Abstract
We hypothesized that 4 weeks of recombinant human erythropoietin (RhEPO) treatment would result in a significant increase in haemoglobin concentration ([Hb]) and arterial blood O2-carrying capacity and that this would (1) increase peak pulmonary oxygen uptake V˙O2 during ramp incremental exercise, and (2) speed V˙O2 kinetics during ‘severe’-, but not ‘moderate’- or ‘heavy’-intensity, step exercise. Fifteen subjects (mean ±s.d. age 25 ± 4 years) were randomly assigned to either an experimental group which received a weekly subcutaneous injection of RhEPO (150 IU kg−1; n = 8), or a control group (CON) which received a weekly subcutaneous injection of sterile saline (10 ml; n = 7) as a placebo, for four weeks. The subjects and the principal researchers were both blind with respect to the group assignment. Before and after the intervention period, all subjects completed a ramp test for determination of the gas exchange threshold (GET) and V˙O2,peak, and a number of identical ‘step’ transitions from ‘unloaded’ cycling to work rates requiring 80% GET (moderate), 70% of the difference between the GET and V˙O2,peak (heavy), and 105% V˙O2,peak (severe) as determined from the initial ramp test. Pulmonary gas exchange was measured breath-by-breath. There were no significant differences between the RhEPO and CON groups for any of the measurements of interest ([Hb], V˙O2,peak V˙O2 kinetics) before the intervention. Four weeks of RhEPO treatment resulted in a 7% increase both in [Hb] (from 15.8 ± 1.0 to 16.9 ± 0.7 g dl−1; P < 0.01) and V˙O2,peak (from 47.5 ± 4.2 to 50.8 ± 10.7 ml kg−1·min−1; P < 0.05), with no significant change in CON. RhEPO had no significant effect on V˙O2 kinetics for moderate (Phase II time constant, from 28 ± 8 to 28 ± 7 s), heavy (from 37 ± 12 to 35 ± 11 s), or severe (from 33 ± 15 to 35 ± 15 s) step exercise. Our results indicate that enhancing blood O2-carrying capacity and thus the potential for muscle O2 delivery with RhEPO treatment enhanced the peak V˙O2 but did not influence V˙O2 kinetics, suggesting that the latter is principally regulated by intracellular (metabolic) factors, even during exercise where the V˙O2 requirement is greater than the V˙O2,peak, at least in young subjects performing upright cycle exercise.
The increase in muscle, and pulmonary, oxygen uptake V˙O2 following a step transition in external work rate (and therefore ATP turnover in the contracting myocytes) is critically dependent upon an effective integration of the cardiovascular and metabolic responses to exercise (Tschakovsky & Hughson, 1999). Rapid increases in both cardiac output and muscle vasodilatation are necessary to ensure that exercising muscle is supplied with sufficient O2 to meet its metabolic requirements (Delp & O'Leary, 2004). In the sudden transition from a lower to a higher metabolic rate, muscle and (following a short time delay) pulmonary V˙O2 rise exponentially towards the expected V˙O2 requirement with a time constant (τ) of approximately 20–40 s (depending on factors such as age, fitness, and exercise modality) such that a ‘steady state’ is typically attained within 2–3 min (Whipp & Mahler, 1980). However, this relatively slow increase in V˙O2 (considered against the immediate increase in work rate) results in the incurrence of an ‘O2 deficit’, the energetic equivalent of which must be supplied by an acceleration of substrate-level phosphorylation (i.e. phosphocreatine splitting and anaerobic glycolysis).
The control determinants of, and the limitations to, the kinetics of the muscle V˙O2 response following the onset of exercise remain a source of considerable debate and controversy (e.g. Tschakovsky & Hughson, 1999; Poole & Jones, 2005). Specifically, the extent to which muscle O2 availability might limit V˙O2 kinetics during exercise is unclear, with some studies suggesting an important role for O2 supply as a limiting factor (Hughson et al. 1993; MacDonald et al. 1997; Grassi et al. 2000; Tordi et al. 2003) and others suggesting that O2 supply is sufficient and that V˙O2 kinetics are principally set by other (intracellular) factors such as an inertia of one or more of the enzymes of oxidative metabolism (Grassi et al. 1998a, b; Burnley et al. 2000; Wilkerson et al. 2004a, b). The potential for O2 supply to limit V˙O2 kinetics is likely to be related to factors such as the age and health status of the subjects tested, the exercise modality (including muscle contraction regimen and position of the exercising muscle relative to the heart), and the exercise intensity (Jones & Poole, 2005). In relation to the latter, it has been reported that pump-perfusing isolated canine muscle at the ‘steady state’ blood flow requirement across the rest-to-exercise transition did not alter muscle V˙O2 kinetics during contractions requiring ∼60% V˙O2,peak (Grassi et al. 1998a), but caused a significant speeding of V˙O2 kinetics at contractions requiring ∼100% V˙O2,peak (Grassi et al. 2000).
A variety of interventions have been used to manipulate muscle O2 availability and to observe the effect on V˙O2 kinetics during exercise in human subjects. Interventions intended to result in a reduction in muscle O2 availability such as the inspiration of hypoxic gas mixtures (e.g. Engelen et al. 1996) or the alteration of body position to reduce muscle perfusion pressure (e.g. MacDonald et al. 1998) have generally resulted in slower V˙O2 kinetics compared to the appropriate control condition. These results suggest a rather delicate balance between O2 supply and O2 utilization which, if perturbed, could result in a reduction in the ability to generate ATP through oxidative phosphorylation across an exercise transient. Conversely, interventions designed to increase muscle O2 availability such as the performance of a prior bout of exercise (Burnley et al. 2000; Koppo & Bouckaert, 2001; Wilkerson et al. 2004b) or the inspiration of hyperoxic gas mixtures (MacDonald et al. 1997; MacDonald et al. 2000) have generally not resulted in a significant speeding of the Phase II pulmonary V˙O2 kinetics (believed to closely reflect the V˙O2 kinetics in the exercising muscles; Grassi et al. 1996; Rossiter et al. 2002). However, these interventions have typically resulted in a reduction in the amplitude of the so-called V˙O2 ‘slow component’ (MacDonald et al. 1997; Burnley et al. 2000; Koppo & Bouckaert, 2001), a slowly developing phase of the V˙O2 kinetics which is only observed at work rates exceeding the lactate threshold, as estimated from the gas exchange threshold (GET; Whipp & Wasserman, 1972).
Connes et al. (2003) have recently reported that 4 weeks of recombinant human erythropoietin (RhEPO) treatment, which caused a ∼10% increase in haemoglobin ([Hb]) concentration (and a similar percentage increase in blood O2-carrying capacity), resulted in a significant speeding of pulmonary V˙O2 kinetics in endurance-trained athletes during cycle exercise at ∼65% V˙O2,peak (τ reduced from a group mean of 36 s to 29 s) with no change in a matched control group. These results are important in that they appear to provide evidence that muscle O2 delivery might represent a significant limitation to V˙O2 kinetics even in young healthy subjects performing submaximal cycle exercise. However, there are a number of possible issues of concern. Firstly, the subjects in this study only performed a single rest-to-exercise transition before and after the intervention period. In studies of this type, the inherently variable nature of pulmonary V˙O2 data dictates that a number (typically 2–6) of transitions be averaged together to enhance the signal-to-noise ratio and improve confidence in the model fit and data interpretation (Lamarra et al. 1987). Secondly, it was not clear whether the exercise intensity studied (65% V˙O2,peak) was in the moderate (<GET) or heavy (>GET but below V˙O2,peak) exercise domain in all of the subjects studied. It is therefore possible that the faster V˙O2 kinetics following RhEPO treatment reported by Connes et al. (2003) actually resulted from a ‘trimming out’ of the V˙O2 slow component, rather than a speeding of the fundamental component V˙O2 kinetics per se.
The purpose of the present study therefore was to examine the influence of 4 weeks of RhEPO treatment on V˙O2 kinetics during cycle exercise in young healthy subjects. We examined the influence of RhEPO treatment on the averaged V˙O2 kinetic response to a number of identical transitions performed at three work rates that were strictly in the moderate (80% GET or ∼45% V˙O2,peak), heavy (70% of the difference between GET and V˙O2,peak or ∼85% V˙O2,peak), and severe (∼105% V˙O2,peak) exercise intensity domains (Whipp & Mahler, 1980; Jones & Poole, 2005). We hypothesized that 4 weeks of RhEPO treatment would: (1) significantly elevate [Hb], haematocrit (Hct), and V˙O2,peak; (2) not significantly influence the Phase II τ during moderate or heavy exercise where there is limited evidence for an O2 availability limitation (Grassi et al. 1998a; Burnley et al. 2000); (3) significantly reduce the amplitude of the V˙O2 slow component during heavy exercise (MacDonald et al. 1997; Burnley et al. 2000); and (4) significantly speed the Phase II V˙O2 kinetics during severe exercise where there is greater likelihood of an O2 availability limitation (Grassi et al. 2000).
