Abstract
The effect of maternal low protein diet in pregnancy on the function of offspring cerebral cytochrome c oxidase (CcO) was investigated in vitro immediately before and after birth, using fetal and neonatal rat pup forebrain tissue. Pregnant rat dams were fed either a control (C, 18% casein n = 22) or low protein (LP, 9% casein n = 14) diet. Cerebral tissues were harvested from pups the day before (E21) and after (P1) birth. A Clarke electrode chamber was used to determine O2 consumption in brain tissue homogenate, under baseline conditions with and without the mitochondrial electron transport chain inhibitor myxothiazol and in the presence of incremental doses of the electron donor N',N',N',N'-tetramethyl-p-phenylenediamide (TMPD) with myxothiazol. Maximal stimulated CcO activity V˙O2max was less in LP versus C pups at both E21 (P < 0.001) and P1 (P < 0.05). At E21 only, sensitivity to electron flux (pEC50) was greater (P < 0.001) in LP compared to C offspring. In addition, V˙O2max was reduced and pEC50 was greater after birth (i.e. P1 versus E21) in C (P < 0.001) but not in LP pups. This is the first report of the effects of maternal dietary imbalance in pregnancy on offspring cerebral metabolic function. The effects may form part of a developmental adaptive response to reduce energy consumption and promote perinatal survival, or to confer advantage in a postnatal environment predicted to be nutritionally poor.
Adaptive changes (e.g. in cardiovascular control or organ growth) by the embryo or fetus to an altered intrauterine environment may improve the immediate chances of survival but be detrimental in subsequent postnatal life. As recently postulated, they might also have no immediate benefit but allow the organism to develop a phenotype designed to optimize its survival in the environment that it predicts it will face postnatally (Gluckman & Hanson, 2004) The consequences on these prenatal effects can influence health in later life (Godfrey & Barker, 2001).
Epidemiological evidence suggests that disease is linked to patterns of reduced fetal growth in which the brain is spared at the expense of the trunk and abdominal viscera (Eriksson et al. 2001) and which may be linked to an altered nutrient environment. Preliminary evidence in late gestation sheep fetuses following undernutrition in early gestation suggests an increase in carotid blood flow (Hawkins, 1999) consistent with a redistribution of fetal combined ventricular output in favour of the brain, and reminiscent of the response to hypoxia in fetal sheep (Jensen & Berger, 1991). Other strategies by which brain growth might be preferentially maintained in the face of an intrauterine challenge include structural adaptations and cellular adaptations, i.e. reducing fetal metabolism (Richardson et al. 1985).
Previous work suggests that low protein diet in pregnant rats has no effect on offspring carcass, liver or brain weight at 20 days of gestation (term, 21 days) (Burdge et al. 2002). Other studies of the reduced protein diet in rats indicate impaired cerebral vascularization (Bennis-Taleb et al. 1999), reduced dendrite numbers, slower sensory cortico-cortico and thalamo-cortico evoked potentials, and elevated biogenic amine levels with associated modification of tryptophan metabolism (Resnick et al. 1979) in offspring. Such structural, functional and metabolic changes in the brain would be expected to reduce energy requirements in the longer term; however, to date it is not known whether they are preceded by a short-term reduction in oxygen consumption as has been observed in response to hypoxaemia in the fetal llama (Llanos et al. 2002). Studies in sheep show that when cerebral oxygen delivery fails it is accompanied by an increase in the oxidation state of CcO (located on the inner mitochondrial membrane as part of the electron transport chain and cellular source of ATP) (Newman et al. 2000) and this indicates that oxidative phosphorylation has been actively reduced. Interestingly, the expression of liver and skeletal muscle mtDNA encoded genes CcO subunits I and III, as well as NADPH dehydrogenase 4, was altered in ∼10- to 20-week-old offspring of dams fed a low protein diet during pregnancy (Park et al. 2003). However, to date the effect of gestational protein restriction on brain metabolism in the fetus has not been investigated.
The rise in arterial PO2 (PaO2) from in utero values to those experienced postnatally initiates the transition in a number of physiological systems which occur at birth. In hepatocytes (Chandel et al. 1997) and cardiomyocytes (Budinger et al. 1998) CcO function is inhibited by chronic exposure to low PaO2 and is restored after reoxygenation. Immediately after birth (5 min to 2 h) ATP concentration in the rat brain is elevated. Over the course of the subsequent days, as the demand for ATP increases (with the increasing dependence of the growing brain on aerobic glycolysis for energy production), the activity of complex II–III, but not that of complexes I or IV (CcO) increases (Bates et al. 1994; Almeida et al. 1999). However, the extent to which fetal adaptations to nutrient restriction affect such changes in brain metabolic function at birth has hitherto not been investigated.
