Abstract
The Kv4.2/4.3 channels are the primary subunits that contribute to the fast-inactivating, voltage-dependent transient outward K+ current (Ito,fast) in the heart. Ito,fast is the critical determinant of the early repolarization of the cardiac action potential and plays an important role in the adaptive remodelling of cardiac myocytes, which usually causes cell volume changes, during myocardial ischaemia, hypertrophy and heart failure. It is not known, however, whether Ito,fast is regulated by cell volume changes. In this study we investigated the molecular mechanism for cell volume regulation of Ito,fast in native mouse left ventricular myocytes. Hyposmotic cell swelling caused a marked increase in densities of the peak Ito,fast and a significant shortening in phase 1 repolarization of the action potential duration. The voltage-dependent gating properties of Ito,fast were, however, not altered by changes in cell volume. In the presence of either protein kinase C (PKC) activator (12,13-dibutyrate) or phosphatase inhibitors (calyculin A and okadaic acid), hyposmotic cell swelling failed to further up-regulate Ito,fast. When expressed in NIH/3T3 cells, both Kv4.2 and Kv4.3 channels were also strongly regulated by cell volume in the same voltage-independent but PKC- and phosphatase-dependent manner as seen in Ito,fast in the native cardiac myocytes. We conclude that Kv4.2/4.3 channels in the heart are regulated by cell volume through a phosphorylation/dephosphorylation pathway mediated by PKC and serine/threonine phosphatase(s). These findings suggest a novel role of Kv4.2/4.3 channels in the adaptive electrical and structural remodelling of cardiac myocytes in response to myocardial hypertrophy, ischaemia and reperfusion.
The Ca2+-independent, fast-inactivating, transient outward K+ current (Ito,1 or Ito,fast) is the critical determinant of the early (phase 1) repolarization of the action potential duration (APD) and excitation–contraction (E–C) coupling of the heart in many mammalian species, including human (Nerbonne & Guo, 2002; Sah et al. 2003). The primary subunits that contribute to Ito,fast are the Kv4.2 or Kv4.3 channels, or a combination of the two (Dixon et al. 1996; Johns et al. 1997; Hoppe et al. 2000). Recently, it has been suggested that decreased expression of Kv4.2/4.3 may be responsible for the decreased Ito,1 density and prolonged APD in many animal models of heart diseases and in human heart failure as well (Takimoto et al. 1997; Kaab et al. 1998; Huang et al. 2000; Kassiri et al. 2002). The mechanism underlying the link between the ionic remodelling (reduction in Ito,1 density and Kv4.2/4.3 expression) and structural remodelling (myocardial hypertrophy and dilated heart failure) remains to be elucidated.
In response to myocardial hypertrophy and heart failure, adaptive remodelling of the cardiac myocytes is closely associated with perturbations of both cell volume and ion channel function (Frey et al. 2004; Duan et al. 2005). Other pathological stress such as myocardial hypoxia, ischaemia and reperfusion also causes alterations in cell volume and ion channel activities due to changes in extracellular osmolarity (Steenbergen et al. 1985; Wright & Rees, 1998; Befroy et al. 1999). Excessive cell volume changes in the heart may cause profound alteration of structural integrity and constancy of the intracellular milieu, which in turn will affect many cellular functions, including: cardiac electrical activity and contractility due to changes in membrane ion permeability (Vandenberg et al. 1996; Wright & Rees, 1998; Befroy et al. 1999; Kocic et al. 2001; Baumgarten & Clemo, 2003; Frey et al. 2004); intracellular metabolism (Wright & Rees, 1998; Kocic et al. 2001; Baumgarten & Clemo, 2003); and multiple intracellular signal cascades including protein kinase C (PKC) and protein phosphatases (Wright & Rees, 1998; Duan et al. 1999a; Lang et al. 2000; Baumgarten & Clemo, 2003; Dorn & Force, 2005). In fact, it has been consistently shown that hyposmotic cell swelling causes significant shortening in APD of both atrial and ventricular myocytes (Vandenberg et al. 1997; Kocic et al. 2001; Duan et al. 2005). Although activation of the volume-regulated Cl− current (ICl,vol) (Duan et al. 1997a, b, 1999a; Vandenberg et al. 1997), the slow delayed rectifier (IK,s) (Rees et al. 1995), the ATP-sensitive K+ current (IK,ATP) (Priebe & Beuckelmann, 1998), and the Cl− inward rectifier (ICl,ir) (Duan et al. 2000), has been reported to contribute to cell swelling-induced APD shortening (Hiraoka et al. 1998; Kocic et al. 2001), the mechanism for the shortening in early phase 1 repolarization of APD remains unclear. It is not known whether Ito,fast is regulated by cell volume changes, and whether the shortening in phase 1 of cardiac APD is due to cell swelling-induced up-regulation of Ito,fast (Vandenberg et al. 1996; Hiraoka et al. 1998; Wright & Rees, 1998; Kocic et al. 2001).
In mouse cardiac myocytes isolated from the apex of left ventricle, previous studies have identified at least four distinct voltage-dependent K+ currents, namely the fast transient outward K+ current (Ito,fast), the ultra-rapidly activating, slowly inactivating delayed rectifier K+ current (IK,slow1), the TEA-sensitive slowly activating delayed rectifier K+ current (IK,slow2) and the non-inactivating steady-state current (Iss). In addition to their distinct molecular identities these K+ currents can be effectively separated for the purpose of functional characterization in cardiac myocytes by using a combination of biophysical and pharmacological approaches (Brouillette et al. 2004). In this study, we adapted these valid approaches to isolate Ito,fast from other K+ currents (e.g. IK,slow1, IK,slow2 and Iss) in cardiac myocytes of mouse left ventricle apex and investigated cell volume regulation of Ito,fast. We found that Ito,fast and its key molecular components, both Kv4.2 and Kv4.3 channels, are strongly regulated by cell volume through a phosphorylation and dephosphorylation mechanism mediated by PKC and serine/threonine phosphatase(s). These results suggest a novel role of Kv4.2/4.3 channels in electrical remodelling caused by cell volume changes such as cell swelling and hypertrophy, and thus provide an alternative mechanism for their function in heart diseases.
Methods
The investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996) and was in accordance with the institutional guidelines for animal care and use approved by the University of Nevada, Reno Institutional Animal Care and Use Committee.
Isolation of single cardiac myocytes
Single cardiac myocytes were isolated from left ventricular apex of adult mice (C57/BL, male, 25–30 g) using a well-established method as previously described (Duan et al. 1993, 1999b, 2000). Briefly, the animals were anaesthetized by injection of sodium phenobarbitone (50 mg kg−1, i.p.) until a surgical level of anaesthesia was confirmed by loss of withdrawal reflex to toe pinch. A thoracotomy was then performed; the heart was quickly removed and perfused in a Langendorff mode, first with normal Tyrode solution and then with Ca2+-free Tyrode solution until heart beating completely ceased. The hearts were then perfused with the same Ca2+-free Tyrode solution but containing 0.04% collagenase type 2 (Worthington, Biochemical, Lakewood, NJ, USA Co.) for 10–15 min. The left ventricular apex was removed and isolated myocytes were harvested. Only Ca2+-tolerant quiescent myocytes with a typical rod-shaped form and clear cross-striations were used for experiments. Cell dimensions (diameter or width and length) were monitored with two calibrated graticules (one for width and the other for length) in the microscope and cell volume was estimated with assumed right cylindrical geometry according to the following equation: V=πL(D/2)2, where V, L and D are cell volume, length and diameter, respectively (Duan et al. 1997a).
Expression of Kv4.2 and Kv4.3 in NIH/3T3 mammalian cells
The cDNA of rat Kv4.3 long form (rKv4.3 l, Genebank accession number AB003587) and rat Kv4.2 (Genebank accession number M59980) (generous gifts from Dr Susumu Ohya, Department of Molecular & Cellular Pharmacology, Nagoya City University, Japan) were transfected into NIH/3T3 cells by Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA). NIH/3T3 cells (American Type Culture Collection, Rockville, MD, USA) were maintained in Dulbecco's modified Eagle's medium supplemented with 5% fetal calf serum (Invitrogen). The day before transfection, cells were trypsinized and plated into 24-well plates at 1.5 × 105 cells per well so that they were 90–95% confluent on the day of transfection. Stable transfectants were selected by 1000 μg ml−1 G418 (Invitrogen). Individual G418-resistant subclones were isolated, expanded and maintained. Cells were grown on sterile glass coverslips for 1 day and then used for patch-clamping experiments.
