Abstract
Apart from its role in elevating red blood cell number, erythropoietin (Epo) exerts protective functions in brain, retina and heart upon ischaemic injury. However, the physiological non-erythroid functions of Epo remain unclear. Here we use a transgenic mouse line (Tg21) constitutively overexpressing human Epo in brain to investigate Epo's impact on ventilation upon hypoxic exposure. Tg21 mice showed improved ventilatory response to severe acute hypoxia and moreover improved ventilatory acclimatization to chronic hypoxic exposure. Furthermore, following bilateral transection of carotid sinus nerves that uncouples the brain from the carotid body, Tg21 mice adapted their ventilation to acute severe hypoxia while chemodenervated wild-type (WT) animals developed a life-threatening apnoea. These results imply that Epo in brain modulates ventilation. Additional analysis revealed that the Epo receptor (EpoR) is expressed in the main brainstem respiratory centres and suggested that Epo stimulates breathing control by alteration of catecholaminergic metabolism in brainstem. The modulation of hypoxic pattern of ventilation after i.v. injection of recombinant human Epo in WT mice and the dense EpoR immunosignal observed in carotid bodies showed that these chemoreceptors are sensitive to plasma levels of Epo. In summary, our results suggest that Epo controls ventilation at the central (brainstem) and peripheral (carotid body) levels. These novel findings are relevant to understanding better respiratory disorders including those occurring at high altitude.
During hypoxic or hypoxaemic conditions, tissue oxygenation and arterial oxygen carrying capacity are up-regulated by two complementary systems, namely (i) the neural respiratory areas (central and peripheral) that lead to increased minute ventilation thereby increasing tissue oxygenation, and (ii) Epo release by the kidney that activates erythropoiesis in bone marrow to augment the oxygen carrying capacity of arterial blood. Despite the fact that both neural respiratory control and Epo-mediated elevation of red blood cells are responsible for keeping arterial oxygen content optimal, no interaction between these systems has been described so far. Interestingly, during the last few years it has been demonstrated that Epo and its receptor (EpoR) are functionally expressed in a variety of tissues, including glial cells and neurones in the brain, and that Epo modulates neural activity (Digicaylioglu et al. 1995; Bernaudin et al. 1999; Marti & Bernaudin, 2003; Gassmann et al. 2003; Marti, 2004). Subsequently, it was recognized that Epo has antiapoptotic (Tanaka et al. 2001), anticytotoxic (Morishita et al. 1997) and antioxidative (Koshimura et al. 1999) functions, and synthesis of Epo in neural cells is increased upon exposure to severely reduced oxygenation (Digicaylioglu et al. 1995; Marti et al. 1996). Overall, there is good evidence that Epo is an important factor in brain-dependent responses to hypoxic/ischaemic insults.
In parallel, neural expressed Epo has been recognized as a potent factor able to modulate release of catecholamines in cells with neural characteristics, such as PC12 cells. This cell line is derived from rat pheochromocytoma and is considered to be a reliable model of peripheral chemosensitive cells (Masuda et al. 1993; Koshimura et al. 1999; Yamamoto et al. 2000; Tanaka et al. 2001). In view of these processes being directly or indirectly involved in respiratory acclimatization to hypoxia and the associated morphological and neurochemical changes in the neural respiratory network, and considering that catecholamines are implicated in respiratory response to acute hypoxia both at the level of peripheral chemoreceptors and in central respiratory areas (Soulage et al. 2003; Hilaire et al. 2004; Soulage et al. 2004), we hypothesized that neural expressed Epo (via respiratory neurones in brainstem) and systemic Epo (via carotid bodies) modulate respiration under conditions of acute and chronic hypoxia. To test this hypothesis, we used our transgenic mouse line (termed Tg21) that overexpresses human Epo in neuronal cells but shows normal haematocrit levels (Wiessner et al. 2001; Kilic et al. 2004). We observed an improved ventilatory response of Tg21 animals exposed to hypoxia compared to the wild-type (WT) controls and detected EpoR protein in central (brainstem) and peripheral organs (carotid bodies). These results suggest that Epo has a crucial role in the fine-tuning of oxygen homeostasis and has decisive implications in respiratory disorders, including those occurring at high altitude, as well as in Epo-mediated doping effects.
Methods
Transgenic mice
The Epo-overexpressing transgenic mouse line termed Tg21 was generated by microinjection of human Epo cDNA driven by the human platelet-derived growth factor (PDGF) B-chain promoter into the pronuclei of fertilized oocytes derived from B6C3 hybrid mice (Ruschitzka et al. 2000). The resulting transgenic mouse line TgN(PDGFBEPO)322ZbZ (Tg21) showed a fourfold increase of Epo levels in brain but not in blood plasma (Wiessner et al. 2001). Transgenic homozygous mice were backcrossed with C57Bl/6 mice for more than six generations to obtain WT littermates. For experimentation, only homozygous and wild-type (WT) males 3–4 months old were used. Transgenic Tg21 mice were identified by PCR using the primers 5′-hPDGF: CCATCTCCAGGTTGAGGC (position 910–927 of the human PDGF promotor) and 3′-hPDGF: GTCTCTGAGAGCCGAGCA (position 1243–1226 of promotor), which generated a 344 basepair fragment. Taq polymerase was obtained from Sigma (D- 1806, Switzerland), and PCR reactions were performed in a Thermo Hybaid PCR Express Cycler (Thermo Labsystems, Finland).
Haemoglobin and haematocrit were quantified using standard methodology. Total brainstem Epo protein concentration was determined using 125I-Epo-based radioimmunoassay (RIA; DiaSorin, Stillwater, MN, USA), according to previously published protocols (Wenger et al. 1998). The lower detection limit of our RIA was 4 U l−1, the intra-assay/interassay variances was < 2% and < 6%, respectively. All Epo determinations were performed in duplicate. Epo concentrations per gram cellular protein always refer to the mean between cellular protein at the beginning and the end of the experiment.
Determination of ventilation by plethysmography
All experiments were performed in accordance with the Swiss and French animal protection laws and institutional guidelines. A whole-body flow-through plethysmograph (EMKA Technologies, France) was used to monitor respiration in unrestrained animals. Mice were placed in a 600-ml chamber continuously supplied with airflow at 0.7–0.8 l min−1 using flow restrictors. Calibration was performed by injection of 1 ml of air, and the signal was amplified and recorded using computer respiratory acquisition software (IOX data acquisition and analysis, EMKA Technologies, France). Ventilation V˙E was calculated as the product of tidal volume (VT) and respiratory rate (RR) and normalized to 100 g of body weight (ml min−1 (100 g)−1).
As soon as the animal familiarized with the new environment (about 1 h), basal ventilation was recorded at 21% O2. Acute hypoxia was achieved by flushing air balanced in N2. The fraction of inspiratory O2(FIO2) in the chamber was gradually decreased from 21% to 10% O2 during 15 min. Respiratory recordings at 10% O2 were performed for 20 min. Then the oxygen concentration in the chamber was further gradually reduced to 6% over a further 15 min and recordings were performed for 20 min with this new hypoxic environment. Body weight was measured routinely after experiments to express VT in ml per 100 g in BTPS (body temperature and pressure, saturated) conditions (previously described in Joseph et al. 2002). RR was defined as respirations per minute (resp min−1). Body temperature in normoxia and hypoxia was analysed using a rectal thermocouple (Fluke Corporation, USA) under all hypoxic conditions following the hypoxia protocol described above.