We hypothesized that cerebral metabolism is reduced in the brain of offspring as a line of defence against inadequate nutrition during gestation, and that this impacts on changes in cerebral metabolism at birth. We used a Clarke electrode to determine changes in partial pressure of oxygen in vitro as a measure of tissue oxygen consumption under baseline conditions and after artificial stimulation of CcO activity using the electron donor TMPD in isolated cerebral tissue from offspring (at the end of gestation and in the immediate postnatal period) of control and low protein fed rat dams.
Methods
Animals
All animal procedures carried out in this study were in accordance with the regulations of the Animals (Scientific Procedures) Act 1986 and approved by the University of Southampton ethical review panel. Virgin Wistar rats (175–200 g, Harlan, UK) were housed in wire metal cages at a temperature of 22°C on a 12 h light–dark cycle. Prior to mating, animals were allowed free access to a standard non-purified laboratory chow diet (CRMX; Special diet services, Cambridge, UK). Virgin rats were date-mated with one of four males. The presence of a vaginal plug was used to identify day 0 (E0) of gestation.
Diet
Pregnant dams were housed individually and fed 30 g a day of an isocaloric diet containing 18% casein (control, C, n = 22) or 9% casein (low protein, LP, n = 14) (Langley & Jackson, 1994), with free access to water.
Brain tissue preparation
Brain tissue was obtained from offspring at either embryonic day 21 (E21: C, n = 13 dams; LP, n = 7 dams), or postnatal day 1 (P1: C, n = 9 dams; LP, n = 7 dams). At embryonic day E21 dams were humanely killed by concussion and cervical dislocation and the pups were then removed and killed by cervical dislocation and decapitation. For P1 tissue, pups were killed as for E21 pups and the dam was subsequently humanely killed. Cerebral hemispheres were quickly dissected free, and kept on ice in physiological salt solution (mm: NaCl, 119; KCl, 4.7; MgSO4, 1.17; KH2PO4, 1.18; NaHCO3, 25; 0.026 mm EDTA; 2.5; and CaCl2, 2.5) solution containing 5 mm glucose. Segments of cerebral cortex (75 mg, approximately the weight of each cerebral hemisphere) were weighed, cut into small pieces, dispersed by aspiration into a pipette, and suspended in 1 ml of PSS in a test tube. Samples were kept on ice until 20 min before their use, when the test tubes were placed in a water bath at 39°C, connected to the water jacket of a Clarke electrode chamber (Rank Brothers Ltd, Cambridge, UK) (Borutaite et al. 2001; Frost et al. 2005).
At the start of each set of experiments the Clarke electrode was calibrated without tissue by filling it with distilled water and without the plunger in place measuring the output of the chamber twice between atmospheric PaO2 and zero over a 15 min period.
Baseline oxygen consumption
To measure baseline brain oxygen consumption V˙O2, brain homogenate (75 mg in 1 ml) was incubated for 20 min with or without 16 μl of 5 mm myxothiazol (which reduces electron flux through complex III) placed in the Clarke electrode chamber. Ten microlitres of 8 mm of ascorbate was added (see below) and all the air bubbles evacuated with the plunger.
TMPD-stimulated respiration data
Tissue V˙O2 was measured in further homogenate samples (75 mg in 1 ml) with randomised concentrations (10–12000 μm) of N′,N′,N′,N′-tetramethyl-p-phenylenediamine (TMPD). TMPD is an electron donor which reduces CcO non-enzymatically; thus when it is used as a substrate, oxygen consumption reflects CcO activity. Experiments were performed under reducing conditions (10 μl of 8 mm of ascorbate to reduce TMPD and prevent its progressive oxidation) and in the presence of myxothiozol (16 μl of 5 mm). Between each sample the chamber was washed out three times with 80% ethanol and three times with distilled H2O to remove traces of myxothiazol and TMPD.
Data acquisition
Analog voltage output from the oxygen electrode was converted to a digital signal (A/D converter, Maclab/8, AD Instruments Pty Ltd, Castle Hill, Australia) and data acquired using Chart software (PowerLab chart, AD instruments Pty Ltd, Castle Hill, Australia).
Drugs and chemicals
CaCl2 was obtained from VWR International Ltd (Lutterworth, Leicestershire, UK) and all other chemicals were obtained from Sigma (Poole, UK).
