s followed by drug-free buffer perfusion for the next 30 min, as detailed by Donoso et al. (1996). In further additional experiments, tissues were previously perfused with100 μmNω-nitro-l-arginine (l-NNA) for 45 min (Boric et al. 1999) to block eNOS activity.
Use of vanilloid TRPV1 receptor antagonists and CB1 receptor antagonists
To identify the involvement of tissue vanilloid TRPV1 or cannabinoid CB1 receptors on the NO release process, several selective receptor antagonists were used to block the release of NO elicited by anandamide, capsaicin or resiniferatoxin, the ultra potent vanilloid (Szolcsanyi et al. 1990) namely the TRPV1 receptor antagonists capsazepine (Dickenson & Dray, 1991; Szallasi & Blumberg, 1999), 5′-iodoresiniferatoxin (Wahl et al. 2001) and SB 366791 (Gunthorpe et al. 2004), and the calcium channel blocker ruthenium red (Amman & Maggi, 1991) as well as the cannabinoid receptor antagonists SR 141716A (Shire et al. 1999) or AM 251 (Gatley et al. 1996; White et al. 2001). The corresponding antagonists were routinely added 30 min prior to testing with the 1 min exposure of 100 nm anandamide, or 100 nm capsaicin or 1 nm resiniferatoxin.
Assessing the possible involvement of the TRPV4 receptor
A battery of metabolic inhibitors were used to study the possible participation of anandamide metabolites on the release of NO through the activation of TRPV4 receptors. Namely, the fatty acid amidohydrolase inhibitor phenylmethylsulphonyl fluoride (PMSF) at 100 μm, the cyclooxygenase inhibitor 10 μm indomethacin, the lipoxigenase inhibitor 10 μm nordihydroguaiaretic acid and the cytochrome P450 inhibitor 10 μm miconazole were added 30 min prior to a 1-min challenge with a 100 nm anandamide pulse.
In addition, the effect on NO release of a 1-min exposure to the selective TRPV4 agonist 1 μm 4α-phorbol-12,13-didecanoate (4αPDD) (Watanabe et al. 2002a, 2003) was also assessed.
Sensory nerve contribution
The possible contribution of sensory nerves to NO production was studied in rat mesenteric beds deprived of sensory nerves. For this purpose, a series of neonatal male rat pups (4–6 g body weight), were injected s.c. with 100 mg kg−1 capsaicin, within the first 24 h after birth (Mupanomunda et al. 1998). Capsaicin was dissolved in 1: 3 ethanol: perfusion buffer. Twenty hours later, a second dose of capsaicin was administered s.c. Pups were grown until reaching 250 g, the weight at which several protocols were performed.
Tissue cGMP determinations
To determine the tissue content of cGMP, mesenteries were homogenized immediately after completing the 1-min agonist application, in 2 ml 10% trichloroacetic acid and centrifuged at 4°C for 30 min at 1700 g. Extractions of the aqueous phase with 8 ml of diethyl ether were performed 4 times. The samples were dried in a Speed-Vac and stored at −20°C until a 10 fmol-threshold radioimmunoassay for acetylated cGMP was performed according to the procedure described by Buvinic et al. (2002). Results are expressed as the production of tissue cGMP (pmol (g tissue)−1). Since pharmacological blockade of the NO/cGMP cascade alters the basal production of cGMP in the rat arterial mesenteric bed (Buvinic & Huidobro-Toro, 2001), the expression of the nucleotide-induced cGMP accumulation over respective basal values was used to standardize the present results. Determinations of cGMP were also performed in mesenteries pretreated during 20 min with 3 μm 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ), to inhibit soluble guanylyl cyclase (Garthwaite et al. 1995; Buvinic & Huidobro-Toro, 2001), prior to the challenge with 100 nm anandamide.
RT-PCR assays
Vanilloid receptor mRNA detection was performed in rat mesenteries manually defatted. Total RNA was extracted following the Chomczynski & Sacchi (1987) procedure. The rat tongue was used as a positive control tissue.
The reverse transcription reaction was performed with 2 μg of total RNA using an oligo-dt primer. The PCR was carried out using forward and reverse primers specific for the rat TPRV1 receptor as described by Anavi-Goffer et al. (2002). After an initial denaturation for 5 min at 94°C, amplifications using 1/10 of the RT products were carried out for 35 cycles as follows: 94°C, 30 s; 55°C, 30 s; 69°C, 3 min. To amplify a 478 bp fragment of the rat TRPV1 receptor, the forward TTCTGCTCAACATGCTCATTG and reverse AATCCTTGAAAACCTCAGC primers were used. Rat mRNA determination of the endothelial cell adhesion molecule (CD31) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was also assessed in the same mesentery preparations. The former served as an endothelial marker, while the latter was used as an internal standard to normalize the material loaded per lane and served as a housekeeper gene. Primers were designed to amplify region 131–481 of CD31 and region 564–1015 of GAPDH, giving rise to PCR products of 350 and 451 base pairs (bp), respectively. PCR products were analysed by electrophoresis in 1% agarose and visualized using ethidium iodide staining. These products were repeated in at least three separate mRNA extracts from separate rat mesenteries. To confirm the identity of the corresponding PCR products, the putative bands having an estimated size, based on the primers used, were isolated for direct sequencing using an ABI-prism sequencing analyser (Applied Biosystems, Foster City, CA, USA). Amplifications without the RT step were performed to exclude possible contamination with genomic DNA.
CGRP Immunohistochemical assays
Mesenteries from either control or adult denervated rats (treated with capsaicin as neonate pups), were prepared for whole-mount CGRP immunochemical analysis following the procedure detailed by Donoso et al. (2002). In essence, isolated and defatted mesenteries were fixed in paraformaldehyde–picric acid in phosphate buffer solution. Tissues were next incubated for 24 h with a commercial antibody for CGRP (1/5000 dilution); as a second antibody a goat immunoglobulin (1/1000 dilution) coupled to biotin was used; the complex was revealed with diaminobenzidine. Parallel studies, omitting the primary or secondary antibody from the incubation solution, evaluated the specificity.
Drugs and chemicals
Anandamide (R)-(+)-methanandamide, olvanil or N-vanilloyloleoylamide, N-arachidonyldopamine (NADA), N-arachidonylglycine, 2-arachidonylglycerol (E)- and (Z)-capsaicin, resiniferatoxin, 5′-iodoresiniferatoxin, SB 366791, AM 251 and capsazepine were purchased from Tocris Bioscience (Evansville, MO, USA). Stocks of 100 μg (100 μl)−1 in absolute ethanol of the endo-cannabinoids and vanilloids were maintained at −20°C, Tyrode buffer dilutions were performed daily prior to each experiment. Saponin, ODQ, l-NNA, indomethacin, nordihydroguaiaretic acid, miconazole and 4α-phorbol 12,13-didecanoate (4αPDD) or phorbol 12,13-didecanoate were purchased from Sigma Chemicals (St Louis, MO, USA). The arachidonic acid cascade inhibitors and the phorbol esters were dissolved in absolute alcohol (1 mg ml−1 stock solutions) and diluted daily in Tyrode solution as required. PMSF was purchased from Sigma; 1 mg ml−1 stock solutions were prepared in dehydrated pure ethanol and used within less than 2 h after preparing each solution. The anti-cGMP antibody was obtained from Calbiochem (La Jolla, CA, USA). Dr M. Mossé (Sanofi, Montpellier, France) provided us with a sample of SR 141716A hydrochloride. All the reagents required for RT-PCR determinations were purchased from Gibco BRL/Life Technologies (CA, USA).
