Abstract
Although cathodal transcranial direct current stimulation (tDCS) decreases cortical excitability, the mechanisms underlying DC-induced changes remain largely unclear. In this study we investigated the effect of cathodal DC stimulation on spontaneous neural activity and on motor responses evoked by stimulation of the central and peripheral nervous system. We studied 17 healthy volunteers. Transcranial magnetic stimulation (TMS) and transcranial electrical stimulation (TES) of the motor area were used to study the effects of cathodal tDCS (1.5 mA, 10 min) on resting motor threshold and motor evoked potentials (MEPs) recorded from the contralateral first dorsal interosseous muscle (FDI). The electroencephalographic (EEG) activity in response to cathodal tDCS was analysed by power spectral density (PSD). Motor axonal excitability changes in response to transcutaneous DC stimulation of the ulnar nerve (0.3 mA, 10 min) were assessed by testing changes in the size of the compound muscle action potential (CMAP) elicited by submaximal nerve stimulation. Cathodal tDCS over the motor area for 10 min increased the motor threshold and decreased the size of MEPs evoked by TMS for at least 60 min after current offset (t0 71.7 ± 5%, t20 50.8 ± 11%, t40 47.7 ± 7.7%, and t60 39.7 ± 6.4%, P < 0.01). The tDCS also significantly decreased the size of MEPs elicited by TES (t0 64 ± 16.4%, P= 0.09; t20 67.6 ± 10.8%, P= 0.06; and t40 58.3 ± 9.9%, P < 0.05). At the same time in the EEG the power of delta (2–4 Hz) and theta (4–7 Hz) rhythms increased (delta 181.1 ± 40.2, P < 0.05; and theta 138.7 ± 27.6, P= 0.07). At the peripheral level cathodal DC stimulation increased the size of the ulnar nerve CMAP (175 ± 34.3%, P < 0.05). Our findings demonstrate that the after-effects of tDCS have a non-synaptic mechanism of action based upon changes in neural membrane function. These changes apart from reflecting local changes in ionic concentrations, could arise from alterations in transmembrane proteins and from electrolysis-related changes in [H+] induced by exposure to constant electric field.
Weak direct currents (DC) delivered through the scalp modulate human brain activity (Priori, 2003). DC delivered at weak intensities (1 mA) and for long periods over the scalp (transcranial DC stimulation or tDCS) induce changes in motor cortical excitability that persist for almost 1 h after current offset and depend on current polarity (Nitsche & Paulus, 2001; Nitsche et al. 2003a; Priori, 2003). Although the long-lasting after-effects of tDCS on the brain are thought to be mediated at a synaptic level by N-methyl-d-aspartate (NMDA) receptors (Liebetanz et al. 2002; Nitsche et al. 2003b; Siebner et al. 2004), the mechanisms underlying DC-induced changes remain largely unclear.
Since cathodal tDCS is a promising technique for treating central nervous system disorders involving increased neuronal excitability, in this study we investigated the effect of cathodal DC stimulation on spontaneous neural activity and on motor responses evoked by stimulation of the central and peripheral nervous systems. Our aim was to investigate the mechanisms underlying the action of cathodal DC on nervous excitability in humans. Using a multiple-experiment approach, in healthy volunteers, we first studied the after-effects of tDCS on the cortico-motorneuronal system by observing changes in motor evoked potentials (MEPs) elicited by transcranial magnetic stimulation (TMS) and electrical stimulation (TES). Second, to see whether DC also influences spontaneous central nervous activity, we assessed the after-effect of tDCS on spontaneous electroencephalographic (EEG) oscillations. Finally, to assess whether the after-effects of DC require the presence of a synapse, we also studied the action of DC on peripheral human motor axons.
Materials and methods
Subjects
Seventeen healthy right-handed volunteers (8 men and 9 women aged 24–40 years; mean ± SD, 29.5 ± 4.5 years) participated in the study. Several subjects participated in more than one experiment. All participants gave their informed consent and the procedures had the approval of the hospital ethical committee. The experimental procedure was in accordance with the declaration of Helsinki.
Direct current (DC) stimulation
DC stimulation was delivered by an electrical stimulator through a constant current unit and an isolation unit (Priori et al. 1998) connected to a pair of electrodes placed on the scalp, one over the right motor cortex (7 cm lateral to the vertex) and the other above the left eyebrow (Experiments 1, 2 and 3) (Nitsche & Paulus, 2000; Nitsche et al. 2003a, b; Priori, 2003), or one over the ulnar nerve at the wrist and the other over the ipsilateral knee (Experiment 4) (Priori et al. 2005). For tDCS the cathode was placed over right motor cortex and the anode above the left eyebrow. Stimulating electrodes were thick (0.3 cm), square (35 cm2) pieces of saline-soaked synthetic sponge. The wide electrode surface avoided the possible harmful effects of high current density. Apart from occasional, transient and short-lasting tingling and burning sensations below the electrodes delivering DC stimulation in a few subjects, DC stimulation strength remained below the conscious cutaneous sensory threshold throughout the experimental session. DC polarity refers to the electrode over the right motor area for Experiments 1, 2 and 3 and over the ulnar nerve for Experiment 4.
Transcranial magnetic stimulation (TMS) and transcranial electrical stimulation (TES)
In Experiment 1 (see next section) TMS was delivered by a Novametrix Magstim 200 stimulator through a flat coil (outer diameter 13.5 cm) centred over the vertex with currents flowing clockwise (viewed from above). MEPs were recorded by standard non-polarizable Ag–AgCl surface electrodes (diameter 9 mm; Meditec, San Paolo di Torrile, Parma, Italy) placed over the belly of the first dorsal interosseous (FDI) muscle and on the skin overlying the first interphalangeal joint of the index finger of the left hand. The resting motor threshold was defined as the lowest intensity able to produce MEPs of ≥ 50 µV in 5 out of 10 consecutive trials of stimulation (Rothwell et al. 1999). Stimulation intensity was 120% of the baseline MEP resting threshold. A total of 24 MEPs were recorded in response to 24 stimuli delivered at 0.15 Hz, and averaged for each time point. The peak-to-peak amplitude was then measured.
