Abstract
We examined the effect of short-term exercise training on skeletal muscle AMP-activated protein kinase (AMPK) signalling and muscle metabolism during prolonged exercise in humans. Eight sedentary males completed 120 min of cycling at 66 ± 1% V˙O2peak, then exercise trained for 10 days, before repeating the exercise bout at the same absolute workload. Participants rested for 72 h before each trial while ingesting a high carbohydrate diet (HCHO). Exercise training significantly (P < 0.05) attenuated exercise-induced increases in skeletal muscle free AMP: ATP ratio and glucose disposal and increased fat oxidation. Exercise training abolished the 9-fold increase in AMPK α2 activity observed during pretraining exercise. Since training increased muscle glycogen content by 93 ± 12% (P < 0.01), we conducted a second experiment in seven sedentary male participants where muscle glycogen content was essentially matched pre- and post-training by exercise and a low CHO diet (LCHO; post-training muscle glycogen 52 ± 7% less than in HCHO, P < 0.001). Despite the difference in muscle glycogen levels in the two studies we obtained very similar results. In both studies the increase in ACCβ Ser221 phosphorylation was reduced during exercise after training. In conclusion, there is little activation of AMPK signalling during prolonged exercise following short-term exercise training suggesting that other factors are important in the regulation of glucose disposal and fat oxidation under these circumstances. It appears that muscle glycogen is not an important regulator of AMPK activation during exercise in humans when exercise is begun with normal or high muscle glycogen levels.
AMP-activated protein kinase (AMPK) is considered an important regulator of basal energy metabolism in the mammalian cell (Winder, 2001; Kemp et al. 2003). AMPK is an αβγ heterotrimer with the α subunit containing the catalytic domain with Thr172 in the activation loop. Both AMPK α1 and α2 are expressed in skeletal muscle and are activated by phosphorylation at Thr172 by the AMPK kinase (AMPKK) LKB1 (Hawley et al. 2003; Woods et al. 2003; Sakamoto et al. 2004; Sakamoto et al. 2005). Once phosphorylated, AMPK can be further activated allosterically by increases in the AMP: ATP ratio (Hardie & Carling, 1997; Sakamoto et al. 2004; Sakamoto et al. 2005). Several lines of evidence suggest that AMPK α2 is the more important isoform in terms of regulation of skeletal muscle metabolism during exercise (Jorgensen et al. 2004). AMPK α2 is activated primarily during exercise at or above ∼60% V˙O2peak (Fujii et al. 2000; Wojtaszewski et al. 2000; Stephens et al. 2002; Chen et al. 2003) whilst AMPK α1 is generally less responsive (Fujii et al. 2000; Wojtaszewski et al. 2000; Stephens et al. 2002; Chen et al. 2003), being substantially activated during maximal sprint-type exercise in humans (Chen et al. 2000), or during intense electrical stimulation in vitro in rat muscle (Hayashi et al. 2000).
It has been suggested that both skeletal muscle glucose uptake and fat oxidation are regulated by AMPK during exercise (Winder, 2001). This is largely based on studies using the nucleoside intermediate 5′aminoimidazole-4-carboxyamide-ribonucleoside (AICAR). AICAR activates AMPK in rat skeletal muscle and increases both glucose uptake and fat oxidation (Merrill et al. 1997; Hayashi et al. 1998; Bergeron et al. 1999; Winder & Holmes, 2000; Raney et al. 2005). However, dissociations between AMPK activation and glucose uptake and fat oxidation have been observed during low intensity contractions in the perfused rat hindlimb (Raney et al. 2005). In addition, skeletal muscle glucose uptake and fat oxidation increase in humans during low intensity exercise even though no increases in skeletal muscle AMPK activity are observed (Wojtaszewski et al. 2002).
Exercise training for as little as 10 days (Mendenhall et al. 1994) increases fat oxidation and reduces glucose clearance during exercise at the same absolute workload (Coggan et al. 1990). There is also an attenuated increase in skeletal muscle free AMP (AMPf) content during exercise after short-term exercise training (Green et al. 1991, 1992; Chesley et al. 1996) which would be expected to diminish the extent of activation of AMPK during exercise. Indeed, in rats there is reduced skeletal muscle AMPK activation during treadmill exercise after exercise training (Hutber et al. 1997; Durante et al. 2002). However, although there were some indications of reduced AMPK signalling, AMPK activity increases similarly during relatively intense exercise (80% V˙O2max) in trained compared with untrained humans (Nielsen et al. 2003). The effect of short-term exercise training on the relationship between AMPK activity, glucose disposal and fat oxidation during exercise has not previously been examined.
Therefore, our aim was to determine the effect of short-term (10 days) exercise training on AMPK signalling and substrate metabolism during prolonged moderate-intensity exercise in sedentary individuals. We hypothesized that during exercise at the same absolute workload the increases in AMPK activity and glucose disposal would be attenuated after training and that the fat oxidation would be higher. To our surprise the ∼8-fold activation of AMPK α2 during exercise before training was abolished after training. Since the pre-exercise muscle glycogen levels were almost double after exercise training, and high muscle glycogen content may attenuate the activation of AMPK during exercise (Derave et al. 2000; Wojtaszewski et al. 2003), we conducted a second series of experiments in different participants where the pre-exercise muscle glycogen content was manipulated to be normal after the exercise training. Despite this manipulation of muscle glycogen we obtained essentially the same results. In both series of experiments, although glucose disposal was reduced during exercise after short-term training it was still substantial when compared to rest, whilst fat oxidation was higher during exercise after training than before training. These results suggest that AMPK is not a critical regulator of skeletal muscle glucose uptake and fat oxidation during exercise in humans and also that muscle glycogen content may not be an important regulator of AMPK activation during exercise.
Methods
Participants
Fifteen healthy, untrained, non-smoker males provided informed written consent to participate in this study, which was approved by the Monash University Standing Committee on Ethics in Research involving Humans and the Human Research Ethics Committee of The University of Melbourne and conducted in accordance with the Declaration of Helsinki. AMPK activity, glucose kinetics and ACCβ phosphorylation were obtained from only 12 participants while muscle metabolites (e.g. muscle glycogen) were conducted on 14 participants since there was insufficient muscle available for metabolite analysis in one participant.
Experimental procedures
Experimental design overview (explained in detail in following sections)
In brief, participants performed a peak pulmonary oxygen consumption test during cycling (V˙O2peak) then 5–7 days later completed the first experimental trial (pre-training) which involved cycling for 120 min or to exhaustion (if unable to complete 120 min) at ∼65% V˙O2peak. Participants then exercise trained for 10 days (10 training sessions over 2 week), before completing a second experimental trial (post-training) which involved cycling for the same amount of time and at the same absolute workload as the first experimental trial. A V˙O2peak test was again conducted as part of the 10th training session. Laboratory conditions were maintained at 20–23°C for all exercise sessions. In the first set of experiments (high carbohydrate; HCHO, n = 8), participants rested for 72 h before each experimental trial while ingesting a high CHO diet. We also performed a second set of experiments (low CHO; LCHO) in seven additional participants where as part of their last training session they completed further exercise designed to deplete muscle glycogen stores and limit muscle glycogen resynthesis prior to the second experimental trial. They also consumed a very low CHO diet as outlined below.
Preliminary exercise testing
V˙O2peak was determined using a graded exercise test to volitional exhaustion on a cycle ergometer (Lode, Gronignen, the Netherlands) as previously described (Chen et al. 2003). The results from the V˙O2peak test were used to determine power outputs that corresponded to 65%, 75% and 90–100% of each individual's V˙O2peak. Participants returned to the laboratory for a 10 min ride at a workload calculated to be ∼65% V˙O2peak to confirm the power output for the experimental trials.