Statistical analysis and data expression
One-way and two-way ANOVA, linear correlation analysis and Student's t test were used throughout. Dunnett's tables for multiple comparisons with a single control were used when appropriate. P-values less than 0.05 were considered statistically significant.
Results
Effects of anandamide on NO release and cGMP levels in the rat mesenteric bed
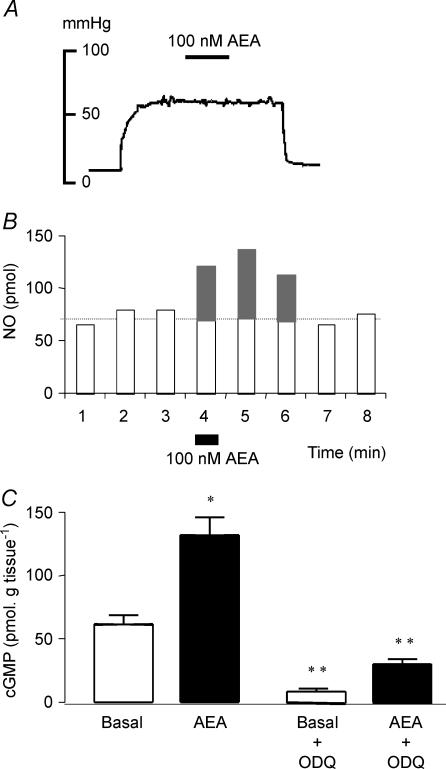
Perfusion with 100 nm anandamide, a concentration that did not cause per se any significant vasodilatation (5.4 ± 2.6 mmHg, n = 4, see a typical tracing in Fig. 1A), elicited the release of NO, which was rapid in onset and lasted up to 2–3 min after its application (Fig. 1B). The mean average NO production elicited by the 1-min exposure to 100 nm anandamide was 165.1 ± 9.2 pmol (n = 36).
Figure 1. Anandamide, 100 nm, elicits a surge of NO and increases cGMP tissue levels but does not vasodilate the arterial mesenteric bed of the rat.
A, representative tracing illustrates the lack of vasodilatation induced by a 4-min perfusion with 100 nm anandamide (AEA) in mesenteries precontracted with 20 μm phenylephrine. B, prototype experiment shows the time course of NO release induced by a 1-min exposure to 100 nm AEA (filled rectangle at min 4). Stimulated NO release was quantified as the integrated NO recovered above the corresponding baseline values, shown in grey. C, tissue levels of cGMP were measured in mesenteric bed homogenates either in the absence (open bars) or immediately after the 1-min perfusion with 100 nm AEA (filled bars). Both the basal and the 100 nm AEA-stimulated increase in cGMP tissue levels were also measured after a 20-min pretreatment with 3 μm ODQ, the inhibitor of soluble guanylyl cyclase. Results in C are the mean ±s.e.m. of 4–6 experiments per group. *P < 0.05 when compared with the corresponding basal value, **P < 0.01 when compared with the corresponding values in the absence of ODQ.
In spite of the lack of vasodilatation, the increase in NO evoked by 100 nm anandamide was paralleled by a twofold increase in the tissue cGMP levels (Fig. 1C) that, as also observed for the basal values, was reduced by 80% (P < 0.01) following pretreatment with the guanylyl cyclase inhibitor, 3 μm ODQ (Fig. 1C).
Release of NO elicited by anandamide and structurally related analogues, capsaicin or resiniferatoxin
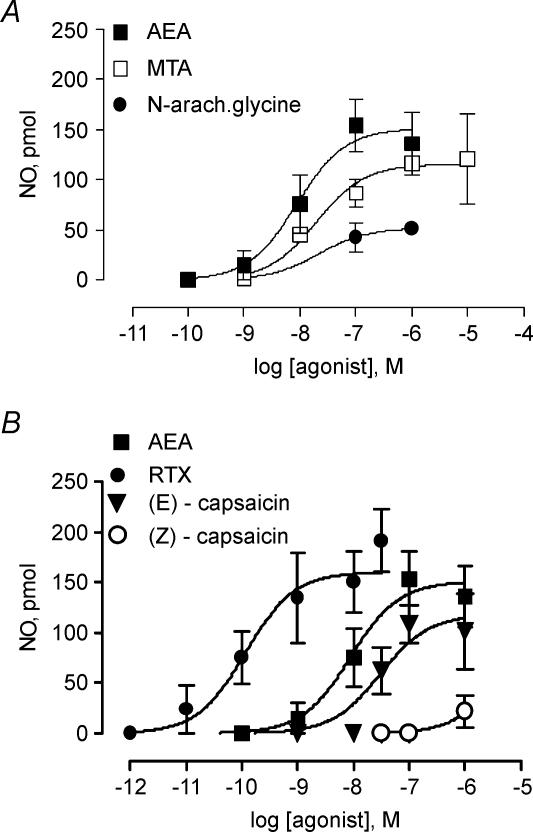
The rise in the release of NO evoked by anandamide was concentration dependent, its EC50 was 8.8 ± 7.1 nm, and its maximal response was achieved with 100 nm AEA (Fig. 2). The effect of anandamide was mimicked, although with a 2.3-fold lower potency, by its synthetic analogue (R)-(+)-methanandamide, whose EC50 was 19.8 ± 14.2 nm (Fig. 2A). The endogenous anandamide analogue, N-arachidonylglycine, induced a modest response, with a maximal NO release, attained with 1 μm that was only 35% of that induced by anandamide (53.8 ± 14.1 pmol, n = 4, P < 0.01, Fig. 2A).
Figure 2. Concentration–response curves for anandamide and its structurally related analogues, resiniferatoxin and the capsaicin isomers.
A, release of NO above baseline levels induced by a 1-min perfusion with increasing concentrations of either anandamide (AEA, n = 30) or its synthetic analogue (R)-(+)-methanandamide (MTA, n = 16) or the endogenous anandamide analogue N-arachidonylglycine (N-arach.glycine, n = 15). B, the release of NO elicited by a 1-min perfusion with increasing concentrations of vanilloid receptor agonists: resiniferatoxin (RTX, n = 20), (E)-capsaicin (n = 23) or its inactive cis isomer (Z)-capsaicin (n = 13) was compared to the AEA concentration–response curve. Symbols represent mean values, bars the s.e.m.
The prototypic vanilloid receptor ligand (E)-capsaicin, but not its inactive cis isomer (Z)-capsaicin, elicited a concentration-dependent rise in the outflow of NO. Compared to anandamide, the (E)-capsaicin concentration–response curve was shifted 3.4-fold to the right; its EC50 was 30.3 ± 23.1 nm and its maximal response achieved 106 ± 9.9 pmol NO (Fig. 2B), a value significantly lower than that elicited by anandamide (P < 0.05). The vanilloid TRPV1 receptor agonist, resiniferatoxin, was 73-fold more potent than anandamide, since its EC50 was 0.12 ± 0.02 nm, with a maximal effect of 153.7 ± 29.5 pmol NO (Fig. 2B), comparable to anandamide.
Table 1 summarizes the data of NO-evoked release elicited by several anandamide analogues perfused at 100 nm each. In contrast to anandamide, 100 nm olvanil, a non-pungent vanilloid receptor agonist, did not evoke significant NO release. In addition, 2-arachidonylglycerol, the alleged endogenous CB2 receptor agonist, did not elicit a significant NO release (19.0 ± 13.2 pmol, n = 4), nor at 100 nm did it interfere with the rise of NO elicited by its simultaneous incubation with 100 nm anandamide (182.2 ± 34.7 pmol, n = 2), allowing us to exclude a preponderant role of the CB2 receptor on the action of anandamide, reinforcing the notion of stringent structural requirements for receptor activation.
Table 1.