Methods
Subjects
Sixteen healthy young subjects (15 male; mean ±s.d. age 25 ± 4 years, body mass 78.0 ± 8.8 kg) volunteered to participate in this study that had received approval from the Manchester Metropolitan University Research Ethics Committee. The subjects were all recreationally active and were fully familiar with laboratory exercise testing procedures. Competitive athletes were strictly excluded from participation in the study. Upon recruitment to the study, all subjects completed a medical health questionnaire and had their blood pressure (BP), [Hb] and Hct measured (see later), in order that individuals with hypertension (systolic BP > 140 mmHg or diastolic BP > 90 mmHg) or an unusually high Hct (> 45%) could be excluded from the study. On test days, the subjects were required to report to the laboratory well hydrated, at least 2 h following the consumption of a light meal and having completed no strenuous exercise in the preceding 24 h.
Experimental overview
The subjects were required to visit the laboratory for exercise testing on five separate occasions both before and after a 4-week intervention period. Briefly, the exercise testing consisted of a ramp incremental exercise test (completed on the first day of testing) and a number of identical ‘step’ transitions from ‘unloaded’ cycling to individualized work rates of moderate- (four repeats), heavy- (two repeats) and severe- (two repeats) intensity (completed on the remaining four days of testing). On each of these visits, the subjects completed one moderate exercise bout followed by one heavy or severe exercise bout. Testing took place in an air conditioned laboratory (maintained at 18°C) and was conducted at the same time of day (±2 h) for each subject. All testing was performed on an electrically braked cycle ergometer (Jaeger Ergoline E800, Germany).
Following completion of the initial set of exercise tests, subjects were randomly assigned to either the experimental group (which received weekly RhEPO injections for 4 weeks; n = 8), or the control group (which received weekly saline injections for 4 weeks as a placebo; n = 7; see below). Both the subjects and the investigators involved in the administration of the exercise tests and the subsequent data analysis were blind to the group assignment.
Exercise tests
On their first visit to the laboratory before and after the intervention period, the subjects completed a ramp incremental exercise test to volitional exhaustion. Following 3 min of baseline cycling at 20 W (the lowest work rate available on the ergometer), the work rate was increased in a ramp fashion until the subject was unable to continue. The ramp rate (20, 25 or 30 W min−1) was selected in order that subjects became exhausted within 10–12 min. The subjects cycled at a self-selected pedal rate (between 70 and 90 rev min−1) and this pedal rate along with the saddle and handlebar heights were recorded and reproduced in subsequent tests. The V˙O2,peak was defined as the highest 30 s mean value recorded before the subject's volitional termination of the test. The GET was determined from a cluster of measures including (1) the first disproportionate increase in carbon dioxide output (V˙CO2) from visual inspection of individual plots of V˙CO2 versus V˙O2; (2) an increase in V˙E/V˙O2 (V˙E, expiratory ventilation) with no increase in V˙E/V˙CO2; and (3) an increase in end-tidal O2 tension with no fall in end-tidal CO2 tension (Wasserman et al. 1994). The work rates that would require 80% of the GET (moderate exercise), 70% of the difference (Δ) between the GET and V˙O2 peak (heavy exercise), and 105% of V˙O2,peak (severe exercise; Wilkerson et al. 2004a, b) were calculated, with account taken of the mean response time of the V˙O2 adaptation to ramp exercise (assumed to approximate two-thirds of the ramp rate; Whipp et al. 1981). The absolute work rates that were calculated in this way from the initial ramp test were used for the step tests that were performed both before and after the intervention period.
On any given ‘step’ test day both before and after the intervention period, subjects completed one moderate exercise bout followed by one heavy or severe exercise bout. In order to increase the signal to noise ratio and enhance the underlying response characteristics, the subjects completed a total of four identical repeat transitions to the moderate work rate and two to each of the heavy and severe work rates. Each of the exercise bouts commenced with 3 min of baseline cycling at 20 W before an abrupt transition to the target work rate which was maintained for 6 min (for moderate and heavy exercise) or until exhaustion (for severe exercise). Within a test session, the exercise bouts were separated by 10 min of recovery. It is known that the completion of a prior bout of moderate exercise has no effect on V˙O2 kinetics during subsequent exercise (e.g. Gerbino et al. 1996; Burnley et al. 2000). During the severe-intensity step tests, the time to exhaustion (defined as the point at which pedal rate dropped by more than 5 rev min−1 below the selected pedal rate for more than 5 s despite strong verbal encouragement) was recorded to the nearest second.
Pulmonary gas exchange and ventilation were measured breath-by-breath throughout all of the exercise tests. The subjects wore a nose clip and breathed through a low dead space, low resistance mouthpiece and volume sensor assembly. Pulmonary gas exchange was measured with a mass spectrometer and volume turbine system (Morgan EX670, Morgan Medical, UK). The system was calibrated prior to each test using gases of known concentration and a precision 3 L calibration syringe. Heart rate (HR) was recorded every 5 s throughout the exercise tests using short-range telemetry (Polar PE 4000, Polar, Finland). A blood sample from a fingertip was collected into a capillary tube immediately before and after one of each of the moderate, heavy and severe exercise bouts and analysed for blood [lactate] using an automated analyser (YSI 1500 Sport, Yellow Springs Instruments, Ohio, USA).
RhEPO and placebo treatments
Once per week for each of 4 weeks, the subjects reported to the laboratory to receive a subcutaneous injection of either RhEPO (experimental group; 150 IU (kg body mass)−1; NeoRecormon, Roche Pharmaceuticals, UK) or sterile saline as a placebo (control group). All injections were administered by a qualified medical practitioner. Both groups of subjects were supplied with tablets containing the recommended daily allowance of iron and vitamin C (Holland and Barrett, Nuneaton, UK) and were instructed to consume one tablet per day. Both groups of subjects were also advised to increase their daily consumption of water.
Immediately before the initial ramp incremental test, twice per week during the 4 week intervention period, and for 2 weeks after receiving the final injection, all subjects had their blood pressure and haematology ([Hb] and Hct) measured. Following a 10 min rest period, blood pressure was measured using a manual sphygmomanometer, and fingertip blood samples were collected into cuvettes and capillary tubes for the determination of [Hb] (B-Hemoglobin, Hemocue Ltd, UK) and, following 8 min of microcentrifugation, Hct (Hawksley Reader, Hawksley, UK), respectively. All blood measurements were made in duplicate. The purpose of these measurements was to track the efficacy of the RhEPO treatment in altering the haematological variables and also to ensure that they did not increase to potentially dangerous levels in our subjects. The post-intervention exercise tests commenced one week following the fourth and final injection, and all testing was completed within the following 10 days.
Modelling procedures
The breath-by-breath V˙O2 data from each test were initially examined to exclude errant breaths caused by coughing, swallowing, sighing, etc. and those values lying more than four standard deviations from the local mean were removed. The breath-by-breath data were subsequently linearly interpolated to provide 1 s-values using custom-designed software. For each subject and each exercise condition, the identical repetitions of each work rate were then time aligned to the start of exercise and ensemble-averaged to reduce the breath-to-breath noise and enhance the underlying physiological response characteristics. The baseline V˙O2 was defined as the mean V˙O2 measured during baseline cycling (20 W) between 150 and 30 s before the start of exercise. The first 20 s of data after the onset of exercise (i.e. the Phase I response) were deleted. Subsequently, a single exponential model was used to analyse the V˙O2 responses to moderate exercise and a bi-exponential model was used for heavy and severe exercise as described in the following equations:
| (1) |
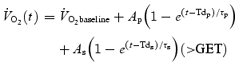 |
(2) |
The parameters of the model were determined using a non-linear least-square algorithm. In the equations above, V˙O2 (t) represents the absolute V˙O2 at a given time t and V˙O2 baseline represents the average V˙O2 in the baseline cycling period. For moderate exercise, the V˙O2 response was appropriately fitted with a single exponential curve that described the amplitude (Ap), the time delay (Tdp), and the time constant (τp) of the fundamental increase in V˙O2 above baseline. For heavy and severe exercise, the V˙O2 response was initially fitted with two exponential curves, one describing the fundamental V˙O2 response and one describing the V˙O2 slow component response. Therefore, this equation also includes terms describing the amplitude of the V˙O2 slow component (As), the time delay before the appearance of the slow component (Tds), and the time constant of the slow component development (τs). Because the asymptotic value (As) of the exponential term describing the V˙O2 slow component may represent a higher value than is actually reached at the end of the exercise, the actual amplitude of the V˙O2 slow component at the end of exercise was determined and termed as As′.
During severe exercise, the exercise duration was typically too short (∼155 s) for a V˙O2 slow component term to be reliably discerned. Where this was the case, the second term in eqn (2)‘dropped out’ and the V˙O2 response was described by a single exponential function with delay term (as for moderate exercise). Because the time to exhaustion during severe exercise was not identical before and after the intervention period, we fitted the data: (1) to the same point in time (given by the time to exhaustion in the shortest bout; Model 1); (2) to the end of exercise in both bouts (Model 2); and (3) to 90 s following the onset of exercise in both bouts (Model 3).
To provide indirect information on cardiac output and muscle blood flow dynamics, we also modelled the HR response to each of the three exercise intensities both before and after the intervention. For this analysis, a mono-exponential model similar to that described in eqn (1) above was used, with the exception that the fitting window commenced at the onset of exercise.