Solutions
For current-clamp and voltage-clamp experiments shown in Fig. 1, the hyposmotic bath solution contained (mm): sodium aspartate, 110; potassium aspartate, 5.4; MgCl2, 0.8; CaCl2, 1; NaCl, 10; glucose, 10; Hepes, 10; pH 7.2, 235 mosmol kg−1. The isosmotic bath solutions were the same except that the osmolarity was adjusted to 305 mosmol kg−1 by adding mannitol. The pipette solution contained (mm): KCl, 20; potassium aspartate, 120; Mg-ATP, 5; EGTA, 10; Hepes, 10; pH 7.2, 290 mosmol kg−1. A high concentration of ATP (5 mm) in the pipette solution was used to prevent the activation of KATP and its contribution to APD. For the rest of the voltage-clamp experiments, bath and pipette solutions were chosen to facilitate Ito recording and to eliminate contaminations from other currents. The standard hyposmotic bath solution contained (mm): N-methyl-d-glucamine (NMDG), 85; l-aspartaric acid, 95; CdCl2, 0.3; MgCl2, 0.8; BaCl2, 2; CaCl2, 1; KCl, 5.4; TEA-Cl, 10; glucose, 10; Hepes, 10; pH 7.2, 235 mosmol kg−1. The isosmotic and hyperosmotic bath solutions were the same as the hyposmotic bath solution except that the osmolarity was adjusted by adding mannitol to 305 and 350 mosmol kg−1, respectively. The standard pipette solution contained (mm): KCl, 20; potassium aspartate, 120; Mg-ATP, 5; EGTA, 10; Hepes, 10; pH 7.2, 290 mosmol kg−1. EGTA (10 mm) was included in the pipette solution and Cd2+ (0.3 mm), Ba2+ (2 mm) and TEA-Cl (10 mm) were present continuously in the bath solution to block L-type Ca2+ current (ICa,L, Ca2+)-dependent outward K+ and Cl− currents, K+ inward rectifiers (IK1), and delayed rectifiers (IK,s, IK,r and IK,slow2), respectively (Duan et al. 1993, 1999b, 2000; Zhou et al. 2003). Extracellular Na+ was eliminated to preclude the contamination by Na+ current (INa). Low Cl− concentrations on both extracellular ([Cl−]o= 21.6 mm) and intracellular ([Cl−]i= 20 mm) sides were used to minimize contamination from the swelling-activated Cl− currents (ICl,swell) and ICl,ir (Duan et al. 1997a, b, 2000) and the Ca2+-activated Cl− current (ICl,Ca or Ito,2) in mouse ventricular myocytes (Xu et al. 2002). The osmolarity of all solutions was measured just before each experiment by the Advanced Microosmometer 3300 (Advanced Instruments, Inc., Norwood, MA, USA). Isosmotic (1T; where T is relative osmolarity) solution was set as 305 mosmol kg−1. Therefore, the hyposmotic solutions were 0.77T (235/305 mosmol kg−1) and the hyperosmotic solutions were 1.15T (350/305 mosmol kg−1). All chemicals were purchased from Sigma.
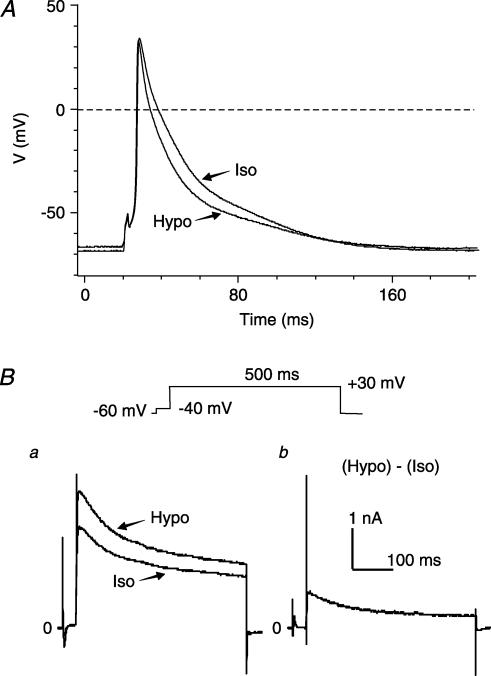
Figure 1. Osmotic regulation of APD and Ito in mouse left ventricular apex myocytes.
A, superimposed representative action potential recordings under isosmotic (Iso, 305 mosmol kg−1) and hyposmotic (Hypo, 235 mosmol kg−1) conditions. B, original traces of whole-cell currents measured in the same cell as in panel A. a, superimposed representative whole-cell current traces recorded with the voltage protocols shown above, under isosmotic (Iso) and hyposmotic (Hypo) conditions. b, hyposmotic stress-sensitive current obtained by subtracting the current recorded under isosmotic (Iso) conditions from that under hyposmotic (Hypo) conditions.
Electrophysiological measurements and data analysis
The tight-seal, whole-cell current-clamp and voltage-clamp techniques as previously described (Hamill et al. 1991; Duan et al. 1997a, b, 1999b, 2000) were used to record action potentials and whole-cell currents, respectively, from single mouse ventricular myocytes. Voltage-clamp and current-clamp recordings were obtained using an Axopatch 200 A patch-clamp amplifier (Axon Instruments, Inc., Union City, CA, USA). Data acquisition and command potentials were controlled by pCLAMP 8.0 software (Axon Instruments). Currents were recorded from a holding potential of −60 mV to a series of test potentials from −50 to +80 mV for 1 s in +10 mV increments at an interval of 10 s. Whole-cell currents were filtered at 1 kHz and sampled at 5 kHz. Action potentials were recorded simultaneously from the same myocytes using a brief stimulus (2-ms depolarizing current) at a frequency of 5 Hz under the same conditions for the recording of outward K+ currents. Recording pipettes were prepared from borosilicate glass electrodes (1.5 mm o.d.) with tip resistance of 1–3 MΩ when filled with pipette solutions. The cell capacitance was calculated by integrating the area under an uncompensated capacitive transient elicited by a 5-mV hyperpolarizing pulse from a holding potential of 0 mV. The whole-cell membrane capacitance of mouse left ventricular myocytes was 116 ± 14 pF (n = 60). To separate individual outward K+ current components, currents were elicited by a +30-mV depolarizing step from a holding potential of −80 mV. The amplitude of test current components was measured as the difference between the peak current and the current level at the end of the voltage pulse. A conventional two-pulse protocol was used to analyse the effects of osmotic stress on the voltage dependence of the inactivation. For Ito,fast, cells were first clamped to different potentials ranging from −100 to 0 mV for 100 ms, and then followed by a 1-s test pulse to +30 mV. The amplitude of test current evoked from each prepulse potential was normalized to the maximal current amplitude (Imax) evoked from −100 mV (at which inactivation was completely removed), and plotted as a function of the inactivating prepulse potential. The inactivation curves obtained from the double-pulse protocol were fitted to the following Boltzmann equation: y∞= 1/{1 + exp [(Vpp−V1/2)/k]}, where Vpp is the potential of the prepulse, V1/2 is the membrane potential at 50% inactivation and k is the slope factor. The time course of recovery from inactivation was determined by a double-pulse stimulus protocol. The first depolarization pulse (P1) was applied from a holding potential of −80 mV to +30 mV for 500 ms (to completely inactivate the currents) followed by a second depolarization pulse (P2) to +30 mV for 500 ms with variable interpulse intervals at the holding potential. The test-current amplitudes evoked by P2 at +30 mV after each recovery period were normalized to the current amplitudes evoked by P1 in the same cell, and plotted against recovery time. Exponential functions were fitted to the data describing the recovery from inactivation. Details are given in Results. All experiments were conducted at room temperature (22–24 °C). To account for differences in cell size, whole-cell currents were normalized to cell capacitance, and the average data were reported as current densities (pA pF−1). Group data are presented as means ±s.e.m. Student's t-test was used to determine statistical significance. A two-tailed probability (P) of ±5% was considered significant.