Chronic hypoxic exposure was performed following an acclimatization protocol, previously described by Prabhakar and colleagues (Malik et al. 2005; Kline et al. 2002). Mice were housed in a homemade hypoxic chamber connected to a gas-mixing pump (Digamix gas mixing pump, type M302 a-F, H. Wösthoff e.H.G, Bochum, Germany) with free access to food and water. The chamber oxygen concentration was gradually reduced from room air to 10% within 30 min and maintained under this condition for three days. One hour after returning the mice to room air, baseline ventilation and hypoxic ventilatory response at 10% and 6% of O2 were recorded by plethysmography as described above.
O2 consumption (V˙O2, ml SPTD min−1 (100 g)−1; STPD: standard temperature and pressure in dry air) and CO2 production (V˙CO2, ml SPTD min−1 (100 g)−1) were measured in normoxia and hypoxia (10% and 6% O2) with an open-circuit system. Each mouse was placed in a chamber where a steady 2 l min−1 flow of air was maintained. The fractions of O2 and CO2 at the inflow and the outflow of the chamber were measured by O2 and CO2 analysers (Qubit Systems Inc., Kingston, Ontario, Canada).
Two thousand units per kilogram of recombinant human Epo (rhEpo; Cilag AG, Schaffhausen, Switzerland) was injected i.v. into WT mice via the tail vein after heat dilatation using an infra-red lamp (100 W light; during 2 min). Subsequently, ventilatory response was evaluated at 10% and 6% O2 as described above. Control WT animals received an injection of saline.
Carotid sinus nerve transection (chemodenervation)
Tg21 and WT mice were subjected to bilateral transection of the carotid sinus nerve as previously described by Roux et al. (2000). In brief anaesthesia was induced with a gas mixture (4% halothane, 70% N2O, and O2) and maintained by reducing the inspired halothane concentration to 1–1.5%. Body temperature of all mice was maintained at 37°C using a temperature-controlled heating pad. To prevent any functional regeneration of chemosensory fibres, the carotid sinus nerves were removed completely from the cranial pole of the carotid body until reaching the branch to the glossopharyngeal nerve. The wound was carefully closed and disinfected with 10% of polividone iodine (Betadine, Asta Medica, Merignac, France). Conscious chemodenervated mice were exposed to hypoxia 5 days after recovery. Sham-operated mice underwent the same procedure but their carotid sinus nerves were left intact.
Detection of EpoR, NK1R and tyrosine hydroxylase
Wild-type and transgenic mice were transcardially perfused with phosphate buffer (0.1 m, pH 7.4), and fixed with 4% paraformaldehyde. Brains and carotid artery bifurcations were removed and immersed in 4% paraformaldehyde for 2 h, cryoprotected in 30% sucrose–phosphate for 48 h (carotid arteries 24 h) at 4°C, and frozen on dry ice. Specimens were serially sectioned (brains at 10 μm; carotid arteries at 8 μm), washed in phosphate-buffered saline (PBS), exposed for 2 h to 1.5% normal goat serum, and incubated with antibodies against EpoR (H-194, Santa Cruz Biotechnology Inc., Lab Force ΔG CH-4208, Nunningen, Switzerland; 1:100) or against tyrosine hydroxylase (TH) (NB300-173, Novus Biologicals, Inc., Littleton, CO, USA; 1:500). EpoR and TH immunostainings were visualized using Vectastain Elite ABC kit (Vector Laboratories, Burlingame, CA, USA) and 3,3′ diaminobenzidine-tetrahydrocloride (Sigma, USA) as chromogene.
For immunofluorescence, postfixed brain sections were incubated for 30 min with PBS–0.1% tween−1% bovine serum albumin (BSA) and incubated for 24 h at 4°C with an antibody against the neurokinin-1 receptor (NK1R; S8305, Sigma, USA; diluted 1:5000) or with an antibody against TH (NB300-173, Novus Biologicals, USA; 1:500). Immunostaining was detected with antirabbit IgG (AlexaFluor 448; Molecular Probes, Eugene, OR, USA; 1:500)
Quantification of catecholamines in brainstem
Brainstem catecholaminergic cell groups were identified in successive transverse brainstem sections (60 μm thick) according to a mouse brain atlas (Paxinos & Watson, 2000) and dissected as described (White & Lawson, 1997). In brief, the following sections were dissected: the A1C1 region, from 20 sections following the one containing the pyramidal tract (caudal to rostral); the A2C2 region, from 12 sections back and two sections following the one containing pyramidal tract; the A5 region, from two sections back and eight sections following the one including the caudal end of the facial nerve; and the A6 region, from four sections back and six sections following the slice containing the caudal end of the facial nerve. Different animals were used to determine noradrenaline (norepinephrine, NE) content and TH activity, the latter determination requiring a previous application of 3-hydroxybenzylhydrazine dihydrochloride (NSD 1015; 75 mg kg−1i.p. in saline solution) (Sigma). Twenty minutes after injection animals were decapitated and the enzymatic activity of TH was indirectly evaluated by measuring the accumulation of l-DOPA during 20 min after the blockade of DOPA decarboxylase with NSD 1015. Both NE and l-DOPA were quantified by HPLC coupled with electrochemical detection as described earlier (Joseph et al. 2000). The mobile phase consisted of 0.1 m potassium phosphate buffer pH 3.0 containing 0.15 mm Na2-EDTA at a flow rate of 0.8 ml min−1. DOPA was measured at +0.65 V. The detection limit, calculated by doubling the noise ratios and expressed in picomoles of injected amounts was < 0.03 pmol and the intra-assay coefficient was 0.2%.
Statistical analysis
Analysis was performed using the StatView software (Abacus Concepts, Berkeley, CA, USA). The reported values are means ±s.d. For simple measurements, data were analysed by one-way ANOVA followed by a post hoc Fisher's PLSD (protected least significant difference) test. For hypoxic ventilation responses, data were analysed by two-way ANOVA for repeated measurements. Differences were considered significant at P < 0.05.