Statistics
The pre- and postintervention responses to each of the three work rates were compared using a two-way (group × time) repeated-measures analysis of variance. The F ratios were considered statistically significant when P < 0.05. Data are presented as mean ±s.d.
Results
One subject in the control group sustained an injury unrelated to the study and was unable to complete the post-intervention tests. Results are therefore presented for eight subjects who underwent RhEPO treatment, and seven subjects who received placebo injections. There were no significant differences between the RhEPO group and control group before the intervention period for any of the measurements of interest (e.g. [Hb], Hct, V˙O2,peak, τp).
Haematological measurements
RhEPO treatment resulted in a 7% increase in [Hb] (from 15.8 ± 1.0 to 16.9 ± 0.7 g dl−1; P < 0.01) and a 12% increase in Hct (from 43 ± 3 to 49 ± 2%; P < 0.01), but there were no significant changes in the control group ([Hb] from 16.0 ± 0.7 to 15.7 ± 0.6 g dl−1 and Hct from 43 ± 3 to 44 ± 3%). The [Hb], Hct and blood pressure values before, during and after the 4 week intervention period are shown for the RhEPO and control groups in Table 1.
Table 1.
Mean ±s.d. haemoglobin, haematocrit and systolic and diastolic blood pressure responses in the control and RhEPO treatment groups
| Baseline | Baseline | Week 1 | Week 2 | Week 3 | Week 4 | Post |
|---|---|---|---|---|---|---|
| Control [Hb] (g dl−1) | 16.0 ± 0.7 | 15.9 ± 0.5 | 15.7 ± 0.8 | 15.7 ± 0.5 | 15.7 ± 0.6 | 15.5 ± 1.1 |
| Control Hct (%) | 43 ± 3 | 44 ± 1 | 45 ± 2 | 44 ± 2 | 44 ± 3 | 44 ± 3 |
| Control BP Sys (mmHg) | 125 ± 7 | 122 ± 9 | 124 ± 7 | 124 ± 8 | 123 ± 7 | 126 ± 7 |
| Control BP Dia (mmHg) | 73 ± 7 | 77 ± 11 | 77 ± 10 | 78 ± 10 | 77 ± 9 | 83 ± 9 |
| RhEPO [Hb] (g dl−1) | 15.8 ± 1.0 | 16.1 ± 1.4 | 16.3 ± 0.9 | 16.8 ± 0.7 * | 16.9 ± 0.7 * | 16.8 ± 0.9* |
| RhEPO Hct (%) | 43 ± 3 | 45 ± 3 | 46 ± 2 | 48 ± 1 * | 49 ± 2 * | 48 ± 2* |
| RhEPO BP Sys (mmHg) | 128 ± 6 | 126 ± 8 | 125 ± 10 | 124 ± 11 | 126 ± 12 | 123 ± 13 |
| RhEPO BP Dia (mmHg) | 76 ± 6 | 79 ± 8 | 81 ± 9 | 82 ± 9 | 82 ± 9 | 85 ± 9 |
Significantly different from the corresponding control value and to RhEPO baseline (P < 0.01); BP Sys, systolic blood pressure; BP Dia, diastolic blood pressure.
Ramp incremental exercise test
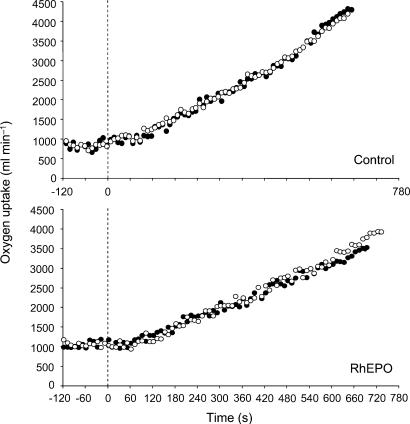
RhEPO treatment resulted in a 7% improvement in V˙O2,peak during the incremental test (from 47.5 ± 11.0 to 50.8 ± 10.7 ml kg−1·min−1; P < 0.05), but there was no significant change in the control group (from 50.1 ± 4.2 to 50.3 ± 3.8 ml kg−1·min−1). The V˙O2 responses to ramp exercise in a representative subject from the control and RhEPO groups are shown in Fig. 1. The improvement in V˙O2,peak in the RhEPO group was associated with a 7% improvement in peak power output (from 311 ± 93 to 332 ± 103 W; P < 0.05), but there was no significant change in the control group (from 364 ± 35 to 365 ± 35 W). There was no significant change in GET in either the RhEPO group (from 1.72 ± 0.67 to 1.69 ± 0.69 l min−1) or the control group (from 2.10 ± 0.40 to 2.07 ± 0.4 l min−1).
Figure 1. Pulmonary V˙O2 response to ramp incremental exercise before (•) and after (○) four weeks of either saline infusion as a placebo (upper panel) or recombinant human erythropoietin treatment (lower panel) in a representative subject from each of the groups.
The vertical broken line indicates the end of the period of baseline cycling (at 20 W). Note the increased V˙O2,peak and extended time to exhaustion following recombinant human erythropoietin treatment.
V˙O2,peak kinetic response to step exercise
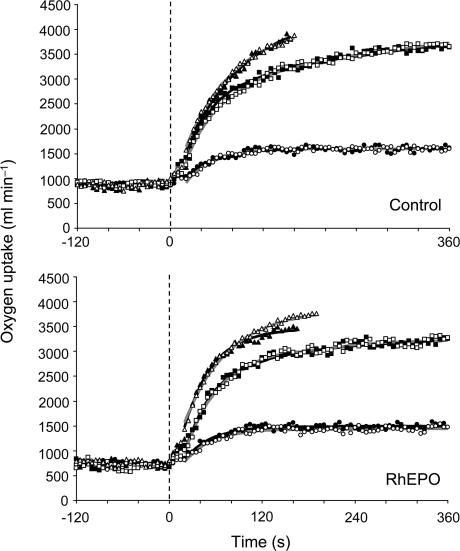
The V˙O2 responses to moderate, heavy and severe step exercise in representative subjects from the RhEPO and control groups are shown in Fig. 2, and the group mean responses are shown in Fig. 3.
Figure 2. Pulmonary V˙O2 response to step exercise of moderate, heavy and severe intensity before (▪) and after (□) four weeks of either saline infusion as a placebo (upper panel) or recombinant human erythropoietin treatment (lower panel) in a representative subject from each of the groups (the same as in Fig. 1).
The vertical broken line indicates the end of the period of baseline cycling (at 20 W). Note the increased V˙O2,peak and extended time to exhaustion during severe exercise following recombinant human erythropoietin treatment.
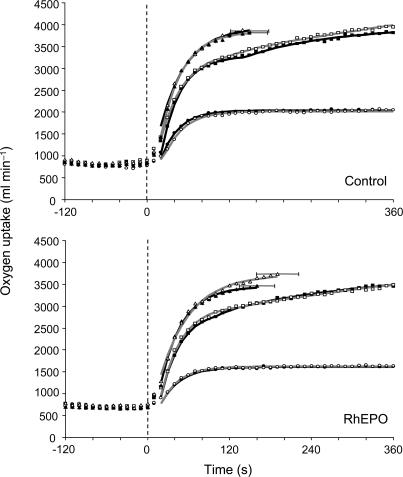
Figure 3. Group mean pulmonary V˙O2 response to step exercise of moderate, heavy and severe intensity before (▪) and after (□) four weeks of either saline infusion as a placebo (upper panel) or recombinant human erythropoietin treatment (lower panel).
For clarity, data are averaged over 10 s periods. The vertical broken line indicates the end of the period of baseline cycling (at 20 W). Black lines (pre-intervention) and grey lines (post-intervention) represent the lines of best fit derived from the mathematical modelling. The standard deviation in the time to exhaustion during severe exercise is also shown. Note the increased V˙O2,peak and extended time to exhaustion during severe exercise following recombinant human erythropoietin treatment.
Table 2 shows the parameters of the V˙O2 kinetic response to moderate-intensity step exercise in the RhEPO and control groups. There were no significant differences in any of the parameters of interest as a result of the RhEPO (or placebo) treatment. Of particular interest was that the τp was not altered with RhEPO treatment (from 28 ± 8 to 28 ± 7 s). The 95% confidence interval (CI) surrounding the estimate of τp was ∼2 ± 1 s.
Table 2.
Mean ±s.d. oxygen uptake kinetics during moderate-intensity step exercise in the control and RhEPO treatment groups
| Control-Pre | Control-Post | RhEPO-Pre | RhEPO-Post | |
|---|---|---|---|---|
| Baseline V˙O2 (l min−1) | 0.83 ± 0.04 | 0.79 ± 0.07 | 0.71 ± 0.12 | 0.74 ± 0.11 |
| Primary time delay (s) | 13 ± 3 | 16 ± 3 | 13 ± 6 | 14 ± 4 |
| Phase II time constant (s) | 27 ± 9 | 27 ± 9 | 28 ± 8 | 28 ± 7 |
| Primary amplitude (l min−1) | 1.23 ± 0.44 | 1.24 ± 0.43 | 0.89 ± 0.32 | 0.88 ± 0.34 |
| Primary ‘gain’ (ml min−1 W−1) | 9.9 ± 0.3 | 10.0 ± 0.5 | 10.3 ± 1.7 | 10.0 ± 1.3 |
The parameters of the V˙O2 kinetic response to heavy-intensity step exercise in the RhEPO and control groups are shown in Table 3. There were no significant differences in any of the parameters of interest as a result of the RhEPO (or placebo) treatment. As for moderate exercise, the τp was not altered with RhEPO treatment (from 37 ± 12 to 35 ± 11 s; 95% CI =∼3 ± 1 s). A V˙O2 slow component term was evident for all subjects both before and after the intervention, and this caused V˙O2 to rise above the expected steady state and, indeed, to approach V˙O2,peak by the end of exercise. However, RhEPO treatment had no significant effect on the V˙O2 slow component amplitude (from 0.46 ± 0.23 to 0.41 ± 0.15 l min−1).