Results
Effects of osmotic stress on APD and transient outward K+ currents in native mouse ventricular myocytes
To examine the cell swelling-induced APD changes in mouse ventricular myocytes and the corresponding changes in whole-cell currents, action potentials and whole-cell currents were recorded from the same cell using alternations of voltage-clamp and current-clamp modes under the same conditions. Representative superimposed action potential traces recorded from a ventricular myocyte exposed to isosmotic (Iso) and hyposmotic (Hypo) solutions for 10 min, respectively, are shown in Fig. 1A. Consistent with previous observations in cardiac myocytes of mouse and several other species (Hamill et al. 1991; Duan et al. 1995, 1997a, b, 1999a; Vandenberg et al. 1997; Kocic et al. 2001), exposure of mouse left ventricular myocytes to hyposmotic perfusates caused cell swelling (∼150% increase in cell size) (Duan et al. 1995, 2000), which was accompanied by a significant shortening in APD in all (five out of five) tested cells. Table 1 summarizes the changes in the action potential parameters under isosmotic and hyposmotic conditions. Hyposmotic cell swelling caused a significant shortening in APD, especially in the early repolarization phases as measured at 5% (APD5) and 10% (APD10) repolarization. APD5 shortened 58 ± 5% and APD10 shortened 38 ± 3%, respectively (n = 5, P < 0.001 versus isosmotic conditions). These changes were accompanied by a significant increase in the outward currents (Fig. 1Ba) under the same hyposmotic conditions. It has been previously reported that activation of several volume-sensitive currents, such as ICl,swell (Duan et al. 1997a, b, 2000; Vandenberg et al. 1997), IK,s (Rees et al. 1995), IK,ATP (Priebe & Beuckelmann, 1998) and ICl,ir (Duan et al. 2000), may be responsible for the swelling-induced shortening in APD but the mechanism for the shortening in early repolarization (APD5 and APD10) remains unclear. As shown in Fig. 1Bb, cell swelling-induced currents include a transient outward current, suggesting that cell swelling-induced increase in transient outward K+ currents may contribute to the shortening in early repolarization.
Table 1.
Effects of hyposmotic cell swelling on action potential in mouse ventricular myocytes
| Resting potential (mV) | APD (ms) | APD25 (ms) | APD75 (ms) | APD90 (ms) | |
|---|---|---|---|---|---|
| Isosmotic | − 69.5 ± 1.6 | 95.1 ± 9.8 | 2.7 ± 0.5 | 18.0 ± 4.4 | 45.2 ± 7.9 |
| Hyposmotic | − 68.5 ± 1.2 | 58.4 ± 8.8** | 1.8 ± 0.4* | 13.7 ± 5.1* | 25.3 ± 7.0** |
P < 0.05
P < 0.01 versus isosmotic (n = 5).
In our experimental conditions, hyposmotic cell swelling failed to cause significant changes in resting membrane potential (RMP) (Table 1) and IK1 (data not shown) in mouse ventricular myocytes. The measured mean RMP of the single adult mouse ventricular myocytes was −69.5 ± 1.6 mV (n = 5, Table 1), which is more positive than the predicted normal RMP (>−80 mV) of ventricular myocytes but is in very good agreement with the value of −64 to −68 mV measured also from adult mouse ventricular myocytes under similar experimental conditions by Nerbonne and colleagues (Barry et al. 1998; Xu et al. 1999a, c). The depolarized RMP recorded from a single ventricular myocyte may be due to a number of reasons, such as: the compositions of the bath and pipette solutions, the experimental temperature, the status of the isolated single adult mouse ventricular myocytes, etc. Therefore caution should be used when interpreting the action potential results recorded from a single cell. In our study of the effects of hyposmotic challenge on the action potentials, therefore, we designed a parallel control group to monitor the changes in action potentials when the cells were exposed to only isosmotic bath solutions for the same time course (at least 30 min). No significant changes in APD and outward current were found in the control group (n = 4, data not shown) while hyposmotic cell swelling caused a significant shortening in APD, especially the early phase of APD (Table 1). These observations are consistent with previous reports from many other laboratories (Vandenberg et al. 1997; Kocic et al. 2001; Duan et al. 2005).
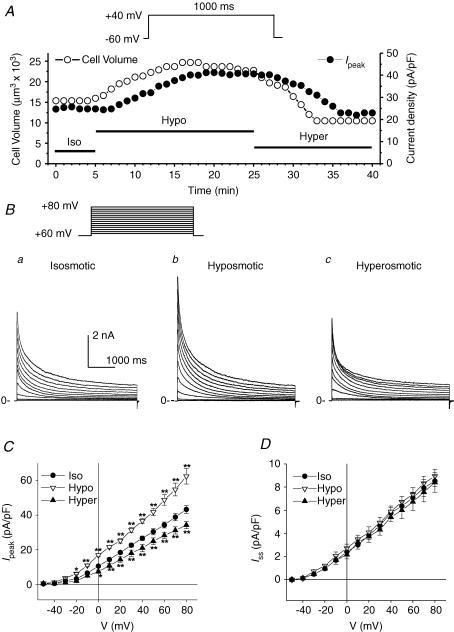
The results shown in Fig. 1Bb are very intriguing because there is currently no information available in the literature about the cell volume regulation of transient outward currents in the heart or any other tissues. Given the well-documented crucial role of the transient outward K+ currents in the early repolarization and E–C coupling (ECC) in human and numerous other animal models (Barry et al. 1998; Kaab et al. 1998; Wang et al. 1999; Yue et al. 1999; Huang et al. 2000), it is very important to determine whether or not these K+ currents in cardiac myocytes are regulated by osmotic stress-induced cell volume changes. We therefore used experimental approaches that are well-established in our laboratory (Duan et al. 1993, 1995, 1997a, b, 1999a, 2000) to study the osmotic regulation of the transient outward currents. Figure 2A shows the time course of osmotic stress on the changes in cell volume and the transient outward peak current recorded in a single cell at +40 mV from a holding potential of −60 mV under isosmotic, hyposmotic and hyperosmotic conditions. Exposure to hyposmotic solutions caused a time-dependent cell swelling and increase in the peak current. As shown in Fig. 2A, cell volume started to increase <1 min after hyposmotic perfusion and reached steady state in 10 min. Peak current started to increase after 3 min perfusion with hyposmotic solutions and reached steady state after 15 min hyposmotic perfusion. In seven cells, hyposmotic challenge increased the mean cell volume from 12 870 ± 1488 to 19 217 ± 2903 μm3 (143 ± 7% increase, P < 0.01, n = 7) and the peak current density from 24.7 ± 0.3 to 41.2 ± 0.4 pA pF−1 (162 ± 7% increase, P < 0.001, n = 7). Hyperosmotic solution caused a time-dependent cell shrinkage (9824 ± 2903 μm3, n = 7) and a decrease in the peak current density (20.4 ± 0.2 pA pF−1, n = 7) after 20-min hyperosmotic perfusion. In all of these cells, there was usually a ∼2-min lag between changes in the peak current and the cell volume. The slower onset of the changes in Ipeak strongly supports the notion that the regulation of Ipeak by osmotic challenges is a result of cell volume changes, which may be working by affecting intracellular and/or cell membrane-bounded (for example, stretch of cytoskeleton) signalling transduction pathways.
Figure 2. Effects of osmotic stress on cell volume and transient outward K+ currents in mouse ventricular apex myocytes.
A, representative time course of changes in cell volume (•, y-axis on the left) and peak transient outward K+ current density (•, y-axis on the right) in a single mouse ventricular myocyte under different osmotic conditions. Cell volume and the whole-cell outward currents elicited by a 1-s depolarizing voltage pulse to +40 mV from a holding potential of −60 mV were continuously monitored every 1 min when the cell was exposed to isosmotic (Iso), hyposmotic (Hypo) and hyperosmotic (Hyper) solutions, respectively. Similar results were observed in seven cells. B, families of whole-cell outward currents elicited by a series of 4.5-s depolarizing voltage steps from a holding potential of −60 mV to potentials between −50 and +80 mV in 10-mV increments (inset on top) under isosmotic (a), hyposmotic (b) and hyperosmotic (c) conditions. C and D, mean I–V curves for Ipeak and Iss in mouse apex myocytes (n = 12) under isosmotic, hyposmotic and hyperosmotic conditions. Ipeak was measured as the peak of outward currents (at 10–50 ms) and Iss was measured at the end of 4.5-s voltage steps. *P < 0.05, **P < 0.01 versus isosmotic conditions.
Figure 2B shows a representative family of outward currents recorded in a myocyte under isosmotic (a), hyposmotic (b) and hyperosmotic (c) conditions. These osmotic stress-regulated outward currents are the Ca2+-independent voltage-gated transient outward K+ currents because: (1) contamination from other ionic channels such as IK,r, IK,s, IK,slow2, IK1, ICa,L, ICl,Ca, INa and ICl,swell were eliminated or minimized by TEA, Ba2+, Cd2+, no Na+, high intracellular EGTA (10 mm) and low concentrations of Cl− (see Methods); and (2) the transient outward currents under hyposmotic conditions were significantly blocked by 4-aminopyridine (4-AP; 5 mm, data not shown). Chiamvimonvant and colleagues have discovered a Ca2+-activated Cl− channel in mouse heart (Xu et al. 2002). The Ca2+-activated Cl− current (ICl,Ca) is also called Ito,2 because its transient activation and inactivation properties are very similar to Ito,fast. Therefore, it is possible that activation of ICl,Ca may also contribute to the shortening in early repolarization of cardiac APD observed in the ventricular myocytes. There is currently no information about the volume regulation of cardiac Ca2+-activated Cl− channels available in any species (Hume et al. 2000; Duan et al. 2005). In the present study, however, the possible contribution of ICl,Ca to the observed cell volume regulation of Ito was eliminated by using a very high intracellular EGTA (10 mm) to buffer intracellular Ca2+ and very low concentrations of Cl− in both pipette and bath solutions (∼20 mm) to minimize Cl− currents.