Results
Transgenic mice maintain high ventilation upon exposure to severe hypoxia
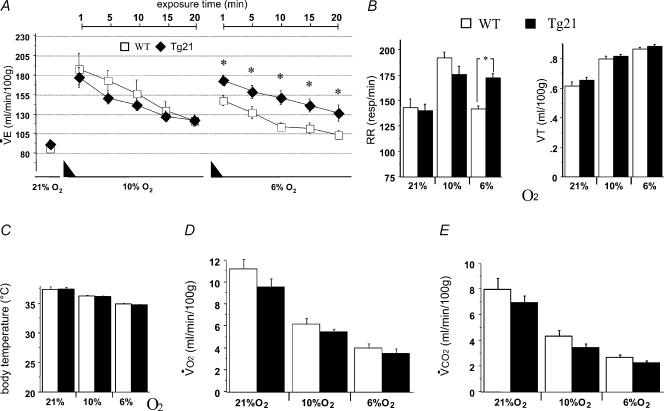
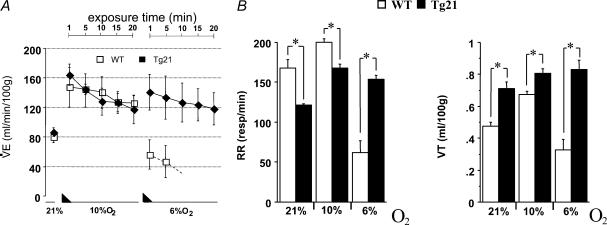
Increased ventilation is the first response to acute reduction of environmental oxygen. After evaluation of ventilation under normoxia, mice were exposed to moderate (10% O2) and severe (6% O2) hypoxia for 20 min each (Fig. 1A). Basal ventilation under normoxia was similar between WT and transgenic mice, and exposure to 10% O2 hypoxia increased the hypoxic ventilatory response (HVR) of both groups equally. However, when exposed to severe hypoxia, HVR decreased drastically in WT mice while the HVR was maintained in transgenic animals. This maintenance of ventilation was due to an elevated respiratory rate (RR; Fig. 1B, left). Note that tidal volume (VT) was similar in WT and Tg21 animals at all conditions tested (Fig. 1B, right). The observed alteration of the HVR at severe hypoxia in Tg21 mice was not due to differences in body temperature (Fig. 1C), oxygen consumption (V˙O2; Fig. 1D), or carbon dioxide production (V˙CO2; Fig. 1E).
Figure 1. The hypoxic ventilatory response (HVR) to acute hypoxia and corresponding changes in body temperature and metabolism.
A and B, hypoxia was achieved in 2 steps of 15 min gradual reduction of FIO2 (represented by the black triangles); first step from 21% to 10% O2, and second step from 10% to 6% O2. RR, VT and V˙E were measured in WT and Tg21 mice during 20 min at 10% and at 6% O2. The bars represent the average of the corresponding parameter during 20 min exposure time. C–E, determination of body temperature (C), V˙O2 (D) and V˙CO2 (E) in WT and Tg21 mice. *P < 0.0001; animals per group = 10–12.
Transgenic mice show enhanced ventilatory acclimatization to chronic hypoxia
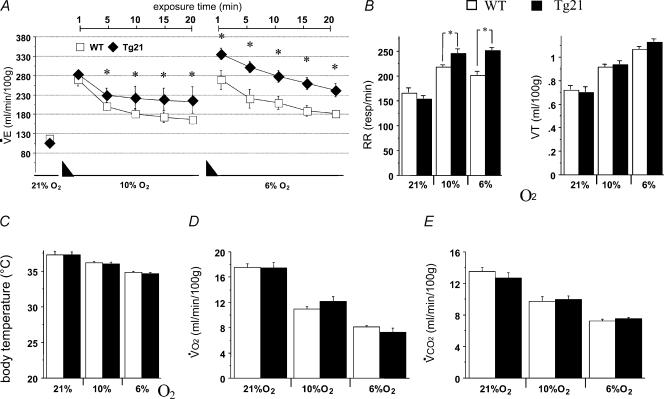
Chronic hypoxia induces ventilatory acclimatization that is manifested by a subsequent augmented response to acute hypoxia (Kline et al. 2002). Since the data shown above revealed increased HVR at 6% O2 in transgenic mice compared to control WT animals, we hypothesized that ventilatory acclimatization to chronic hypoxia would be also facilitated in Tg21 mice. To test this notion, we measured ventilatory responses to acute hypoxia in Tg21 and WT mice after a 3-day exposure to normobaric hypoxia (FIO210%). After this period the haemoglobin concentration increased in WT and Tg21 to similar levels (from 13.8 ± 0.6 versus 14.3 ± 0.3 g dl−1 to 19.2 ± 0.7 versus 19.8 ± 0.4 g dl−1; n.s.). However, while WT control and Tg21 mice presented comparable normoxic basal ventilation, the HVR of cerebral Epo-overexpressing Tg21 mice increased already at 10% O2 (Fig. 2A). More interestingly, in the severe hypoxic condition (6% O2), acclimatized WT mice were not able to elevate their HVR, while acclimatized Tg21 mice showed an additional stimulation of ventilation, resulting in a supplementary augmentation of the HVR (Fig. 2A). The increased HVR observed at 10% and 6% O2 in Tg21 mice, once again, was due to an increase in RR rather than VT (Fig. 2B). As observed previously, HVR in Tg21 mice was not caused by differences in body temperature (Fig. 2C) or metabolic rate (V˙O2, V˙CO2) (Fig. 2D and E). Considering that Tg21 mice have transgenic overexpression of hEpo in brain, these data support the hypothesis that brain-derived Epo directly affects neural respiratory control to modulate ventilation under acute and chronic hypoxic exposure.
Figure 2. Effect of chronic hypoxia (3 days at 10% O2) on the HVR, body temperature and metabolism.
A and B, after chronic hypoxia, ventilation in normoxia and in acute hypoxia was evaluated. Hypoxia was achieved with a gradual reduction of FIO2 (black triangles), from 21% to 10% O2 (in 15 min) and from 10% to 6% O2 (in 15 min). HVR was evaluated during 20 min at 10% and at 6% O2. C–E, determination of body temperature (C), V˙O2 (D) and V˙CO2 (E) in WT and Tg21 mice. *P < 0.0001; animals per group = 10–12.
EpoR is present in main respiratory areas of the brainstem
In a first attempt to understand the involvement of Epo in the HVR, we focused on the impact of Epo and its receptor (EpoR) in the brainstem. Brainstem contains the basic circuitry for rhythm generation (rhythmogenesis) and is a target of relevant sensory afferents (Hilaire & Duron, 1999). Quantification by radioimmunoassay (RIA) showed that transgenic Tg21 mice have a 3-fold overexpression of Epo in brainstem compared to WT animals (57.4 ± 5.2 versus 19.1 ± 2.6 mU Epo (mg protein)−1). Of note, the Tg21 line shows no elevation of plasma Epo levels keeping the transgenic haematocrit (41.1%± 2.1%versus 40.2%± 1.8%) and the haemoglobin content (13.8 ± 0.6 versus 14.3 ± 0.3 g dl−1) in the normal range.
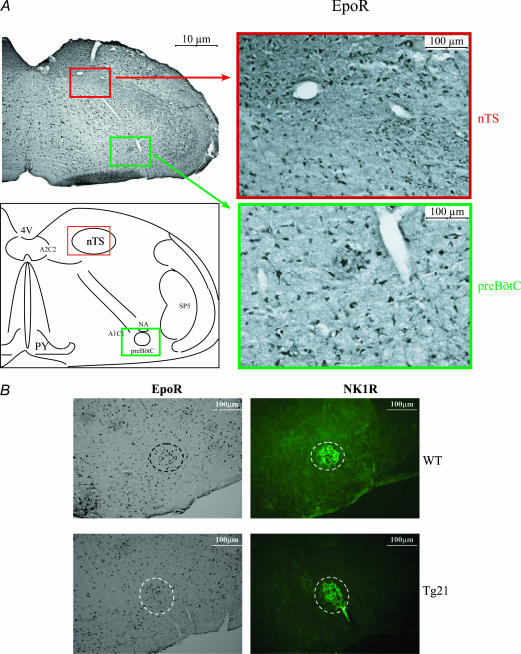
Subsequently, immunohistochemical analysis revealed that EpoR is widely expressed in the whole brainstem, including the main respiratory areas nucleus tractus solitarii (nTS) and the pre-Bötzinger complex (preBötC) (Fig. 3A). In particular, the preBötC is considered the principal site of respiratory rhythmogenesis (Onimaru & Homma, 2003; Blanchi et al. 2003; Di Pasquale et al. 1992). The presence of EpoR in this area further supports the evidence that Epo participates in neural respiratory control. Immunohistochemical analysis revealed that neurokinin-1 receptor (NK1R) and EpoR are expressed in the same areas in WT and Tg21 mice (Fig. 3B). Since the anatomical localization of the preBötC is defined by expression of NK1R (Blanchi et al. 2003), this result reflects the fact that EpoR are also expressed in the respiratory centre.