In Experiment 2, TES was delivered by a Digitimer D180 stimulator connected to a pair of non-polarizable Ag–AgCl electrodes over the scalp: the anode placed above the motor cortex and the cathode above the vertex. Stimulation intensity was 120% of the baseline MEP active threshold (50 µs time constant). A total of 8 TES stimuli were delivered at 0.15 Hz for each time point, MEPs were recorded and the peak-to-peak amplitude was measured. The ongoing voluntary EMG activity was monitored to ensure the same level before and after tDCS.
The EMG signals were preamplified, amplified, band-passed from 20 Hz to 5 kHz, acquired and stored by a Nikolet Viking IVP.
EEG recordings
In Experiment 3 (see next section) we used a bipolar montage (C4–Fp2; C3–Fp1) (Jasper, 1958). EEG signals were filtered at 2–1000 Hz and amplified 30 000× using a Cambridge 1902 signal analyser (Cambridge Electronic Design, Cambridge, England); A-to-D converted at 2500 Hz through a Cambridge 1401 device (Cambridge Electronic Design), and monitored on-line by a dedicated software (Signal software, version 1.80, Cambridge Electronic Design). All further data were analysed with the Matlab software (version 6.0, The Mathworks, Natick, MA, USA). The signal power over time was investigated using the fast Fourier transform (FFT) algorithm; FFT estimates the power spectral density (PSD) using Welch's averaged periodogram method. EEG signals were down-sampled off-line at 500 Hz, then segmented and averaged. The signal was divided into ∼360 non-overlapping epochs, each of 512 samples (∼1 s), then detrended and windowed by a Hanning window. To ensure a stationary signal, windows were kept narrow, with a frequency resolution of 0.97 Hz; the magnitude squared of the discrete FFTs of the sections was averaged to obtain the PSD. We analysed only spectral frequencies ranging from 2 to 45 Hz, divided into subranges: 2–4 Hz (delta), 4–7 Hz (theta), 8–13 Hz (alpha), and 14–45 Hz (beta/gamma). EEG epochs contaminated by eye movements or EMG activity were rejected.
Test nerve stimulation and analysis of compound muscle action potentials (CMAPs)
In Experiment 4 (see next section) test square current pulses lasting 1 ms were delivered to the ulnar nerve at 5-s intervals and the CMAP amplitude was measured peak-to-peak. Supramaximal CMAP, motor threshold and stimulus–response curves were studied before and after transcutaneous DC stimulation. Stimuli were delivered at suprathreshold intensity for stimulus–response curves (125%, 150%, 175%, and 200% of nerve motor threshold). CMAPs were recorded through surface electrodes placed over the FDI muscle. The EMG signal was preamplified, amplified, band-passed at 100 Hz to 10 kHz, acquired and stored by a Nikolet Viking IVP.
Experimental procedures
Experiment 1
Seven subjects underwent tDCS and five of them underwent sham stimulation. The two conditions were tested in random order and on different days. At least 2 weeks elapsed between sessions. Whereas the investigators knew the kind of conditioning stimulation, the subjects were unaware of it. Subjects sat in a comfortable chair with the elbow slightly flexed. The motor threshold was measured, then MEPs were recorded. Target muscle relaxation was monitored by auditory feedback of the high-gain amplified EMG signal. The conditioning stimulus was a cathodal DC of 1.5 mA delivered for 10 min (0.026 C cm−2) over the contralateral motor cortex. Resting motor threshold and MEPs were recorded at the tDCS offset and every 20 min for 1 h.
Experiment 2
In six subjects, TES was delivered at near-threshold intensity (120% of active threshold before tDCS) during a mild voluntary contraction to magnify the D-wave-related component of the MEP (Day et al. 1989; Mills, 1999). MEPs were recorded after tDCS offset and every 20 min for 40 min, and the peak-to-peak amplitude was calculated. The EMG voluntary activity was monitored to ensure the same level of voluntary contraction before and after tDCS.
Experiment 3
Six subjects underwent EEG recordings before and after tDCS. The subjects were studied at rest with the eyes closed. The conditioning stimulus was a cathodal DC delivered at 1.5 mA for 10 min (0.026 C cm−2). A 6-min trial of EEG signal was recorded before tDCS. After tDCS offset, the EEG signal was recorded in separate 6-min files for 1 h. To minimize the decrease of arousal, intervals of ∼2 min with eyes open elapsed between each 6-min recording. Each file was analysed by PSD. The effects of cathodal tDCS were assessed only on the right motor cortex. To rule out possible non-specific effects five subjects underwent sham stimulation in the same experimental setting. After tDCS and after sham stimulation subjects reported similar, mild drowsiness. The two experimental conditions were tested in random order and at least 2 weeks elapsed between sessions. Whereas the investigators knew the kind of conditioning stimulation, the subjects were unaware of it.
Experiment 4
To assess whether the after-effects of tDCS depended on the presence of synapses, we used the same tDCS protocol but delivered stimulation over the ulnar nerve proximally to the wrist. The peripheral-nerve model away from the motor point involves no synapses and reflects axonal excitability alone. Polarizing electrodes for conditioning stimulation were placed one over the nerve above the wrist (at least 10 cm away from the motor point of the FDI muscle) and other over the ipsilateral knee. Before and after polarization, test nerve stimulation was delivered at the same point with a bipolar stimulator. We studied the effect of cathodal DC (polarity on ulnar nerve) delivered at 0.3 mA for 10 min (0.0051 C cm−2, 7 subjects). Supramaximal CMAP, motor threshold and stimulus–response curves were recorded before and 2 min after DC offset. Six subjects underwent the same protocol before and after peripheral sham stimulation. The two conditions were tested in random order and at least 2 weeks elapsed between sessions. Whereas the investigators knew the kind of conditioning stimulation, the subjects were unaware of it.
Statistical analysis
Values in the text are means ±s.e.m.
Experiment 1
The mean value of motor threshold and MEP amplitude were calculated for each time point. Threshold and MEP amplitudes after tDCS were expressed as a percentage of baseline values (baseline = 100%). A repeated-measures one-way ANOVA of raw data was used to compare the independent variables (tDCS or sham stimulation and time course), and the dependent variable (MEP amplitude). In addition, in five subjects a two-way repeated-measures ANOVA was used to compare the effects of tDCS with those of sham stimulation. The Wilcoxon signed rank test was used to compare values at each time point and baseline. The non-parametric Wilcoxon test was used because it does not assume normal distributions or homogeneity of the variances.