Effects of the vanilloid receptor antagonist capsazepine on the NO release induced by 100 nm of the anandamide analogues
| Control | 100 nm capsazepine | |
|---|---|---|
| (R-(+)-Methanandamide (4) | 86.7 ± 13.5 | 22.5 ± 19.4* |
| N-Arachidonylglycine (4) | 42.7 ± 14.6 | 42.5 ± 7.9 |
| N-Arachidonyldopamine (4) | 117.5 ± 4.7 | 28.1 ± 16.2* |
| Olvanil (4) | 13.7 ± 8.4 | — |
The data are ΔNO in pmol (means ±s.e.m.). Values of n are shown in parentheses.
P < 0.01, when compared to the corresponding control value.
Involvement of endothelium, NO synthase and TRP channels in the NO release elicited by anandamide and capsaicin
The release of NO induced by either anandamide or capsaicin was prevented by endothelium removal (90% reduction versus non-treated controls, n = 4), NO synthase inhibition with 100 μml-NNA (89% reduction, n = 4) or ruthenium red, a non-selective vanilloid channel blocker (83% reduction, n = 4); see values in Table 2. These results suggest that the source of NO is endothelial and in all likelihood derived from a specific enzymatic pathway likely to be mediated by the activation of vanilloid endothelial TRP channels.
Table 2.
Effects of endothelium removal, NO synthase blockade and of vanilloid receptor antagonism on NO release elicited by anandamide and capsaicin
| 100 nm anandamide | 100 nm capsaicin | |
|---|---|---|
| Control | 156.3 ± 20.5 (9) | 108.4 ± 19.2 (6) |
| Denuded endothelium | 14.0 ± 14.0* (4) | 16.7 ± 16.7* (4) |
| 100 μml-NNA | 17.3 ± 5.80* (4) | 0* (4) |
| 0.3 μm ruthenium red | 27.2 ± 10.3* (6) | 19.7 ± 16.7* (4) |
Methodological details are described under Methods.
P < 0.05 when compared to the corresponding control value.
Studies with TRPV1 and CB1 receptor antagonists
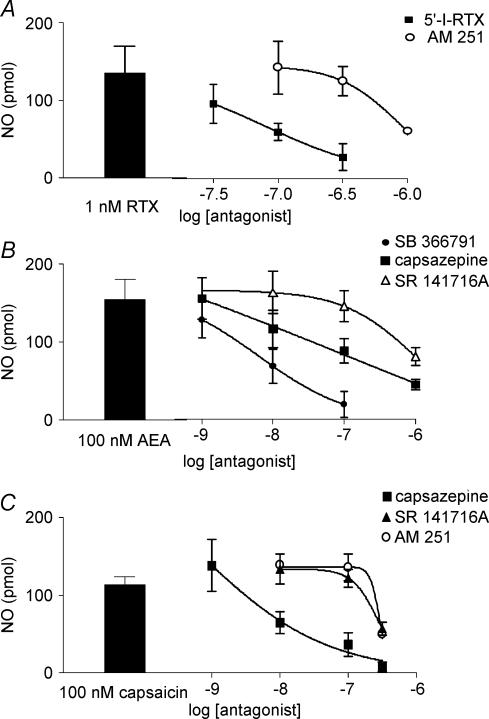
The rise in NO production elicited by anandamide as well as by vanilloid ligands was blocked by a variety of TRPV1 receptor antagonists, clarifying that it is likely that the TRPV1 receptor mediates the NO released. The median inhibitory concentration of the vanilloid receptor antagonist 5′-iodoresiniferatoxin to reduce the 1 nm resiniferatoxin-induced NO release was 79.4 nm (Fig. 3A); however, at 100 nm it annulled the anandamide whereas it reduced significantly by 50% the capsaicin-induced NO production (P < 0.05, Table 3). Likewise, the approximate median effective inhibitory concentration of SB 366791, a selective TRPV1 receptor antagonist, was 6.8 nm (Fig. 3B). The vanilloid receptor antagonist capsazepine was at least 6.5-fold less potent, while the cannabinoid receptor antagonist SR 141716A was the least potent with an approximate median inhibitory concentration of 940 nm (Fig. 3B). Furthermore, SB 366791 more than halved the rise in NO production elicited by 1 nm resiniferatoxin but reduced by 80–90% that elicited by anandamide or capsaicin (P < 0.05, Table 3). Likewise, the median inhibitory concentration of capsazepine to block the standard of 100 nm capsaicin was 13.9 nm while that of SR 141716A or its iodo substituted analogue (AM 251), two claimed selective cannabinoid receptor antagonists, was 276.7 and 289.7 nm, respectively (Fig. 3C and Table 3). AM 251 was slightly more potent in blocking the effect of anandamide than the vanilloids; at 300 nm it reduced 50% (P < 0.05, n = 4) the burst of NO elicited by capsaicin, while resiniferatoxin was the least sensitive (Table 3). The median inhibitory concentration of AM 251 to block the 1 nm resiniferatoxin was 0.94 μm (Fig. 3A).
Figure 3. Effects of TRPV1 and cannabinoid CB1 receptor antagonists on the release of NO elicited by resiniferatoxin, anandamide or capsaicin.
A, 5′-iodoresiniferatoxin-induced (5′-I-RTX) and AM 251 blocked concentration-dependently the NO release elicited by a 1-min perfusion with 1 nm resiniferatoxin (RTX, n = 18). B, comparative study of the potency of SB 366791, capsazepine or SR 141716A to block the NO production elicited by a 1-min pulse application of 100 nm anandamide (AEA). C, capsazepine, SR 141716A and AM 251 reduced concentration-dependently the NO production elicited by a 1-min perfusion with 100 nm capsaicin. Left side columns denote the NO produced in separate control experiments by a 1-min perfusion with 1 nm resiniferatoxin (RTX), or 100 nm of either anandamide (AEA) or capsaicin alone (panels A, B and C, respectively). The antagonists were perfused 30 min before and during the 1-min incubation with the agonists. Results are the mean ±s.e.m. of at least 4 separate experiments per concentration of each antagonist assayed in the presence of either anandamide or capsaicin.
Table 3.
Effect of TRPV1 and CB1 receptors antagonists on the NO production evoked by prototype vanilloid and cannabinoids agonists
| 1 nm resiniferatoxin | 100 nm anandamide | 100 nm capsaicin | |
|---|---|---|---|
| Untreated | 134.8 ± 44.5 (4) | 175.6 ± 13.9 (5) | 93 ± 9.9 (6) |
| 100 nm I-RTX | 58.8 ± 10.9* (4) | 8.2 ± 7.5** (4) | 45.2 ± 13.5* (6) |
| 100 nm SB 366791 | 52.7 ± 19.9* (4) | 32.0 ± 19.9** (7) | 29.3 ± 9.5** (4) |
| 100 nm AM 251 | 142.2 ± 33.8 (4) | 35.9 ± 11.2** (4) | 119.5 ± 15.3 (4) |
| 300 nm AM 251 | 124.8 ± 18.9 (2) | nd | 43.5 ± 2.4* (2) |
The data are ΔNO in pmol (means ±s.e.m.).
P < 0.05,
P < 0.01 as compared to the untreated tissues.
The release of NO elicited by either the synthetic anandamide analogue (R)-(+)-methanandamide or the endogenous vanilloid ligand N-arachidonyldopamine (NADA) was reduced by 100 nm capsazepine (Table 1), while the outflow of NO induced by 100 nmN-arachidonylglycine, an endogenous anandamide analogue was rather resistant to capsazepine (Table 1).