Dietary and exercise control
In the HCHO group participants were instructed to refrain from any formal exercise in the 72 h prior to the experimental trials. During the study period the participants' only formal exercise was that of the prescribed supervised exercise training schedule. To ensure sufficient quantity of carbohydrate in their diet, HCHO participants were provided with a commercially available soft drink to supplement their normal diet prior to the trials (2.5 l; 250 g of carbohydrate over 2 days). On the day prior to the first experimental trial participants were instructed to record all food and fluids consumed and to refrain from ingesting caffeine and alcohol. Participants were given a photocopy of this dietary record and asked to consume the same diet before the second experimental trial.
In the second set of experiments (low carbohydrate, LCHO group, n = 7), participants were given a diet very low in CHO (9.6 ± 0.6% CHO; 1.2 ± 0.1 g CHO (kg body weight)−1; 62.3 ± 1.3% fat, 28.1 ± 1.0% protein; 13.8 ± 0.3 MJ) over the 48 h prior to both experimental trials in order to lower post-training muscle glycogen levels compared with the HCHO experiments. In addition, 20 min following the V˙O2peak test on training day 10, LCHO participants cycled for 30 min at 70% V˙O2peak, then completed 30 min of arm ergometry at 25 W, followed by four to five 30 s cycling sprints which were separated by 3 min of rest. This V˙O2peak test and muscle glycogen depletion protocol was performed 24–48 h before the post-training experimental trial (24 h: n = 3, 48 h: n = 4).
Experimental trials
In the pre- and post-training experimental trials of both series of experiments (HCHO and LCHO), participants fasted from 23.00 h then reported to the laboratory at either 07.00 h or 08.00 h and commenced exercise at either 10.00 h or 11.00 h (same time for both experimental trials).
A catheter was inserted into an antecubital vein for the infusion of the non-radioactive glucose tracer [6,6-2H]glucose (Cambridge Isotope Laboratories, MA, USA) and another catheter inserted into the contralateral forearm for blood sampling. The blood-sampling catheter was kept patent with regular flushing with sterile saline. A bolus of 45.2 ± 0.2 μmol kg−1 (mean ±s.e.m.) of the glucose tracer was administered immediately prior to commencing a 120 min pre-exercise constant infusion (0.57 ± 0.02 μmol kg−1 min−1) which was continued for the duration of the exercise bout. Blood samples were obtained immediately prior to the commencement of the infusion and at 30 min, 10 min and immediately prior to the start of exercise. The exercise protocol in HCHO consisted of cycling for 120 min at a constant workload averaging 66 ± 1% of V˙O2peak (149 ± 12 W). In LCHO, participants cycled at a constant workload that required 67 ± 1% of V˙O2peak (125 ± 5 W) for 120 min or until exhaustion (mean: 108 ± 7 min).
Participants ingested 8 ml (kg b.w.)−1 of water just prior to exercise and 2 ml (kg b.w.)−1 every 15 min during exercise. Blood was sampled every 15 min during exercise for glucose kinetics. Expired air was sampled into Douglas bags for ∼15 min at rest, and for 3 min each 15 min of exercise. Heart rate was monitored throughout exercise using a heart rate monitor (Polar Favour, Oulu, Finland). Muscle was obtained from the vastus lateralis muscle under local anaesthesia using the percutaneous needle biopsy technique, with suction. Prior to commencing exercise three separate incisions were made approximately 1 cm apart then muscle was sampled at rest, after 30 min of cycling, and immediately following the completion of exercise. One leg was chosen at random for the muscle biopsies of the pretraining trial then the other leg used for the post-training trial. Samples were frozen in liquid nitrogen within 4–6 s of inserting the needle at rest and within 8–12 s of the participant stopping exercise.
Short-term exercise training regimen
The exercise training protocol employed involved 10 cycling bouts over 2 week and closely replicated the design of Mendenhall et al. (1994): 2 days per week of 45 min of cycling at ∼75% V˙O2peak (HCHO: 203 ± 13 W; LCHO: 167 ± 9 W); 1 day per week of 60–90 min at ∼75% V˙O2peak ; 2 days per week of 6 × 5 min work bouts at 90–100% of V˙O2peak (HCHO: 244 ± 14 W; LCHO: 208 ± 12 W) with 3 min active recovery at ∼40% V˙O2peak (Mendenhall et al. 1994). The final exercise training session involved a V˙O2peak test, followed, after a 30 min rest, by a further 30 min of cycling at 75% of V˙O2peak in HCHO. The LCHO group performed the same final training session as in HCHO but with the addition of interval cycling and arm-cranking, as described above.
Analytical techniques
Calculation of rates of whole body substrate utilization
Whole body rates of carbohydrate and fat oxidation (g min−1) were calculated from V˙CO2 and V˙O2 using non-protein respiratory exchange ratio (RER) values (Peronnet & Massicotte, 1991). The calorimetric data should be interpreted with some caution since protein oxidation may have been quite substantial during exercise in LCHO.
Blood analysis
Plasma glucose and lactate were determined using an automated glucose oxidase and l-lactate oxidase method, respectively (YSI 2300 Stat, Yellow Springs, OH, USA), Glucose kinetics were estimated, as previously described (Chen et al. 2003), using a modified one-pool, non-steady-state model (Steele et al. 1956) which has been validated (Radziuk et al. 1978). Rates of plasma glucose appearance (glucose Ra) and glucose disappearance (glucose Rd) were determined from the changes in percentage enrichment of [6,6-2H]glucose and plasma glucose concentration. Glucose clearance rate (glucose CR) was calculated by dividing the glucose Rd by the plasma glucose concentration. During exercise at ∼60% of V˙O2max, 90–95% of tracer-determined glucose Rd is oxidized in both the trained and untrained state (Coggan et al. 1990; Jeukendrup et al. 1999).
Muscle analysis
A portion (∼20 mg) of each muscle sample was freeze-dried then analysed for muscle glycogen, ATP, creatine phosphate, creatine and lactate as previously described (Chen et al. 2003). Free ADP and AMPf (μmol (kg d.m.)−1) were calculated as outlined previously (Chen et al. 2000). Free AMP was converted from μmol (kg d.m.)−1 to μm (where μm= (μmol (kg d.m.)−1/3.308) × 1.11) (Hultman & Sahlin, 1980).
AMPK α1 activity, AMPK α2 activity and ACCβ phosphorylation at Ser221 were measured as previously described (Chen et al. 2000, 2003) using ∼70 mg of each frozen muscle sample. The polyclonal antipeptide antibodies to α1 and α2 were raised to non-conserved regions of the AMPK isoforms, α1 (amino acid sequence 373–390 of rat AMPK α1) and α2 (351–366 and 490–516 of rat AMPK α2). For AMPK α2 Thr172 phosphorylation, AMPK α2 was immunoprecipitated, subjected to SDS-PAGE then immunoblotted for antiphospho-AMPK-Thr172 or anti-AMPK α2 and subsequently detected using horseradish peroxidase-conjugated protein G. Given that insufficient muscle biopsy material was available for analysis of AMPK-Thr172 in HCHO, only data for LCHO were obtained. Furthermore, due to technical difficulties AMPK-Thr172 was determined in only four LCHO participants.
Statistical analysis
The two trials in the two groups (HCHO and LCHO) were compared using two factor repeated measures analysis of variance (ANOVA) utilizing the statistical package SPSS. Time and training (pre-compared with post-training) were the within subjects factors, and group (HCHO and LCHO) was the between subjects factor. If the ANOVA was significant (P < 0.05), specific differences were located using Fisher's least significant difference (LSD) test. To compare the relationships between muscle glycogen concentrations and muscle AMPK α1 and AMPK α2 activities, the slope of regression lines for data from individual subjects across time (0, 30 and end) both pre- and post-training was determined using the least squares method. The means of these derived slopes were then compared to zero using a t test.