Experiment 2
The mean value of MEP amplitude were calculated for each time point. MEP amplitudes after tDCS were expressed as percentage of baseline values. A repeated-measures one-way ANOVA of raw data was used to compare the independent variables (tDCS, and time course), and the dependent variable (MEP amplitude). The Wilcoxon signed rank test was used to compare values at each time point and baseline.
Experiment 3
The PSD was expressed as a percentage of the mean baseline value. In five subjects a two-way repeated-measures ANOVA was used to compare the effects of tDCS with those of sham stimulation The Wilcoxon signed rank test was used to compare PSD values at each time point and baseline.
Experiment 4
CMAP amplitude at each stimulus value of the stimulus–response curve was normalized as a percentage of the baseline CMAP. A repeated-measures one-way ANOVA was used to compare the independent variables (DC, sham stimulation and stimulus intensity as a percentage of motor threshold), and the dependent variable (CMAP amplitude). In addition, in six subjects the two-way repeated-measures ANOVA was used to compare the effects of tDCS with those of sham stimulation. The Wilcoxon signed rank test was used to compare each single value with baseline.
Results
None of the subjects complained of adverse reactions to scalp or peripheral nerve DC stimulation.
Experiment 1: after-effects of tDCS on motor responses evoked by TMS
After cathodal tDCS offset the amplitude of MEPs at rest decreased significantly: the decrease persisted for at least 1 h (t0 71.7 ± 5%, P < 0.01; t20 50.8 ± 11%, P < 0.01; t40 47.7 ± 7.7%, P < 0.01; t60 39.7 ± 6.4%, P < 0.01, Wilcoxon signed rank test) (Fig. 1B and C). Conversely, the resting motor threshold increased significantly (t0 105.9 ± 2.2%, P < 0.05; t20 109.3 ± 4.6%, P= 0.054; t40 105.6 ± 3.4%, P < 0.05; t60 108.6 ± 1.8%, P < 0.01, Wilcoxon signed rank test) (Fig. 1A). The repeated-measures one-way ANOVA disclosed a significant main effect of tDCS at 0, 20, 40, and 60 min (MEPs, F= 9.776, d.f. = 4, P < 0.0001; Bonferroni post test, t0P > 0.05, t20P < 0.01, t40P < 0.001, t60P < 0.001) and the repeated-measures two-way ANOVA disclosed a significant main effect of tDCS on MEPs and threshold (Table 1). In four subjects MEP amplitude decreased by > 50% and in three subjects by > 30%. In the five subjects studied with sham tDCS, MEP amplitudes and thresholds remained unchanged (Fig. 1A and B). The resting motor threshold was 40.3 ± 2.4 % (percentage of maximal stimulator output), control MEP amplitude was 2.1 ± 0.6 mV during tDCS and 2.3 ± 0.8 mV for sham stimulation.
Figure 1. Effect of cathodal transcranial direct current stimulation (tDCS) on resting motor threshold (A) and on motor evoked potentials (MEP amplitude) (B,C) elicited by transcranial magnetic stimulation (TMS).
MEP amplitude is expressed as a percentage of the control unconditioned response; resting motor threshold is expressed as a percentage of control threshold; error bars show s.e.m. Time axis shows minutes after the end of tDCS or sham stimulation; □, tDCS (n = 7 subjects); Δ, sham stimulation (n = 5). *P < 0.05, **P < 0.01 Wilcoxon signed rank test. MEP recordings from a representative subject (C) showing the decrease in MEP amplitude after cathodal tDCS. Each trace is the average of 24 sweeps. t0 (0 min after tDCS), t20 (20 min after tDCS), t40 (40 min after tDCS), t60 (60 min after tDCS). Note the persistent decrease in MEP amplitude and persistent increase in resting motor threshold after cathodal tDCS but not after sham stimulation.
Table 1.
Results of the two-way repeated-measures ANOVAs
| Variables | d.f. | F-values | P-values |
|---|---|---|---|
| Experiment 1 | |||
| MEPs | |||
| Intervention | 1 | 62.02 | < 0.0001* |
| Time course | 3 | 1.432 | 0.2703 |
| Intervention × time course | — | — | 0.1294 |
| Threshold | |||
| Intervention | 1 | 15.29 | 0.0012* |
| Time course | 3 | 0.3926 | 0.76 |
| Intervention × time course | — | — | 0.8233 |
| Experiment 3 (Right hemisphere) | |||
| Total spectrum | |||
| Intervention | 1 | 7.182 | 0.0164* |
| Time course | 3 | 0.05 | 0.98 |
| Intervention × time course | — | — | 0.7861 |
| Delta | |||
| Intervention | 1 | 13.26 | 0.0022* |
| Time course | 3 | 0.322 | 0.809 |
| Intervention × time course | — | — | 0.778 |
| Theta | |||
| Intervention | 1 | 9.767 | 0.0065* |
| Time course | 3 | 0.0291 | 0.993 |
| Intervention × time course | — | — | 0.88 |
| Alpha | |||
| Intervention | 1 | 3.626 | 0.075 |
| Time course | 3 | 0.199 | 0.895 |
| Intervention × time course | — | — | 0.9856 |
| Beta/gamma | |||
| Intervention | 1 | 0.4338 | 0.5195 |
| Time course | 3 | 0.6829 | 0.5754 |
| Intervention × time course | — | — | 0.4339 |
| Experiment 3 Left hemisphere | |||
| Total spectrum | |||
| Intervention | 1 | 0.3 | 0.5912 |
| Time course | 3 | 0.3433 | 0.7944 |
| Intervention × time course | — | — | 0.9428 |
| Delta | |||
| Intervention | 1 | 1.319 | 0.2676 |
| Time course | 3 | 0.09347 | 0.9626 |
| Intervention × time course | — | — | 0.7384 |
| Theta | |||
| Intervention | 1 | 1.185 | 0.2924 |
| Time course | 3 | 0.4909 | 0.6936 |
| Intervention × time course | — | — | 0.7055 |
| Alpha | |||
| Intervention | 1 | 0.9187 | 0.3521 |
| Time course | 3 | 1.906 | 0.1693 |
| Intervention × time course | — | — | 0.9898 |
| Beta/gamma | |||
| Intervention | 1 | 0.744 | 0.6241 |
| Time course | 3 | 0.0564 | 0.9817 |
| Intervention × time course | — | — | 0.9650 |
| Experiment 4 | |||
| CMAP | |||
| Intervention | 1 | 0.486 | 0.003* |
| Time course | 3 | 1.695 | 1.3902 |
| Intervention × time course | — | — | 0.0141* |
significant.