None of these receptor blockers elicited per se a meaningful release of NO. Control experiments revealed that the sole perfusion for 1 min with one of the drugs 100 nm 5′iodo-resiniferatoxin, SB 366791, AM 251 or capsazepine or 1000 nm SR 141716A did not cause a significant outflow of NO; in fact, the rise in NO elicited by 100 nm of these drugs was 23 ± 13.3 (n = 4), 8.6 ± 5, 10.4 ± 7.2 (n = 4), 5.7 ± 3.6 (n = 4) and 12.4 ± 2 pmol NO (n = 3), respectively. 5′-Iodoresiniferatoxin up to 1 μm was devoid of agonist activity; it failed to elicit a burst of luminal NO.
Lack of significant influence of TRPV4 receptors in the anandamide-induced NO burst
To investigate the role of TRPV4 receptor activation in the anandamide-induced NO production, we perfused mesenteries with 1 μm 4αPDD, a purported selective activator of the TRPV4 receptor channel. This compound did not elicit a rise in luminal NO (12 ± 7.7 pmol NO, n = 6), while 1 μm PDD significantly elicited a burst of 100.2 ± 14 pmol NO (n = 4, P < 0.01), revealing that protein kinase C activation acts as an intermediate enzymatic step in the NO production. Furthermore, to discard the possibility that anandamide is metabolized to arachidonic acid, which could rapidly be further transformed to epoxyeicosatrienoic acids, alleged endogenous TRPV4 receptor ligands, we next blocked several enzymes of this metabolic pathway. Application of 100 nm anandamide to mesenteries pretreated with 100 μm PMSF, 10 μm indomethacin, 10 μm nordihydroguaiaretic acid, 10 μm indomethacin plus nordihydroguaiaretic, or 10 μm miconazole did not significantly modify the 100 nm anandamide-induced rise in NO production, allowing to discard a primary role of TRPV4 receptor channels in the anandamide-induced NO production. The results of these studies are summarized in Table 4.
Table 4.
Role of several inhibitors involved in the arachidonic acid cascade to generate putative TRPV4 active metabolites
| NO (pmol) | n | |
|---|---|---|
| 100 nm AEA | 159.0 ± 27.2 | 7 |
| +100 μm PMSF | 121.2 ± 31.7 | 4 |
| +10 μm indomethacin | 148.3 ± 14.0 | 4 |
| +10 μm nordihydroguaiaretic acid (NHGA) | 168.7 ± 16.6 | 4 |
| +10 μm indomethacin +10 μm NHGA | 175.8 ± 49.2 | 4 |
| +10 μm (±)-miconazole | 138.9 ± 31.2 | 3 |
The data are means ±s.e.m.
RT-PCR analysis of TRPV1 receptor RNA expression in rat mesenteries
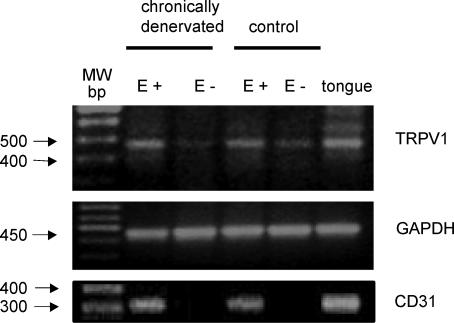
To test the possibility that the putative vanilloid receptors involved in the release of NO could be located in endo-thelial cells, TRPV1 mRNA expression was studied in intact and endothelium-denuded mesenteric bed homogenates. A single clear band of the expected size was observed for the rat TRPV1 vanilloid receptor in mesenteries with an intact endothelial cell layer (E+, Fig. 4). This band was reduced to a very low level in endothelium-denuded preparations. To preclude the presence of the sensory nerves, RT-PCR studies were also performed in chronically denervated mesenteries isolated from adult rats treated with capsaicin at birth. Intact denervated tissues showed a PCR product of the expected size for the rat TRPV1 receptor that was essentially obliterated upon shedding of the endothelial cell layer from chronically denervated rat mesenteries (Fig. 4).
Figure 4. RT-PCR analysis of the mRNA coding for the vanilloid TRPV1 receptor expressed in rat mesenteries.
Representative ethidium bromide stained agarose gel of reversed transcribed and PCR-amplified fragments of the expected size for the vanilloid TRPV1 receptor mRNA. RT-PCR analysis was performed by using TRPV1-specific primers to examine expression in mRNA extracted from mesenteries isolated from adult controls and adult chronically denervated rats (neonatal capsaicin treatment) either with intact (E+), or without endothelium (E−). Total rat tongue mRNA was used as a positive control tissue. While GAPDH was used as an internal standard to control gel loading, CD31 served as the endothelium marker. Left lane indicates the molecular weight markers. The mRNA used for the TRPV1, CD31 and GAPDH identification proceeded from a same rat mesentery. Identical results were attained in three protocols using separate intact or endothelium denuded rat mesenteries.
The PCR product for GAPDH, observed in mesenteric mRNA extracts with or without endothelium showed a similar intensity (Fig. 4), while the mRNA for CD31, was identified only in E+ mesenteries, i.e. those with intact endothelium (Fig. 4). Sequencing confirmed more than 99% identity of these products with the corresponding cDNA for the rat TRPV1 receptor. Total tongue mRNA served as a control tissue. As a further control, protocols performed in the absence of cDNA did not yield PCR products. Likewise, protocols carried out in the absence of the RT-step did not yield PCR products, confirming the absence of genomic DNA contamination (data not shown).
Effects of chronic denervation on CGRP-immunoreactivity and on the NO release induced by anandamide and by the vanilloid receptor agonist capsaicin
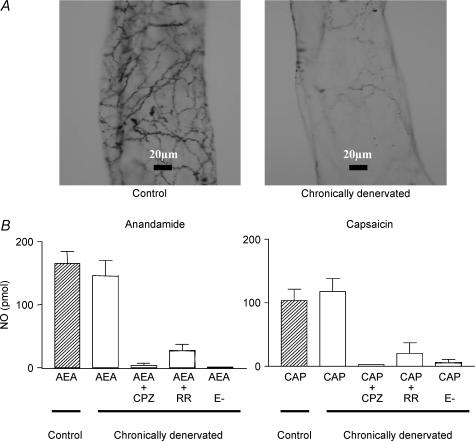
Chronic denervation of the myenteric plexus sensory nerves, notoriously reduced the CGRP immunoreactivity as compared to mesenteries from adult control rats (Fig. 5A). However, sensory nerve denervation did not significantly modify the NO released by 100 nm anandamide or 100 nm capsaicin (Fig. 5B). Furthermore, capsazepine or ruthenium red significantly reduced the outflow of NO elicited by either anandamide or capsaicin in chronically denervated mesenteries (Fig. 5B). Moreover, the magnitude of blockade caused by the antagonists was similar to that observed with these drugs in control mesenteries (compare data from Table 2 and Fig. 5B). Removal of the endothelial cell layer abolished the release of NO caused by the agonists in the chronically denervated tissues (Fig. 5B), to the same magnitude as that produced in the control mesenteries.
Figure 5. Chronic sensory nerve denervation reduced myenteric CGRP immunoreactivity but not the anandamide- or capsaicin-induced NO release.
A, representative whole-mount sections of myenteric CGRP-immunostained nerve terminals from adult control (left image) and denervated adult rats (right panel). Neonatal capsaicin treatment was performed on days 1 and 2 after birth as described under methods. B, control values of NO release elicited by 1-min exposure to either anandamide (AEA, left panel) or capsaicin (CAP, right panel) are depicted as hatched bars. Open bars represent the outflow of NO after chronic denervation. The vanilloid TRPV1 receptor antagonist 100 nm capsazepine (CPZ), or 0.3 μm ruthenium red (RR), the TRPV1 channel blocker, was added 20 min before and during the 1-min incubation with the agonists. When indicated (E−) the endothelium was removed by perfusing 0.1% saponin for 55 s. Results are the mean ±s.e.m. of at least 4 separate experiments per group. P < 0.01 when compared to the NO release induced by the agonists alone in denervated mesenteries.