Table 3.
Mean ±s.d. oxygen uptake kinetics during heavy-intensity step exercise in the control and RhEPO treatment groups
| Control-Pre | Control-Post | RhEPO-Pre | RhEPO-Post | |
|---|---|---|---|---|
| Baseline V˙O2 (l min−1) | 0.83 ± 0.46 | 0.81 ± 0.10 | 0.71 ± 0.12 | 0.73 ± 0.11 |
| Primary time delay (s) | 10 ± 6 | 11 ± 4 | 11 ± 5 | 9 ± 7 |
| Phase II time constant (s) | 29 ± 10 | 28 ± 9 | 37 ± 12 | 35 ± 11 |
| Primary amplitude (l min−1) | 2.52 ± 0.25 | 2.46 ± 0.31 | 2.31 ± 0.70 | 2.33 ± 0.74 |
| Primary ‘gain’ (ml min−1 W−1) | 9.0 ± 1.1 | 8.7 ± 0.7 | 9.9 ± 1.0 | 10.0 ± 0.9 |
| Slow phase time delay (s) | 129 ± 48 | 116 ± 38 | 143 ± 44 | 160 ± 41 |
| V˙O2 SC amplitude (l min−1) | 0.66 ± 0.25 | 0.68 ± 0.21 | 0.46 ± 0.23 | 0.41 ± 0.15 |
SC, slow component.
The parameters of the V˙O2 kinetic response to severe-intensity step exercise in both the RhEPO and control groups and for Model 1 and Model 2 are shown in Table 4. RhEPO treatment had no significant effect on τp during severe exercise either when Model 1 (from 33 ± 15 to 34 ± 15 s; 95% CI =∼3 ± 2 s), Model 2 (from 33 ± 15 to 35 ± 15 s; 95% CI =∼3 ± 2 s), or Model 3 (from 29 ± 11 to 29 ± 10 s; 95% CI =∼5 ± 3 s) was used. A V˙O2 slow component was evident in one of the seven subjects before and after the placebo treatment, and in two of the eight subjects before and after the RhEPO treatment. The time to exhaustion during severe exercise was extended by 22% following RhEPO treatment (from 157 ± 26 to 192 ± 31 s; P < 0.01), but there was no significant change in the control group (from 158 ± 25 to 152 ± 28 s). The increased time to exhaustion in the RhEPO group was associated with a significantly increased end-exercise V˙O2 (from 3.51 ± 0.87 to 3.66 ± 0.94 l min−1; P < 0.05).
Table 4.
Mean ±s.d. oxygen uptake kinetics during severe-intensity step exercise in the control and RhEPO treatment groups
| Control-Pre | Control-Post | RhEPO-Pre | RhEPO-Post (Model 1) | RhEPO-Post (Model 2) | |
|---|---|---|---|---|---|
| Baseline V˙O2 (l min−1) | 0.88 ± 0.08 | 0.82 ± 0.09 | 0.70 ± 0.10 | 0.75 ± 0.12 | 0.75 ± 0.12 |
| Primary time delay (s) | 4 ± 6 | 6 ± 8 | 8 ± 6 | 9 ± 6 | 8 ± 6 |
| Phase II time constant (s) | 40 ± 21 | 36 ± 18 | 33 ± 15 | 34 ± 15 | 35 ± 15 |
| Primary amplitude (l min−1) | 2.99 ± 0.34 | 2.99 ± 0.30 | 2.66 ± 0.71 | 2.68 ± 0.74 | 2.74 ± 0.73 |
| Primary ‘gain’ (ml min−1 W−1) | 8.3 ± 0.9 | 8.3 ± 0.8 | 8.8 ± 0.9 | 8.8 ± 0.6 | 9.0 ± 0.6 |
| End-exercise V˙O2 (l min−1) | 3.91 ± 0.33 | 3.84 ± 0.37 | 3.51 ± 0.87 | 3.57 ± 0.90 | 3.66 ± 0.94* |
Significantly greater than the RhEPO-Pre condition (P < 0.05). Model 1 = data modelled to the same point in time (given by the time to exhaustion in the RhEPO-Pre condition); and (2) data modelled to the end of exercise in both bouts.
Blood [lactate] and heart rate response to step exercise
The Δblood [lactate], end-exercise HR values, and HR kinetics for moderate, heavy and severe exercise in the control and RhEPO groups are shown in Table 5. The Δblood [lactate], end-exercise HR, and HR kinetics were not significantly altered as a consequence of the intervention either in the control group or the RhEPO group, at any of the three intensities studied.
Table 5.
Mean ±s.d.Δblood[lactate], end-exercise heart rate and heart rate kinetics during moderate, heavy and severe exercise in the control and RhEPO treatment groups
| Control-Pre | Control-Post | RhEPO-Pre | RhEPO-Post | |
|---|---|---|---|---|
| Moderate exercise | ||||
| ΔBlood [lactate] (mm) | 0.3 ± 0.3 | 0.4 ± 0.3 | 0.2 ± 0.3 | 0.4 ± 0.4 |
| End-exercise HR (beats min−1) | 120 ± 13 | 125 ± 13 | 115 ± 12 | 113 ± 11 |
| HR time constant (s) | 24 ± 10 | 33 ± 18 | 23 ± 17 | 26 ± 15 |
| Heavy exercise | ||||
| ΔBlood[lactate] (mm) | 6.9 ± 1.6 | 7.0 ± 0.8 | 6.5 ± 2.1 | 5.5 ± 1.5 |
| End-exercise HR (beatsmin−1) | 176 ± 8 | 177 ± 8 | 174 ± 12 | 172 ± 13 |
| HR time constant (s) | 44 ± 17 | 38 ± 8 | 50 ± 19 | 49 ± 17 |
| Severe exercise | ||||
| ΔBlood[lactate] (mm) | 5.4 ± 1.7 | 4.4 ± 2.0 | 3.9 ± 1.5 | 4.5 ± 1.6 |
| End-exercise HR (beats min−1) | 178 ± 7 | 177 ± 7 | 180 ± 6 | 182 ± 6 |
| HR time constant (s) | 37 ± 14 | 45 ± 20 | 53 ± 29 | 64 ± 31 |
Discussion
This is the first study to comprehensively investigate the influence of RhEPO treatment on the pulmonary V˙O2 response to both ramp exercise and step exercise (in each of the moderate, heavy, and severe exercise intensity domains) in the same healthy subjects. The principal results of this study were that RhEPO treatment: (1) resulted in a significant increase in [Hb] and Hct, and a significant increase in V˙O2,peak during the ramp incremental exercise test (consistent with our first hypothesis); (2) did not significantly influence τp during moderate or heavy-intensity exercise (consistent with our second hypothesis); (3) did not significantly influence the amplitude of the V˙O2 slow component (not consistent with our third hypothesis), and (4) increased time to exhaustion and the V˙O2 attained at exhaustion during severe-intensity exercise, but did not significantly influence τp (not consistent with our fourth hypothesis). The results demonstrate that an increased blood O2-carrying capacity, and therefore a greater potential for O2 supply to muscle, resulted in a greater peak rate of oxidative energy metabolism (increased V˙O2,peak) and an enhanced exercise tolerance during severe exercise, but did not alter the kinetics of the V˙O2 response following the onset of moderate, heavy or severe-intensity step exercise. These data are consistent with the notion that the fundamental component (Phase II) V˙O2 kinetics are principally regulated/limited by factors intrinsic to the myocytes rather than by muscle O2 availability, at least in the exercise model used in the present study. Moreover, the data indicate that enhancing blood O2-carrying capacity does not alter V˙O2 kinetics (despite increasing V˙O2,peak) even at ‘supramaximal’ work rates where the required V˙O2 exceeds the V˙O2,peak.