Figure 3. Immunohistochemical localization of EpoR in the caudal brainstem.
A, an anatomical overview and the ubiquitous expression of EpoR. Nucleus tractus solitarii (nTS; red box) and pre-Bötzinger complex (preBötC; green box) areas are shown in higher magnification. B, the expression of EpoR in the preBötC area. The preBötC was visualized by immunohistochemistry of EpoR and NK1R in serial brainstem sections obtained from WT and Tg21 mice. In both WT and Tg21 mice EpoR and NK1R colocalized in the preBötC neurones. Abbreviations: 4V, 4th ventricle; nTS, nucleus tractus solitarii; PY, pyramidal decussation; A1C1 and A2C2, catecholaminergic areas in the medulla oblongata; preBötC, pre-Bötzinger complex; NA, nucleus ambiguus; SP5, spinal trigeminal tract.
Evidence that Epo alters catecholaminergic levels in respiratory brainstem areas
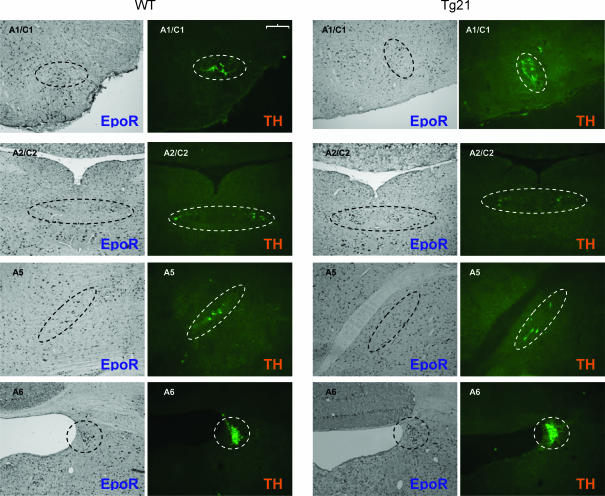
Catecholaminergic neural cell groups have been reported to modulate the HVR (Soulage et al. 2003; Hilaire et al. 2004; Soulage et al. 2004). Since Epo increases dopamine release and TH activity in cells with neural characteristics (Masuda et al. 1993; Koshimura et al. 1999; Yamamoto et al. 2000; Tanaka et al. 2001), we hypothesized that catecholaminergic cell groups in Tg21 brainstems alter monoamine turnover thereby contributing to the observed changes in HVR. To address this question we first examined the expression of EpoR in catecholaminergic areas of the brainstem (A1C1 and A2C2 in the medulla oblongata, and A5 and in A6 the pons). Immunohistochemical analysis revealed that EpoR is also located in all TH-positive cell groups in the brainstem (Fig. 4).
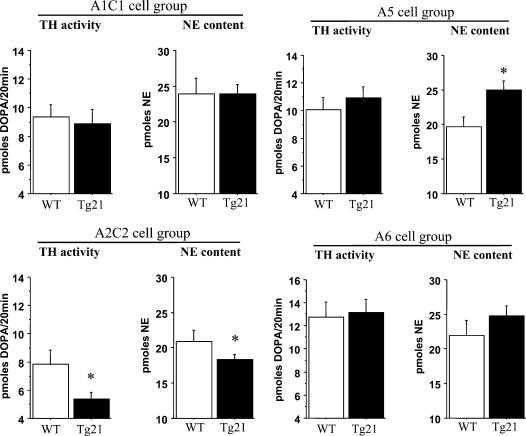
Figure 4. Catecholaminergic cell groups in brainstem (A1C1, A2C2 in medulla oblongata; A5, A6 in pons) were localized by immunodetection of TH in WT and Tg21 mice.
The coexpression of TH and EpoR could be demonstrated in all analysed brain structures in serial brainstem sections of 10 μm thickness. Scale bar = 100 μm
The level of catecholamines in these areas was quantified by HPLC analysis. Noradrenaline (NE) content and TH activity (the latter assessed by accumulation of L-DOPA during 20 min after the blockage of a DOPA decarboxylase inhibitor) were determined. A1C1 and A6 neural groups showed similar NE levels and TH activity in WT and Tg21 mice (Fig. 5). However, compared to WT control, A2C2 exhibited lower NE content and TH activity in transgenic animals. In contrast A5 revealed higher NE content, but similar TH activity, in Tg21 mice. Since previous publications showed that lower medullar but higher pontine catecholamine levels increases the respiratory rate (Hilaire & Duron, 1999; Dick & Coles, 2000), the present data show that overexpression of Epo alters the brainstem catecholamine turnover in Tg21 mice and suggest that this contributes to increased acute and chronic HVR via augmentation of the RR.
Figure 5. In vivo TH activity and NE levels in catecholaminergic groups in the brainstem (A1C1, A2C2 in the medulla oblongata; A5 and A6 in the pons) were determined by HPLC.
TH activity is expressed as picomoles of DOPA formed in 20 min of blockage of DOPA decarboxylase. Significant differences were found in A2C2 and A5 cell groups: in A2C2 the TH activity and NE content were decreased and in A5 the NE content was increased in Tg21 mice. *P < 0.05. Animals per group = 10–12.
Severe hypoxia is life-threatening in chemodenervated WT but not Tg21 mice
Bilateral transection of carotid sinus nerves (chemodenervation) is a common approach to study the oxygen sensing process in brainstem in the absence of peripheral interference (Pascual et al. 2004). Note that respiratory stimulation occurs in chemodenervated animals exposed to moderate hypoxia. However, this response is followed by depression when hypoxia becomes severe (Neubauer et al. 1990). To define the impact of cerebral Epo in modulating the HVR, we evaluated ventilation in normoxic and hypoxic conditions in chemodenervated WT and Tg21 mice. Basal ventilation under normoxia was similar in both chemodenervated mouse lines (Fig. 6A). Likewise, the HVR at 10% O2, although reduced compared to intact animals, was comparable in both groups. However, when exposed to severe hypoxia (6% O2) WT mice had a very pronounced respiratory depression which prompted us to abort the hypoxic exposure. In contrast, despite severe hypoxia, transgenic animals preserved the level of ventilation previously observed at 10% O2 (Fig. 6A). The ventilatory pattern showed that while Tg21 mice elevated VT (but reduced RR), compared to WT animals kept at normoxic and moderate hypoxic conditions, both parameters dropped in WT mice when exposed to severe hypoxia (Fig. 6B). In contrast, Tg21 mice showed no reduction of VT or RR at severe hypoxia. These data indicate that Epo overexpression in the brainstem of Tg21 mice allows maintenance of the respiratory activity even under severe hypoxic conditions despite the lack of information from the peripheral chemoreceptors. Evidently, the presence of Epo in the brain rescues the chemodenervated transgenic animal from life-threatening respiratory depression occurring upon exposure to severe hypoxic conditions.