Figure 2C shows the mean current–voltage (I–V) relationship of osmotic-regulated peak (Ipeak) and steady-state (Iss) components of the outward K+ current. The density of Ipeak was measured as the difference between the peak current and the current level at the end of the voltage steps then normalized with the cell capacitance. The mean current density of Ipeak was increased significantly by hyposmotic-induced cell swelling over the range of −30 to +80 mV, and the subsequent hyperosmotic cell shrinkage decreased the mean current density of Ipeak significantly over the range of 0 to +80 mV. For example, the current density of Ipeak at +40 mV was 26.6 ± 1.3, 36.7 ± 1.6 and 21 ± 2.1 pA pF−1 (n = 12, P < 0.01), under isosmotic, hyposmotic and hyperosmotic conditions, respectively. The non-inactivating steady-state current that remains at the end of 4.5-s voltage steps is referred to as Iss (Xu et al. 1999b). In contrast to Ipeak, and as shown in Fig. 2D, Iss was not affected by changes in osmotic conditions. For example, at +40 mV, the current densities of Iss were 5.6 ± 0.5, 5.8 ± 0.5 and 5.4 ± 0.4 pA pF−1 under isosmotic, hyposmotic and hyperosmotic conditions, respectively (n = 12, NS).
Effects of hyposmotic cell swelling on Ito,fast in mouse left ventricular apex myocytes
In mouse left ventricular apex, voltage-dependent outward K+ currents are composed of at least four K+ currents: Ito,fast, IK,slow1, IK,slow2 and Iss (Xu et al. 1999b; Zhou et al. 2003). While IK,slow2 can be selectively eliminated by 10 mm of TEA (Zhou et al. 2003), the separation of Ito,fast from IK,slow1 and Iss was relatively more difficult. In this study, we adapted an approach established by Brouillette and colleagues to further effectively separate Ito,fast from IK,slow1 and Iss in mouse ventricular myocytes (Brouillette et al. 2004). Because Ito,fast inactivates much more rapidly than the other outward K+ current components (IK,slow1 and Iss), it could be separated from those K+ currents by using ‘inactivating’ prepulses (Fig. 3A and B) (Brouillette et al. 2004). In our experimental conditions Ito,fast in mouse left ventricular apex myocytes inactivated completely at membrane potentials more positive than −20 mV (see Fig. 4). The time constant of Ito,fast inactivation was 31 ± 7 ms (at +10 mV, n = 7). In addition, previous studies have shown that 10–100 μm of 4-AP can selectively block IK,slow1 without significant effects on Ito,fast in mouse ventricular myocytes (Xu et al. 1999b; Brouillette et al. 2004). In the present study, we also performed additional experiments with different concentrations of 4-AP (10, 50 and 100 μm) under isosmotic and hyposmotic conditions and found that 50 μm 4-AP was able to selectively block IK,slow1 with insignificant blocking effects on Ito,fast (see Fig. 3A). The osmotic challenge does not change the concentration–effect curves of 4-AP. Therefore, a combination of a 100-ms ‘inactivating’ prepulse to −20 mV and 50 μm of 4-AP in the bath solutions was used to effectively separate Ito,fast from other K+ currents in the ventricular myocytes.
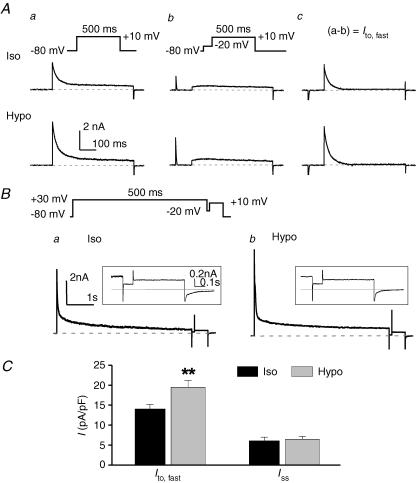
Figure 3. Hyposmotic cell swelling increases Ito,fast but not Iss in mouse left ventricular apex myocytes.
A, effects of osmotic stress on Ito,fast. Whole-cell currents were recorded in the presence of 50 μm 4-AP from the same cell with voltage protocols shown on the top under isosmotic (Iso, upper traces) and hyposmotic (Hypo, lower traces) conditions. Cells were held at −80 mV, and currents were elicited by a 500-ms depolarizing voltage pulse to +10 mV (a) or by a 500-ms depolarizing step preceded by a 100-ms ‘inactivating’ prepulse to −20 mV to inactivate Ito,fast.(b). Ito,fast was measured from the prepulse-sensitive difference currents obtained by subtracting the currents in panel b from the currents in panel a (a−b) under corresponding osmotic conditions (c). B, effects of hyposmotic cell swelling on Iss. The voltage protocol (shown on the top) consists of a 5-s, +30-mV step and a 0.75-s, +10-mV step, which is interposed by a 100-ms ‘inactivating’ prepulse at −20 mV. The membrane currents elicited by the depolarization step from −20 mV to +10 mV are Iss. Representative Iss recorded under isosmotic (Iso) and hyposmotic (Hypo) conditions are shown on expanded time and current scales in panels a and b. The dashed lines indicate zero current. Representative whole-cell current traces are shown in the inset. C, mean current densities of Ito,fast (Ac, n = 7) and Iss (B, n = 6) in mouse left ventricular apex myocytes under isosmotic (black bars) and hyposmotic (grey bars) conditions. **P < 0.01 versus isosmotic condition.
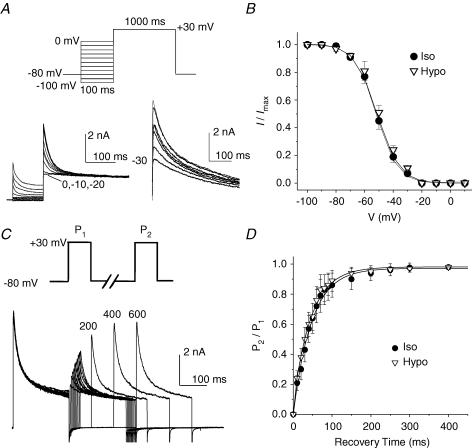
Figure 4. Effects of osmotic stress on voltage-dependent gating properties of Ito,fast in mouse ventricular apex myocytes.
A, the voltage dependence of steady-state inactivation of Ito,fast. Outward K+ currents were recorded during 1-s depolarization to +30 mV after 100-ms prepulses to potentials between −100 and 0 mV (protocol shown on the top) in the presence of 50 μm 4-AP. The representative current traces recorded under hyposmotic conditions are shown on the left. The test pulse currents obtained with the −20, −10 and 0 mV prepulses were superimposed on each other. The difference currents were obtained by subtraction of the test pulse currents recorded with the −20-mV prepulse from those recorded with prepulses between −100 and 0 mV. No inactivating component was observed with prepulses of −20, −10 and 0 mV. Numbers next to current traces indicate the corresponding potential of the inactivating prepulse. B, mean steady-state voltage-dependent inactivation curve of Ito,fast under isosmotic and hyposmotic conditions (n = 6). Peak Ito,fast recorded at +30 mV was normalized to the corresponding current amplitude measured at −100 mV. C and D, effects of cell swelling on the recovery from inactivation of Ito,fast; C, representative current traces recorded using a double-pulse voltage protocol (shown on the top); D, recovery of Ito,fast from inactivation under isosmotic and hyposmotic conditions (n = 7).
Figure 3Ab shows the currents recorded by a depolarizing step from a 100-ms ‘inactivating’ prepulse of −20 mV to +10 mV for 500 ms in the presence of 50 μm 4-AP under isosmotic (Iso, upper traces) and hyposmotic (Hypo, lower traces) conditions. Subtraction of the traces recorded with the inactivating prepulse protocol (Fig. 3Ab) from those without the prepulse (Fig. 3Aa) revealed a prepulse-sensitive current (Fig. 3Ac). This fast activating and inactivating prepulse-sensitive current corresponds to the Ito,fast as previously described in mouse ventricular myocytes (Xu et al. 1999b; Brouillette et al. 2004). As shown in Fig. 3Ac, hyposmotic cell swelling significantly increased Ito,fast. The decaying phase of prepulse-sensitive current was well fitted by a single exponential function with mean values of the time constant of 27 ± 4 and 25 ± 3 ms under isosmotic and hyposmotic conditions, respectively (n = 7, P > 0.05), further confirming that this current is the fast inactivating Ito,fast. The inactivation kinetics of Ito,fast was not affected by cell swelling.