Results
Participant characteristics, pulmonary gas measures and plasma lactate concentration
As shown in Table 1, the only difference observed between the HCHO and LCHO groups before exercise training was for V˙O2peak (absolute). However, this appeared largely to be due to differences in body weight as there were no significant differences when V˙O2 was expressed in ml kg−1 min−1 (P= 0.14). The short-term training protocol significantly increased V˙O2peak in HCHO (5 ± 1%, P < 0.05), but not LCHO participants (5 ± 2%, P= 0.06). There was no significant change in body weight after training in HCHO, but a small but significant increase in body weight was observed in LCHO (P < 0.05).
Table 1.
Participant characteristics pre- and post-10-days of exercise training
| Parameter | Trial | HCHO | LCHO |
|---|---|---|---|
| Age (years) | Pre | 23 ± 2 | 22 ± 1 |
| Post | — | — | |
| Weight (kg) | Pre | 82.4 ± 4.9 | 73.8 ± 2.3 |
| Post | 82.7 ± 4.6 | 74.3 ± 2.3 * | |
| Height (m) | Pre | 1.85 ± 0.03 | 1.79 ± 0.01 |
| Post | — | — | |
| BMI | Pre | 24.8 ± 0.7 | 22.9 ± 0.6 |
| Post | 24.9 ± 0.7 | 23.1 ± 0.5 | |
| V˙O2 (l min−1) | Pre | 3.78 ± 0.18 | 3.15 ± 0.10‡ |
| Post | 4.00 ± 0.17* | 3.22 ± 0.13‡ | |
| V˙E (ml kg−1 min−1) | Pre | 46.4 ± 1.9 | 42.8 ± 1.1 |
| Post | 48.8 ± 1.8* | 43.5 ± 1.3‡ |
Values are means ±s.e.m. for n = 8 (HCHO) and n = 7 (LCHO). Pre, before training; Post, after training; BMI, body mass index; V˙O2, oxygen consumption; HCHO, high carbohydrate diet; LCHO, low carbohydrate diet.
Significantly different from corresponding pre-training value, P < 0.05;
significantly different from HCHO group, P < 0.05.
V˙O2 during exercise averaged 66 ± 1% and 67 ± 1% (HCHO and LCHO, respectively) of pretraining V˙O2peak before training, and was significantly lower (P < 0.05) after training (HCHO = 63 ± 1% and LCHO = 65 ± 1% of pretraining V˙O2peak). The post-training exercise V˙O2 equated to 60 ± 1% (HCHO) and 63 ± 3% (LCHO) of post-training V˙O2peak. Table 2 summarizes the pulmonary gas measures, estimated rates of substrate oxidation and plasma lactate concentrations during the HCHO and LCHO experimental trials. After exercise training, ventilation, RER and CHO oxidation during exercise were all significantly lower, whilst fat oxidation was significantly higher in both HCHO and LCHO compared with during exercise before training. Plasma lactate concentration was higher during exercise before compared with after short-term exercise training in both HCHO and LCHO.
Table 2.
Respiratory gas exchange measures, calculated substrate oxidation and plasma lactate concentration at rest and during exercise (average for the exercise bout) at 66 ± 1% (HCHO) and 67 ± 1% (LCHO) of pre-training V˙O2peak conducted pre- and post-10-days of exercise training
| HCHO | LCHO | ||||
|---|---|---|---|---|---|
| Parameter | Trial | Rest | Exercise | Rest | Exercise |
| V˙O2 (l min−1) | Pre | 0.32 ± 0.01 | 2.47 ± 0.15# | 0.33 ± 0.02 | 2.13 ± 0.08# |
| Post | 0.33 ± 0.02 | 2.36 ± 0.15# | 0.31 ± 0.03 | 2.05 ± 0.08# | |
| RER | Pre | 0.86 ± 0.01 | 0.96 ± 0.01# | 0.82 ± 0.03 | 0.92 ± 0.01‡ |
| Post | 0.87 ± 0.02 | 0.93 ± 0.01*# | 0.79 ± 0.01‡ | 0.87 ± 0.01*#‡ | |
| V˙E (l min−1) | Pre | 8.6 ± 0.5 | 55.1 ± 4.7# | 9.8 ± 0.7 | 53.7 ± 3.7# |
| Post | 9.8 ± 0.7 | 48.1 ± 3.7*# | 8.0 ± 0.7 | 45.5 ± 3.2*# | |
| CHOox (μmol kg−1 min−1) | Pre | 16 ± 2 | 197 ± 13# | 13 ± 3 | 158 ± 8 #‡ |
| Post | 18 ± 2 | 166 ± 12*# | 10 ± 2‡ | 117 ± 9 *#‡ | |
| Fatox (μmol kg−1 min−1) | Pre | 3.1 ± 0.3 | 7.5 ± 1.1# | 4.9 ± 0.9 | 13.5 ± 1.8 #‡ |
| Post | 3.0 ± 0.4 | 11.3 ± 1.1*# | 5.0 ± 0.7 | 20.9 ± 1.8 *#‡ | |
| Plasma lactate (mmol l−1) | Pre | 1.4 ± 0.3 | 3.2 ± 0.5# | 0.9 ± 0.0 | 3.6 ± 0.3 # |
| Post | 1.3 ± 0.3 | 2.5 ± 0.5*# | 1.0 ± 0.1 | 2.4 ± 0.3*# | |
Values are means ±s.e.m. for n = 8 (HCHO) and n = 7 (LCHO). Pre, before training; Post, after training; V˙O2, oxygen consumption; RER, respiratory exchange ratio; V˙E, ventilation; CHOox, carbohydrate oxidation; Fatox, fat oxidation; HCHO, high carbohydrate diet; LCHO, low carbohydrate diet.
Significantly different from corresponding pre-training value, P < 0.05;
significantly different from rest value, P < 0.05;
significantly different from corresponding HCHO, P < 0.05.
Glucose kinetics
In the HCHO group, plasma glucose concentration decreased (P < 0.05) during exercise before training and was better maintained (P < 0.05) during exercise after training (Table 3). Glucose Ra, glucose Rd and glucose CR increased (P < 0.05) throughout the exercise bout in both HCHO trials, with these increases during exercise being attenuated (P < 0.05) post-training (Table 3). In the LCHO group, plasma glucose concentration did not change from rest during exercise in either trial (Table 3). Glucose Ra, glucose Rd, and glucose CR increased throughout exercise in both trials, with glucose Ra and glucose Rd significantly attenuated during exercise post-training (P < 0.05). Pre- and post-training rest and exercise plasma glucose concentrations were significantly higher in LCHO than HCHO (P < 0.05).
Table 3.