Discussion
The present study shows that 100 nm anandamide, a concentration that does not elicit vasodilatation, provoked a rapid and transient release of endothelial NO with a concomitant increase in tissue cGMP levels. The anandamide-induced rise in luminal NO was antagonized by the highly potent and selective TRPV1 receptor antagonists 5′-iodoresiniferatoxin, SB 366791 or capsazepine, suggesting that an endothelial TRPV1 receptor might mediate the NO production. This finding is unrelated to previous evidence with classical vasodilators that act through the activation of the NO/cGMP pathway. For instance, in our laboratory we consistently observed the production of NO elicited by either the muscarinic cholinergic agonist acetylcholine or the purinergic agonists 2-MeSADP or UTP (Buvinic et al. 2002), ligands that elicited the same magnitude of luminally released NO as anandamide but is indeed linked to vasodilatation of the mesenteric vascular tree. Although less potent than anandamide in eliciting NO release, 100 nm capsaicin did produce a significant vasodilatation in the rat mesenteric territory (20 ± 2.9 mmHg). This relaxation could be due to the higher potency of (E)-capsaicin to induce CGRP release from myenteric sensory nerves (Zygmunt et al. 1999), a finding that is supported by additional experiments showing a significant reduction in the capsaicin-induced vasodilatation in denervated rats (data not shown). Notwithstanding, 10- to 100-fold larger concentrations of anandamide elicited a concentration-dependent vasodilatation of this vascular territory (data not shown). In rat bladder urothelial cells, vanilloid receptor activation elicited a much larger production of NO (Birder et al. 2001), a finding likely to be related to the denser expression of TRPV1 receptors, which in this tissue is derived from both afferent nerves and by the urothelial cells themselves.
The fact that nanomolar concentrations of the synthetic anandamide analogue (R)-(+)-methanandamide also elicited a concentration-dependent release of NO is likely to indicate that a common site exists for the action of these two structurally related arachidonic acid derivatives. Since the TRPV1 receptor agonists resiniferatoxin and (E)-capsaicin but not its inactive analogue (Z)-capsaicin mimicked the release of NO elicited by anandamide, the possibility exists that the TRPV1 vanilloid receptors are the target site linked to the outflow of NO that we now report. Therefore, we also argue that the recently identified endo-vanilloid N-arachidonyldopamine (NADA; Huang et al. 2002) might also activate the TRPV1 receptor channel acting at a common site. We are aware that the potency of anandamide is 3-fold larger than capsaicin, and that resiniferatoxin is 300-fold more potent that capsaicin, values that might be at variance with TRPV1 receptor pharmacology, an issue that might suggest the involvement of other receptors, such as an endothelial CB1 receptor.
Several arguments support our interpretation favouring a major role of the TRPV1 receptor channel in the anandamide-induced NO release. The endogenous anandamide analogue N-arachidonylglycine, which is devoid of affinity for the TRPV1 receptor (Huang et al. 2001), elicited only a modest release of NO, much lower than that evoked by anandamide. In addition, the non-pungent capsaicin analogue olvanil was also unable to induce NO release. In this respect, Stebbins et al. (2003) showed marked differences in the ability of olvanil to stimulate TRPV1 receptors, such as lack of contractile effects in the guinea pig airways. Therefore, we infer that these vanilloid TRPV1 receptors must interact at a site that recognizes specific structural requirements of the ligands. Moreover, the concentration of anandamide required to evoke NO release is nanomolar, a finding compatible with tissue levels of anandamide. For instance, anandamide is found in rat brain at a concentration of 30 pmol g−1 and in human brain at a range between 55 and 100 pmol g−1. Furthermore, around 10 pmol g−1 are present in the human spleen and heart and levels within the nanomolar range have also been detected in serum, plasma and cerebrospinal fluid of rats and humans (Martin et al. 1999; Giuffrida et al. 2000).
The participation of vanilloid receptors in the anandamide-evoked NO release is clearly supported by the observation that the highly selective TRPV1 receptor antagonists 5′-iodoresiniferatoxin or SB 366791 blocked and even abrogated the NO production induced by resiniferatoxin, anandamide or capsaicin. It is worth noting that the addition of an iodine atom at the vanilloid moeity of resiniferatoxin yields a compound with an antagonist profile; its potency to reduce NO production in our study is compatible with the in vivo results reported by Undem & Kollarik (2002) who studied vagal sensory C-fibre activation. Moreover, the SB 366791 potency to reduce the anandamide-induced NO production that we now report is entirely compatible with its potency to inhibit the activation of the hTRPV1 receptor by 100 nm capsaicin (Gunthorpe et al. 2004). Capsazepine also produced a concentration-dependent reduction of either anandamide- or capsaicin-induced release of NO, albeit at concentrations 10 times higher. In addition, capsazepine also reduced the release of NO induced by nanomolar concentrations of the synthetic anandamide analogue (R)-(+)-methanandamide and by NADA, an endogenous vanilloid ligand, but it failed to antagonize the NO release elicited by N-arachidonylglycine, that was reported as a weak vanilloid receptor ligand (Huang et al. 2001). Moreover, the finding that ruthenium red, a non-selective channel blocker, also reduced the outflow of NO elicited by either anandamide or capsaicin gives additional support to our notion that the vanilloid TRPV1 receptor is a target of cannabinoids and vanilloids for NO production.
Although the involvement of cannabinoid CB1 receptors in the release of NO evoked by anandamide and capsaicin could arise from the observation that micromolar concentrations of the CB1 receptor antagonist SR 141716 A or AM 251 reduced the outflow of NO induced by both agonists, a non-specific antagonism caused by the high concentration used of these drugs cannot be entirely precluded. This is in keeping with the finding of De Petrocellis et al. (2001) that micromolar concentrations of SR 141716 A, a rather selective CB1 receptor antagonist, inhibited the TRPV1-mediated Ca2+ increase caused by anandamide and capsaicin in human endothelial kidney cells expressing the TRPV1 receptor. In view of our observations that 5′-iodoresiniferatoxin, SB 366791 and capsazepine are remarkably more potent than SR 141716A in antagonizing the anandamide-induced outflow of NO, in addition to the fact that AM 251 blocked the anandamide-induced NO burst as well as that elicited by capsaicin or resiniferatoxin, we propose that the effect of anandamide and structurally related analogues is mainly mediated by TRPV1 receptor activation. The role of endo-thelial CB1 receptors (Liu et al. 2000) on the release of endothelial NO elicited by anandamide cannot be entirely precluded since this receptor might also promote NO production; however, we propose that its contribution might be minor. In this respect, we have not ignored the proposal of a functional cross-talk between CB1 receptors and the TRPV1 channel (Kim et al. 2005) based on the observation that, in addition to capsazepine, the rather selective CB1 receptor antagonist AM 251 was also able to prevent Ca2+ influx elicited by capsaicin in mesencephalic cultured neurones.