Data and statistical analysis
The decline in PaO2 of the brain homogenate as a result of tissue respiration was monitored by the Clarke electrode in the sealed chamber until all the oxygen in the sample had been used. The slope of the decline of PaO2 in the brain homogenate was determined between 35 and 19 mmHg using linear regression (GraphPad Prism v 3.0, San Diego, CA, USA) and used a measure of tissue V˙O2.
At each concentration of TMPD, baseline V˙O2 (derived from myxothiaxol control values) was subtracted from measured V˙O2max and this was expressed as a percentage of the non-myxothiazol control value. TMPD dose–response curves were fitted using non-linear regression (GraphPad Prism v 3.0). Vmax and pEC50 (dose of TMPD required to produce 50% of the maximum response) values were derived from individual dose–response curves as indices of maximal CcO activity and sensitivity to electron flux, respectively (Rang & Dale, 1987; Chandel et al. 1997). All values are expressed as mean ± S.E.M. and data were analysed using one- and two-way ANOVA followed by Newman-Keuls post hoc analysis where appropriate. Significant difference was accepted where P < 0.05.
Results
Number of pups in each litter
The lower protein group was found to have on average a larger number of pups in each litter (P= 0.0347): 18%; 6.227 ± 0.3998 (n = 22), 9%; 7.500 ± 0.3593 (n = 14).
Baseline oxygen consumption and myxothiazol inhibition
There was no significant difference in baseline V˙O2 between the C and LP offspring (E21: C group 0.001964 ± 8.9160e-005 mm O2 min−1, n = 13; LP group 0.002008 ± 0.0001186 mm O2 min−1, n = 7; P1: C group 0.002046 ± 0.0001126 mm O2 min−1, n = 9; LP group 0.001894 ± 7.8510e-005 mm O2 min−1, n = 7). At both ages, the addition of 5 mm of myxothiazol caused a similar degree of V˙O2 reduction in the four groups (E21: C group, 76.9 ± 1.1%; LP group, 73.5 ± 1.3%; P1: C group, 73.2 ± 3%; LP group, 73.0 ± 2.0%).
TMPD dose–response curves
Effect of diet
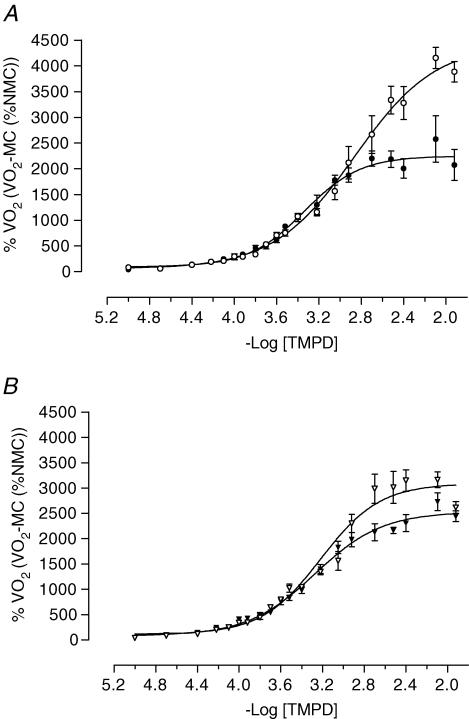
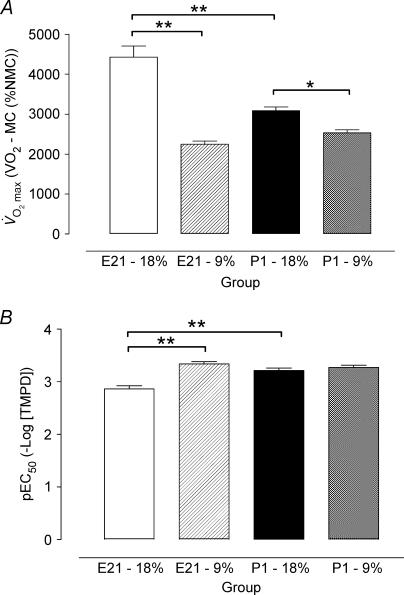
On day E21 V˙O2max was less (P < 0.001) and pEC50 was greater (P < 0.001) in LP compared with C offspring. At P1 V˙O2max was lower in LP compared with C offspring (P < 0.05), but there was no difference between groups in pEC50 (Figs 1 and 2).
Figure 1. The effect of maternal low protein diet on oxygen consumption before and after birth.