Plasma glucose concentration, rate of glucose appearance (glucose Ra), rate of glucose disappearance (glucose Rd), and glucose clearance rate (glucose CR) before and during exercise (average for the exercise bout) at 66 ± 1% (HCHO) and 67 ± 1% (LCHO) of pre-training V˙O2peak conducted pre- and post-10-days of exercise training
| HCHO | LCHO | ||||
|---|---|---|---|---|---|
| Parameter | Trial | Rest | Exercise | Rest | Exercise |
| Glucose (mmol l−1) | Pre | 4.8 ± 0.1 | 4.4 ± 0.1# | 5.2 ± 0.1‡ | 5.2 ± 0.2‡ |
| Post | 4.9 ± 0.2 | 4.7 ± 0.2* | 5.3 ± 0.1‡ | 5.1 ± 0.2‡ | |
| Glucose Ra(μmol kg−1 min−1) | Pre | 10.9 ± 1.3 | 30.1 ± 3.3# | 14.4 ± 2.5 | 33.5 ± 1.2# |
| Post | 11.7 ± 1.3 | 26.8 ± 3.7*# | 14.4 ± 1.5 | 31.5 ± 1.5*# | |
| Glucose Rd(μmol kg−1 min−1) | Pre | 10.6 ± 1.4 | 31.0 ± 3.5# | 13.7 ± 2.3 | 34.8 ± 1.4# |
| Post | 11.7 ± 1.3 | 27.1 ± 3.8*# | 14.6 ± 1.5 | 31.9 ± 1.6*# | |
| Glucose CR(ml kg−1 min−1) | Pre | 2.3 ± 0.3 | 7.4 ± 0.9# | 2.8 ± 0.5 | 6.8 ± 0.3# |
| Post | 2.5 ± 0.3 | 6.0 ± 1.0*# | 2.9 ± 0.3 | 6.5 ± 0.4# | |
Data are means ±s.e.m., n = 5 for HCHO and n = 7 for LCHO; Pre, before training; Post, after training; HCHO, high carbohydrate diet; LCHO, low carbohydrate diet.
Significantly different from corresponding pre-training value, P < 0.05;
significant different from rest value, P < 0.05;
significantly different from corresponding HCHO, P < 0.05.
Muscle metabolites
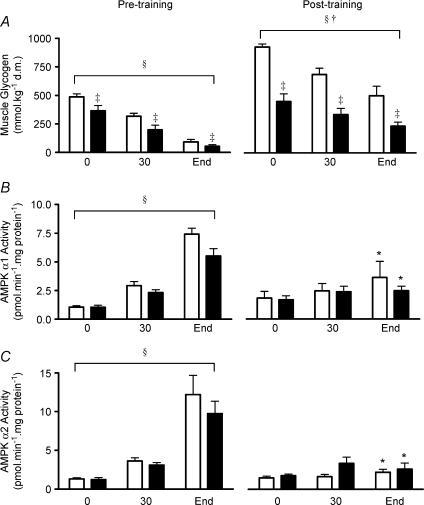
Muscle glycogen levels at rest were substantially higher after exercise training in HCHO than LCHO (927 ± 28 versus 450 ± 68 mmol (kg d.m.)−1, respectively; P < 0.01; Fig. 1A). Exercise training was associated with an increase in pre-exercise muscle glycogen levels in both groups (P < 0.01 for HCHO and P < 0.05 for LCHO). Muscle glycogen levels at the end of exercise after training were significantly lower in LCHO compared with HCHO (500 ± 85 versus 235 ± 34 mmol (kg d.m.)−1 for HCHO and LCHO, respectively). Post-training muscle glycogen levels at rest in LCHO were not significantly different from pretraining muscle glycogen levels at rest in HCHO. An unpaired t test revealed that the decrease in muscle glycogen content from rest to the end of exercise was greater in HCHO post-training compared with LCHO post-training (P= 0.046) and was also greater in HCHO pre-training compared with LCHO post-training (P= 0.02). There was no significant difference (unpaired t test) in the net muscle glycogen use between HCHO pre-training versus HCHO post-training (P= 0.70), LCHO pre-training versus LCHO post-training (P= 0.24) or HCHO pre-training versus LCHO pre-training (P= 0.10).
Figure 1. Muscle glycogen content (A), AMPK α1 activity (B), and AMPK α2 activity (C) at rest (0 min), after 30 min, and at the completion of exercise (End) at 66 ± 1% (HCHO □) and 67 ± 1% (LCHO ▪) of pretraining V˙O2peak conducted pre- and post-10-days of exercise training.
Data are means ±s.e.m. For muscle glycogen, n = 7 (HCHO and LCHO); for AMPK activity n = 5 (HCHO) and n = 7 (LCHO). *Significantly different from corresponding pre-training value, P < 0.001; §significant time effect (in both HCHO and LCHO), P < 0.01; †significant trial effect (pre-training versus post-training in both HCHO and LCHO), P < 0.01; ‡significantly different from HCHO, P < 0.001.
Muscle Cr, ADPf, AMPf and the AMPf: ATP ratio all progressively increased, whilst PCr progressively decreased, during exercise in both groups during both the pre- and post-exercise training trials (P < 0.05; Table 4). The increases in muscle Cr, ADPf, AMPf and the AMPf: ATP ratio, and the decrease in PCr during exercise were significantly attenuated after exercise training in both groups (P < 0.05; Table 4). Muscle lactate levels also increased significantly in both trials in both groups with the increase attenuated after exercise training (P < 0.05; Table 4). Muscle ATP content for HCHO and LCHO was unaffected by exercise in both exercise trials (Table 4), but ATP was significantly lower after short-term training.
Table 4.
Measured and calculated muscle metabolites at rest (0 min), 30 min and after the cessation of exercise (End) at 66 ± 1% (HCHO) and 67 ± 1% (LCHO) of pre-training V˙O2peak conducted pre- and post-10-days of exercise training
| HCHO | LCHO | ||||||
|---|---|---|---|---|---|---|---|
| Metabolite | Trial | 0 min | 30 min | End | 0 min | 30 min | End |
| Lactate (mmol (kg d.m.)−1) | Pre | 2.3 ± 0.1 | 17.0 ± 5.7# | 6.8 ± 1.3 # | 4.2 ± 0.7 | 25.4 ± 6.5# | 10.8 ± 3.0# |
| Post | 2.2 ± 0.3 | 6.9 ± 2.3*# | 6.2 ± 0.9*# | 1.8 ± 0.3 | 11.6 ± 5.9*# | 5.5 ± 0.9*# | |
| PCr (mmol (kg d.m.)−1) | Pre | 95.9 ± 3.9 | 54.1 ± 4.5# | 40.4 ± 2.6# | 85.7 ± 3.3 | 43.6 ± 6.3# | 33.6 ± 5.8# |
| Post | 104.5 ± 2.8 | 83.8 ± 7.4*# | 71.8 ± 6.3*# | 87.1 ± 3.3 | 58.0 ± 8.1*# | 62.1 ± 5.2*# | |
| Cr (mmol (kg d.m.)−1) | Pre | 46.2 ± 0.5 | 88.9 ± 5.7# | 102.7 ± 4.6# | 50.5 ± 4.2 | 92.5 ± 10.4# | 102.6 ± 7.0# |
| Post | 46.5 ± 2.8 | 67.3 ± 5.4*# | 79.2 ± 7.7*# | 51.2 ± 3.3 | 78.8 ± 9.9*# | 76.2 ± 6.6*# | |
| ATP (mmol (kg d.m.)−1) | Pre | 26.6 ± 0.6 | 26.0 ± 0.6 | 26.0 ± 1.5 | 25.5 ± 1.9 | 22.9 ± 2.3 | 25.1 ± 3.5 |
| Post | 25.1 ± 1.5* | 24.6 ± 0.5* | 26.8 ± 1.2 | 20.3 ± 1.7* | 20.8 ± 1.7* | 19.8 ± 1.4* | |
| ADPf (μmol (kg d.m.)−1) | Pre | 130 ± 6 | 365 ± 30# | 645 ± 59# | 149 ± 19 | 377 ± 49# | 775 ± 114# |
| Post | 116 ± 13 | 200 ± 29*# | 318 ± 67*# | 122 ± 17 | 278 ± 50*# | 243 ± 32*# | |
| AMPf (μmol (kg d.m.)−1) | Pre | 0.7 ± 0.1 | 5.5 ± 0.8# | 17.3 ± 2.3# | 1.0 ± 0.2 | 7.6 ± 2.1# | 33.9 ± 11.4# |
| Post | 0.6 ± 0.1 | 2.0 ± 0.7*# | 4.9 ± 2.0*# | 0.8 ± 0.2 | 4.9 ± 2.0*# | 3.8 ± 1.4*# | |
| AMPf: ATP ratio | Pre | 0.03 ± 0.00 | 0.21 ± 0.03# | 0.67 ± 0.08# | 0.04 ± 0.01 | 0.38 ± 0.13# | 1.72 ± 0.64# |
| Post | 0.02 ± 0.00 | 0.08 ± 0.03*# | 0.18 ± 0.08*# | 0.04 ± 0.01 | 0.26 ± 0.12*# | 0.22 ± 0.10*# | |
Values are means ±s.e.m. for n = 7 (HCHO and LCHO). Pre, before training; Post, after training; PCr, creatine phosphate; Cr, creatine; ATP, adenosine triphosphate; ADPf, free adenosine diphosphate; AMPf, free adenosine monophosphate; d.m, dry muscle.