The results shown in Figs 2 and 3A indicate that Ito,fast, but not Iss, was regulated by hyposmotic cell swelling or hyperosmotic cell shrinkage. To further confirm this, as shown in Fig. 3B, a combination of 50 μm 4-AP and an inactivating prepulse (Fig. 3B, inset on top) was used to separate Iss from IK,slow1 and Ito,fast, respectively. The voltage protocol involves a long depolarizing voltage step from a holding potential of −80 mV to +30 mV for 5 s, which is sufficient to completely inactivate IK,slow1 (Wang et al. 1999; Xu et al. 1999b; Brouillette et al. 2004), followed by a brief 100-ms ‘gap’ at −20 mV, which inactivates Ito,fast. The current activated by the second depolarizing step from −20 mV to +10 mV for 750 ms was the non-inactivating current Iss as shown with expanded time and current scales in the insets. The amplitude of Iss under isosmotic conditions (Fig. 3Ba) was unchanged after exposure to hyposmotic solutions (Fig. 3Bb). Similar results were observed when the same voltage protocol was applied to the same cells in the absence of 50 μm 4-AP (data not shown). Figure 3C summarizes the mean current densities of Ito,fast (as measured in Fig. 3Ac) and Iss (as measured in Fig. 3Ba and b) obtained under isosmotic (black bars) and hyposmotic (grey bars) conditions, respectively. Consistent with previous reports (Xu et al. 1999b), Ito,fast is a major contributor to the peak outward K+ currents under both osmotic conditions, while Iss was significantly smaller than Ito,fast. The current density of Ito,fast was significantly increased from 14.1 ± 1.1 to 19.5 ± 1.5 pA pF−1 (n = 7, P < 0.01) by hyposmotic cell swelling, whereas the current density of Iss was not changed after exposure to hyposmotic solution (6.1 ± 0.9 and 6.5 ± 0.7 pA pF−1 under isosmotic and hyposmotic conditions, respectively; n = 6, P > 0.05). When hyperosmotic perfusion was applied to the cells first, there was a significant decrease in the current amplitude of Ito,fast in mouse ventricular myocytes. For example, the mean current density of Ito,fast at +10 mV was decreased from 15.2 ± 1.2 to 10.5 ± 1.5 pA pF−1 (n = 4, P < 0.01) by hyperosmotic cell shrinkage.
The above results indicate that: (1) Ito,fast in native mouse ventricular myocytes can be effectively separated from other outward K+ current components (IK,slow1 and Iss) using a combination of voltage protocols and pharmacological tools; (2) hyposmotic cell swelling activates Ito,fast but not Iss, suggesting that specific activation of Ito,fast may be responsible for the shortening in early repolarization (APD5 and APD10).
Effects of hyposmotic cell swelling on voltage-dependent gating properties of Ito,fast
We next tested whether the regulation of Ito,fast by cell volume changes is due to changes in the voltage-dependent gating mechanism. The steady-state voltage-dependent inactivation of Ito,fast was examined using the double-pulse protocol (see Methods) in the presence of 50 μm 4-AP as previously described (Brouillette et al. 2004), under isosmotic and hyposmotic conditions (Fig. 4A). As shown by the current traces on the left in Fig. 4A, the test pulse currents obtained with inactivating prepulses of 0, −10 and −20 mV were superimposed. The current traces on the right in Fig. 4A were the difference currents obtained from the subtraction of the currents recorded with the prepulse of −20 mV from those with prepulses from −100 to 0 mV. No inactivating components were observed at prepulses of −20, −10 and 0 mV, indicating that Ito,fast could be inactivated completely at a membrane voltage more positive than −20 mV. These results also confirm that it is optimal to choose −20 mV as the inactivating prepulse for separation of Ito,fast. Figure 4B shows the mean steady-state inactivation curve of Ito,fast averaged from six cells. Consistent with previous observations in mouse ventricular myocytes (Xu et al. 1999b; Brouillette et al. 2004), the steady-state inactivation of Ito,fast was best fitted by a single Boltzmann function with a derived V1/2 of −48.5 ± 0.6 mV and slope factor (k) of 7.7 ± 0.4 mV under isosmotic conditions. Hyposmotic cell swelling failed to alter both parameters (−49.7 ± 1.3 and 7.0 ± 0.4 mV, respectively; n = 6, P > 0.05).
The process of Ito,fast recovery from inactivation under isosmotic and hyposmotic conditions was examined using a double-pulse protocol in the presence of 50 μm 4-AP (see Methods) (Brouillette et al. 2004). Figure 4C shows representative current traces recorded under hyposmotic conditions. Figure 4D summarizes the time course of recovery of Ito,fast from inactivation. Under isosmotic conditions the recovery process of Ito,fast was best fitted by a single exponential function with a mean time constant (τ) of 49 ± 6 ms. Exposure to hyposmotic solutions failed to significantly alter the recovery process (τ= 46 ± 7 ms, n = 7, P > 0.05). Since the recovery of Ito,fast from inactivation is a voltage-dependent process (Brouillette et al. 2004) we further examined whether osmotic challenge affects the kinetics of recovery from inactivation at different holding potentials. The recovery of Ito,fast was significantly faster at more hyperpolarized potentials under isosmotic conditions. The mean time constants (τ) were 49 ± 6, 68 ± 5 and 127 ± 6 ms (n = 7) at −80, −70 and −60 mV, respectively. No significant changes in the recovery process were observed when the cells were exposed to hyposmotic solutions. The corresponding mean τ values were 46 ± 7, 64 ± 7 and 119 ± 8 ms at holding potentials of −80, −70 and −60 mV, respectively (n = 7, P > 0.05).
Taken together, these results indicate that cell swelling increased Ito,fast current density through a mechanism that was independent of the voltage-gating properties of the channel.
Role of PKC and phosphatases in the regulation of Ito,fast by hyposmotic cell swelling
As described above in Fig. 2A, the onset of changes in Ito,fast was slower than changes in the cell volume, suggesting that an intracellular signalling transduction pathway may be involved in the volume regulation of the channels. Indeed, numerous previous studies have reported that changes in protein phosphorylation/dephosphorylation activities are closely related to changes in cell volume in a variety of cell types of different species (for review see Duan et al. 1999a; Lang et al. 2000; Zhong et al. 2002; Baumgarten & Clemo, 2003). In some cells, including cardiac cells, hyposmotic cell swelling induces protein dephosphorylation whereas cell shrinkage causes protein phosphorylation due to altered activities of protein kinases and/or phosphatases (Duan et al. 1995, 1999a; Duan & Hume, 2000; Ellershaw et al. 2000; Lang et al. 2000; Rutledge et al. 2001; Zhong et al. 2002; Ellershaw et al. 2002). In cardiac and many non-cardiac cells, for example, volume regulation of Cl− channels is linked to PKC phosphorylation and dephosphorylation (Duan et al. 1995, 1999a; Lang et al. 2000; Ellershaw et al. 2002; Zhong et al. 2002; Baumgarten & Clemo, 2003). It is noteworthy that both native Ito in cardiac myocytes and the expressed Kv4.2 and Kv4.3 currents have been previously reported to be regulated by PKC (Nakamura et al. 1997; Po et al. 2001; Shimoni & Liu, 2003) or protein phosphatases (Caballero et al. 2004). Therefore, we tested whether volume regulation of native Ito,fast in mouse heart is also mediated through a phosphorylation/dephosphorylation mechanism.