Values are mean ±s.e.m. TMPD dose–response curves. A, the day before birth (E21) in control (•, n = 13) and low protein (•, n = 7) groups; B, the day after birth (P1) in control (▴, n = 9) and low protein (▴, n = 7) groups. MC, myxothiazol control; NMC, non-myxothiazol control.
Figure 2. Statistical summary of oxygen consumption before and after birth.
Values are mean ±s.e.m.A, maximal stimulated response to TMPD V˙O2max and B, sensitivity (pEC50) in control (open bar: E21, n = 13; filled bar: P1, n = 9) and low protein (hatched bar: E21, n = 7; chequered bar: P1, n = 7) groups. *P < 0.05 and **P < 0.001, significant difference between diet or age by ANOVA and Newman-Keuls post hoc test. MC, myxothiazol control; NMC, non-myxothiazol control.
Effect of birth
In C offspring, V˙O2max was higher (P < 0.001) and pEC50 was lower (P < 0.001) before compared with after birth. In contrast, in LP offspring there was no difference in the Vmax or pEC50 between E21 and P1 (Figs 1 and 2).
Discussion
To date the mechanisms by which the nervous system may be influenced by events in early life, such as reduced nutrient supply, have been little investigated. This is the first study to demonstrate a decrease in brain V˙O2max from offspring of mothers fed a low protein diet throughout gestation, indicative of altered CcO activity and which may have implications for cerebral metabolic function. Moreover, the maternal gestational diet may influence the physiological transitions that occur at birth because we find that the reduction in V˙O2max in control offspring after birth does not occur in low protein offspring.
Effect of maternal diet on offspring cerebral metabolism
Our data show that LP offspring before birth have a higher pEC50 and a lower V˙O2max relative to C offspring. These differences persist into very early postnatal life. Such effects on electron transport (i.e. altered sensitivity to electron flux and maximal electron transport capacity, respectively) are likely to be reflected in altered brain metabolic function since CcO is the terminal enzyme of mitochondrial oxidative phosphorylation and is tightly coupled to ATP generation, although this remains to be determined. Altered CcO enzyme activity in the LP group could reflect altered activity of this enzyme, or a reduction in its amount. Indeed, previous studies have shown a decrease in cerebral protein synthesis in pups from a maternal or neonatal low protein diet (Banay-Schwartz et al. 1978; Burdge et al. 2002). Previously, a 35% reduction in CcO activity has been associated with learning impairment in the rodent (Bennett et al. 1992). Our study showed a 49% and 20% reduction in maximal CcO activity in the LP group before and after birth, respectively. Dendrites have a high concentration of CcO and interestingly offspring of rats fed a low protein during gestation have reduced numbers of dendrites (Resnick et al. 1979) as well as lower sensory cortico-cortical and thalamo-cortical evoked potentials. Taken together these studies suggest that there may be long-term implications for cerebral function in offspring of dams fed the low protein diet, but this remains to be determined.
In previous studies using the same low protein diet (9 versus 18% casein) in the rat no significant difference was observed between dietary groups in fetal carcass, liver or brain weights at day 20 of gestation (Burdge et al. 2002). However, the observed reduction in delivery of docosahexaenoic acid from the mother to the fetus, and reduced accumulation in the fetal brain, in the low protein group were postulated to be of import in subsequent brain development and function (Burdge et al. 2002). Assessment of brain development in terms of dendrite and synaptic formation should be considered in future studies. Maintenance of brain growth in the face of reduced maternal nutrient supply may involve a compensatory increase in cerebral blood flow (and hence nutrient delivery) and/or altered brain activity (and hence metabolic demand). It is therefore of great interest that we have demonstrated altered cerebral CcO activity in the protein-restricted offspring in the present study. The short- and long-term implications of these physiological changes are unknown but epidemiological studies suggest that undernutrition and growth in early life are linked to neurological, cognitive and emotional development (Davies & Stewart, 1975; Hoek et al. 1998; Gale et al. 2003).