Figure 6. Ventilatory responses after bilateral transection of the carotid sinus nerve (chemodenervation) in WT and Tg21 mice.
The graph shows V˙E, VT and RR under normoxia and hypoxia in both groups of animals. Note that while chemodenervated WT mice showed a very pronounced respiratory depression (A) due to a drop in both VT and RR (B), chemodenervated Tg21 maintained ventilation at severe hypoxia (6% O2); WT mice were removed from the plethysmographic chamber after 5–10 min of severe hypoxia (6% O2). Black triangles indicate 15 min of gradual reduction of FIO2. *P < 0.01. Animals per group = 6–7.
Evidence that plasma Epo modulates the hypoxic ventilatory pattern via EpoR in carotid bodies
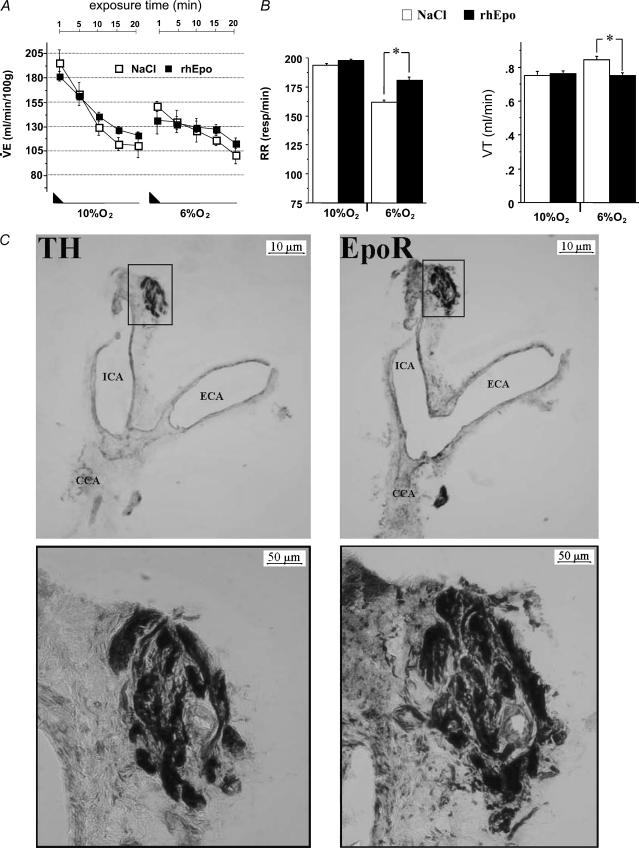
Apart from its postulated role in central regulation of the HVR, we suspected that blood plasma Epo also participates in the regulation of ventilation. The rationale comes from the fact that upon exposure to Epo, non-erythroid cells displaying neuronal characteristics and carrying the EpoR – such as PC12 cells – showed increased intracellular Ca2+ concentration, dopamine release, TH activity and membrane depolarization (Assandri et al. 1999; Koshimura et al. 1999; Yamamoto et al. 2000; Tanaka et al. 2001). As PC12 cells mimic carotid body type I cells (Tanaka et al. 2001), we hypothesized that these peripheral chemoreceptors were also activated by Epo. To address this question we measured the HVR in WT mice upon injection of 2000 U kg−1 rhEpo (Fig. 7A). Control WT animals received 0.9% NaCl solution. While there were no alterations observed at moderate hypoxia, Epo-injected animals showed lower VT but higher RR than saline injected controls under severe hypoxia (Fig. 7B). These data suggested that Epo has an impact on carotid body cells, most probably by binding to the EpoR. Thus, to determine whether EpoR is present in the carotid body, we performed immunostaining in 8 μm serial lateral sections from the carotid bifurcation. TH staining was used to identify the glomus cells on one section, and EpoR staining was performed on the subsequent one. Figure 7C shows a dense staining of EpoR in the carotid body, apparently localized within islets of chemosensitive cells. These data imply that peripheral chemoreceptors can be activated by plasma Epo upon its binding to EpoR.
Figure 7. Systemic Epo modulates HVR.
A, HVR at 10% and 6% O2 evaluated by a stepwise reduction of oxygen (black triangles) in WT mice after i.v. injection of recombinant human Epo (rhEpo, 2000 U kg−1); control animals were injected with saline solution. B, although V˙E did not differ between Epo and saline injected mice there was a change in respiratory pattern at 6% O2 (increase in RR and a slight reduction in VT). *P < 0.007; animals per group = 8. C, identification of carotid body cells in the carotid bifurcation was achieved by immunodetection of TH. Co-expression of TH and EpoR was performed using serial sections of 8 μm thickness. Magnifications of the boxed areas are shown on the right. CB cells are positive for both TH and EpoR. Abbreviations: ICA, ECA, CCA: Internal, external and common carotid artery, respectively.
Discussion
Apart from red blood cell production, Epo exerts protective functions in different organs including brain, retina and heart upon ischaemic injury (Gassmann et al. 2003; Grasso et al. 2004; Grimm et al. 2005). So far, however, non-erythropoietic physiological roles of Epo remain elusive. In this work we state that brain-derived Epo modulates the HVR upon acute hypoxia and more strongly after acclimatization to chronic hypoxia (3 days; 10% O2). We show that EpoRs are present in the main respiratory areas in the brainstem such as preBötC, nTS and catecholaminergic cell groups. Concerning the latter, our results suggest that Epo alters the catecholamine turnover allowing an increase of RR in hypoxia even when animals are uncoupled from peripheral stimulation. Furthermore, by injecting rhEpo into WT mice we demonstrate that carotid bodies are influenced by Epo in the plasma and in turn modulate the HVR. Of note, carotid bodies express the EpoR. Taken together, these observations indicate that Epo is a crucial factor that modulates neural respiratory control by acting both in the central and peripheral nervous system.
Our experimental protocol was adapted from previous ones (Roux et al. 2000; Kline et al. 2002; Soulage et al. 2003) and includes the measurement of ventilation at three conditions: normoxia, moderate hypoxia (10% O2) and severe hypoxia (6% O2). While the first two conditions are common in ventilatory assessments, evaluation of severe hypoxia (6% O2) is rare, especially because this oxygen concentration is lethal to man. Mice, however, show an extraordinary capacity to cope with this extreme environment. As such, in a previous work in which the expression of HIF-1α was evaluated, mice were exposed to severe hypoxia after 60 min of gradual reduction of FIO2, from 21 to 6% O2. Subsequently, mice were kept in severe hypoxic conditions for up to 12 h of 6% O2 without animal losses (Stroka et al. 2001). Based on this observation, a similar protocol was developed for the present work. Moderate hypoxia was reached after 15 min of gradual reduction of FIO2, and severe hypoxia after an additional 35 min (see Methods). Once at 6% O2, the animals were kept in this condition for 20 further minutes, and then restored to a normoxic atmosphere.