Significantly different from corresponding pre-training value, P < 0.05;
significantly different from rest value, P < 0.05.
AMPK activity
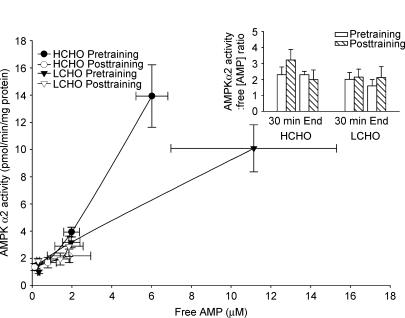
Skeletal muscle AMPK α1 and α2 activity increased progressively during exercise before training in both HCHO and LCHO. These increases in AMPK α1 and α2 activity during exercise were abolished after short-term training with both AMPK α1 and α2 activity being significantly lower at the end of exercise post-training compared with pre-training in both groups (P < 0.05, Fig. 1B,C). Despite the participants in LCHO exercising at a lower absolute workload (LCHO: 125 ± 5 W; HCHO: 149 ± 12 W) and for a shorter exercise time (LCHO: 108 ± 7 min; HCHO: 120 ± 0 min) than HCHO, AMPK signalling during exercise was close to identical in the two groups. Before exercise training, the mean slope of the regression between muscle glycogen and AMPK α1 activity was −0.016 ± 0.002 (n = 11), and for glycogen and AMPK α2 activity, it was −0.032 ± 0.006 (n = 11). In both cases the slopes were significantly different from zero (P < 0.001) indicating a significant relationship between the parameters. After exercise training, the mean slopes of the regression lines were not different from zero, being −0.006 ± 0.004 for glycogen and AMPK α1, and 0.003 ± 0.004 for glycogen and AMPK α2. These data suggest that the previously reported relationship between muscle glycogen concentrations and AMPK activity is lost following training The relationship between AMPK α2 activity and AMPf (μm) and the AMPK α2 activity: AMPf ratio (arbitrary units, inset) in HCHO and LCHO both pre- and post-training is shown in Fig. 2. Note that in Fig. 2 data were only used when there was both AMPK α2 activity and AMPf for a given participant, therefore n = 7 for LCHO and n = 4 for HCHO since there was no AMPf measurement for one participant in HCHO. The AMPK α2 activity: AMPf ratio was not significantly different between training status (pre- versus post-training), group (HCHO and LCHO) or exercise time (30 min and end). Therefore in the present study the relationship between skeletal muscle AMPf content and AMPK α2 activity during exercise was not affected by training status, muscle glycogen content or exercise time. These results are consistent with AMPf concentrations being the primary determinant of AMPK activity during exercise.
Figure 2. Relationship between skeletal muscle AMPK α2 activity and AMPf (μM) at rest, after 30 min and at the completion of exercise at 66 ± 1% (HCHO) and 67 ± 1% (LCHO) of pretraining V˙O2peak conducted pre- and post-10-days of exercise training.
Inset shows ratio of AMPK α2 activity (pmol min−1 (mg protein)−1) to AMPf (μM) after 30 min of exercise and at the completion of exercise at 66 ± 1% (HCHO) and 67 ± 1% (LCHO) of pre-training V˙O2peak conducted pre- and post-10-days of exercise training. n = 7 for LCHO and n = 4 for HCHO since there was no AMPf measurement for one participant in HCHO.
ACCβ phosphorylation and AMPK α2 Thr-172 phosphorylation
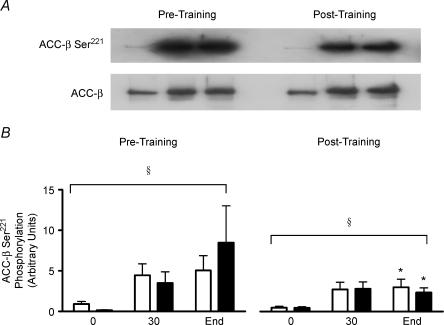
In both the HCHO and LCHO groups, ACCβ phosphorylation increased significantly (P < 0.05) during exercise both before and after short-term exercise training (Fig. 3B). However, the increase in ACCβ phosphorylation during exercise was significantly (P < 0.05) attenuated after exercise training in both groups. The content of muscle ACCβ was unaffected by exercise and exercise training in both groups (data not shown).
Figure 3. ACC-β Ser221 phosphorylation at rest (0 min), after 30 min and at the completion of exercise (End) at 66 ± 1% (HCHO □) and 67 ± 1% (LCHO ▪) of pretraining V˙O2peak conducted pre- and post-10-days of exercise training.
Affinity purified muscle ACCβ were immunoblotted with phospho-peptide antibody ACC-β-Ser221 and horseradish peroxidase-conjugated Streptavidin. A, representative immunoblot. B, mean quantitative densitometric data. Data are means ±s.e.m.n = 5 (HCHO) and n = 7 (LCHO), except for 0 where n = 6 for LCHO. *Significantly different from corresponding pre-training value, P < 0.05; §significant time effect (in both HCHO and LCHO), P < 0.01.
In LCHO, Thr172 phosphorylation of AMPK α2 progressively increased (P < 0.05) during exercise before training (11-fold increase at the end of exercise, data not shown). After exercise training this increase in Thr172 phosphorylation during exercise was abolished (P < 0.05) and at the end of exercise Thr172 phosphorylation was significantly lower post- compared with pre-exercise training.
Discussion
The results of the present study indicate a clear dissociation between skeletal muscle AMPK activity and glucose Rd during exercise in humans. The increase in glucose disposal during exercise was significantly attenuated after short-term training (Table 3) but was still very substantial despite there being no increase in skeletal muscle AMPK activity during exercise after training (Fig. 1). Along similar lines it has been shown in humans that glucose uptake increases during low intensity exercise without an increase in skeletal muscle AMPK activity (Wojtaszewski et al. 2002). Studies in AMPK dominant negative mice (Mu et al. 2001), AMPK knock-out mice (Jorgensen et al. 2004) and LKB1 knock-out mice (Sakamoto et al. 2005) indicate a substantial AMPK-independent component to contraction-stimulated glucose uptake. There may be fibre type considerations since in rat fast muscle AMPK and calcium–calmodulin-dependent protein kinase (CaMK) are equally involved in the regulation of glucose uptake (Wright et al. 2004) but in slow muscle AMPK is not involved at all (Derave et al. 2000; Wright et al. 2005).
Based on the genetically modified mice studies, it is possible that AMPK plays a partial role in the regulation of glucose uptake during exercise in humans such that the reduction in glucose Rd during exercise after training associated with lack of AMPK activation could be viewed as representing the role of AMPK in contraction-stimulated glucose uptake and consequently that AMPK is mainly important in untrained muscles. It is also possible that AMPK plays a greater role in skeletal muscle glucose uptake during more intense exercise.