Experiment 2: after-effects of tDCS on motor responses evoked by TES
After tDCS offset the amplitude of MEPs elicited by TES during a slight voluntary contraction decreased significantly at 40 min, and tended to decrease at earlier time points (t0 64 ± 16.4%, P= 0.09; t20 67.6 ± 10.8%, P= 0.06; t40 58.3 ± 9.9%, P= 0.03, Wilcoxon signed rank test) (Fig. 2). The repeated-measures one-way ANOVA disclosed a significant effect of tDCS on MEPs (F= 3.41, d.f. = 3, P= 0.045). In one subject with a higher active motor threshold the tDCS-induced depressive effect was less pronounced. In conclusion tMS and TES detected similar tDCS-induced depressive effects.
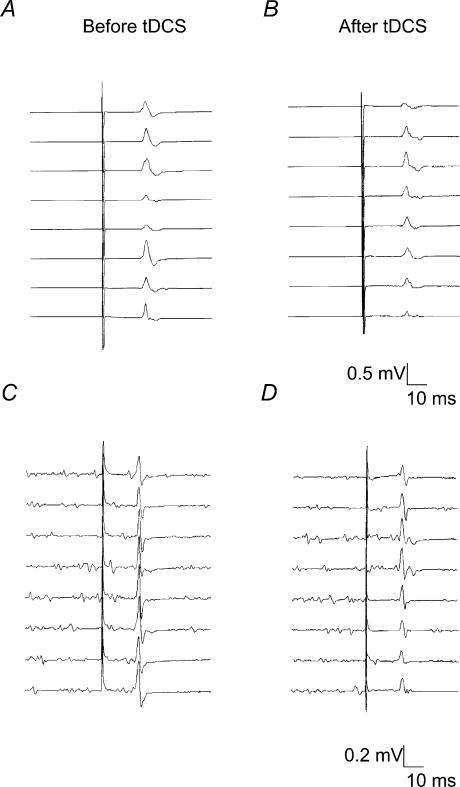
Figure 2. Effect of cathodal transcranial direct current stimulation (tDCS) on motor evoked potentials (MEPs) elicited by transcranial magnetic stimulation (TMS) and transcranial electrical stimulation (TES).
Consecutive non-averaged MEP recordings from a representative subject showing the persistent decrease in MEP amplitude 40 min after cathodal tDCS offset. A and B MEPs evoked by TMS. C and D MEPs evoked by TES. Note that the level of EMG activity in the 40 ms before test stimulus was similar in C and D.
Experiment 3: after-effects of tDCS on EEG rhythms
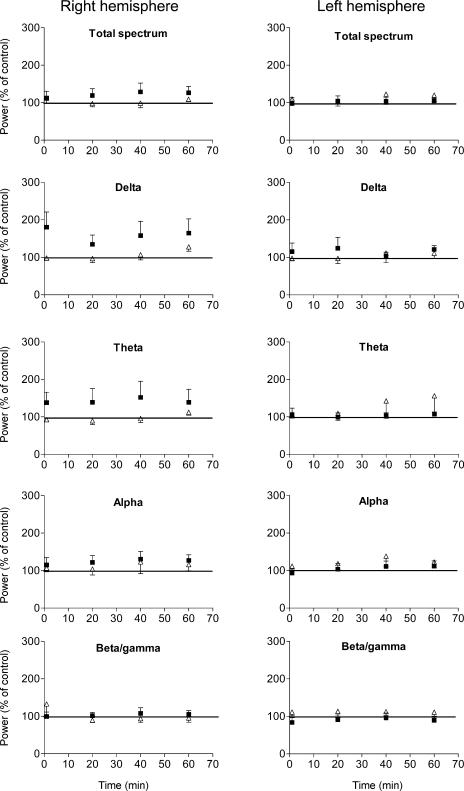
We found no significant lateralization of baseline total EEG power or individual EEG rhythms. In the right cathodally stimulated hemisphere the two-way ANOVA revealed that the power in the alpha- and beta/gamma-rhythms remained statistically unchanged (Fig. 3, Table 1), whereas there was a significant effect of tDCS on total power, delta and theta activity. The Wilcoxon signed rank test disclosed a significant main effect of tDCS on delta rhythm compared to baseline (Table 2). No statistical difference was found even when beta and gamma activity were separately analysed. Conversely, on the left, anodally stimulated hemisphere, power in the delta-, theta-, alpha- and beta-rhythms remained statistically unchanged (Tables 1 and 2). After sham stimulation in five subjects EEG indexes remained statistically unchanged on the right hemisphere and on the left hemisphere (Tables 1 and 2).
Figure 3. Effect of transcranial direct current stimulation (tDCS) and sham tDCS on the power of EEG rhythms.
▪: power spectral density (PSD) after tDCS offset (tDCS: right hemisphere cathodal polarity, left hemisphere anodal polarity, six subjects); Δ, PSD after sham stimulation (five subjects). Y-axes: power expressed as a percentage of the control value estimated on the EEG signal acquired for 30 min before scalp tDCS. Error bars show the s.e.m. Note the increased delta and theta rhythms after cathodal tDCS.
Table 2.