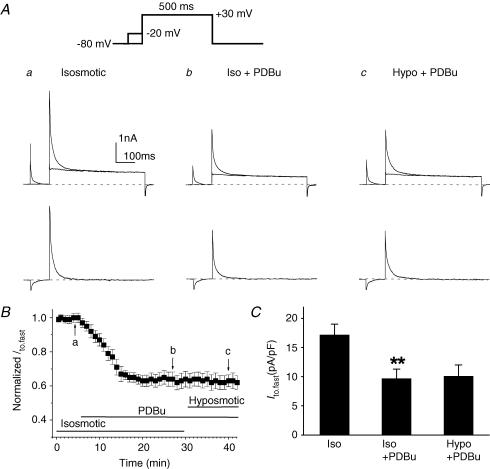
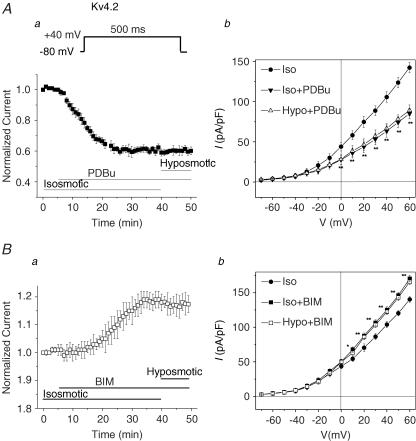
Figure 5 shows the effects of a PKC activator, phorbol-12,13-dibutyrate (PDBu, 100 nm), on Ito,fast in mouse left ventricular apex myocytes. As described above in Fig. 3A, Ito,fast was measured by subtracting the current recorded with an inactivating prepulse at −20 mV for 100 ms from that recorded without the prepulse in the presence of 50 μm 4-AP. In Fig. 5A, the representative current traces recorded with and without the prepulses are superimposed (upper traces). The corresponding difference currents (Ito,fast) yielded from the subtraction under isosmotic (a), isosmotic + PDBu (b) and hyposmotic + PDBu (c) conditions are shown in the lower panels. When the cells were exposed to isosmotic solutions for 5 min, activation of PKC by PDBu caused a time-dependent inhibition of the peak Ito,fast, which reached the steady state within 10 min. Subsequent exposure of the cells to hyposmotic solutions in the presence of PDBu failed to increase Ito,fast (Fig. 5B). Similar results were observed in seven cells. At +30 mV, for example, PDBu significantly inhibited Ito,fast (from 17.2 ± 1.8 to 9.7 ± 1.6 pA pF−1, n = 7, P < 0.01) under isosmotic conditions and prevented further activation by hyposmotic cell swelling (10.1 ± 1.9 pA pF−1, n = 7, P > 0.05). The mean current densities of Ito,fast recorded at +30 mV under corresponding conditions to those in Fig. 5A and B are shown in Fig. 5C. When cells were treated with 100 nm 4α-phorbol 12, 13-didecanoate (4α-PDD; an inactive form of PDBu), however, no inhibition of Ito,fast by 4α-PDD was observed and hyposmotic cell swelling caused a similar increase in Ito,fast as seen in the absence of either 4α-PDD or PDBu (data not shown); this suggests that the observed effect of PDBu on volume regulation of Ito,fast is not due to a non-specific effect of phorbol esters.
Figure 5. Effects of PKC activator (PDBu) on Ito,fast in mouse left ventricular apex myocytes.
A, representative current traces of Ito,fast (lower traces in each panel) obtained from the subtraction of currents recorded with the inactivating prepulse from those recorded without the inactivating prepulse (upper superimposed traces) in the presence of 50 μm 4-AP. Voltage protocols are shown on the top. The same cell was consecutively exposed to isosmotic solution (a), isosmotic + PDBu (100 nm, b), and hyposmotic + PDBu (100 nm, c). PDBu decreased Ito,fast under isosmotic conditions and subsequent hyposmotic perfusion failed to further activate Ito,fast. B, time course of changes in normalized Ito,fast at +30 mV when cells were exposed consecutively to isosmotic, isosmotic + PDBu, and hyposmotic + PDBu solutions. Peak Ito,fast was recorded every 1 min then normalized to the initial corresponding value at time 0 (n = 7). C, mean peak current densities of Ito,fast recorded when cells were exposed to isosmotic, isosmotic + PDBu, and hyposmotic + PDBu solutions. Currents were obtained using the protocols shown in panel A (n = 7). **P < 0.01 versus isosmotic (Iso) condition.
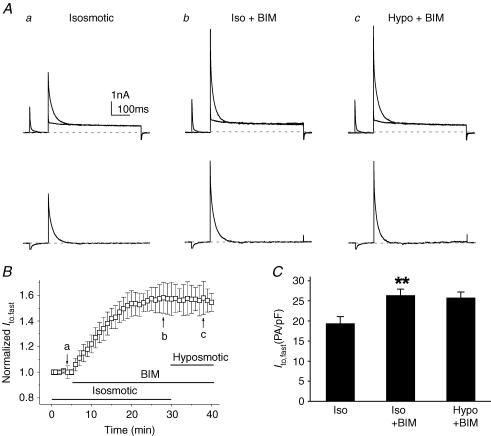
To further test whether PKC is indeed involved in the volume regulation of Ito,fast in cardiac myocytes, we examined the effect of a PKC inhibitor, bisindolylmaleimide (BIM; 100 nm), on cell volume regulation of Ito,fast. As shown in Fig. 6, exposure of the cells to BIM under isosmotic condition caused a significant increase in Ito,fast in a time-dependent fashion (Fig. 6B). At +30 mV, Ito,fast was increased from 19.4 ± 1.7 to 26.4 ± 1.5 pA pF−1 (n = 6, P < 0.01) and reached the steady state within 20 min. In the presence of BIM, however, hyposmotic cell swelling failed to further increase Ito,fast (25.8 ± 1.4 pA pF−1, n = 7, P > 0.05 versus isosmotic + BIM) (Fig. 6C). These results suggest that cell volume regulation of Ito,fast in mouse ventricular myocytes may be linked to a PKC-mediated phosphorylation and dephosphorylation activity.
Figure 6. Effects of PKC inhibitor (BIM) on Ito,fast in mouse left ventricular apex myocytes.
A, representative current traces of Ito,fast recorded from a ventricular myocyte using the same voltage protocols as shown in Fig. 5A in the presence of 50 μm 4-AP when the same cell was consecutively exposed to isosmotic (a), isosmotic + BIM (100 nm, b), and hyposmotic + BIM (c) solutions. B. time course of changes in normalized Ito,fast at +30 mV when cells were exposed consecutively to isosmotic, isosmotic + BIM, and hyposmotic + BIM solutions. The amplitudes of Ito,fast were recorded every 1 min and then normalized to the initial corresponding value at time 0 (n = 7). C, mean peak current densities of Ito,fast (n = 7) recorded at +30 mV when cells were exposed to isosmotic, isosmotic + BIM (Iso + BIM), and hyposmotic + BIM (Hypo + BIM) solutions using protocols shown in Fig. 5A. **P < 0.01 versus isosmotic (Iso) conditions.
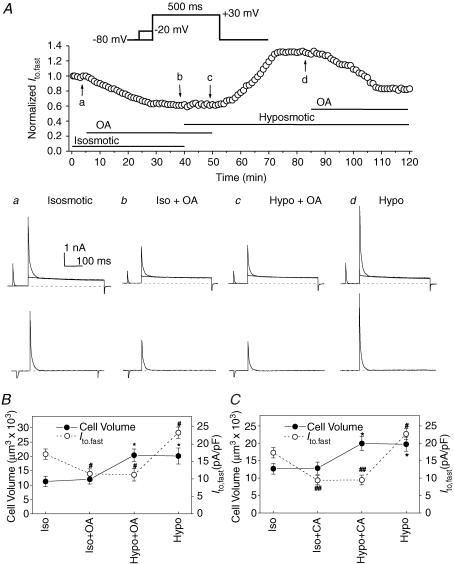
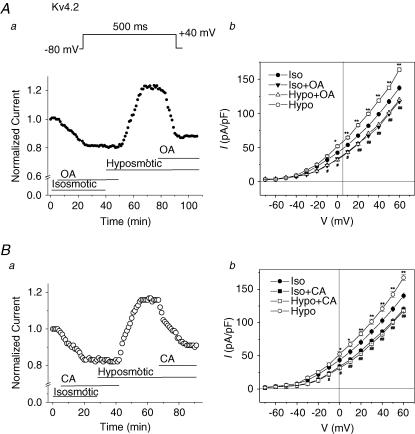
In intact cells, protein phosphorylation processes are reversibly controlled by protein kinases and protein phosphatases (Hunter, 1995; Herzig & Neumann, 2000). The balance of kinase and phosphatase activities directly determines the net level of protein phosphorylation (Cohen, 1992). Both protein kinases and phosphatases have been reported to be subject to regulation by cell volume (Jennings & Schulz, 1991; Lang et al. 1998; Duan et al. 1999a). To further test the hypothesis that a balance between channel protein phosphorylation and dephosphorylation may be the key regulatory event responsible for Ito,fast regulation by cell volume in the face of osmotic perturbation, we studied the effects of two highly potent serine/threonine protein phosphatase inhibitors: okadaic acid (Cohen, 1992; Duan et al. 1999a) and calyculin A (Ishihara et al. 1989; Duan et al. 1999a), on the osmotic regulation of mouse ventricular Ito,fast. As shown in Fig. 7A, the current amplitude of Ito,fast started to decrease after the cell was exposed to okadaic acid (OA; 100 nm) for >2 min in the isosmotic bath solution and reached the steady-state level after ∼25 min (Fig. 7Ab). In the presence of OA, subsequent exposure of the cell to hyposmotic solution for 10 min caused no changes in Ito,fast (Fig. 7Ac). Washout of OA with hyposmotic bath solution for >10 min increased Ito,fast (Fig. 7Ad). Re-application of OA in the same hyposmotic solution inhibited Ito,fast again. Figure 7B summarizes the relationship between changes in mean Ito,fast current densities and cell volume of six different mouse ventricular myocytes under isosmotic, isosmotic + OA, hyposmotic + OA, and hyposmotic conditions. Under isosmotic conditions, exposure of the cells to OA caused a significant decrease (from 17.1 ± 1.5 to 11.5 ± 1.4 pA pF−1, n = 6, P < 0.05) in Ito,fast but no significant changes in cell volume (12 032 ± 1671 μm3versus 11 274 ± 1816 μm3, n = 6, P > 0.05). In the presence of OA, subsequent exposure of the cells to hyposmotic solution caused a significant increase in cell volume (20 416 ± 2174 μm3, n = 6, P < 0.05) but no further significant changes in Ito,fast (11.2 ± 1.8 pA pF−1, n = 6, NS). Washout of OA with hyposmotic solution, however, caused a significant increase in Ito,fast (23.3 ± 1.6 pA pF−1, n = 6, P < 0.05) while the cell volume remained almost unchanged (20 145 ± 2786 μm3, P > 0.05). Similar results were also observed when another more potent phosphatase inhibitor, calyculin A (CA, 20 nm), was used under identical conditions to those described above for OA (Fig. 7C). These data strongly indicate that inhibition of serine/thronine phosphatases (types 1 and 2A; PP1 and PP2A) not only inhibited Ito,fast but also prevented the effect of hyposmotic cell swelling on the channel in the intact cardiac myocytes.