Another issue that captured our attention was the finding of endothelial TRPV4 receptors (Watanabe et al. 2002a) and the possibility that anandamide directly or indirectly, through epoxyeicosatrienoic acid metabolites, putative endogenous ligands of the TRPV4 receptor channel (Watanabe et al. 2003), might act on TRPV4 receptors. Therefore we ascertained the possible participation of TRPV4 receptors as putative targets of an indirect effect of anandamide. Several arguments in the present study allow us to discard a major role of the TRPV4 receptor channel as an actor in NO production. First of all, 4αPDD, the classical TRPV4 receptor activator (Watanabe et al. 2003; Andrade et al. 2005), did not elicit NO release, failing to mimic the action of anandamide. Second, a battery of enzyme inhibitors successfully used by Watanabe et al. (2003) to block different steps of the anandamide metabolism to arachidonic acid and subsequent active metabolites, did not attenuate anandamide NO production, suggesting that this metabolic route is apparently not involved in NO production. We reasoned that if anandamide is metabolized in the mesenteries to active arachidonic acid-derived products, and that these metabolites are indirectly involved in its activity, the stepwise inhibition of key enzymes in the arachidonic acid cascade should reflect a reduction in NO production at some stage. This was not the case, contrary to the studies of Watanabe et al. (2003). Moreover (R)-(+)-methanandamide behaved as a partial agonist in our bioassay system, in spite of its failure to activate the TRPV4 receptor (Watanabe et al. 2003) and its being a non-hydrolysable derivative of anandamide. Altogether, the collection of these findings allows us to discard that the endothelial TRPV4 receptor plays a major role in NO production, although at present this possibility cannot be totally excluded.
The observation that 4PDD, an activator of protein kinase C, but not its inactive enantiomer 4αPDD (Fukushima et al. 1996) elicited a significant rise in NO production led us to conclude that protein kinase C activation is a distal step in receptor-mediated transduction signalling pathways, and likely a crossed way common to several receptor mechanisms. Future studies should detail whether the effect of 4PDD is extended to other phorbol esters, and in addition, whether blockade of protein kinase C interferes with the cannabinoid- and vanilloid-induced NO production.
Regarding the source of NO, the fact that endo-thelium removal prevented the NO release elicited by either anandamide or capsaicin strongly supports the involvement of the endothelium in the NO production. In this regard, although it was suggested that only neuronal NO has a role in sensory nerve modulation (Harris et al. 2002), a tonic release of NO from the endothelium may have an inhibitory modulator effect on sensory neurotransmission in the rat mesenteric bed (Ralevic, 2002). Moreover, in addition to a tonic NO production, our results indicate that TRPV1 receptor agonists could also elicit a stimulated release of NO from the endothelium not linked to vasodilatation that could in turn contribute to sensory nerve modulation. On the other hand, since the NO synthase inhibitor l-NNA reduced the outflow of NO induced by either anandamide or capsaicin, we infer that this enzyme is involved in the production of NO. Because endothelial NO synthase activity is dependent on intracellular Ca2+ (Toda & Okamura, 2003), the activation of a putative endothelial TRPV1 channel could be directly linked to the rise in intracellular Ca2+ leading to the subsequent activation of the endothelial NO synthase.
Our proposal of an endothelial TRPV1 receptor linked to the release of NO is supported by the observation that, in control mesenteries, the mRNA coding for the TRPV1 receptor was notoriously reduced after removal of the endothelial cell layer. In addition, in mesenteries isolated from adult chronically denervated rats by neonatal capsaicin treatment, a remnant PCR product for TRPV1 receptor was still present and was also significantly reduced after endothelium removal, further suggesting the presence of an endothelial TRPV1 receptor. In this respect, endothelial TRPV1 receptors have been recently found in human brain microvasculature (Golech et al. 2004) and it has become increasingly apparent that TRPV1 receptors are expressed in cells other that primary afferent neurones with as yet undefined physiological roles (Birder et al. 2001; Caterina, 2003).
We have not ignored that vanilloid TRPV1 receptors from sensory nerves could participate as an additional source of NO production or alternatively induce the release of neuromodulators such as CGRP, which could in its turn stimulate endothelial NO release. In this regard, the release of CGRP from stimulated capsaicin-sensitive neurones and subsequent increase in endothelial NO has been described in rat gastric mucosa and rabbit ear (Whittle et al. 1992; Holzer, 1998; Susuki et al. 1998). Nevertheless, in our experiments, the involvement of sensory nerves may be disregarded on the basis that the release of NO induced by either anandamide or capsaicin was unmodified after chronic sensory nerve degeneration. The possibility that this could be due to an incomplete denervation cannot be discarded. However, this possibility is rendered unlikely by the observation that the CGRP immunoreactivity, the vasodilator peptide coupled to the activation of TRPV1 receptors, was largely reduced in the mesenteries of chronically denervated rats. In addition, and as observed for control mesenteries, the release of NO elicited by anandamide and capsaicin was also reduced by either endothelium removal or the vanilloid receptor antagonists capsazepine and ruthenium red in mesenteries isolated from chronically denervated rats.
Regarding the role of the NO released by anandamide through TRPV1 endothelial receptor activation, a modulatory function could be speculated on. Thus, NO has been shown to produce actions other than vascular smooth muscle relaxation, such as control of the release of several neurotransmiters in brain and non-vascular tissues (Toda & Okamura, 2003). A modulator role for NO where capsaicin-induced increase in NO production is required for CGRP release has been proposed in rat spinal cord (Garry et al. 2000) and in rabbit skin (Hughes & Brain, 1994). It has also been described that NO through cGMP elevation may function as an inhibitory neurotransmitter and might be involved in sensory nerve desensitization (Lopshire & Nicol, 1997; Kopp et al. 2001).
Regarding the role of the TRPV1 receptor and the NO produced, the possibility exists that nanomolar concentrations of anandamide could act an autoregulator pathway in vascular endothelial cells. It has been suggested that after anandamide biosynthesis its release might be dependent on the same putative membrane transporter proposed to underlie its cellular uptake, which may act bidirectionally (De Petrocellis et al. 2004). This membrane transporter has been shown to be activated by NO in human endothelial cells (Maccarrone et al. 2000; De Petrocellis et al. 2001). Hence, it could be speculated that anandamide, by stimulating endothelial NO production, could modulate either its own release or its own uptake. Whereas our results suggest the participation of endo-thelial vanilloid TRPV1 receptors in the release of NO, it is of interest to note that Deusch et al. (1997) and Maccarrone et al. (2000) had advanced that the NO released by anandamide could involve cannabinoid receptor stimulation.
In sum, the present results demonstrate that nanomolar concentrations of anandamide devoid of vascular effects, elicit nevertheless a rapid and transient release of NO mediated mainly through the activation of TRPV1 vanilloid receptors localized at the vascular endothelium of the rat mesentery. The implications of the present findings for the nervous system and the pathophysiological relevance of the anandamide-induced transient NO release open new opportunities for research. Further studies will evaluate the physiological significance of the endothelial TRPV1 receptors and assess whether other vascular beds also express these endothelial channels, which might be relevant for endothelial cell communication and/or vascular tone regulation in health and disease.
Acknowledgments
This work was funded by FONDAP grant 13980001; the MIFAB Institute also contributed with funds to support the Centre. ANCYPT grant 05-14107 as well as Fundación Antorchas grants 14022-112 and 14156-3 allowed interactions between the Chilean and Argentinian laboratories. We thank Professor F. Torrealba for the CGRP immunochemical assays, and Sanofi-Synthélabo Recherche laboratories for a sample of SR 141716A.