AMPK activation has been implicated in the increase in fat oxidation that occurs during submaximal exercise via the phosphorylation and inactivation of ACCβ and the resultant lowering of malonyl CoA levels (Winder & Hardie, 1996; Vavvas et al. 1997; Park et al. 2002). However, in both experimental series of the current study whole body rates of fat oxidation during exercise were not closely associated with AMPK activation or ACCβ phosphorylation during exercise since fat oxidation was higher during exercise after exercise training (Table 2) while AMPK activation and ACCβ phosphorylation were substantially lower (Figs 1B and C and 3B). In addition, dissociations between AMPK activity, ACCβ phosphorylation and fat oxidation have been observed with increases in exercise intensity in humans (Chen et al. 2003) and during prolonged low intensity (45% V˙O2max) exercise in humans (Wojtaszewski et al. 2002). Finally, skeletal muscle fat oxidation increases during low intensity contractions in the perfused rat hindlimb without any increase in AMPK activity or decrease in ACC activity (Raney et al. 2005).
We found that the ∼10-fold increase in AMPK α2 activity observed during prolonged exercise before training was abolished during exercise at the same absolute workload following short-term exercise training in previously sedentary individuals (Fig. 1C). This was the case whether the post-training exercise was begun with either high (HCHO group) or normal (LCHO group) muscle glycogen levels (Fig. 1A). We were surprised by this finding since exercise training in rats attenuates, but does not abolish, the activation of skeletal muscle AMPK during treadmill exercise (Durante et al. 2002). In addition, AMPK α2 activity increases similarly during 20 min of exercise at 80% V˙O2max in endurance-trained athletes compared with untrained individuals, although there is an attenuation of the exercise-induced increases in AMPK Thr172 phosphorylation and ACCβ phosphorylation in the endurance-trained participants (Nielsen et al. 2003). It is not likely that substantial activation of AMPK would have occurred if we had examined exercise at the same relative exercise intensity after the short-term exercise training since in the LCHO group there was no significant increase in V˙O2peak after the exercise training.
As was expected (Green et al. 1992; Chesley et al. 1996) the short-term exercise training programme attenuated the increase in skeletal muscle AMPf: ATP ratio and the decrease in PCr during exercise (Table 4). This lessened muscle energy imbalance is likely to have accounted, at least in part, for the lack of increase in AMPK α2 activity during exercise after training. Indeed, the ratio between muscle AMPK α2 activity and muscle AMPf during exercise (Fig. 2) was similar in HCHO and LCHO both before and after exercise training.
It is possible though that factors in addition to the AMPf content play a role in the activation of AMPK during exercise since we have previously (Chen et al. 2003) observed activation of AMPK α2 during exercise at ∼60% V˙O2peak with similar levels of AMPf as we measured after training in the present study (at 60–63% of post-training V˙O2peak). Indeed, in the present study the response of AMPK α2 to a given level of AMPf during exercise before short-term training differed substantially in HCHO compared with LCHO (Fig. 2). In addition, the calculated concentration of AMPf at the end of exercise post-training was 1.9 μm in HCHO and 1.3 μm in LCHO (Fig. 2) and since the concentration of cytosolic AMP necessary to cause half-maximal activation of AMPK in isolated perfused rat heart is approximately 1.8 μm (Frederich & Balschi, 2002), one would have expected some activation of AMPK during exercise after training.
Some studies suggest that the muscle glycogen content influences the degree of AMPK activation during contractions, with high pre-exercise muscle glycogen levels attenuating the extent of AMPK activation. Skeletal muscle AMPK activity increases less during exercise in humans (Wojtaszewski et al. 2003) and during contractions in perfused rat soleus muscles (Derave et al. 2000) when begun with high muscle glycogen levels compared with low muscle glycogen levels. However, after exercise training in the present study there was no relationship between AMPK activation during exercise and muscle glycogen content over a wide range of muscle glycogen levels (Fig. 1). The resting levels of muscle glycogen in HCHO pretraining were not different from LCHO post-training (Fig. 1A), but AMPK increased ∼8-fold during exercise in HCHO pretraining while there was no increase in AMPK activity during exercise in LCHO post-training (Fig. 1B and C). These results suggest that muscle glycogen does not regulate AMPK activation during exercise in humans. In support of this, although the β subunit of AMPK targets AMPK to glycogen via its glycogen binding domain (Hudson et al. 2003; Polekhina et al. 2003), addition of glycogen to purified rat liver AMPK does not directly alter AMPK activity (Polekhina et al. 2003).
The α2 adrenergic agonist phenylephrine increases AMPK α2 activity in mouse soleus muscle in vitro (Minokoshi et al. 2002). Therefore, it cannot be ruled out that attenuated increases in plasma catecholamines during exercise after short-term exercise training (Mendenhall et al. 1994) contributed to the reduced AMPK activation during exercise. However, we recently found dissociations between plasma catecholamine concentrations and AMPK activity during exercise in humans in hypoxic conditions compared with the same absolute and relative workload under normoxic conditions (Wadley et al. unpublished observations).
Surprisingly, a substantial increase in skeletal muscle AMPK α1 activity was observed during prolonged exercise before exercise training in both series of experiments (Fig. 1B). Although we (Chen et al. 2003) and others (Roepstorff et al. 2004) have found an approximate doubling of AMPK α1 activity during exercise at 60–65% V˙O2peak in humans, other studies (Fujii et al. 2000; Wojtaszewski et al. 2000; Wojtaszewski et al. 2002; Nielsen et al. 2003; Wojtaszewski et al. 2003), including one of our own (Stephens et al. 2002), have found no suggestion of an increase in AMPK α1 activity during submaximal exercise (50–80% V˙O2peak) in humans. The reason for the much larger increase in AMPK α1 activity during exercise in the present study is unclear but may be related to the use of sedentary participants in this study. It is likely that the sedentary individuals were particularly sensitive to activation of AMPK α1 due to their low fitness levels and the associated large increases in muscle energy imbalance during the prolonged exercise (Table 4). Most previous studies did not specifically utilize sedentary participants. There was no significant increase in AMPK α1 activity during exercise after the exercise training in the present study, as was the case with AMPK α2 activity (Fig. 1B).
In conclusion, short-term exercise training in humans abolished the ∼10-fold increase in AMPK activity observed during prolonged exercise before training. Since glucose Rd during exercise was only partially suppressed after exercise training and fat oxidation during exercise was higher after exercise training these results suggest that AMPK activation is not critical to the regulation of these processes, particularly following short-term exercise training. In addition, there was little relationship between muscle glycogen content and the degree of activation of AMPK during exercise after exercise training. These findings place in doubt the importance of AMPK in the regulation of skeletal muscle metabolism during exercise and also suggest that muscle glycogen may not be an important regulator of AMPK activation during exercise in humans.
Acknowledgments
The authors would like to thank the participants for taking part in this study and also Dr Rodney Snow, Dr Domenic Caredi, Dr Alistair Simpson, Dr Katharina Beyer, Dr Glenn Wadley and Dr Chanchira Wasuntarawat for their technical assistance and David Caddy for his statistical assistance. This work was supported by grants from the National Health and Medical Research Council (NHMRC) of Australia (G.K.M. and B.E.K.), Diabetes Australia (G.K.M.), the Australian Research Council (ARC; B.E.K.) and the National Heart Foundation of Australia (B.E.K.). B.E.K. is an ARC Federation Fellow.