Figure 7. Effects of serine/threonine protein phosphatase inhibitors, okadaic acid and calyculin A, on Ito,fast in mouse left ventricular myocytes.
A, whole-cell currents were monitored continuously in the presence of 50 μm 4-AP (voltage-clamp protocols shown on the top, also see Fig. 3A for details). Top panel, Ito,fast was recorded every 1 min then normalized to the initial corresponding value at time 0 when cardiac myocytes were consecutively exposed to isosmotic, isosmotic + OA (100 nm), hyposmotic + OA (100 nm), and hyposmotic solutions. Changes in perfusion solutions were started when the changes in current amplitude reached the steady-state level. a–d, the representative superimposed current traces (upper) and the prepulse-sensitive difference current (Ito,fast) traces (lower) recorded from the same cell as shown in the top panel at the time points indicated by the arrows. B, changes in mean peak Ito,fast current densities (•, y-axis on the left) and cell volume (•, y-axis on the right) recorded when cells were consecutively exposed to isosmotic (Iso), isosmotic +100 nm OA (Iso + OA), hyposmotic +100 nm OA (Hypo + OA), and hyposmotic (Hypo) solutions (n = 6). Currents were obtained using the protocols described in panel A (*P < 0.05 when compared with cell volume under control (Iso) conditions; #P < 0.05 when compared with peak current density under control (Iso) conditions). C, changes in mean peak Ito,fast current densities (•) and cell volume (•) recorded from cardiac myocytes when they were consecutively exposed to isosmotic (Iso), isosmotic + 20 nm CA (Iso + CA), hyposmotic + 20 nm CA (Hypo + CA), and hyposmotic (Hypo) solutions (n = 5, *P < 0.05 when compared with cell volume under control (Iso) conditions; #P < 0.05, ##P < 0.01 when compared with peak current density under control (Iso) conditions).
Taken together, these results indicate that phosphorylation of the channel, by either increase in the protein kinase activity or decrease in the phosphatase activity, caused an inhibition of the channel activity; dephosphorylation of the channel, by increase in the phosphatase activity or decrease in the protein kinase activity, caused an activation of the channels; these results suggest that the balance of PKC–phosphatase activity may be constantly regulated by cell volume and may be the key regulatory process responsible for the regulation of Ito,fast by cell volume in the face of osmotic challenges.
Volume regulation of Kv4.2 and Kv4.3 channels expressed in NIH/3T3 cells
To further investigate the molecular mechanism underlying the volume regulation of Ito,fast in cardiac myocytes, we examined whether cell volume changes also regulate Kv4.2 and Kv4.3 channels since the primary subunits that contribute to Ito,fast are the Kv4.2 or Kv4.3 channels, or a combination of the two (Dixon et al. 1996; Johns et al. 1997; Hoppe et al. 2000).
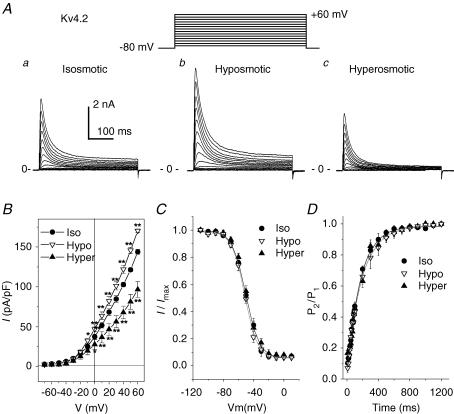
As shown in Fig. 8A, expression of Kv4.2 in NIH/3T3 cells yielded large transient outward currents when the cells were depolarized from a holding potential of −80 mV to a variety membrane potentials in increments of +10 mV under isosmotic conditions (Fig. 8Aa); in contrast, only very small endogenous voltage-gated outward currents were recorded from untransfected cells (data not shown). The expressed Kv4.2 currents were markedly blocked by 5 mm 4-AP (data not shown). These results are consistent with previous studies from other investigators (Ohya et al. 1997; Hatano et al. 2003). Exposure of the cells to hyposmotic solutions increased Kv4.2 currents (Fig. 8Ab) and subsequent exposure to hyperosmotic solutions decreased the currents (Fig. 8Ac). Similar results were observed in 12 cells under identical conditions and the mean I–V curves of the Kv4.2 currents under isosmotic, hyposmotic and hyperosmotic conditions are shown in Fig. 8B (n = 12). The mean current density of Kv4.2 channels was increased significantly by hyposmotic swelling over the range −10 to +60 mV, switch from hyposmotic perfusion to hyperosmotic perfusion decreased the current amplitude significantly over the range 0 to +60 mV. Consistent with observations in the endogenous Ito,fast in native cardiac myocytes (Fig. 4), no significant effects of osmotic stress on the voltage dependence of steady-state inactivation of Kv4.2 channels were observed (Fig. 8C). The V1/2 values of Kv4.2 channels were −36.6 ± 0.6, −34.7 ± 0.5 and −33.9 ± 1.2 mV and k values of Kv4.2 channels were −6.3 ± 0.2, −7.7 ± 0.6, and −7.7 ± 0.3 mV under isosmotic, hyposmotic and hyperosmotic conditions, respectively (n = 6, P > 0.05). As shown in Fig. 8D, the recovery kinetics of Kv4.2 channels was not significantly altered by cell volume changes. The time constants (τ) were 303 ± 43, 317 ± 41 and 291 ± 38 ms under isosmotic, hyposmotic and hyperosmotic conditions, respectively (n = 7, NS).
Figure 8. Effects of osmotic stress on Kv4.2 channels in NIH/3T3 cells.
A, representative whole-cell currents recorded from NIH/3T3 cells expressing the rat Kv4.2 gene under isosmotic (a), hyposmotic (b) and hyperosmotic (c) conditions. Kv4.2 currents were elicited by a series of 400-ms depolarizing voltage steps (inset). B, mean I–V curves of Kv4.2 channels (n = 12) recorded under isosmotic, hyposmotic and hyperosmotic conditions. C, mean steady-state voltage-dependent inactivation curves of Kv4.2 (n = 6) obtained from a double-pulse protocol (inset in A,) under isosmotic, hyposmotic and hyperosmotic conditions. D, recovery from inactivation was examined using a double-pulse protocol with different recovery times (see Methods). Mean normalized currents (n = 7) under isosmotic, hyposmotic and hyperosmotic conditions were plotted against recovery time. The kinetics of recovery from inactivation was a single exponential process (continuous lines) with a time constant (τ) of 303 ± 43, 317 ± 41 and 291 ± 38 ms (P > 0.05) under isosmotic, hyposmotic and hyperosmotic conditions, respectively.
Similarly, Kv4.3 channels expressed in NIH/3T3 cells were also regulated by changes in cell volume. When measured at +40 mV, for example, exposure of the cells to hyposmotic solutions increased Kv4.3 current density from 44.5 ± 3.4 to 72.2 ± 9.1 pA pF−1 (n = 10, P < 0.01), and subsequent exposure to hyperosmotic solutions decreased Kv4.3 current density to 39.0 ± 3.2 pA pF−1 (P < 0.01). Consistent with the observations in native Ito,fast and expressed Kv4.2 channels, neither the voltage-dependent inactivation nor the recovery from inactivation of the expressed Kv4.3 channels were altered by cell volume changes (data not shown).