Effect of transcranial direct current stimulation (tDCS) and sham tDCS on the power of EEG rhythms
| Power, right hemisphere | Power, left hemisphere | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total spectrum | Delta | Theta | Alpha | Beta/ gamma | Total spectrum | Delta | Theta | Alpha | Beta/ gamma | |
| tDCS | ||||||||||
| t0 | 112.4 ± 18 | 181.1 ± 40.2* | 138.7 ± 27.6 | 115.1 ± 19.7 | 99.61 ± 11.8 | 98.2 ± 15.1 | 115.4 ± 22.7 | 106.1 ± 17.3 | 93.3 ± 11.3 | 83.7 ± 12.6 |
| t20 | 119.2 ± 18.2 | 134.5 ± 24.8 | 139 ± 36.4 | 121.9 ± 18 | 101.6 ± 8.9 | 104.7 ± 13.3 | 124.4 ± 29.3 | 99 ± 11.6 | 103.5 ± 14.7 | 91.4 ± 12.2 |
| t40 | 128.9 ± 23.5 | 158 ± 38.1 | 152.1 ± 43 | 130.7 ± 20.5 | 107.7 ± 15 | 103.9 ± 9.8 | 103.7 ± 9.9 | 104.2 ± 7.8 | 111.2 ± 14.2 | 95.8 ± 13.1 |
| t60 | 126.3 ± 17.3 | 164.7 ± 37.8 | 139.2 ± 34.3 | 127.1 ± 15.4 | 105.2 ± 10.5 | 105 ± 7.4 | 121.1 ± 10 | 107.4 ± 5.5 | 111.9 ± 14 | 89.5 ± 10.7 |
| Sham | ||||||||||
| t0 | 112.2 ± 13.3 | 98.2 ± 6.4 | 92.7 ± 5.9 | 106.9 ± 9.9 | 132.9 ± 33.9 | 110.2 ± 5.8 | 97.5 ± 6.5 | 107.1 ± 10.4 | 111.6 ± 7.6 | 111.6 ± 10 |
| t20 | 97.9 ± 8.7 | 96.7 ± 10.8 | 89.6 ± 10.3 | 104.2 ± 15.8 | 89.9 ± 6.5 | 104.3 ± 13.4 | 97.3 ± 13.7 | 109.3 ± 19.4 | 118.6 ± 15.8 | 113.4 ± 17.2 |
| t40 | 98.9 ± 12.2 | 106.8 ± 13.5 | 95.8 ± 10.9 | 123.1 ± 31.1 | 93.8 ± 10.4 | 122.8 ± 22.4 | 111.8 ± 25.4 | 142.7 ± 46.7 | 138.4 ± 28.5 | 112.5 ± 10.4 |
| t60 | 109.2 ± 6.4 | 127.8 ± 11.7 | 112.3 ± 7.7 | 116.2 ± 16.8 | 95.5 ± 11.4 | 120.2 ± 17.2 | 111.1 ± 14.4 | 156.3 ± 42.8 | 122.9 ± 15.6 | 111.1 ± 13.7 |
Times after tDCS or sham stimulation: t0, 0 min; t20, 20 min; t40, 40 min; and t60, 60 min. Values are means ±s.e.m.
P < 0.05 Wilcoxon signed rank test, compared with baseline.
Experiment 4: after-effects of tDCS on peripheral axonal excitability
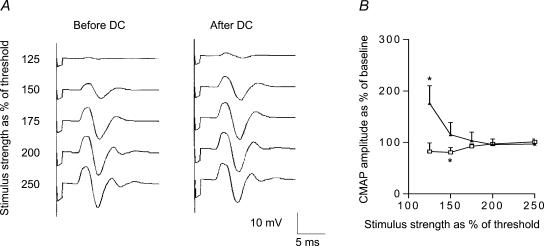
The stimulus–response curve for cathodal polarization showed an upward shift (Fig. 4), indicating increased excitability of peripheral motor axons. The one-way repeated-measures ANOVA and Wilcoxon signed rank test disclosed a significant effect of polarity on CMAP at just above the threshold (F= 6.01, d.f. = 3, P= 0.005; amplitude 175 ± 34.3%, P= 0.04), but no significant effect at stronger test stimulus intensities (Fig. 4). The repeated-measures two-way ANOVA disclosed a significant main effect of tDCS on CMAP (Table 1). Sham stimulation induced only a minimal decrease in excitability (80.6 ± 9%, P < 0.05) at low test stimulation intensities. Axonal threshold before and after nerve polarization did not differ significantly (baseline threshold 4.1 ± 0.4 mA, threshold after tDCS 4.1 ± 0.5 mA, P= 0.85, Wilcoxon signed rank test).
Figure 4. Effect of cathodal transcutaneous direct current (DC) stimulation and sham stimulation on the excitability of ulnar motor axons.
A, CMAP recordings from a representative subject showing the increase in CMAP amplitude just above the threshold in ulnar nerve after cathodal transcutaneous DC stimulation. B, Δ: cathodal polarization (n = 7 subjects); □: sham stimulation (n = 6); Y-axis: compound muscle action potential (CMAP) size expressed as a percentage of the control unconditioned response; X-axis: test stimulation strength expressed as percentage (%) of motor threshold; error bars: s.e.m. Note the increased excitability of low-threshold motor axons after cathodal DC offset. *P < 0.05 Wilcoxon signed rank test. Note the persistent increase in CMAP size after cathodal transcutaneous DC stimulation but not after sham stimulation.
Discussion
Our experiments in healthy volunteers show that cathodal DC stimulation induces functional after-effects in human central and peripheral nervous system. When we applied cathodal tDCS over the scalp, after the current offset motor cortical excitability in response to TMS and TES decreased and the spontaneous slow EEG rhythms (theta and delta) increased. When we applied cathodal DC stimulation over the peripheral nerve, after the current offset motor axonal excitability increased.
The effect of cathodal tDCS on responses evoked by motor cortical stimulation
After cathodal tDCS – but not after sham tDCS – the size of the test MEP evoked by TMS decreased, whereas the resting motor threshold increased for ∼1 h. Because, to our knowledge, changes in motor threshold are an unreported finding, the present experiments in healthy subjects expand previous studies using a similar placement for stimulating electrodes and showing that cathodal tDCS decreases the MEP size (Nitsche et al. 2003a; Priori, 2003). Changes in TMS-motor threshold and TMS-MEP amplitude could arise through different mechanisms. This hypothesis agrees with pharmacological studies showing that motor threshold reflects neuronal membrane excitability, which is mainly dependent on ion channel conductance, whereas MEP size reflects the excitability of intracortical interneuronal network, which is principally dependent on synaptic activity (Ziemann et al. 1996).