References
- Bergeron R, Russell RR, 3rd, Young LH, Ren JM, Marcucci M, Lee A, Shulman GI. Effect of AMPK activation on muscle glucose metabolism in conscious rats. Am J Physiol. 1999;276:E938–E944. doi: 10.1152/ajpendo.1999.276.5.E938. [DOI] [PubMed] [Google Scholar]
- Chen ZP, McConell GK, Michell BJ, Snow RJ, Canny BJ, Kemp BE. AMPK signaling in contracting human skeletal muscle: acetyl-CoA carboxylase and NO synthase phosphorylation. Am J Physiol Endocrinol Metab. 2000;279:E1202–E1206. doi: 10.1152/ajpendo.2000.279.5.E1202. [DOI] [PubMed] [Google Scholar]
- Chen Z-P, Stephens TJ, Murthy S, Canny BJ, Hargreaves M, Witters LA, Kemp BE, McConell GK. Effect of exercise intensity on skeletal muscle AMPK signaling in humans. Diabetes. 2003;52:2205–2212. doi: 10.2337/diabetes.52.9.2205. [DOI] [PubMed] [Google Scholar]
- Chesley A, Heigenhauser GJ, Spriet LL. Regulation of muscle glycogen phosphorylase activity following short-term endurance training. Am J Physiol. 1996;270:E328–E335. doi: 10.1152/ajpendo.1996.270.2.E328. [DOI] [PubMed] [Google Scholar]
- Coggan AR, Kohrt WM, Spina RJ, Bier DM, Holloszy JO. Endurance training decreases plasma glucose turnover and oxidation during moderate-intensity exercise in men. J Appl Physiol. 1990;68:990–996. doi: 10.1152/jappl.1990.68.3.990. [DOI] [PubMed] [Google Scholar]
- Derave W, Ai H, Ihlemann J, Witters LA, Kristiansen S, Richter EA, Ploug T. Dissociation of AMP-activated protein kinase activation and glucose transport in contracting slow-twitch muscle. Diabetes. 2000;49:1281–1287. doi: 10.2337/diabetes.49.8.1281. [DOI] [PubMed] [Google Scholar]
- Durante PE, Mustard KJ, Park SH, Winder WW, Hardie DG. Effects of endurance training on activity and expression of AMP-activated protein kinase isoforms in rat muscles. Am J Physiol Endocrinol Metab. 2002;283:E178–E186. doi: 10.1152/ajpendo.00404.2001. [DOI] [PubMed] [Google Scholar]
- Frederich M, Balschi JA. The relationship between AMP-activated protein kinase activity and AMP concentration in the isolated perfused rat heart. J Biol Chem. 2002;277:1928–1932. doi: 10.1074/jbc.M107128200. [DOI] [PubMed] [Google Scholar]
- Fujii N, Hayashi T, Hirshman MF, Smith JT, Habinowski SA, Kaijser L, Mu J, Ljungqvist O, Birnbaum MJ, Witters LA, Thorell A, Goodyear LJ. Exercise induces isoform-specific increase in 5′AMP-activated protein kinase activity in human skeletal muscle. Biochem Biophys Res Commun. 2000;273:1150–1155. doi: 10.1006/bbrc.2000.3073. [DOI] [PubMed] [Google Scholar]
- Green HJ, Helyar R, Ball-Burnett M, Kowalchuk N, Symon S, Farrance B. Metabolic adaptations to training precede changes in muscle mitochondrial capacity. J Appl Physiol. 1992;72:484–491. doi: 10.1152/jappl.1992.72.2.484. [DOI] [PubMed] [Google Scholar]
- Green HJ, Jones S, Ball-Burnett ME, Smith D, Livesey J, Farrance BW. Early muscular and metabolic adaptations to prolonged exercise training in humans. J Appl Physiol. 1991;70:2032–2038. doi: 10.1152/jappl.1991.70.5.2032. [DOI] [PubMed] [Google Scholar]
- Hardie DG, Carling D. The AMP-activated protein kinase – fuel gauge of the mammalian cell? Eur J Biochem. 1997;246:259–273. doi: 10.1111/j.1432-1033.1997.00259.x. [DOI] [PubMed] [Google Scholar]
- Hawley SA, Boudeau J, Reid JL, Mustard KJ, Udd L, Makela TP, Alessi DR, Hardie DG. Complexes between the LKB1 tumor suppressor, STRADalpha/beta and MO25alpha/beta are upstream kinases in the AMP-activated protein kinase cascade. J Biol. 2003;2:28. doi: 10.1186/1475-4924-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Hirshman MF, Fujii N, Habinowski SA, Witters LA, Goodyear LJ. Metabolic stress and altered glucose transport: activation of AMP- activated protein kinase as a unifying coupling mechanism. Diabetes. 2000;49:527–531. doi: 10.2337/diabetes.49.4.527. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Hirshman MF, Kurth EJ, Winder WW, Goodyear LJ. Evidence for 5′-AMP-activated protein kinase mediation of the effect of muscle contraction on glucose transport. Diabetes. 1998;47:1369–1373. doi: 10.2337/diab.47.8.1369. [DOI] [PubMed] [Google Scholar]
- Hudson ER, Pan DA, James J, Lucocq JM, Hawley SA, Green KA, Baba O, Terashima T, Hardie DG. A novel domain in AMP-activated protein kinase causes glycogen storage bodies similar to those seen in hereditary cardiac arrhythmias. Curr Biol. 2003;13:861–866. doi: 10.1016/s0960-9822(03)00249-5. [DOI] [PubMed] [Google Scholar]
- Hultman E, Sahlin K. Acid-base balance during exercise. Exerc Sport Sci Rev. 1980;8:41–128. [PubMed] [Google Scholar]
- Hutber CA, Rasmussen BB, Winder WW. Endurance training attenuates the decrease in skeletal muscle malonyl-CoA with exercise. J Appl Physiol. 1997;83:1917–1922. doi: 10.1152/jappl.1997.83.6.1917. [DOI] [PubMed] [Google Scholar]
- Jeukendrup AE, Raben A, Gijsen A, Stegen JH, Brouns F, Saris WH, Wagenmakers AJ. Glucose kinetics during prolonged exercise in highly trained human subjects: effect of glucose ingestion. J Physiol. 1999;515:579–589. doi: 10.1111/j.1469-7793.1999.579ac.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen SB, Viollet B, Andreelli F, Frosig C, Birk JB, Schjerling P, Vaulont S, Richter EA, Wojtaszewski JF. Knockout of the alpha2 but not alpha1 5′-AMP-activated protein kinase isoform abolishes 5-aminoimidazole-4-carboxamide-1-beta-4-ribofuranosidebut not contraction-induced glucose uptake in skeletal muscle. J Biol Chem. 2004;279:1070–1079. doi: 10.1074/jbc.M306205200. [DOI] [PubMed] [Google Scholar]
- Kemp BE, Stapleton D, Campbell DJ, Chen ZP, Murthy S, Walter M, Gupta A, Adams JJ, Katsis F, Van Denderen B, Jennings IG, Iseli T, Michell BJ, Witters LA. AMP-activated protein kinase, super metabolic regulator. Biochem Soc Trans. 2003;31:162–168. doi: 10.1042/bst0310162. [DOI] [PubMed] [Google Scholar]
- Mendenhall LA, Swanson SC, Habash DL, Coggan AR. Ten days of exercise training reduces glucose production and utilization during moderate-intensity exercise. Am J Physiol. 1994;266:E136–E143. doi: 10.1152/ajpendo.1994.266.1.E136. [DOI] [PubMed] [Google Scholar]
- Merrill GF, Kurth EJ, Hardie DG, Winder WW. AICA riboside increases AMP-activated protein kinase, fatty acid oxidation, and glucose uptake in rat muscle. Am J Physiol. 1997;273:E1107–E1112. doi: 10.1152/ajpendo.1997.273.6.E1107. [DOI] [PubMed] [Google Scholar]