References
- Amman R, Maggi CA. Ruthenium red as a capsaicin antagonist. Life Sci. 1991;49:849–859. doi: 10.1016/0024-3205(91)90169-c. [DOI] [PubMed] [Google Scholar]
- Anavi-Goffer S, McKay NG, Ashford MLJ, Coutts A. Vanilloid receptor type1-immunoreactivity is expressed by intrinsic afferent neurones in the guinea-pig myenteric plexus. Neurosc Lett. 2002;319:53–57. doi: 10.1016/s0304-3940(01)02480-6. [DOI] [PubMed] [Google Scholar]
- Andrade YN, Fernándes J, Vázquez E, Fernández-Fernández JM, Arniges M, Sánchez TM, Villalón M, Valverde MA. TRPV4 channel is involved in the coupling of fluid viscosity changes to epithelial ciliary activity. J Cell Biol. 2005 doi: 10.1083/jcb.200409070. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birder LA, Kanai AJ, de Groat WC, Kiss S, Nealen M, Burke NE, Dineley KE, Watkins S, Reynolds IJ, Caterina MJ. Vanilloid receptor expression suggest a sensory role for urinary epithelial cells. Proc Natl Acad Sci U S A. 2001;98:13396–13401. doi: 10.1073/pnas.231243698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boric MP, Figueroa XF, Donoso MV, Paredes A, Poblete IM, Huidobro-Toro JP. Rise in endothelium-derived NO after stimulation of rat perivascular sympathetic mesenteric nerves. Am J Physiol. 1999;277:H1027–H1035. doi: 10.1152/ajpheart.1999.277.3.H1027. [DOI] [PubMed] [Google Scholar]
- Buvinic S, Briones R, Huidobro-Toro JP. P2Y1 and P2Y2 receptors are coupled to the NO/cGMP pathway to vasodilate the rat arterial mesenteric bed. Br J Pharmacol. 2002;136:847–856. doi: 10.1038/sj.bjp.0704789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buvinic S, Huidobro-Toro JP. Basal tone release of nitric oxide coupled to cGMP production regulates vascular reactivity of the rat mesenteric bed. Eur J Pharmacol. 2001;424:221–227. doi: 10.1016/s0014-2999(01)01165-7. [DOI] [PubMed] [Google Scholar]
- Caterina MJ. Vanilloid receptors take a TRP beyond the sensory afferent. Pain. 2003;105:5–9. doi: 10.1016/s0304-3959(03)00259-8. [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–225. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- De Petrocellis LD, Bisogno T, Maccarrone M, Davis JB, Finazzi-Agro A, Di Marzo V. The activity of anandamide at vanilloid VR1 receptors requires facilitated transport across the cell membrane and is limited by intracellular metabolism. J Biol Chem. 2001;276:12856–12863. doi: 10.1074/jbc.M008555200. [DOI] [PubMed] [Google Scholar]
- De Petrocellis L, Cascio MG, Di Marzo V. The endocannabinoid system: a general view and latest additions. Br J Pharmacol. 2004;141:765–774. doi: 10.1038/sj.bjp.0705666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deusch DG, Goligorsky MS, Schmid PC, Krebsbach RJ, Schmid HHO, Das SK. Production and physiological actions of anandamide in the rat vasculature of the rat kidney. J Clin Invest. 1997;100:1538–1546. doi: 10.1172/JCI119677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- Dickenson AH, Dray A. Selective antagonism of capsaicin by capsazepine: evidence for a spinal receptor site in capsaicin-induced antinociception. Br J Pharmacol. 1991;104:1045–1049. doi: 10.1111/j.1476-5381.1991.tb12547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoso MV, Carvajal A, Paredes A, Tomic A, Koening C, Huidobro-Toro JP. α2-Adrenoceptors control the release of noradrenaline but not neuropeptide Y from perivascular nerves terminals. Peptides. 2002;23:1663–1671. doi: 10.1016/s0196-9781(02)00108-0. [DOI] [PubMed] [Google Scholar]
- Donoso MV, Faundez H, Rosa G, Fournier A, Edvinsson L, Huidobro-Toro JP. Pharmacological characterization of ETA receptors in the vascular smooth muscle comparing its analogous distribution in the rat mesentery artery and in the rat mesenteric vein. Peptides. 1996;17:1145–1153. doi: 10.1016/s0196-9781(96)00188-x. [DOI] [PubMed] [Google Scholar]
- Figueroa XF, Poblete MI, Boric MP, Mendizabal VE, Adler-Graschinsky E, Huidobro-Toro JP. Clonidine-induced nitric oxide-dependent vasorelaxation mediated by endothelial α2-adrenoceptor activation. Br J Pharmacol. 2001;134:957–968. doi: 10.1038/sj.bjp.0704320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima Y, Asano T, Takagiri H, Aihara M, Saitoh T, Anai M, Funaki M, Ogihara T, Inukai K, Matsuhashi N, Oka Y, Yazaki Y, Sugano K. Interaction between the two signal transduction systems of the histamine H2 receptor: desensitization and sensitising effects of histamine stimulation on histamine-dependent cAMP production in Chinese hamster ovary cells. Biochem J. 1996;320:27–32. doi: 10.1042/bj3200027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garry MG, Walton LP, Davis MA. Capsaicin-evoked release of immunoreactive calcitonin gene-related peptide from the spinal cord is mediated by nitric oxide but not by cyclic GMP. Brain Res. 2000;861:208–219. doi: 10.1016/s0006-8993(99)02448-8. [DOI] [PubMed] [Google Scholar]
- Garthwaite J, Southam E, Boulton CL, Nielsen EB, Schmidt K, Mayer B. Potent and selective inhibition of nitric oxide-sensitive guanylyl cyclase by 1H-[1,2,4]oxadiazolo[4,3a]quinoxalin-1-one. Mol Pharmacol. 1995;48:184–188. [PubMed] [Google Scholar]
- Gatley SJ, Gifford AN, Volkow ND, Lan R, Makriyannis A. 125I-labelled AM 251: a radioiodinated ligand which binds in vivo to mouse brain cannabinoid CB1 receptors. Eur J Pharmacol. 1996;307:331–338. doi: 10.1016/0014-2999(96)00279-8. [DOI] [PubMed] [Google Scholar]
- Giuffrida A, Rodriguez de Fonseca F, Piomelli D. Quantification of bioactive acylethanolamides in rat plasma by electrospray mass spectrometry. Anal Biochem. 2000;280:87–93. doi: 10.1006/abio.2000.4509. [DOI] [PubMed] [Google Scholar]
- Golech SA, McCarron RM, Chen Y, Bembry J, Lenz F, Mechoulam R, Shohami E, Spatz M. Human brain endothelium: coexpression and function of vanilloid and endocannabinoid receptors. Mol Brain Res. 2004;132:87–92. doi: 10.1016/j.molbrainres.2004.08.025. [DOI] [PubMed] [Google Scholar]
- Gunthorpe MJ, Rami HK, Jerman JC, Smart D, Gill CH, Soffin EM, Hannan SL, Lappin SC, Egerton J, Smith GD, Worby A, Howett L, Owen D, Nasir S, Davies CH, Thompson M, Wyman PA, Randall AD, Davis JB. Identification and characterisation of SB-366791, a potent and selective vanilloid receptor (VR1/TRPV1) antagonist. Neuropharmacology. 2004;46:133–149. doi: 10.1016/s0028-3908(03)00305-8. [DOI] [PubMed] [Google Scholar]
- Harris D, McCulloch AI, Kendall DA, Randall MD. Characterization of vasorelaxant responses to anandamide in the rat mesenteric arterial bed. J Physiol. 2002;539:898–902. doi: 10.1113/jphysiol.2001.013489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzer P. Neural emergency system in the stomach. Gastroenterology. 1998;114:823–839. doi: 10.1016/s0016-5085(98)70597-9. [DOI] [PubMed] [Google Scholar]
- Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, Felder CC, Herkenham M, Mackie K, Martin BB, Mechoulam R, Pertwee RG. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev. 2002;54:161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- Huang SM, Bisogno T, Petros TJ, Chang SY, Savitsanos PA, Zipkin RE, Sivakumar R, Coop A, Maeda DY, De Petrocellis L, Burstein S, Di Marzo V, Walker JM. Identification of a new class of molecules, the arachidonylaminoacids, and characterization of one member that inhibits pain. J Biol Chem. 2001;276:42639–42644. doi: 10.1074/jbc.M107351200. [DOI] [PubMed] [Google Scholar]
- Huang SM, Bisogno T, Trevisani M, Al-Hayani A, De Petrocellis L, Fezza F, Tognetto M, Petros TJ, Krey JF, Chu CJ, Miller JD, Davies SN, Gepetti P, Walker JM, Di Marzo V. An endogenous capsaicin-like substance with high potency at recombinant and native vanilloid VR1 receptors. Proc Natl Acad Sci U S A. 2002;99:8400–8405. doi: 10.1073/pnas.122196999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes SR, Brain SD. Nitric oxide-dependent release of vasodilator quantities of calcitonin-gene related peptide from capsaicin sensitive nerves in rabbit skin. Br J Pharmacol. 1994;111:425–430. doi: 10.1111/j.1476-5381.1994.tb14752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SR, Lee DY, Chung ES, Oh UT, Kim SU, Jin BK. Transient receptor potential vanilloid subtype 1 mediates cell death of mesencephalic dopaminergic neurons in vivo and in vitro. J Neurosci. 2005;23:662–671. doi: 10.1523/JNEUROSCI.4166-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp UC, Cicha MZ, Smith LA, Hokfelt T. Nitric oxide modulates renal sensory nerve fibers by mechanisms related to substance P receptor activation. Am J Physiol Regul Integr Comp Physiol. 2001;281:R279–R290. doi: 10.1152/ajpregu.2001.281.1.R279. [DOI] [PubMed] [Google Scholar]
- Liu J, Gao B, Mirshahi F, Sanyan AJ, Khanolkar AD, Makriyannis A, Kunos G. Functional CB1 cannabinoid receptors in human endothelial cells. Biochem J. 2000;346:835–840. [PMC free article] [PubMed] [Google Scholar]
- Lopshire JC, Nicol GD. Activation and recovery of the PGE2-mediated sensitization of the capsaicin response in rat sensory neurons. J Neurophysiol. 1997;78:3154–3164. doi: 10.1152/jn.1997.78.6.3154. [DOI] [PubMed] [Google Scholar]
- Maccarrone M, Bari M, Lorenzon T, Bisogno T, Di Marzo V, Finazzi-Agro A. Ananadamide uptake by human endothelial cells and its regulation by nitric oxide. J Biol Chem. 2000;275:13484–13492. doi: 10.1074/jbc.275.18.13484. [DOI] [PubMed] [Google Scholar]
- Martin BR, Mechoulam R, Razdan RK. Discovery and characterization of endogenous cannabinoids. Life Sci. 1999;65:573–595. doi: 10.1016/s0024-3205(99)00281-7. [DOI] [PubMed] [Google Scholar]
- Mupanomunda MM, Wang Y, Bukoski RD. Effect of chronic sensory denervation of isolated mesenteric resistance arteries. Am J Physiol. 1998;274:H1655–HH1661. doi: 10.1152/ajpheart.1998.274.5.H1655. [DOI] [PubMed] [Google Scholar]
- Ralevic V. Endothelial nitric oxide modulates perivascular sensory neurotransmission in the rat isolated mesenteric arterial bed. Br J Pharmacol. 2002;137:19–28. doi: 10.1038/sj.bjp.0704837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross RA. Anandamide and vanilloid TRPV1 receptors. Br J Pharmacol. 2003;140:790–801. doi: 10.1038/sj.bjp.0705467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shire D, Calandra B, Bouaboula M, Barth F, Rinaldi-Carmona M, Casellas P, Ferrara P. Cannabinoid receptor interactions with the antagonists SR 141716A and SR 144528. Life Sci. 1999;65:627–635. doi: 10.1016/s0024-3205(99)00285-4. [DOI] [PubMed] [Google Scholar]
- Stebbins KJ, Carr MJ, Pedapati EV, Ellis JL. Effect of olvanil on the afferent and efferent function of capsaicin-sensitive C-fibers in guinea pig airways. Eur J Pharmacol. 2003;471:205–211. doi: 10.1016/s0014-2999(03)01833-8. [DOI] [PubMed] [Google Scholar]
- Susuki T, Wada S, Tomisawa N, Kamata R, Saito S, Sato I, Sugawara E, Tachikawa E, Kobayashi H. A possible role of nitric oxide formation in vasodilation of rabbit ear artery induced by a topically applied capsaicin analogue. J Vet Med Sci. 1998;60:691–697. doi: 10.1292/jvms.60.691. [DOI] [PubMed] [Google Scholar]
- Szallasi A, Blumberg PM. Vanilloid (capsaicin) receptors and mechanisms. Pharmacol Rev. 1999;51:159–212. [PubMed] [Google Scholar]
- Szolcsanyi J, Szallasi A, Szallasi Z, Joo F, Blumberg PM. Resiniferatoxin: an ultra potent modulator of capsaicin-sensitive primary afferens neurons. J Pharmacol Exp Ther. 1990;255:923–928. [PubMed] [Google Scholar]
- Toda N, Okamura T. The pharmacology of nitric oxide in the peripheral nervous system of blood vessels. Pharmacol Rev. 2003;55:271–324. doi: 10.1124/pr.55.2.3. [DOI] [PubMed] [Google Scholar]
- Undem BJ, Kollarik M. Characterization of the vanilloid 1 receptor antagonist iodo-resiniferatoxin on the afferent and efferent function of vagal sensory C-fibers. J Pharmacol Expther. 2002;303:716–722. doi: 10.1124/jpet.102.039727. [DOI] [PubMed] [Google Scholar]
- Van Der Stelt M, Di Marzo V. Endovanilloids. Eur J Biochem. 2004;271:1827–1834. doi: 10.1111/j.1432-1033.2004.04081.x. [DOI] [PubMed] [Google Scholar]
- Wahl P, Foged C, Tullin S, Thomsen C. Iodo-resiniferatoxin, a new potent vanilloid receptor antagonist. Mol Pharmacol. 2001;59:9–15. doi: 10.1124/mol.59.1.9. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Davis JB, Smart D, Jerman JC, Smith GD, Hayes P, Vriens J, Cairns W, Wissenbach U, Prenen J, Flockerzi V, Droogmans G, Benham CD, Nilius B. Activation of TRPV4 channels (hVRL-2/mTRP12) by phorbol derivatives. J Biol Chem. 2002a;277:13569–13577. doi: 10.1074/jbc.M200062200. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Vriens J, Prenen J, Droogmans G, Voets T, Nilius B. Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature. 2003;424:434–438. doi: 10.1038/nature01807. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Vriens J, Suh SH, Benham CD, Droogmans G, Nilius B. Heat evoked activation of TRPV4 channels in a HEK293 cell expression system and in native mouse aorta endothelial cells. J Biol Chem. 2002b;277:47044–47051. doi: 10.1074/jbc.M208277200. [DOI] [PubMed] [Google Scholar]
- White R, Ho WSV, Bottrill FE, Ford WR, Hiley CR. Mechanisms of anandamide-induced vasorelaxation in rat isolated coronary arteries. Br J Pharmacol. 2001;134:921–924. doi: 10.1038/sj.bjp.0704333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle BJR, Lopez-Belmonte J, Moncada S. Nitric oxide mediates rat mucosal vasodilation induced by intragastric capsaicin. Eur J Pharmacol. 1992;281:339–341. doi: 10.1016/0014-2999(92)90188-a. [DOI] [PubMed] [Google Scholar]
- Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sorgard M, Di Marzo V, Julius D, Hogestatt ED. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]