To understand whether tDCS-induced after-effects involve only intracortical networks, we tested cortico-motorneuronal excitability using TES at near-threshold intensity and during slight tonic voluntary contraction. These experimental conditions guarantee that the component of the descending volley along the cortico-motorneuronal connection due to direct axonal activation (D-wave) maximally contributes to the evoked response in the target muscle (Mills, 1999). The D-wave arises from the direct activation of the corticospinal axons in the white matter, below the cerebral cortex (Patton & Amassian, 1954; Inghilleri et al. 1990). Hence, responses evoked under these experimental conditions maximally reflect the excitability of cortico-motorneuronal fibres. In our experiments the MEP amplitude evoked by TES decreased significantly for at least 40 min, therefore showing that the long-lasting after-effects of cathodal tDCS involve cortico-motorneuronal axons below the cerebral cortex and spread from or bypass cortical synaptic networks. A non-synaptic mechanism of tDCS contrasts with previous reports by others (Liebetanz et al. 2002; Nitsche et al. 2003a) who found no change in MEPs evoked by TES. The discrepancy might reflect the different total charge density of tDCS and other methodological differences. Although the intensity and experimental design these investigators used for TES is not clear, a higher test stimulus strength excites corticospinal axons at a deeper site in the brain, probably far away from the site involved by tDCS. In one subject we studied, who had a higher active motor threshold than the other subjects, tDCS induced a less pronounced effect, suggesting that a higher test TES intensity that excites axons at a deeper site may fail to disclose a tDCS-induced change. In conclusion, although we cannot rule out the possibility of concomitant persistent changes in the strength of synaptic transmission within the motor cortical circuitry, our data demonstrate that the after-effects of scalp cathodal tDCS involve changes in axonal excitability.
The effects of cathodal tDCS on spontaneous EEG activity
To see whether DCs also influence spontaneous central nervous activity, besides studying the after-effects of cathodal tDCS on responses evoked at the level of motor cortex by a test external stimulation – TMS and TES – we also assessed changes in the spontaneous oscillatory activity of the same cortical areas. We found that theta and delta rhythms specifically increased in the cathodally polarized motor cortex, demonstrating that excitability changes extend beyond neural structures activated by TMS or TES, and also involve physiological local network oscillators. Our EEG findings after tDCS agree with the increased slow EEG activity (3–8 Hz) after cathodal polarization of the cat cerebral cortex (Creutzfeldt et al. 1962). Although no data are yet available about resting oscillatory activity of human motor cortex in response to tDCS, Antal et al. (2004) after cathodal tDCS and visual stimulation observed decreased beta and gamma power of the EEG signal from the occipital cortex, but they made no mention of slower rhythms. In a different experimental setting using anodal tDCS during sleep Marshall et al. (2004) reported an enhanced generation of slow oscillatory EEG. Slow waves in the EEG in general correlate with behavioural inhibition, a relaxed, less active state, and with sleep. EEG recordings show a progressive slowing from alertness to sleep. Slow EEG rhythm increases also after several drugs and in disorders affecting the cerebral cortex. Most anaesthetic agents and antiepileptic drugs at high doses elicit EEG slowing (Sloan, 1998; Rampil, 1995). After a stroke, delta and theta rhythms increase over the ischaemic area as result of decreasing blood flow and changes in the neuronal membrane potential (Luu et al. 2001). The focal increase or synchronization of slow EEG rhythms we observed after cathodal tDCS therefore reflects reduced cortical activity. A depression of neuronal activity receives support from a study conducted by Baudewig et al. (2001) who, combining fMRI and tDCS, observed decreased motor cortical activation after cathodal tDCS. Because frontal intermittent rhythmic delta activity is associated with encephalopathy (Watemberg et al. 2002), the observed increase in frontal delta activity might further support a metabolic mechanism in explaining the after-effects of cathodal tDCS.
The effects of cathodal tDCS on peripheral motor axons
To further assess whether cathodal tDCS can also elicit non-synaptic excitability changes, using a simple, non-synaptic system we tested the effects of DC on the excitability of peripheral motor axons. We found that cathodal DC significantly influenced the excitability of low-threshold peripheral motor axons, thus suggesting that cathodal tDCS could change excitability also through non-synaptic mechanisms. Surprisingly, however, the changes were in the opposite direction: after cathodal DC stimulation axonal excitability did not decrease as it did after scalp tDCS, but increased. Although others have studied the excitability of sensory and motor axons during the passage of DC (Kiernan & Bostock, 2000; Burke et al. 2001; Lin et al. 2002), no data are available about the after-effects of human peripheral nerve polarization. The long-lasting after-effect of cathodal DC on nerve was observed by Lorente de Nó (1947) in frog motor axons. Delivering DC for hours elicited a conduction block lasting for more than an hour after current offset (Lorente de Nó, 1947). An intriguing issue is that the excitability changes at cortical and peripheral levels take diametrically opposite directions. Although the peripheral-nerve model is obviously simpler than the cerebral-cortex model, the different behaviour could reflect, besides the presence of synapses, specific homeostatic systems (glial cells versus Schwann cells) and the different geometry of the electric field. Yet, increased or decreased neuronal excitability depend on the orientation of the excitable tissue with respect to the electric field, and the distance from the polarizing electrodes. Small differences in the electrode placement over the scalp can result in diametrically opposite effects of tDCS on motor evoked potentials (Priori, 2003). Terzuolo & Bullock (1956) observed that the response to the application of a constant electric field to cardiac ganglion of lobster could vary depending on the orientation of the electric field: the effect of polarization could be reversed by tilting the electric field with respect to the axo-dendritic axis. Purpura & McMurtry (1965) reported similar findings in the cat cerebral cortex. Cathodal polarization induced an opposite effect on spontaneous firing rates in different neuronal cells (Purpura & McMurtry, 1965). In our experiments, the axonal orientation relative to the electric field differed by ∼90° because peripheral axons ran parallel to the overlying skin, whereas corticospinal axons lay perpendicular to it where tDCS was delivered.
Possible mechanisms of tDCS action on the human brain
The after-effects of tDCS could arise through several basic mechanisms that concur to alter neural membrane function. A prolonged constant electric field, apart from locally changing ionic concentrations, could induce migration of transmembrane proteins (similarly to gel electrophoresis), cause their steric and conformational changes, and locally alter the tissue acid–base balance. The demonstrated redistribution of membrane proteins on cultured cells (Jaffe, 1977) and migration of the acetylcholine receptor (Stollberg & Fraser, 1988) in response to externally applied electric field, support the possibility of transmembrane protein or channel migration. Changes in the properties and number of ion channels may affect the propagation of neuronal activity and contribute to non-synaptic plasticity (Debanne et al. 2003). Finally, an important phenomenon induced by DC on tissues is water electrolysis (Loeb & Gans, 1986). In pure water, H+ dissociation is low (∼10−7 mol l−1). However, it is influenced by weak acid/base copresence; H+ and OH− generated by electrolysis and dissociation of weak acid in solution could change the acid–base balance by inducing acidosis or alkalosis that, in turn, remarkably affect membrane, receptor, and cell function (Chesler, 2003). Because changes in intracellular pH and [Ca2+] are tightly correlated and anodal polarization has been shown to increase Ca2+ (Islam et al. 1995), cathodal polarization might also influence [Ca2+], thus shifting the pH.