Influence of RhEPO on haematological measurements and V˙O2,peak
As expected, four weeks of RhEPO treatment (150 IU (kg body mass)−1 week−1) resulted in a significant increase in both [Hb] and Hct, with no change in the control group. The time course and magnitude of the effects of RhEPO on [Hb] and Hct in our study was similar to those reported in previous studies (Berglund & Ekblom, 1991; Birkeland et al. 2000; Parisotto et al. 2000; Russell et al. 2002; Connes et al. 2003), and can be calculated to have improved the arterial blood O2-carrying capacity by ∼7%. With the assumption that muscle blood flow was not attenuated during exercise following RhEPO treatment (see ‘Limitations’), this would represent an increase in the bulk O2 delivery to muscle also of the order of 7%. Interestingly, the V˙O2,peak and peak power output measured during the ramp incremental test were also increased by ∼7% in the RhEPO group, with no change in the control group in whom there was no change in [Hb] or Hct (Fig. 1). The 7% improvement in V˙O2,peak with RhEPO treatment is very similar to that reported previously (Ekblom & Berglund, 1991; Birkeland et al. 2000; Parisotto et al. 2000; Russell et al. 2002; Connes et al. 2003), and supports the notion that the V˙O2,peak is primarily limited by the capacity of the cardiovascular system to deliver O2 to the exercising muscles (Saltin & Strange, 1992; Wagner, 2000; Gonzalez-Alonso & Calbet, 2003). It is not clear whether microvascular haematocrit was enhanced in line with systemic haematocrit following RhEPO treatment but, if so, then it is possible that an enhanced diffusional O2 conductance might have contributed to the increased V˙O2,peak (Wagner, 1996; Wagner, 2000).
The significant increases in [Hb], Hct and V˙O2,peak in the RhEPO group in the present study indicate that our experimental intervention was successful in enhancing arterial blood O2-carrying capacity, and therefore the potential for an increased muscle O2 delivery during exercise. Interestingly, despite the significant increase in V˙O2,peak with RhEPO treatment, there was no significant change in the GET. Although some earlier studies suggested that the GET might be sensitive to muscle O2 availability, the present study suggests that other factors are more important, a conclusion that is consistent with another recent report in which V˙O2,peak was reduced following inhibition of nitric oxide synthase, with no change in the GET (Jones et al. 2004).
Influence of RhEPO on Phase II V˙O2 kinetics
Despite the enhanced potential for an increase in O2 delivery to muscle, we could discern no significant effect of RhEPO treatment on τp at any of the three exercise intensities we studied (Figs 2 and 3). The lack of a significant effect of RhEPO treatment on blood lactate accumulation provides indirect support to our finding that V˙O2 kinetics (and therefore the O2 deficit) were not altered by the intervention. However, an important issue when considering our results is the sensitivity of our methods to detect a speeding of V˙O2 kinetics following RhEPO treatment if one had actually occurred. In this respect, we were careful to average together the responses to an appropriate number of identical step transitions to each of the moderate, heavy and severe work rates in order to reduce breath-by-breath variability, and increase confidence in the physiological interpretations derived from our model fits (Lamarra et al. 1987). The mean 95% CIs surrounding our estimates of τp were ±2 s, ±3 s and ±3 s for moderate, heavy, and severe exercise, respectively. These CIs suggest that we would have been able to discern all but very small changes in V˙O2 kinetics resulting from the intervention. Our data therefore strongly suggest that RhEPO treatment does not alter τp during moderate, heavy or severe-intensity upright cycle exercise in young healthy subjects.
The extent to which muscle O2 availability potentially limits V˙O2 kinetics during ‘submaximal’ (i.e. moderate and heavy) exercise has been extensively debated (Tschakovsky & Hughson, 1999; Poole & Jones, 2005). It has been demonstrated that O2 availability can limit V˙O2 kinetics in a number of situations, for example in exercise modalities where muscle perfusion pressure might be reduced, for example in supine cycling (Hughson et al. 1993; Koga et al. 1999) and arm cranking (Hughson & Inman, 1986; Koppo & Bouckaert, 2005), and also in the elderly and individuals with disease conditions that affect the cardiovascular system (Poole et al. 2005). Additionally, the inspiration of hypoxic gas mixtures results in a lengthening of τp (Engelen et al. 1996), indicating that V˙O2 kinetics become slower when muscle O2 delivery is sufficiently reduced. However, in exercise modalities where the exercising muscle mass is below the level of the heart, there is evidence that muscle O2 supply does not normally limit V˙O2 kinetics. For example, most studies appear to indicate that cardiac output and muscle blood flow kinetics are faster than muscle V˙O2 kinetics in the transition from a very low to a higher metabolic rate (De Cort et al. 1991; MacDonald et al. 1998; Bangsbo et al. 2000), suggesting that O2 supply to muscle is probably in excess of its requirements across the rest-to-exercise transient.
‘Priming’ bouts of prior exercise have been used extensively in an effort to manipulate (enhance) muscle O2 availability before and during a subsequent bout of heavy exercise (Jones et al. 2003a). It has been suggested that the performance of a prior bout of high-intensity exercise (which elicits a metabolic acidosis) enhances muscle O2 availability by increasing muscle vasodilatation, and by right-shifting the O2 dissociation curve (Gerbino et al. 1996; MacDonald et al. 1997). Several studies have indeed shown that muscle blood flow and/or oxygenation is/are enhanced during exercise when it is preceded by such a ‘priming’ exercise bout (Krustrup et al. 2001; Hughson et al. 2003). Despite this, however, the vast majority of studies indicate that the performance of prior high-intensity exercise has no effect on τp during subsequent moderate or heavy exercise, at least in young healthy subjects performing upright cycle exercise (Jones et al. 2003a). Another intervention that has been used with the goal of enhancing muscle O2 availability is the inspiration of hyperoxic gas mixtures. However, to date, hyperoxia has not resulted in a reduction in τp (MacDonald et al. 1997, 2000), muscle substrate phosphorylation (Evans et al. 2001; Savasi et al. 2002), or muscle [phosphocreatine] kinetics (Haseler et al. 2004). Our data are consistent with these previous studies in suggesting that an enhanced potential for muscle O2 delivery (with RhEPO treatment) does not lead to a speeding of the Phase II V˙O2 kinetics during moderate or heavy exercise.
It is generally considered that O2 availability might represent a progressively more important limitation to V˙O2 kinetics at exercise intensities exceeding the GET (Gerbino et al. 1996; Tordi et al. 2003; Poole & Jones, 2005). Indeed, it has been reported that pump perfusing isolated canine muscle at the ‘steady state’ blood flow requirement did not alter muscle V˙O2 kinetics during contractions requiring ∼65% V˙O2,peak (Grassi et al. 1998a), but caused a significant speeding of V˙O2 kinetics at contractions requiring ∼100% V˙O2,peak (τp reduced from ∼17 to ∼12 s; Grassi et al. 2000). These latter data suggest that muscle O2 delivery, along with an inherent oxidative metabolic inertia, might limit V˙O2 kinetics at near-maximal exercise intensities. However, our results did not support our hypothesis that τp would be reduced during severe exercise following RhEPO treatment. Rather, RhEPO treatment resulted in a 22% increase in the time to exhaustion, and a 5% increase in the absolute V˙O2 attained at the end of exercise (consistent with the increased V˙O2,peak measured in the ramp incremental test), but without altering the kinetics of the V˙O2 response (Figs 2 and 3). These data indicate that an enhancement of muscle O2 delivery increases the maximum rate of O2 consumption (V˙O2,peak), but does not speed the rate at which V˙O2 increases towards this maximum. The present data are consistent with our previous study in which we reported that the performance of prior sprint exercise (which resulted in enhanced muscle O2 availability as determined using near infra-red spectroscopy) had no significant effect on τp but increased the V˙O2 achieved at exhaustion during subsequent severe-intensity exercise (Wilkerson et al. 2004b). This apparent dissociation between the effect of an altered muscle O2 delivery on V˙O2,peak and on V˙O2 kinetics is also evident in the study of Nybo et al. (2001), who reported that hyperthermia resulted in a significant reduction in V˙O2,peak but without altering the rate at which V˙O2 increased towards the peak value. Collectively, these studies indicate that V˙O2,peak is limited by muscle O2 delivery, but that V˙O2 kinetics are principally regulated by intracellular (metabolic) factors, even during severe-intensity cycle exercise.
Our results contrast with the recent study of Connes et al. (2003). These authors reported that 4 weeks of RhEPO treatment (which had similar effects on [Hb], Hct and V˙O2,peak as our study) resulted in a significant 18% reduction in τp during moderate-intensity exercise (65% V˙O2,peak) in nine endurance athletes. There was no change in τp in a control group who received saline injections as a placebo. The reason for the different results between our study and that of Connes et al. (2003) is not clear. One obvious difference between the two studies is the training status of the recruited subjects: endurance-trained athletes in theirs (V˙O2,peak ∼64 ml kg−1 min−1), and recreationally active individuals in ours (V˙O2,peak ∼49 ml kg−1 min−1). It has been suggested that subjects with higher aerobic fitness might be more sensitive to O2 delivery limitations to V˙O2,peak than subjects of lower aerobic fitness (Wagner, 2000), although whether this is also true for V˙O2 kinetics is not known. It should also be considered that trained subjects might be more sensitive to a potential alteration in microvascular haematocrit with RhEPO treatment (and therefore an enhanced diffusive O2 conductance) compared to untrained subjects. Another possible reason for the difference between the two studies is that the subjects in the study of Connes et al. (2003) only completed a single rest-to-exercise transition before and after the intervention period. The selected exercise intensity of 65% V˙O2,peak lay close to the GET in these subjects as evidenced by the fact that three subjects (of nine) in the experimental group and two subjects (of seven) in the control group demonstrated a V˙O2 slow component. Connes et al. (2003) modelled the V˙O2 data with either single or double exponential terms with delays, with the fit commencing at the onset of exercise. It is possible that these procedures did not provide adequate confidence in the model fit (Whipp & Rossiter, 2005). The surprisingly large τp values reported by Connes et al. (2003) for their endurance-trained subjects (∼35 s in the control condition when values of ∼15 s might have been expected; e.g. Koppo et al. 2004) is consistent with this suggestion.