The impact of brain-derived Epo on ventilation in Tg21 mice exposed to the abovementioned hypoxic conditions is summarized as follows: (i) respiratory modulation in acute hypoxia occurs only under severe conditions (6% O2), (ii) hypoxic exposure followed by an acclimatization period (3 days; 10% O2) enhanced ventilation at both moderate (10% O2) and severe (6% O2) hypoxia, and (iii) all the mentioned ventilatory responses occur mainly due to increased RR rather than elevation of VT. Interestingly, while 10% O2 increases ventilation in both groups upon acute hypoxia, WT mice show a ventilatory depression when hypoxia becomes severe (e.g. 6%, Fig. 1A). Clearly the ability to cope with severe hypoxia in WT animals is greatly impaired. This drop in HVR under severe hypoxia does not occur in transgenic animals. These animals preserve, even under severe hypoxia, the elevated HVR reached at 10% O2. When exposed to chronic hypoxia, the HVR is different. After 3-days of normobaric hypoxia both groups show increased ventilation when kept at normoxia or exposed to hypoxia. However, transgenic mice revealed higher stimulation of HVR at both moderate and severe hypoxia. The important difference between the results obtained under acute and chronic hypoxia is that brain-derived Epo preserves high ventilation under severe acute hypoxia whereas upon chronic hypoxic exposure it stimulates ventilation under moderate and severe hypoxia. Assessment of ventilation was performed in unrestrained mice using whole body plethysmography that enables the non-invasive determination of the respiratory response in conscious mice under normoxic and hypoxic conditions. Oxygen consumption V˙O2, carbon dioxide production V˙CO2, and body temperature showed no differences between WT and Tg21 at normoxic and hypoxic conditions. Considering that V˙O2 and V˙CO2 reflect the levels of arterial blood gases (e.g. the arterial gas pressure is proportional to the metabolic rate, and inversely proportional to minute ventilation, as described by the equation of the alveolar gas; Mortola et al. 1995), we assume that arterial blood gases were similar between both mouse groups. Taken together, our data imply that brain-derived Epo is a physiologically relevant factor in the process of acclimatization to chronic hypoxia.
Bilateral transection of carotid sinus nerves (chemodenervation) disconnects the brain from its main peripheral sensor. This allows assessment of the impact of brain Epo on ventilation in the absence of carotid body information relay. Hypoxic response in conscious chemodenervated animals is characterized by a respiratory stimulation, primarily manifested as tachypnoea upon mild to moderate hypoxia that is followed by a ventilatory depression when hypoxia becomes severe (Neubauer et al. 1990). Indeed, while WT mice experienced a dramatic respiratory depression under severe hypoxia, transgenic mice maintained elevated ventilation that allowed their survival. This result implies that cerebrally overexpressed Epo produces a constitutive and peripheral-independent stimulation of the central respiratory network under hypoxia.
Concerning the mechanism regulating preservation and stimulation of ventilation in transgenic mice, it is important to note that Tg21 always showed higher RR than WT animals. Beside the cells controlling respiratory rhythmogenesis, it is known that neighbouring catecholaminergic groups increase inhibitory or decrease stimulatory input in the brainstem (Blanchi et al. 2003). In keeping with this, it was found that catecholaminergic cells are potent modulators of ventilation in hypoxia (Soulage et al. 2003; Hilaire et al. 2004; Soulage et al. 2004). The medullary and pontine areas in the brainstem that contain major clusters of noradrenergic neurones are the A6 cell group in the locus ceruleus, A5 in the ventrolateral pons, A2C2 in the caudal nTS, and A1C1 in the ventrolateral medulla. We found that EpoR is localized in all these catecholaminergic areas and furthermore, compared to WT control, transgenic mice express different catecholaminergic activity that most probably contributes to increased RR in hypoxia. As such, it was shown that higher content of noradrenaline in A5 increases RR (Hilaire & Duron, 1999; Dick & Coles, 2000). In line with these findings our measurements show that compared to WT, transgenic A5 posses higher levels of NE. Similarly, it was shown that lower NE content in medullar A2C2 and A1C1 groups is also associated with augmentation of RR (Champagnat et al. 1979). Accordingly, our measurements show lower NE content and diminished TH activity in transgenic A2C2 compared to WT. Since our results are consistent with previous demonstrations that Epo increases catecholamine synthesis and release in neural noradrenergic cells (Assandri et al. 1999; Koshimura et al. 1999; Tanaka et al. 2001), it is tempting to speculate that Epo overexpression modulates the catecholaminergic synthesis in brainstem. Once in hypoxia, this alteration would affect the ventilatory response via increasing the respiratory rate.
Apart from central regulation, carotid bodies are the main organs sensing arterial decrease of oxygen content and mediate the integrated cardiorespiratory responses to hypoxaemia (Prabhakar, 2000). Chemosensitive cells within the carotid body synthesize and store a variety of neuromodulators and/or transmitters that once released during hypoxia exposure initiate and/or modulate the response of the carotid sinus nerve (Shirahata & Sham, 1999; Pardal & López-Barneo, 2002). PC12 cells are considered to be a reliable model for the study of carotid body cells (Yamamoto et al. 2000; Tanaka et al. 2001). We and others demonstrated that PC12 cells express EpoR, and that Epo stimulates dopamine release via activation of Ca2+ channels (Asandri et al. 1999; Koshimura et al. 1999). These observations led to the hypothesis that plasma Epo modulates the HVR via the carotid body chemoreflex pathway. This hypothesis is supported by our first demonstration that EpoR is expressed by carotid bodies. In addition, based on the notion that an intact blood–brain barrier excludes large glycosylated molecules such as Epo (Brines et al. 2000; Marti & Bernaudin, 2003; Marti et al. 2004), the i.v. injection of rhEpo in WT mice induced upon severe hypoxia a significant increase in RR that was compensated by a significantly lower VT compared with saline-injected mice. Consequently, these results indicate that plasma Epo participates in the modulation of ventilation under hypoxic conditions. One might speculate that carotid body cells are able to synthesize their own Epo. Several decades ago, however, some authors found that cat and rabbit carotid bodies do not produce Epo under normoxic or hypoxic conditions (Hansen et al. 1973; Paulo et al. 1973). Considering the high level of Epo in plasma and more, carotid bodies are among the most irrigated organs in the organism (5–6 folds/brain, Gonzales et al. 1994) these results are consistent. Thus carotid body may simply be influenced by circulating Epo levels through the EpoR expression.
In conclusion, we found that Epo is a key factor that modulates neural respiratory control in hypoxia by acting on both the central nervous system and peripheral chemoreceptors. Under acute severe hypoxia (6% O2) and after long-term hypoxia, transgenic mice overexpressing Epo in the brain but not WT littermates showed increased HVR that correlates with the catecholamine level in the brainstem's noradrenergic cell groups. Analogously, we demonstrate that HVR is modulated in WT animals upon systemic Epo administration, most probably via EpoR located in carotid bodies. Epo is thus a ‘multitarget’ factor of general oxygen homeostasis which increases red cell mass and modulates HVR, carotid body activity and hypoxic ventilatory acclimatization. In view of Epo having been successfully administrated to patients suffering from stroke (Ehrenreich et al. 2002), considering that Epo is physiologically expressed in the brain in an oxygen-dependent manner, and keeping in mind that an adequate chemoreflex function is a key component of respiratory physiology, our findings suggest that Epo has potential clinical implications in respiratory responses evoked by environmental (i.e. high altitude) and pathological conditions, and thereby has an impact on several million people worldwide.