The long-lasting after-effects of tDCS on the brain are thought to be mediated at synaptic level by NMDA receptors (Liebetanz et al. 2002; Nitsche et al. 2003b; Siebner et al. 2004). Together with our findings, several observations nevertheless suggest that the after-effects of tDCS do not arise from NMDA receptor synaptic involvement alone. Yet, although NMDA receptors are present on peripheral axons (Kinkelin et al. 2000), they are unreported on central axons. Finally, Liebetanz et al. (2002) and Nitsche et al. (2003b) hypothesized NMDA receptor-mediated synaptic long-term depression (LTD) as a possible mechanism. Their results should, however, be interpreted cautiously because dextromethorphan at higher doses can also non-specifically inhibit voltage-dependent non-NMDA channels (Netzer et al. 1993; Liebetanz et al. 2002). Nonetheless, tDCS-induced alterations in normal membrane function can also lead to NMDA system dysfunction as a non-causal epiphenomenon through several different mechanisms, as non-synaptic after-effects on membrane dynamics could also prime NMDA receptor-mediated changes in synaptic signalling (Shouval et al. 2002; Lei et al. 2003). Changes in pH below the polarizing electrode can also affect the membrane function outside the synapse directly and indirectly, thus changing the activity of the NMDA system (Tang et al. 1990).
Overall, the after-effects of cathodal tDCS in the human brain arise through non-synaptic mechanisms, possibly involving alterations in transmembrane protein and changes in pH. These findings can be useful in studies designed to validate cathodal tDCS as a technique for treating central nervous system disorders.
Acknowledgments
This work was supported by University of Milan, by Fondazione IRCCS Ospedale Maggiore Policlinico and by Associazione Amici del Centro Dino Ferrari. Dr Ardolino has been partly supported by Istituto Clinico Humanitas IRCCS. The authors wish to thank B. Meda and M. Pellegrini for developing the algorithm for EEG analysis, C. Ghiroldi, F. Gregorini, and S. Mrakic for technical assistance.
References
- Antal A, Varga ET, Kincses TZ, Nitsche MA, Paulus W. Oscillatory brain activity and transcranial direct current stimulation in humans. Neuroreport. 2004;15:1307–1310. doi: 10.1097/01.wnr.0000127460.08361.84. [DOI] [PubMed] [Google Scholar]
- Baudewig J, Nitsche MA, Paulus W, Frahm J. Regional modulation of BOLD MRI responses to human sensorimotor activation by transcranial direct current stimulation. Magn Reson Med. 2001;45:196–201. doi: 10.1002/1522-2594(200102)45:2<196::aid-mrm1026>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Burke D, Kiernan MC, Bostock H. Excitability of human axons. Clin Neurophysiol. 2001;112:1575–1585. doi: 10.1016/s1388-2457(01)00595-8. [DOI] [PubMed] [Google Scholar]
- Chesler M. Regulation and modulation of pH in the brain. Physiol Rev. 2003;83:1183–1221. doi: 10.1152/physrev.00010.2003. [DOI] [PubMed] [Google Scholar]
- Creutzfeldt OD, Fromm GH, Kapp H. Influence of transcortical DC-currents on cortical neuronal activity. Exp Neurol. 1962;5:436–452. doi: 10.1016/0014-4886(62)90056-0. [DOI] [PubMed] [Google Scholar]
- Day BL, Dressler D, Maertens de Noordhout A, Marsden CD, Nakashima K, Rothwell JC, et al. Electric magnetic stimulation of human motor cortex: surface EMG and single motor unit responses. J Physiol. 1989;412:449–473. doi: 10.1113/jphysiol.1989.sp017626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debanne D, Daoudal G, Sourdet V, Russier M. Brain plasticity and ion channels. J Physiol Paris. 2003;97:403–414. doi: 10.1016/j.jphysparis.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Inghilleri M, Berardelli A, Cruccu G, Priori A, Manfredi M. Motor potentials evoked by paired cortical stimuli. Electroencephalogr Clin Neurophysiol. 1990;77:382–389. doi: 10.1016/0168-5597(90)90060-q. [DOI] [PubMed] [Google Scholar]
- Islam N, Aftabuddin M, Moriwaki A, Hattori Y, Hori Y. Increase in the calcium level following anodal polarization in the rat brain. Brain Res. 1995;684:206–208. doi: 10.1016/0006-8993(95)00434-r. [DOI] [PubMed] [Google Scholar]
- Jaffe LF. Electrophoresis along cell membranes. Nature. 1977;265:600–602. doi: 10.1038/265600a0. [DOI] [PubMed] [Google Scholar]
- Jasper HH. The ten-twenty electrode system of the International Federation. Electroencephalographalogr Clin Neurophysiol. 1958;10:371–375. [PubMed] [Google Scholar]
- Kiernan MC, Bostock H. Effect of membrane polarization and ischaemia on excitability properties of human motor axons. Brain. 2000;123:2542–2551. doi: 10.1093/brain/123.12.2542. [DOI] [PubMed] [Google Scholar]
- Kinkelin I, Bröker E-B, Koltzenburg M, Carlton SM. Localization of ionotropic glutamate receptors in peripheral axons of human skin. Neurosci Lett. 2000;28:149–152. doi: 10.1016/s0304-3940(00)00944-7. [DOI] [PubMed] [Google Scholar]
- Lei S, Pelkey KA, Topolnik L, Congar P, Lacaille J-C, McBain CJ. Depolarization-induced long-term depression at hippocampal mossy fiber-CA3 pyramidal neuron synapses. J Neurosci. 2003;23:9786–9795. doi: 10.1523/JNEUROSCI.23-30-09786.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebetanz D, Nitsche M, Tergau F, Paulus W. Pharmacological approach to the mechanisms of transcranial DC-stimulation-induced after-effects of human motor cortex. Brain. 2002;125:2238–2247. doi: 10.1093/brain/awf238. [DOI] [PubMed] [Google Scholar]