Overall, our results are consistent with the notion that muscle O2 delivery is sufficient for the metabolic requirements during moderate, heavy and severe upright cycle exercise in young healthy subjects, such that V˙O2 kinetics are principally regulated and/or limited by intracellular processes. It has been proposed that an inertia of the oxidative metabolic processes within the myocytes (such as relatively slow activation of one or more of the key oxidative enzymes) is principally responsible for the relatively slow rate at which V˙O2 increases following the onset of exercise (relative to the, assumed, immediate step increase in metabolic demand) (Cerretelli et al. 1980; Whipp & Mahler, 1980). The control of muscle oxidative metabolism appears to be intricately linked to the splitting of high-energy phosphates in the cytosol. For example, Rossiter et al. (2002) have reported a close agreement between the time constant for the fall in muscle [phosphocreatine] following the onset of exercise and the time constant for the rise in pulmonary V˙O2 in Phase II. Moreover, Kindig et al. (2005) have shown that acute inhibition of creatine kinase (CK) in single isolated myocytes resulted in a significant speeding of intracellular (Pi02) kinetics (equivalent to faster V˙O2 kinetics in this model). One other source of oxidative metabolic inertia appears to be the influence of nitric oxide (NO) on mitochondrial function: in vivo, NO has been demonstrated both to inhibit a number of key oxidative enzymes and to compete with O2 for the binding site at cytochrome c oxidase, the final electron acceptor in the electron transport chain (Reid, 1998). Inhibiting the production of nitric ixide synthase (NOS) (with the drug l-NAME) has been shown to result in a significant reduction in τp both in the horse (Kindig et al. 2001, 2002) and in humans (Jones et al. 2003b; Wilkerson et al. 2004a).
Influence of RhEPO on the V˙O2 slow component
In the present study, RhEPO treatment had no significant effect on the amplitude of the V˙O2 ‘slow component’ during heavy or severe exercise. This is in contrast to other interventions such as priming exercise (Gerbino et al. 1996; MacDonald et al. 1997; Burnley et al. 2000) and hyperoxic gas breathing (MacDonald et al. 1997; Haseler et al. 2004), which have consistently resulted in reductions in the amplitude of the V˙O2 slow component. The physiology of the V˙O2 slow component phenomenon remains somewhat obscure, but there is growing evidence that it is linked either directly or indirectly to the recruitment of type II muscle fibres at higher work rates and/or to metabolic changes occurring within the initially recruited fibres (Jones et al. 2005). It is possible that an enhanced muscle O2 supply and/or improved distribution of O2 within working muscles resulting from prior exercise or hyperoxic gas breathing interventions, might attenuate the V˙O2 slow component by reducing fatigue in initially recruited fibres and therefore limiting the requirement for the recruitment of additional (higher-order) fibres as exercise continues. That we observed no effect of RhEPO treatment on the V˙O2 slow component therefore suggests either that muscle O2 delivery was not enhanced following RhEPO treatment during heavy exercise (see ‘Limitations’) or that the V˙O2 slow component is not sensitive to enhanced muscle O2 delivery. Presumably, the principal effect of RhEPO treatment is to enhance muscle O2 availability by improving (the potential for) convective O2 delivery to muscle, whereas the principle effect of hyperoxic gas breathing is to improve the potential for O2 diffusion from the blood to the myocytes by increasing mean capillary PO2 (Hogan et al. 1992). Prior exercise models potentially enhance both convective and diffusive O2 delivery through the effects of the residual metabolic acidosis on muscle vasodilatation and the oxyhaemoglobin dissociation curve, although simultaneous ‘priming’ effects on muscle metabolic processes might be more important (Jones et al. 2003a). That the V˙O2 slow component is attenuated by both hyperoxia (MacDonald et al. 1997) and prior heavy exercise (Gerbino et al. 1996; Burnley et al. 2000), but not by RhEPO treatment (present study) or by fixing blood flow at the required steady state level across the transient (Grassi et al. 2000), suggests that the slow component might be related in some manner to peripheral O2 diffusion, rather than bulk O2 delivery, limitations.
Influence of RhEPO on exercise tolerance and performance
To our knowledge, this is the first study to investigate the influence of RhEPO treatment on exercise performance. In addition to the ∼7% improvement in V˙O2,peak and peak power output attained on the ramp incremental test, the time to exhaustion during severe exercise (∼105% V˙O2,peak) was extended by 35 s (or 22%) following RhEPO treatment, an effect that was associated with the attainment of a higher V˙O2 at the end of exercise. A 22% increase in time to exhaustion at a constant work rate is equivalent to a ∼1–2% improvement in time trial performance over an equivalent distance (Hopkins et al. 1999). This is similar to the effect predicted by Birkeland et al. (2000) and translates into a reduction in the time to run 1500 m of ∼3 s in an elite athlete, an effect that is certainly likely to be meaningful.
We would stress that although our results do not support a role for an enhanced blood O2-carrying capacity in speeding V˙O2 kinetics in young healthy subjects possessing normal haematology (pre-test [Hb] and Hct of ∼16.0 g dl−1 and ∼43%, respectively), it remains possible that RhEPO treatment could speed V˙O2 kinetics during submaximal exercise and hence contribute to enhanced exercise tolerance and improved quality of life in clinical conditions in which blood O2-carrying capacity, and hence muscle O2 delivery, is impaired (e.g. anaemia, renal failure, Marrades et al. 1996). Indeed, even in the absence of a speeding of V˙O2 kinetics, the enhanced V˙O2,peak with RhEPO treatment should be beneficial to exercise tolerance, since any absolute work rate would require a lower fraction of the new V˙O2,peak.
Limitations
It should, of course, be considered that despite the enhanced blood O2-carrying capacity and increased V˙O2,peak following RhEPO treatment, there was actually no significant alteration in muscle O2 delivery across the transient during step exercise. For example, some studies (e.g. Welch et al. 1977), though by no means all (e.g. Knight et al. 1993), have indicated that muscle blood flow is reduced during the inspiration of hyperoxic gas mixtures, such that O2 delivery to muscle might not be appreciably changed. We did not measure muscle blood flow and therefore it cannot be discounted that a similar effect occurred in the present study. However, it has been reported that the reduction in leg blood flow was not sufficient to fully cancel out the increased O2-carrying capacity following RhEPO treatment in chronic renal failure patients such that muscle O2 delivery was significantly enhanced (Marrades et al. 1996). Moreover, to the extent that HR kinetics reflect the kinetics of cardiac output and muscle blood flow, our measurements suggest that RhEPO treatment had a rather limited effect on the cardiovascular response to step exercise. The absolute HR at any specific time point was typically 1–2% lower after, compared to before, RhEPO treatment (much less than the 7% improvement in arterial O2-carrying capacity). A similarly small reduction in HR during submaximal exercise following RhEPO treatment has been reported previously (Russell et al. 2002; Connes et al. 2003). Collectively, these results, along with the significant improvement in V˙O2,peak, suggest that muscle O2 delivery was probably enhanced following RhEPO treatment.
Our subjects only received 4 weeks of RhEPO treatment because we wished to avoid possible medical complications arising from the achievement of very high [Hb] and Hct values (>17 g dl−1 and >50%, respectively). It is possible that a greater muscle O2 delivery might have been achieved if we had used a higher dose of RhEPO or a longer treatment period, and that this might have influenced the V˙O2 kinetics. However, any positive effect of an increased O2-carrying capacity in this situation might be counterbalanced by the negative effect of an increased blood viscosity (Linde et al. 1992).
Conclusions
In summary, we have shown for the first time that 4 weeks of RhEPO treatment, which resulted in a significant increase in [Hb] and V˙O2,peak, did not significantly influence V˙O2 kinetics during moderate-, heavy-, or severe-intensity step cycle exercise. During severe exercise, the time to exhaustion and the V˙O2 attained at the end of exercise were significantly increased by RhEPO treatment, with no change in τp, suggesting that muscle O2 delivery limits the highest attainable V˙O2 but not the dynamic adaptation of V˙O2 to a step change in work rate. Our results are therefore consistent with the notion that V˙O2 kinetics are principally regulated by intracellular (metabolic) factors and not by O2 supply, at least during upright cycle exercise in healthy young subjects, and even when the imposed work rate has a V˙O2 requirement that exceeds the V˙O2,peak.
Acknowledgments
The authors express their gratitude to Roche Pharmaceuticals Ltd for their generous support.