Acknowledgments
The authors wish to thank Stephan Keller, Beat Grenacher, Gisele Höpfl, Isabelle Desbaillets, Edith Schneider and Jacqueline Pequignot for their superb assistance and Christian Bauer and Thomas Gorr for fruitful discussions. J.S., O.O.O., J.V. and M.G. are supported by grants from the Swiss National Science Foundation and the EUgrant EUROXY(No 502932).
References
- Assandri R, Egger M, Gassmann M, Niggli E, Bauer C, Forster I, et al. Erythropoietin modulates intracellular calcium in a human neuroblastoma cell line. J Physiol. 1999;516:343–352. doi: 10.1111/j.1469-7793.1999.0343v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernaudin M, Marti HH, Roussel S, Divoux D, Nouvelot A, MacKenzie ET, et al. A potential role for erythropoietin in focal permanent cerebral ischemia in mice. J Cereb Blood Flow Metab. 1999;19:643–651. doi: 10.1097/00004647-199906000-00007. [DOI] [PubMed] [Google Scholar]
- Blanchi B, Kelly LM, Viemari JC, Lafon I, Burnet H, Bevengut M, et al. MafB deficiency causes defective respiratory rhythmogenesis and fatal central apnea at birth. Nat Neurosci. 2003;6:1091–1100. doi: 10.1038/nn1129. [DOI] [PubMed] [Google Scholar]
- Brines ML, Ghezzi P, Keenan S, Agnello D, de Lanerolle NC, Cerami C, et al. Erythropoietin crosses the blood–brain barrier to protect against experimental brain injury. Proc Natl Acad Sci U S A. 2000;97:10526–10531. doi: 10.1073/pnas.97.19.10526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagnat J, Denavit-Saubie M, Henry JL, Leviel V. Catecholaminergic depressant effects on bulbar respiratory mechanisms. Brain Res. 1979;160:57–68. doi: 10.1016/0006-8993(79)90600-0. [DOI] [PubMed] [Google Scholar]
- Di Pasquale E, Monteau R, Hilaire G. In vitro study of central respiratory-like activity of the fetal rat. Exp Brain Res. 1992;89:459–464. doi: 10.1007/BF00228263. [DOI] [PubMed] [Google Scholar]
- Dick TE, Coles SK. Ventrolateral pons mediates short-term depression of respiratory frequency after brief hypoxia. Respir Physiol. 2000;121:87–100. doi: 10.1016/s0034-5687(00)00121-3. [DOI] [PubMed] [Google Scholar]
- Digicaylioglu M, Bichet S, Marti HH, Wenger RH, Rivas LA, Bauer C, et al. Localization of specific erythropoietin binding sites in defined areas of the mouse brain. Non-erythroid functions of erythropoietin. Proc Natl Acad Sci U S A. 1995;92:3717–3720. doi: 10.1073/pnas.92.9.3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrenreich H, Hasselblatt M, Dembowski C, Cepek L, Lewczuk P, Stiefel M, et al. Erythropoietin therapy for acute stroke is both safe and beneficial. Mol Med. 2002;8:495–505. [PMC free article] [PubMed] [Google Scholar]
- Gassmann M, Heinicke K, Soliz J, Ogunshola OO. Non-erythroid functions of erythropoietin. Adv Exp Med Biol. 2003;543:323–330. doi: 10.1007/978-1-4419-8997-0_22. [DOI] [PubMed] [Google Scholar]
- Gonzalez C, Almaraz L, Obeso A, Rigual R. Carotid body chemoreceptors: from natural stimuli to sensory discharges. Physiol Rev. 1994;74:829–898. doi: 10.1152/physrev.1994.74.4.829. [DOI] [PubMed] [Google Scholar]
- Grasso G, Sfacteria A, Cerami A, Brines M. Erythropoietin as a tissue-protective cytokine in brain injury: what do we know and where do we go? Neuroscientist. 2004;10:93–98. doi: 10.1177/1073858403259187. [DOI] [PubMed] [Google Scholar]
- Grimm C, Hermann D, Bogdanova A, Hotop S, Kilic U, Wenzel A, et al. Neuroprotection by hypoxic preconditioning: HIF-1 and erythropoietin protect from retinal degeneration. Semin Cell Dev Biol. 2005 doi: 10.1016/j.semcdb.2005.03.004. (in press) [DOI] [PubMed] [Google Scholar]
- Hansen AJ, Fogh J, Mollgard K, Sorensen SC. Evidence against erythropoietin production by the carotid body. Respir Physiol. 1973;18:101–106. doi: 10.1016/0034-5687(73)90025-x. [DOI] [PubMed] [Google Scholar]
- Hilaire G, Duron B. Maturation of the mammalian respiratory system. Physiol Rev. 1999;79:325–360. doi: 10.1152/physrev.1999.79.2.325. [DOI] [PubMed] [Google Scholar]
- Hilaire G, Viemari JC, Coulon P, Simonneau M, Bevengut M. Modulation of the respiratory rhythm generator by the pontine noradrenergic A5 and A6 groups in rodents. Respir Physiol Neurobiol. 2004;143:187–197. doi: 10.1016/j.resp.2004.04.016. [DOI] [PubMed] [Google Scholar]
- Joseph V, Soliz J, Pequignot J, Sempore B, Cottet-Emard JM, Dalmaz Y, et al. Gender differentiation of the chemoreflex during growth at high altitude: functional and neurochemical studies. Am J Physiol. 2000;278:R806–R816. doi: 10.1152/ajpregu.2000.278.4.R806. [DOI] [PubMed] [Google Scholar]
- Joseph V, Soliz J, Soria R, Pequignot J, Favier R, Spielvogel H, et al. Dopaminergic metabolism in carotid bodies and high-altitude acclimatization in female rats. Am J Physiol. 2002;282:R765–R773. doi: 10.1152/ajpregu.00398.2001. [DOI] [PubMed] [Google Scholar]
- Kilic U, Kilic E, Soliz J, Bassetti CI, Gassmann M, Hermann DM. Erythropoietin protects from axotomy-induced degeneration of retinal ganglion cells by activating ERK-1/-2. FASEB J. 2004;19:249–251. doi: 10.1096/fj.04-2493fje. [DOI] [PubMed] [Google Scholar]
- Kline DD, Peng YJ, Manalo DJ, Semenza GL, Prabhakar NR. Defective carotid body function and impaired ventilatory responses to chronic hypoxia in mice partially deficient for hypoxia-inducible factor 1 alpha. Proc Natl Acad Sci U S A. 2002;99:821–826. doi: 10.1073/pnas.022634199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshimura K, Murakami Y, Sohmiya M, Tanaka J, Kato Y. Effects of erythropoietin on neuronal activity. J Neurochem. 1999;72:2565–2572. doi: 10.1046/j.1471-4159.1999.0722565.x. [DOI] [PubMed] [Google Scholar]
- Malik MT, Peng YJ, Kline DD, Adhikary G, Prabhakar NR. Impaired ventilatory acclimatization to hypoxia in mice lacking the immediate early gene fos B. Respir Physiol Neurobiol. 2005;145:23–31. doi: 10.1016/j.resp.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Marti HH. Erythropoietin and the hypoxic brain. J Exp Biol. 2004;207:3233–3242. doi: 10.1242/jeb.01049. [DOI] [PubMed] [Google Scholar]