- Lin CS-Y, Kuwabara S, Cappelen-Smith C, Burke D. Responses of human sensory and motor axons to the release of ischaemia and to hyperpolarizing currents. J Physiol. 2002;541:1025–1039. doi: 10.1113/jphysiol.2002.017848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb GE, Gans C. Electromyography for Experimentalists. London: The University of Chicago Press; 1986. [Google Scholar]
- Lorente de Nó R. Studies from the Rockefeller Institute for Medical Research. Vol. 131. New York: Rockefeller Institute for Medical Research; 1947. A study of nerve physiology; pp. 334–389. [PubMed] [Google Scholar]
- Luu P, Tucker DM, Englander R, Lockfeld A, Lutsep H, Oken B. Localizing acute stroke-related EEG changes: assessing the effects of spatial undersampling. J Clin Neurophysiol. 2001;18:302–317. doi: 10.1097/00004691-200107000-00002. [DOI] [PubMed] [Google Scholar]
- Marshall L, Mölle M, Hallschmid M, Born J. Transcranial direct current stimulation during sleep improves declarative memory. J Neurosci. 2004;24:9985–9992. doi: 10.1523/JNEUROSCI.2725-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills KR. Magnetic Stimulation of the Human Nervous System. New York: Oxford University Press; 1999. [Google Scholar]
- Netzer R, Pflimlin P, Trube G. Dextromethorphan blocks N-methyl-d-aspartate-induced currents and voltage-operated inward currents in cultured cortical neurons. Eur J Pharmacol. 1993;238:209–216. doi: 10.1016/0014-2999(93)90849-d. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Fricke K, Henschke U, Schlitterlau A, Liebetanz D, Lang N, et al. Pharmacological modulation of cortical excitability shifts induced by transcranial DC stimulation. J Physiol. 2003b;553:293–301. doi: 10.1113/jphysiol.2003.049916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Nitsche MS, Klein CC, Tergau F, Rothwell JC, Paulus W. Level of action of cathodal DC polarization induced inhibition of the human motor cortex. Clin Neurophysiol. 2003a;114:600–604. doi: 10.1016/s1388-2457(02)00412-1. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 2000;527:633–639. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology. 2001;57:1899–1901. doi: 10.1212/wnl.57.10.1899. [DOI] [PubMed] [Google Scholar]
- Patton HD, Amassian VE. Single and multiple unit analysis of cortical stage of pyramidal tract activation. J Neurophysiol. 1954;17:345–363. doi: 10.1152/jn.1954.17.4.345. [DOI] [PubMed] [Google Scholar]
- Priori A. Brain polarization in humans: a reappraisal of an old tool for prolonged non-invasive modulation of brain excitability. Clin Neurophysiol. 2003;114:589–595. doi: 10.1016/s1388-2457(02)00437-6. [DOI] [PubMed] [Google Scholar]
- Priori A, Berardelli A, Rona S, Accornero N, Manfredi M. Polarization of the human motor cortex through the scalp. Neuroreport. 1998;9:2257–2260. doi: 10.1097/00001756-199807130-00020. [DOI] [PubMed] [Google Scholar]
- Priori A, Bossi B, Ardolino G, Bertolasi L, Carpo M, Nobile-Orazio E, et al. Pathophysiological heterogeneity of conduction blocks in multifocal motor neuropathy. Brain. 2005;128:1642–1648. doi: 10.1093/brain/awh513. [DOI] [PubMed] [Google Scholar]
- Purpura DP, McMurtry JG. Intracellular activities and evoked potential changes during polarization of motor cortex. J Neurophysiol. 1965;28:166–185. doi: 10.1152/jn.1965.28.1.166. [DOI] [PubMed] [Google Scholar]
- Rampil IJ. Electroencephalogram. In: Albin MA, editor. Textbook of Neuroanesthesia with Neurosurgical and Neuroscience Perspectives. New York: McGraw-Hill; 1995. pp. 193–220. [Google Scholar]
- Rothwell JC, Hallet M, Berardelli A, Eisen A, Rossini P, Paulus W. Magnetic stimulation: motor evoked potentials. The International Federation of Clinical Neurophysiology. Electroencephalogr Clin Neurophysiol Supplement. 1999;52:97–103. [PubMed] [Google Scholar]
- Shouval HZ, Bear MF, Cooper LN. A unified model of NMDA receptor-dependent bidirectional synaptic plasticity. Proc Natl Acad Sci. 2002;99:10831–10836. doi: 10.1073/pnas.152343099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebner HR, Lang N, Rizzo V, Nitsche MA, Paulus W, Lemon RN, et al. Preconditioning of low-frequency repetitive transcranial magnetic stimulation with transcranial direct current stimulation: evidence for homeostatic plasticity in the human motor cortex. J Neurosci. 2004;24:3379–3385. doi: 10.1523/JNEUROSCI.5316-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan TB. Anesthetic effects on electrophysiological recordings. J Clin Neurophysiol. 1998;15:217–226. doi: 10.1097/00004691-199805000-00005. [DOI] [PubMed] [Google Scholar]
- Stollberg J, Fraser SE. Acetylcholine receptors and concanavalin A-binding sites on cultured Xenopus muscle cells: electrophoresis, diffusion, and aggregation. J Cell Biol. 1988;107:1397–1408. doi: 10.1083/jcb.107.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang C-M, Dichter M, Morad M. Modulation of the N-methyl-d-aspartate channel by extracellular H+ Proc Natl Acad Sci. 1990;87:6445–6449. doi: 10.1073/pnas.87.16.6445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terzuolo CA, Bullock TH. Measurement of imposed voltage gradient adequate to modulate neuronal firing. Proc Natl Acad Sci. 1956;42:687–693. doi: 10.1073/pnas.42.9.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watemberg N, Alehan F, Dabby R, Lerman-Sagie T, Pavot P, Towne A. Clinical and radiologic correlates of frontal intermittent rhythmic delta activity. J Clin Neurophysiol. 2002;19:535–539. doi: 10.1097/00004691-200212000-00006. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Lonnecker S, Steinhoff BJ, Paulus W. Effects of antiepileptic drugs on motor cortex excitability in humans: a transcranial magnetic stimulation study. Ann Neurol. 1996;40:367–378. doi: 10.1002/ana.410400306. [DOI] [PubMed] [Google Scholar]