References
- Bangsbo J, Krustrup P, Gonzalez-Alonso J, Saltin B. Muscle oxygen kinetics at onset of intense dynamic exercise in humans. Am J Physiol. 2000;279:R899–R906. doi: 10.1152/ajpregu.2000.279.3.R899. [DOI] [PubMed] [Google Scholar]
- Berglund B, Ekblom B. Ffect of recombinant human erythropoietin treatment on blood pressure and some haematological parameters in healthy men. J Intern Med. 1991;229:125–130. doi: 10.1111/j.1365-2796.1991.tb00319.x. [DOI] [PubMed] [Google Scholar]
- Birkeland KI, Stray-Gundersen J, Hemmersbach P, Hallen J, Haug E, Bahr R. Effect of RhEPO administration on serum levels of sTfR and cycling performance. Med Sci Sports Exerc. 2000;32:1238–1243. doi: 10.1097/00005768-200007000-00009. [DOI] [PubMed] [Google Scholar]
- Burnley M, Jones AM, Carter H, Doust JH. Effect of prior heavy exercise on phase II pulmonary oxygen uptake kinetics and the slow component during heavy exercise in humans. J Appl Physiol. 2000;89:1387–1396. doi: 10.1152/jappl.2000.89.4.1387. [DOI] [PubMed] [Google Scholar]
- Cerretelli P, Rennie DW, Pendergast DP. Kinetics of metabolic transients during exercise. In: Cerretelli P, Whipp BJ, editors. Exercise Bioenergetics and Gas Exchange. Amsterdam: Elsevier; 1980. pp. 187–209. [Google Scholar]
- Connes P, Perrey S, Varrat A, Prefaut C, Caillaud C. Faster oxygen uptake kinetics at the onset of submaximal cycling exercise following 4 weeks recombinant human erythropoietin (r-HuEPO) treatment. Pflugers Arch. 2003;447:231–238. doi: 10.1007/s00424-003-1174-0. [DOI] [PubMed] [Google Scholar]
- De Cort SC, Innes JA, Barstow TJ, Guz A. Cardiac output, oxygen consumption and arteriovenous oxygen difference following a sudden rise in exercise level in humans. J Physiol. 1991;441:501–512. doi: 10.1113/jphysiol.1991.sp018764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delp MD, O'Leary DS. Integrative control of the skeletal muscle microcirculation in the maintenance of arterial pressure during exercise. J Appl Physiol. 2004;97:1112–1118. doi: 10.1152/japplphysiol.00147.2003. [DOI] [PubMed] [Google Scholar]
- Ekblom B, Berglund B. Effect of erythropoietin administration on maximal aerobic power. Scand J Med Sci Sports. 1991;1:88–93. [Google Scholar]
- Engelen M, Porszasz J, Riley M, Wasserman K, Maehara K, Barstow TJ. Effects of hypoxic hypoxia on O2 uptake and heart rate kinetics during heavy exercise. J Appl Physiol. 1996;81:2500–2508. doi: 10.1152/jappl.1996.81.6.2500. [DOI] [PubMed] [Google Scholar]
- Evans MK, Savasi I, Heigenhauser GJ, Spriet LL. Effects of acetate infusion and hyperoxia on muscle substrate phosphorylation after onset of moderate exercise. Am J Physiol. 2001;281:E1144–E1150. doi: 10.1152/ajpendo.2001.281.6.E1144. [DOI] [PubMed] [Google Scholar]
- Gerbino A, Ward SA, Whipp BJ. Effects of prior exercise on pulmonary gas-exchange kinetics during high-intensity exercise in humans. J Appl Physiol. 1996;80:99–107. doi: 10.1152/jappl.1996.80.1.99. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Alonso J, Calbet JAL. Reductions in systemic and skeletal muscle blood flow and oxygen delivery limit maximal aerobic capacity in humans. Circulation. 2003;107:824–830. doi: 10.1161/01.cir.0000049746.29175.3f. [DOI] [PubMed] [Google Scholar]
- Grassi B, Gladden LB, Samaja M, Stary CM, Hogan MC. Faster adjustment of O2 delivery does not affect V˙O2 on-kinetics in isolated in situ canine muscle. J Appl Physiol. 1998a;85:1394–1403. doi: 10.1152/jappl.1998.85.4.1394. [DOI] [PubMed] [Google Scholar]
- Grassi B, Gladden LB, Stary CM, Wagner PD, Hogan MC. Peripheral O2 diffusion does not affect V˙O2 on-kinetics in isolated in situ canine muscle. J Appl Physiol. 1998b;85:1404–1412. doi: 10.1152/jappl.1998.85.4.1404. [DOI] [PubMed] [Google Scholar]
- Grassi B, Hogan MC, Kelley KM, Aschenbach WG, Hamann JJ, Evans RK, Patillo RE, Gladden LB. Role of convective O2 delivery in determining V˙O2 on-kinetics in canine muscle contracting at peak V˙O2. J Appl Physiol. 2000;89:1293–1301. doi: 10.1152/jappl.2000.89.4.1293. [DOI] [PubMed] [Google Scholar]
- Grassi B, Poole DC, Richardson RS, Knight DR, Erickson BK, Wagner PD. Muscle O2 uptake kinetics in humans: implications for metabolic control. J Appl Physiol. 1996;80:988–998. doi: 10.1152/jappl.1996.80.3.988. [DOI] [PubMed] [Google Scholar]
- Haseler LJ, Kindig CA, Richardson RS, Hogan MC. The role of oxygen in determining phosphocreatine onset kinetics in exercising humans. J Physiol. 2004;558:985–992. doi: 10.1113/jphysiol.2004.062042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan MC, Willford DC, Keipert PE, Faithfull NS, Wagner PD. Increased plasma O2 solubility improves O2 uptake of in situ dog muscle working maximally. J Appl Physiol. 1992;73:2470–2475. doi: 10.1152/jappl.1992.73.6.2470. [DOI] [PubMed] [Google Scholar]
- Hopkins WG, Hawley JA, Burke LM. Design and analysis of research on sport performance enhancement. Med Sci Sports Exerc. 1999;31:472–485. doi: 10.1097/00005768-199903000-00018. [DOI] [PubMed] [Google Scholar]
- Hughson RL, Cochrane JE, Butler GC. Faster O2 uptake kinetics at onset of supine exercise with than without lower body negative pressure. J Appl Physiol. 1993;75:1962–1967. doi: 10.1152/jappl.1993.75.5.1962. [DOI] [PubMed] [Google Scholar]
- Hughson RL, Inman MD. Faster kinetics of V˙O2 during arm exercise with circulatory occlusion of the legs. Int J Sports Med. 1986;7:22–25. doi: 10.1055/s-2008-1025729. [DOI] [PubMed] [Google Scholar]
- Hughson RL, Schijvens H, Burrows S, Devitt D, Betik AC, Hopman MTE. Blood flow and metabolic control at the onset of heavy exercise. Int J Sport Health Sci. 2003;1:1–9. [Google Scholar]
- Jones AM, Koppo K, Burnley M. Effects of prior exercise on metabolic and gas exchange responses to exercise. Sports Med. 2003a;33:949–971. doi: 10.2165/00007256-200333130-00002. [DOI] [PubMed] [Google Scholar]
- Jones AM, Poole DC. Oxygen uptake dynamics: from muscle to mouth. Med Sci Sports Exerc. 2005 doi: 10.1249/01.mss.0000177466.01232.7e. (in press) [DOI] [PubMed] [Google Scholar]
- Jones AM, Pringle JSM, Carter H. Influence of muscle fibre type and motor unit recruitment on V˙O2 kinetics. In: Jones AM, Poole DC, editors. Oxygen Uptake Kinetics in Sport, Exercise and Medicine. London: Routledge; 2005. pp. 261–293. [Google Scholar]
- Jones AM, Wilkerson DP, Campbell IT. Nitric oxide synthase inhibition with l-NAME reduces maximal oxygen uptake but not gas exchange threshold during incremental cycle exercise in man. J Physiol. 2004;560:329–338. doi: 10.1113/jphysiol.2004.065664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AM, Wilkerson DP, Koppo K, Wilmshurst S, Campbell IT. Inhibition of nitric oxide synthase by l-NAME speeds phase II pulmonary V˙O2 kinetics in the transition to moderate-intensity exercise in man. J Physiol. 2003b;552:265–272. doi: 10.1113/jphysiol.2003.045799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindig CA, Howlett RA, Stary CM, Walsh B, Hogan MC. Effects of acute creatine kinase inhibition on metabolism and tension development in isolated single myocytes. J Appl Physiol. 2005;98:541–549. doi: 10.1152/japplphysiol.00354.2004. [DOI] [PubMed] [Google Scholar]
- Kindig CA, McDonough P, Erickson HH, Poole DC. Effect of l-NAME on oxygen uptake kinetics during heavy-intensity exercise in the horse. J Appl Physiol. 2001;91:891–896. doi: 10.1152/jappl.2001.91.2.891. [DOI] [PubMed] [Google Scholar]
- Kindig CA, McDonough P, Erickson HH, Poole DC. Nitric oxide synthase inhibition speeds oxygen uptake kinetics in horses during moderate domain running. Respir Physiol Neurobiol. 2002;132:169–178. doi: 10.1016/s1569-9048(02)00068-x. [DOI] [PubMed] [Google Scholar]