- Marti HH, Bernaudin M. Function of erythropoietin in the brain. In: Jelkmann W, editor. Erythropoietin: Molecular Biology and Clinical Use. Johnson City, TN, USA: F. P. Graham Publishing Co; 2003. pp. 195–215. [Google Scholar]
- Marti HH, Wenger RH, Rivas LA, Straumann U, Digicaylioglu M, Henn V, et al. Erythropoietin gene expression in human, monkey and murine brain. Eur J Neurosci. 1996;8:666–676. doi: 10.1111/j.1460-9568.1996.tb01252.x. [DOI] [PubMed] [Google Scholar]
- Masuda S, Nagao M, Takahata K, Konishi Y, Gallyas FJ, Tabira T, et al. Functional erythropoietin receptor of the cells with neural characteristics. Comparison with receptor properties of erythroid cells. J Biol Chem. 1993;268:11208–11216. [PubMed] [Google Scholar]
- Morishita E, Masuda S, Nagao M, Yasuda Y, Sasaki R. Erythropoietin receptor is expressed in rat hippocampal and cerebral cortical neurons, and erythropoietin prevents in vitro glutamate-induced neuronal death. Neuroscience. 1997;76:105–116. doi: 10.1016/s0306-4522(96)00306-5. [DOI] [PubMed] [Google Scholar]
- Mortola J, Gautier H. Interaction between metabolism and ventilation: effects of respiratory gases and temperature. In: Dempsey JA, Pack AI, editors. Regulation of Breathing. 2nd edn. New York: Marcel Deekker; 1995. pp. 1011–1064. [Google Scholar]
- Neubauer JA, Melton JE, Edelman NH. Modulation of respiration during brain hypoxia. J Appl Physiol. 1990;68:441–451. doi: 10.1152/jappl.1990.68.2.441. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Homma I. A novel functional neuron group for respiratory rhythm generation in the ventral medulla. J Neurosci. 2003;23:1478–1486. doi: 10.1523/JNEUROSCI.23-04-01478.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardal R, Lopez-Barneo J. Low glucose-sensing cells in the carotid body. Nat Neurosci. 2002;5:197–198. doi: 10.1038/nn812. [DOI] [PubMed] [Google Scholar]
- Pascual O, Roux JC, Soulage C, Morin-Surun MP, Denavit-Saubie M, Pequignot JM. Carotid chemodenervation approach to study oxygen sensing in brain stem catecholaminergic cells. Meth Enzymol. 2004;381:422–449. doi: 10.1016/S0076-6879(04)81029-2. [DOI] [PubMed] [Google Scholar]
- Paulo LG, Fink GD, Roh BL, Fisher JW. Influence of carotid body ablation on erythropoietin production in rabbits. Am J Physiol. 1973;224:442–444. doi: 10.1152/ajplegacy.1973.224.2.442. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Mouse Brain in Stereotaxic Coordinates. San Diego: Academic Press Ltd; 2000. [Google Scholar]
- Prabhakar NR. Oxygen sensing by the carotid body chemoreceptors. J Appl Physiol. 2000;88:2287–2295. doi: 10.1152/jappl.2000.88.6.2287. [DOI] [PubMed] [Google Scholar]
- Roux JC, Pequignot JM, Dumas S, Pascual O, Ghilini G, Pequignot J, et al. O2-sensing after carotid chemodenervation: hypoxic ventilatory responsiveness and upregulation of tyrosine hydroxylase mRNA in brainstem catecholaminergic cells. Eur J Neurosci. 2000;12:3181–3190. doi: 10.1046/j.1460-9568.2000.00208.x. [DOI] [PubMed] [Google Scholar]
- Ruschitzka FT, Wenger RH, Stallmach T, Quaschning T, de Wit C, Wagner K, et al. Nitric oxide prevents cardiovascular disease and determines survival in polyglobulic mice overexpressing erythropoietin. Proc Natl Acad Sci U S A. 2000;97:11609–11613. doi: 10.1073/pnas.97.21.11609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirahata M, Sham JS. Roles of ion channels in carotid body chemotransmission of acute hypoxia. Jpn J Physiol. 1999;49:213–228. doi: 10.2170/jjphysiol.49.213. [DOI] [PubMed] [Google Scholar]
- Soulage C, Pascual O, Roux JC, Denavit-Saubie M, Pequignot JM. Chemosensory inputs and neural remodeling in carotid body and brainstem catecholaminergic cells. Adv Exp Med Biol. 2004;551:53–58. doi: 10.1007/0-387-27023-x_9. [DOI] [PubMed] [Google Scholar]
- Soulage C, Perrin D, Cottet-Emard JM, Pequignot JM. A6 noradrenergic cell group modulates the hypoxic ventilatory response. Adv Exp Med Biol. 2003;536:481–487. doi: 10.1007/978-1-4419-9280-2_61. [DOI] [PubMed] [Google Scholar]
- Stroka DM, Burkhardt T, Desbaillets I, Wenger RH, Neil DA, Bauer C, et al. HIF-1 is expressed in normoxic tissue and displays an organ-specific regulation under systemic hypoxia. FASEB J. 2001;15:2445–2453. doi: 10.1096/fj.01-0125com. [DOI] [PubMed] [Google Scholar]
- Tanaka J, Koshimura K, Sohmiya M, Murakami Y, Kato Y. Involvement of tetrahydrobiopterin in trophic effect of erythropoietin on PC12 cells. Biochem Biophys Res Commun. 2001;289:358–362. doi: 10.1006/bbrc.2001.6002. [DOI] [PubMed] [Google Scholar]
- Wenger RH, Marti HH, Bauer C, Gassmann M. Optimal erythropoietin expression in human hepatoma cell lines requires activation of multiple signalling pathways. Int J Mol Med. 1998;2:317–324. doi: 10.3892/ijmm.2.3.317. [DOI] [PubMed] [Google Scholar]
- White LD, Lawson EE. Effects of chronic prenatal hypoxia on tyrosine hydroxylase and phenylethanolamine N-methyltransferase messenger RNA and protein levels in medulla oblongata of postnatal rat. Pediatr Res. 1997;42:455–462. doi: 10.1203/00006450-199710000-00006. [DOI] [PubMed] [Google Scholar]
- Wiessner C, Allegrini PR, Ekatodramis D, Jewell UR, Stallmach T, Gassmann M. Increased cerebral infarct volumes in polyglobulic mice overexpressing erythropoietin. J Cereb Blood Flow Metab. 2001;21:857–864. doi: 10.1097/00004647-200107000-00011. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Koshimura K, Kawaguchi M, Sohmiya M, Murakami Y, Kato Y. Stimulating effect of erythropoietin on the release of dopamine and acetylcholine from the rat brain slice. Neurosci Lett. 2000;292:131–133. doi: 10.1016/s0304-3940(00)01441-5. [DOI] [PubMed] [Google Scholar]