- Minokoshi Y, Kim YB, Peroni OD, Fryer LG, Muller C, Carling D, Kahn BB. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature. 2002;415:339–343. doi: 10.1038/415339a. [DOI] [PubMed] [Google Scholar]
- Mu J, Brozinick JT, Jr, Valladares O, Bucan M, Birnbaum MJ. A role for AMP-activated protein kinase in contraction- and hypoxia- regulated glucose transport in skeletal muscle. Mol Cell. 2001;7:1085–1094. doi: 10.1016/s1097-2765(01)00251-9. [DOI] [PubMed] [Google Scholar]
- Nielsen JN, Mustard KJ, Graham DA, Yu H, MacDonald CS, Pilegaard H, Goodyear LJ, Hardie DG, Richter EA, Wojtaszewski JF. 5′-AMP-activated protein kinase activity and subunit expression in exercise-trained human skeletal muscle. J Appl Physiol. 2003;94:631–641. doi: 10.1152/japplphysiol.00642.2002. [DOI] [PubMed] [Google Scholar]
- Park SH, Gammon SR, Knippers JD, Paulsen SR, Rubink DS, Winder WW. Phosphorylation-activity relationships of AMPK and acetyl-CoA carboxylase in muscle. J Appl Physiol. 2002;92:2475–2482. doi: 10.1152/japplphysiol.00071.2002. [DOI] [PubMed] [Google Scholar]
- Peronnet F, Massicotte D. Table of nonprotein respiratory quotient: an update. Can J Sport Sci. 1991;16:23–29. [PubMed] [Google Scholar]
- Polekhina G, Gupta A, Michell BJ, van Denderen B, Murthy S, Feil SC, Jennings IG, Campbell DJ, Witters LA, Parker MW, Kemp BE, Stapleton D. AMPK beta subunit targets metabolic stress sensing to glycogen. Curr Biol. 2003;13:867–871. doi: 10.1016/s0960-9822(03)00292-6. [DOI] [PubMed] [Google Scholar]
- Radziuk J, Norwich KH, Vranic M. Experimental validation of measurements of glucose turnover in nonsteady state. Am J Physiol. 1978;234:E84–E93. doi: 10.1152/ajpendo.1978.234.1.E84. [DOI] [PubMed] [Google Scholar]
- Raney MA, Yee AJ, Todd MK, Turcotte LP. AMPK activation is not critical in the regulation of muscle FA uptake and oxidation during low-intensity muscle contraction. Am J Physiol Endocrinol Metab. 2005;288:E592–E598. doi: 10.1152/ajpendo.00301.2004. [DOI] [PubMed] [Google Scholar]
- Roepstorff C, Vistisen B, Donsmark M, Nielsen JN, Galbo H, Green KA, Hardie DG, Wojtaszewski JF, Richter EA, Kiens B. Regulation of hormone-sensitive lipase activity and Ser563 and Ser565 phosphorylation in human skeletal muscle during exercise. J Physiol. 2004;560:551–562. doi: 10.1113/jphysiol.2004.066480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto K, Goransson O, Hardie DG, Alessi DR. Activity of LKB1 and AMPK-related kinases in skeletal muscle: effects of contraction, phenformin, and AICAR. Am J Physiol Endocrinol Metab. 2004;287:E310–E317. doi: 10.1152/ajpendo.00074.2004. [DOI] [PubMed] [Google Scholar]
- Sakamoto K, McCarthy A, Smith D, Green KA, Grahame Hardie D, Ashworth A, Alessi DR. Deficiency of LKB1 in skeletal muscle prevents AMPK activation and glucose uptake during contraction. EMBO J. 2005;24:1810–1820. doi: 10.1038/sj.emboj.7600667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele R, Wall JS, de Bodo RC, Altszuler N. Measurement of the size and turnover rate of body glucose pool by the isotope dilution method. Am J Physiol. 1956;187:15–24. doi: 10.1152/ajplegacy.1956.187.1.15. [DOI] [PubMed] [Google Scholar]
- Stephens TJ, Chen ZP, Canny BJ, Michell BJ, Kemp BE, McConell GK. Progressive increase in human skeletal muscle AMPK α2 activity and ACC phosphorylation during exercise. Am J Physiol Endocrinol Metab. 2002;282:E688–E694. doi: 10.1152/ajpendo.00101.2001. [DOI] [PubMed] [Google Scholar]
- Vavvas D, Apazidis A, Saha AK, Gamble J, Patel A, Kemp BE, Witters LA, Ruderman NB. Contraction-induced changes in acetyl-CoA carboxylase and 5′-AMP-activated kinase in skeletal muscle. J Biol Chem. 1997;272:13255–13261. doi: 10.1074/jbc.272.20.13255. [DOI] [PubMed] [Google Scholar]
- Winder WW. Energy-sensing and signaling by AMP-activated protein kinase in skeletal muscle. J Appl Physiol. 2001;91:1017–1028. doi: 10.1152/jappl.2001.91.3.1017. [DOI] [PubMed] [Google Scholar]
- Winder WW, Hardie DG. Inactivation of acetyl-CoA carboxylase and activation of AMP-activated protein kinase in muscle during exercise. Am J Physiol. 1996;270:E299–E304. doi: 10.1152/ajpendo.1996.270.2.E299. [DOI] [PubMed] [Google Scholar]
- Winder WW, Holmes BF. Insulin stimulation of glucose uptake fails to decrease palmitate oxidation in muscle if AMPK is activated. J Appl Physiol. 2000;89:2430–2437. doi: 10.1152/jappl.2000.89.6.2430. [DOI] [PubMed] [Google Scholar]
- Wojtaszewski JF, MacDonald C, Nielsen JN, Hellsten Y, Hardie D, Kemp BEB, Richter EA. Regulation of 5′AMP-activated protein kinase activity and substrate utilization in exercising human skeletal muscle. Am J Physiol Endocrinol Metab. 2003;284:E813–E822. doi: 10.1152/ajpendo.00436.2002. [DOI] [PubMed] [Google Scholar]
- Wojtaszewski JF, Mourtzakis M, Hillig T, Saltin B, Pilegaard H. Dissociation of AMPK activity and ACCbeta phosphorylation in human muscle during prolonged exercise. Biochem Biophys Res Commun. 2002;298:309–316. doi: 10.1016/s0006-291x(02)02465-8. [DOI] [PubMed] [Google Scholar]
- Wojtaszewski JF, Nielsen P, Hansen BF, Richter EA, Kiens B. Isoform-specific and exercise intensity-dependent activation of 5′-AMP-activated protein kinase in human skeletal muscle. J Physiol. 2000;528:221–226. doi: 10.1111/j.1469-7793.2000.t01-1-00221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods A, Johnstone SR, Dickerson K, Leiper FC, Fryer LG, Neumann D, Schlattner U, Wallimann T, Carlson M, Carling D. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr Biol. 2003;13:2004–2008. doi: 10.1016/j.cub.2003.10.031. [DOI] [PubMed] [Google Scholar]
- Wright DC, Geiger PC, Holloszy JO, Han DH. Contraction- and hypoxia-stimulated glucose transport is mediated by a Ca2+-dependent mechanism in slow-twitch rat soleus muscle. Am J Physiol Endocrinol Metab. 2005;288:E1062–E1066. doi: 10.1152/ajpendo.00561.2004. [DOI] [PubMed] [Google Scholar]
- Wright DC, Hucker KA, Holloszy JO, Han DH. Ca2+ and AMPK both mediate stimulation of glucose transport by muscle contractions. Diabetes. 2004;53:330–335. doi: 10.2337/diabetes.53.2.330. [DOI] [PubMed] [Google Scholar]