Transition at birth
We have demonstrated that despite no change in baseline V˙O2 there is a significant reduction in V˙O2max after birth in C group offspring. This is likely to reflect the capacity of the brain for oxidative phosphorylation. In the sheep an increase in arterial and sagittal vein PaO2 occurs in the immediate neonatal period (Richardson et al. 1989) and this is likely to be reflected in cerebral tissue oxygenation. Similar observations have not been made for the rat but it seems unlikely that the reduced maximal CcO activity we observed after birth can be accounted for by changes in oxygenation. Moreover, metabolism in hepatocytes (Chandel et al. 1997) and cardiomyocytes (Budinger et al. 1998) incubated in a low PaO2 environment is elevated by increasing ambient PaO2 and use of near-infrared spectroscopy in adult rats in vivo showed that a reduction in CcO only occurs when brain oxyhaemoglobin concentration is extremely impaired (by ∼60% (Hoshi et al. 1997)). Interestingly, the low protein offspring have a lower V˙O2max at E21 compared with control and this is not reduced further following the transition to extra-uterine life. These data suggest that the alteration in CcO function at birth is affected by maternal diet throughout gestation although the underlying mechanisms remain to be determined.
Conclusions
Results obtained in this study support our original hypotheses, and provide the first direct evidence that protein restriction in utero results in a reduced maximal fetal cerebral CcO activity, both pre- and postnatally, and alters the normal transition in cerebral CcO activity at birth. These responses may be part of a developmental adaptive response to reduce energy consumption to promote perinatal survival or development in an environment predicted to be nutritionally poor. Whether or not the effect of a gestational low protein diet on cerebral CcO activity persists in later postnatal life and its possible effects on cerebral function remain to be determined.
Acknowledgments
M.A.H. was supported by The British Heart Foundation.
References
- Almeida A, Bolanos JP, Medina JM. Nitric oxide mediates brain mitochondrial maturation immediately after birth. FEBS Lett. 1999;452:290–294. doi: 10.1016/s0014-5793(99)00628-6. [DOI] [PubMed] [Google Scholar]
- Banay-Schwartz M, Zanchin G, De Guzman T, Sershen H, Lajtha A. Decrease in cerebral protein synthesis on a low protein diet. Vopr Biokhim Mozga. 1978;13:113–126. [PubMed] [Google Scholar]
- Bates TE, Almeida A, Heales SJ, Clark JB. Postnatal development of the complexes of the electron transport chain in isolated rat brain mitochondria. Dev Neurosci. 1994;16:321–327. doi: 10.1159/000112126. [DOI] [PubMed] [Google Scholar]
- Bennett MC, Diamond DM, Stryker SL, Parks JK, Parker WD., Jr Cytochrome oxidase inhibition: a novel animal model of Alzheimer's disease. J Geriatr Psychiatry Neurol. 1992;5:93–101. doi: 10.1177/002383099200500206. [DOI] [PubMed] [Google Scholar]
- Bennis-Taleb N, Remacle C, Hoet JJ, Reusens B. A low-protein isocaloric diet during gestation affects brain development and alters permanently cerebral cortex blood vessels in rat offspring. J Nutr. 1999;129:1613–1619. doi: 10.1093/jn/129.8.1613. [DOI] [PubMed] [Google Scholar]
- Borutaite V, Matthias A, Harris H, Moncada S, Brown GC. Reversible inhibition of cellular respiration by nitric oxide in vascular inflammation. Am J Physiol Heart Circ Physiol. 2001;281:H2256–H2260. doi: 10.1152/ajpheart.2001.281.6.H2256. [DOI] [PubMed] [Google Scholar]
- Budinger GR, Duranteau J, Chandel NS, Schumacker PT. Hibernation during hypoxia in cardiomyocytes. Role of mitochondria as the O2 sensor. J Biol Chem. 1998;273:3320–3326. doi: 10.1074/jbc.273.6.3320. [DOI] [PubMed] [Google Scholar]
- Burdge GC, Dunn RL, Wootton SA, Jackson AA. Effect of reduced dietary protein intake on hepatic and plasma essential fatty acid concentrations in the adult female rat: effect of pregnancy and consequences for accumulation of arachidonic and docosahexaenoic acids in fetal liver and brain. Br J Nutr. 2002;88:379–387. doi: 10.1079/BJN2002664. [DOI] [PubMed] [Google Scholar]