- Knight DR, Schaffartzik W, Poole DC, Hogan MC, Bebout DE, Wagner PD. Effects of hyperoxia on maximal leg O2 supply and utilization in men. J Appl Physiol. 1993;75:2586–2594. doi: 10.1152/jappl.1993.75.6.2586. [DOI] [PubMed] [Google Scholar]
- Koga S, Shiojiri T, Shibasaki M, Kondo N, Fukuba Y, Barstow TJ. Kinetics of oxygen uptake during supine and upright heavy exercise. J Appl Physiol. 1999;87:253–260. doi: 10.1152/jappl.1999.87.1.253. [DOI] [PubMed] [Google Scholar]
- Koppo K, Bouckaert J. The effect of prior high-intensity cycling exercise on the V˙O2 kinetics during high-intensity cycling exercise is situated at the additional slow component. Int J Sports Med. 2001;22:21–26. doi: 10.1055/s-2001-11335. [DOI] [PubMed] [Google Scholar]
- Koppo K, Bouckaert J. Prior arm exercise speeds the V˙O2 kinetics during arm exercise above the heart level. Med Sci Sports Exerc. 2005;37:613–619. doi: 10.1249/01.mss.0000159013.20244.f8. [DOI] [PubMed] [Google Scholar]
- Koppo K, Bouckaert J, Jones AM. Effects of training status and exercise intensity on phase II V˙O2 kinetics. Med Sci Sports Exerc. 2004;36:225–232. doi: 10.1249/01.MSS.0000113473.48220.20. [DOI] [PubMed] [Google Scholar]
- Krustrup P, Gonzalez-Alonso J, Quistorff B, Bangsbo J. Muscle heat production and anaerobic energy turnover during repeated intense dynamic exercise in humans. J Physiol. 2001;536:947–956. doi: 10.1111/j.1469-7793.2001.00947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamarra N, Whipp BJ, Ward SA, Wasserman K. Effect of interbreath fluctuations on characterizing exercise gas exchange kinetics. J Appl Physiol. 1987;62 doi: 10.1152/jappl.1987.62.5.2003. [DOI] [PubMed] [Google Scholar]
- Linde T, Sandhagen B, Danielson BG, Wikstrom B. Impaired erythrocyte fluidity during treatment of renal anaemia with erythropoietin. J Intern Med. 1992;231:601–606. doi: 10.1111/j.1365-2796.1992.tb01246.x. [DOI] [PubMed] [Google Scholar]
- MacDonald MJ, Pedersen PK, Hughson RL. Acceleration of V˙O2 kinetics in heavy submaximal exercise by hyperoxia and prior high-intensity exercise. J Appl Physiol. 1997;83:1318–1325. doi: 10.1152/jappl.1997.83.4.1318. [DOI] [PubMed] [Google Scholar]
- MacDonald MJ, Shoemaker JK, Tschakovsky ME, Hughson RL. Alveolar oxygen uptake and femoral artery blood flow dynamics in upright and supine leg exercise in humans. J Appl Physiol. 1998;85:1622–1628. doi: 10.1152/jappl.1998.85.5.1622. [DOI] [PubMed] [Google Scholar]
- MacDonald MJ, Tarnopolsky MA, Hughson RL. Effect of hyperoxia and hypoxia on leg blood flow and pulmonary and leg oxygen uptake at the onset of kicking exercise. Can J Physiol Pharmacol. 2000;78:67–74. doi: 10.1139/cjpp-78-1-67. [DOI] [PubMed] [Google Scholar]
- Marrades RM, Roca J, Campistol JM, Diaz O, Barbera JA, Torregrosa JV, Masclans JR, Cobos A, Rodriguez-Roisin R, Wagner PD. Effects of erythropoietin on muscle O2 transport during exercise in patients with chronic renal failure. J Clin Invest. 1996b;97:2092–2100. doi: 10.1172/JCI118646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nybo L, Jensen T, Nielsen B, Gonzalez-Alonso J. Effects of marked hyperthermia with and without dehydration on V˙O2 kinetics during intense exercise. J Appl Physiol. 2001;90:1057–1064. doi: 10.1152/jappl.2001.90.3.1057. [DOI] [PubMed] [Google Scholar]
- Parisotto R, Gore CJ, Emslie KR, Ashenden MJ, Brugnara C, Howe C, Martin DT, Trout GJ, Hahn AG. A novel method utilising markers of altered erythropoiesis for the detection of recombinant human erythropoietin abuse in athletes. Haematologica. 2000;85:564–572. [PubMed] [Google Scholar]
- Poole DC, Jones AM. Towards an understanding of the mechanistic bases of V˙O2 kinetics. In: Jones AM, Poole DC, editors. Oxygen Uptake Kinetics in Sport, Exercise and Medicine. London: Routledge; 2005. pp. 298–328. [Google Scholar]
- Poole DC, Kindig CA, Behnke BJ. V˙O2 kinetics in different disease states. In: Jones AM, Poole DC, editors. Oxygen Uptake Kinetics in Sport, Exercise and Medicine. London: Routledge; 2005. pp. 298–328. [Google Scholar]
- Reid MB. Role of nitric oxide in skeletal muscle: synthesis, distribution and functional importance. Acta Physiol Scand. 1998;162:401–409. doi: 10.1046/j.1365-201X.1998.0303f.x. [DOI] [PubMed] [Google Scholar]
- Rossiter HB, Ward SA, Kowalchuk JM, Howe FA, Griffiths JR, Whipp BJ. Dynamic asymmetry of phosphocreatine concentration and O2 uptake between the on- and off-transients of moderate- and high-intensity exercise in humans. J Physiol. 2002;541:991–1002. doi: 10.1113/jphysiol.2001.012910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell G, Gore CJ, Ashenden MJ, Parisotto R, Hahn AG. Effects of prolonged low doses of recombinant human erythropoietin during submaximal and maximal exercise. Eur J Appl Physiol. 2002;86:442–449. doi: 10.1007/s00421-001-0560-6. [DOI] [PubMed] [Google Scholar]
- Saltin B, Strange S. Maximal oxygen uptake: ‘old’ and ‘new’ arguments for a cardiovascular limitation. Med Sci Sports Exerc. 1992;24:30–37. [PubMed] [Google Scholar]
- Savasi I, Evans MK, Heigenhauser GJ, Spriet LL. Skeletal muscle metabolism is unaffected by DCA infusion and hyperoxia after onset of intense aerobic exercise. Am J Physiol. 2002;283:E108–E115. doi: 10.1152/ajpendo.00337.2001. [DOI] [PubMed] [Google Scholar]
- Tordi N, Perrey S, Harvey A, Hughson RL. Oxygen uptake kinetics during two bouts of heavy cycling separated by fatiguing sprint exercise in humans. J Appl Physiol. 2003;94:533–541. doi: 10.1152/japplphysiol.00532.2002. [DOI] [PubMed] [Google Scholar]
- Tschakovsky ME, Hughson RL. Interaction of factors determining oxygen uptake at the onset of exercise. J Appl Physiol. 1999;86:1101–1113. doi: 10.1152/jappl.1999.86.4.1101. [DOI] [PubMed] [Google Scholar]
- Wagner PD. Determinants of maximal oxygen transport and utilization. Annu Rev Physiol. 1996;58:21–50. doi: 10.1146/annurev.ph.58.030196.000321. [DOI] [PubMed] [Google Scholar]
- Wagner PD. New ideas on limitations to V˙O2max. Exerc Sport Sci Rev. 2000;28:10–14. [PubMed] [Google Scholar]
- Wasserman K, Hansen JE, Sue DY, Whipp BJ, Casaburi R. Principles of Exercise Testing and Prescription. Philadelphia: Lea & Febiger; 1994. [Google Scholar]
- Welch HG, Bonde-Petersen F, Graham T, Klausen K, Secher N. Effects of hyperoxia on leg blood flow and metabolism during exercise. J Appl Physiol. 1977;42:385–390. doi: 10.1152/jappl.1977.42.3.385. [DOI] [PubMed] [Google Scholar]
- Whipp BJ, Davis JA, Torres F, Wasserman K. A test to determine parameters of aerobic function during exercise. J Appl Physiol. 1981;50:217–221. doi: 10.1152/jappl.1981.50.1.217. [DOI] [PubMed] [Google Scholar]
- Whipp BJ, Mahler M. Dynamics of pulmonary gas exchange during exercise. In: West JB, editor. Pulmonary Gas Exchange. New York: Academic Press; 1980. pp. 33–96. [Google Scholar]
- Whipp BJ, Rossiter HB. The kinetics of oxygen uptake: physiological inferences from the parameters. In: Jones AM, Poole DC, editors. Oxygen Uptake Kinetics in Sport, Exercise and Medicine. London: Routledge; 2005. pp. 62–114. [Google Scholar]
- Whipp BJ, Wasserman K. Oxygen uptake kinetics for various intensities of constant-load work. J Appl Physiol. 1972;33:351–356. doi: 10.1152/jappl.1972.33.3.351. [DOI] [PubMed] [Google Scholar]
- Wilkerson DP, Campbell IT, Jones AM. Influence of nitric oxide synthase inhibition on pulmonary O2 uptake kinetics during supra-maximal exercise in humans. J Physiol. 2004a;561:623–635. doi: 10.1113/jphysiol.2004.071894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkerson DP, Koppo K, Barstow TJ, Jones AM. Effect of prior multiple-sprint exercise on pulmonary O2 uptake kinetics following the onset of perimaximal exercise. J Appl Physiol. 2004b;97:1227–1236. doi: 10.1152/japplphysiol.01325.2003. [DOI] [PubMed] [Google Scholar]