- Chandel NS, Budinger GR, Choe SH, Schumacker PT. Cellular respiration during hypoxia. Role of cytochrome oxidase as the oxygen sensor in hepatocytes. J Biol Chem. 1997;272:18808–18816. doi: 10.1074/jbc.272.30.18808. [DOI] [PubMed] [Google Scholar]
- Davies PA, Stewart AL. Low-birth-weight infants: neurological sequelae and later intelligence. Br Med. 1975;31:85–91. doi: 10.1093/oxfordjournals.bmb.a071248. [DOI] [PubMed] [Google Scholar]
- Eriksson JG, Forsen T, Tuomilehto J, Osmond C, Barker DJ. Early growth and coronary heart disease in later life: longitudinal study. BMJ. 2001;322:949–953. doi: 10.1136/bmj.322.7292.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost MT, Wang Q, Moncada S, Singer M. Hypoxia accelerates nitric oxide-dependent inhibition of mitochondrial complex I in activated macrophages. Am J Physiol Regul Integr Comp Physiol. 2005;288:R394–R400. doi: 10.1152/ajpregu.00504.2004. [DOI] [PubMed] [Google Scholar]
- Gale CR, Walton S, Martyn CN. Foetal and postnatal head growth and risk of cognitive decline in old age. Brain. 2003;126:2273–2278. doi: 10.1093/brain/awg225. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA. Living with the past: evolution, development, and patterns of disease. Science. 2004;305:1733–1736. doi: 10.1126/science.1095292. [DOI] [PubMed] [Google Scholar]
- Godfrey KM, Barker DJ. Fetal programming and adult health. Public Health Nutr. 2001;4:611–624. doi: 10.1079/phn2001145. [DOI] [PubMed] [Google Scholar]
- Hawkins P. University College London; 1999. Nutritional influences in development of the cardiovascular system and the hypothalamic-pituitary-adrenal axis. PhD thesis. [Google Scholar]
- Hoek HW, Brown AS, Susser E. The Dutch famine and schizophrenia spectrum disorders. Soc Psychiatry Psychiatr Epidemiol. 1998;33:373–379. doi: 10.1007/s001270050068. [DOI] [PubMed] [Google Scholar]
- Hoshi Y, Hazeki O, Kakihana Y, Tamura M. Redox behavior of cytochrome oxidase in the rat brain measured by near-infrared spectroscopy. J Appl Physiol. 1997;83:1842–1848. doi: 10.1152/jappl.1997.83.6.1842. [DOI] [PubMed] [Google Scholar]
- Jensen A, Berger R. Fetal circulatory responses to oxygen lack. J Dev Physiol. 1991;16:181–207. [PubMed] [Google Scholar]
- Langley SC, Jackson AA. Increased systolic blood pressure in adult rats induced by fetal exposure to maternal low protein diets. Clin Sci (Colch) 1994;86:217–222. doi: 10.1042/cs0860217. [DOI] [PubMed] [Google Scholar]
- Llanos AJ, Riquelme RA, Sanhueza EM, Herrera E, Cabello G, Giussani DA, et al. Regional brain blood flow and cerebral hemispheric oxygen consumption during acute hypoxaemia in the llama fetus. J Physiol. 2002;538:975–983. doi: 10.1113/jphysiol.2001.013230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman JP, Peebles DM, Harding SR, Springett R, Hanson MA. Hemodynamic and metabolic responses to moderate asphyxia in brain and skeletal muscle of late-gestation fetal sheep. J Appl Physiol. 2000;88:82–90. doi: 10.1152/jappl.2000.88.1.82. [DOI] [PubMed] [Google Scholar]
- Park KS, Kim SK, Kim MS, Cho EY, Lee JH, Lee KU, et al. Fetal and early postnatal protein malnutrition cause long-term changes in rat liver and muscle mitochondria. J Nutr. 2003;133:3085–3090. doi: 10.1093/jn/133.10.3085. [DOI] [PubMed] [Google Scholar]
- Rang HP, Dale MM. Measurement in pharmacology. In: Rang HP, Dale MM, editors. Pharmacology. 1st. Edinburgh: Churchill Livingstone; 1987. pp. 35–56. [Google Scholar]
- Resnick O, Miller M, Forbes W, Hall R, Kemper T, Bronzino J, et al. Developmental protein malnutrition: influences on the central nervous system of the rat. Neurosci Biobehav Rev. 1979;3:233–246. doi: 10.1016/0149-7634(79)90011-3. [DOI] [PubMed] [Google Scholar]
- Richardson BS, Carmichael L, Homan J, Gagnon R. Cerebral oxidative metabolism in lambs during perinatal period: relationship to electrocortical state. Am J Physiol. 1989;257:R1251–R1257. doi: 10.1152/ajpregu.1989.257.5.R1251. [DOI] [PubMed] [Google Scholar]
- Richardson B, Patrick J, Abduljabbar H. Cerebral oxidative metabolism in the fetal lamb: relationship to electrocortical state. Am J Obstet Gynecol. 1985;153:426–431. doi: 10.1016/0002-9378(85)90081-x. [DOI] [PubMed] [Google Scholar]