Role of PKC and phosphatases in cell volume regulation of Kv4.2 and Kv4.3 channels
We further examined the effects of PKC activator (PDBu), PKC inhibitor (BIM), and phosphatase inhibitors (OA and CA) on volume regulation of Kv4.2 and Kv4.3 channels under the same conditions as described for endo-genous Ito,fast in native mouse cardiac myocytes. As shown in Fig. 9A, PDBu (100 nm) caused a time-dependent inhibition of Kv4.2 currents under isosmotic conditions and prevented the activation of the current by hyposmotic cell swelling (Fig. 9Aa). Similar results were observed in six different cells. The mean I–V curves under isosmotic, isosmotic + PDBu, and hyposmotic + PDBu are summarized in Fig. 9Ab. Similar results were also observed in the Kv4.3 channels expressed in NIH/3T3 cells. Activation of PKC by PDBu reduced Kv4.3 currents from 47.4 ± 2.5 to 32.0 ± 3.3 pA pF−1 at +40 mV (n = 7, P < 0.05) under isosmotic conditions. No further increase in Kv4.3 currents was observed (34.5 ± 3.7 pA pF−1, P > 0.05) when cells were exposed to hyposmotic solution in the presence of PDBu.
Figure 9. Effects of PKC activator (PDBu) and inhibitor (BIM) on Kv4.2 currents in NIH/3T3 cells.
A, effect of PDBu (100 nm) on peak Kv4.2 currents. a, time course of changes in normalized peak Kv4.2 currents (n = 6) measured at +40 mV under isosmotic, isosmotic + PDBu, and hyposmotic + PDBu conditions. b, I–V curves of Kv4.2 channels (n = 7) recorded using a protocol as described in Fig. 5 in isosmotic (Iso), isosmotic + PDBu (Iso + PDBu), and hyposmotic + PDBu (Hypo + PDBu) solutions. PDBu inhibited the currents under isosmotic conditions and prevented further activation by hyposmotic cell swelling. B, effects of BIM on Kv4.2 channels. a, time course of changes in normalized peak Kv4.2 currents recorded at +40 mV under isosmotic, isosmotic + BIM, and hyposmotic + BIM conditions (n = 5). b, mean I–V curves of Kv4.2 channels (n = 5) in isosmotic (Iso), isosmotic + BIM (Iso + BIM), and hyposmotic + BIM (Hypo + BIM) solutions. BIM caused a time-dependent increase in Kv4.2 currents even under isosmotic conditions and prevented further activation by hyposmotic cell swelling.
When the cells were exposed to BIM (100 nm), on the other hand, a time-dependent increase in peak Kv4.2 current between +10 mV and 60 mV was observed (Fig. 9B). The peak Kv4.2 current densities at +40 mV were increased from 103.9 ± 4.5 to 125.1 ± 4.6 pA pF−1 (n = 5, P < 0.01). In the presence of BIM, no further increase in Kv4.2 currents was observed when the cells were perfused with hyposmotic solutions for >10 min (122.3 ± 3.7 pA pF−1 at +40 mV, P > 0.05). The mean I–V curves recorded from five different cells under isosmotic, isosmotic + BIM, and hyposmotic + BIM conditions are summarized in Fig. 9Bb. Similarly, inhibition of PKC by BIM also increased Kv4.3 currents at +40 mV from 44.2 ± 3.5 to 61.7 ± 3.3 pA pF−1 (n = 6, P < 0.01) under isosmotic conditions. Subsequent hyposmotic cell swelling in the presence of BIM failed to further increase the current (63.5 ± 4.2 pA pF−1, n = 6, P > 0.05).
As shown in Fig. 10, both OA (Fig. 10A) and CA (Fig. 10B) caused a time-dependent inhibition of Kv4.2 currents under isosmotic conditions and prevented further activation of the currents by hyposmotic cell swelling. Similar effects of OA and CA on the Kv4.3 channels expressed in NIH/3T3 cells were also observed. For example, under isosmotic conditions, OA (100 nm) reduced the current amplitude of Kv4.3 at +40 mV from 45.2 ± 3.1 to 33.1 ± 2.9 pA pF−1 (n = 4, P < 0.05) and no further increase in Kv4.3 currents was observed when cells were exposed to hyposmotic solutions in the presence of OA (35.7 ± 2.9 pA pF−1, n = 4, NS). Similarly, CA (20 nm) reduced Kv4.3 current amplitude from 47.3 ± 2.8 to 36.1 ± 2.7 pA pF−1 (n = 4, P < 0.05) and no further increase in Kv4.3 currents was observed when cells were exposed to hyposmotic solutions in the presence of CA (36.3 ± 2.8 pA pF−1, n = 4, P > 0.05).
Figure 10. Effects of serine/threonine phosphatase inhibitors on volume regulation of Kv4.2 currents in NIH/3T3 cells.
A, effect of OA (100 nm) on peak Kv4.2 currents. a, time course of changes in normalized peak Kv4.2 currents measured at +40 mV under isosmotic, isosmotic + OA, and hyposmotic + OA conditions. b, I–V curves of Kv4.2 channels (n = 5) recorded using a protocol as described in Fig. 5 in isosmotic (Iso), isosmotic + OA (Iso + PDBu), and hyposmotic + OA (Hypo + OA) solutions. OA inhibited the currents under isosmotic conditions and prevented further activation by hyposmotic cell swelling. B, effects of CA (20 nm) on Kv4.2 channels. a, time course of changes in normalized peak Kv4.2 currents recorded at +40 mV under isosmotic, isosmotic + CA, and hyposmotic + CA conditions. b, mean I–V curves of Kv4.2 channels (n = 5) in isosmotic (Iso), isosmotic + CA (Iso + CA), and hyposmotic + CA (Hypo + BIM) solutions. CA inhibited the currents under isosmotic conditions and prevented further activation by hyposmotic cell swelling.
Discussion
Our results provide the following evidence for a novel regulation mechanism for Kv4.2/4.3 K+ channels in the heart: (1) Ito,fast in native mouse left ventricular apex myocytes, and its key molecular counterparts Kv4.2/4.3 channels expressed in NIH/3T3 cells, are strongly regulated by cell volume changes which may be responsible for hyposmotic cell swelling-induced shortening in early repolarization of APD in cardiac myocytes; (2) changes in cell volume alter the amplitude of these currents with no effect on their voltage-dependent gating properties including activation, inactivation and recovery from inactivation; (3) cell volume regulation of these channels is closely coupled to a phosphorylation/dephosphorylation process mediated by PKC and serine/thronine phosphatases. These findings are potentially very important because the Kv4.2/4.3-encoded Ito,fast plays a crucial role in APD repolarization and ECC in the heart, and changes in expression and function of Kv4.2/4.3 are regarded as hallmark features of diseased myocardium in human and numerous other animal models (Barry et al. 1998; Kaab et al. 1998; Wang et al. 1999; Yue et al. 1999; Huang et al. 2000).
Volume regulation of ion channels and adaptive remodelling of cardiac myocytes
Perturbations in cell volume and ion channel function are closely associated during adaptive structural and electrical remodelling of the cardiac myocytes such as hypertrophic cell volume increase caused by pressure overload or changes in extracellular osmolarity caused by myocardial hypoxia, ischaemia and reperfusion (Steenbergen et al. 1985; Wright & Rees, 1998; Befroy et al. 1999; Baumgarten & Clemo, 2003; Frey et al. 2004; Duan et al. 2005). It has been demonstrated that cardiac myocytes are able to avoid excessive cell volume changes through the regulation of loss or gain of intracellular ions or other osmolytes (Duan et al. 1999a; Hume et al. 2000; Lang et al. 2000; Baumgarten & Clemo, 2003). Several ionic mechanisms such as ICl,swell (Duan et al. 1995, 1997a, b, 1999a; Vandenberg et al. 1997; Hiraoka et al. 1998), IK,s (Rees et al. 1995), IK,ATP (Priebe & Beuckelmann, 1998) and ICl,ir (Duan et al. 2000), have been previously reported to be responsible for the hyposmotic cell swelling-induced changes in membrane ion permeability and various cellular functions, including cardiac electrical activity and contractility (Vandenberg et al. 1996; Wright & Rees, 1998; Befroy et al. 1999; Kocic et al. 2001; Baumgarten & Clemo, 2003; Frey et al. 2004). It has been recently reported that volume-regulated K+ transport or flux across the plasma membrane plays a crucial role in the regulation of the apoptotic volume decrease and programmed cell death (apoptosis) (Remillard & Yuan, 2004). Increasing evidence suggests that apoptosis may be a driving force in the transition from compensated hypertrophy to failure in the work-overloaded myocardium. In this study we found that Ito,fast is regulated by cell volume changes and cell swelling-induced up-regulation of Ito,fast may be responsible for the shortening in early (phase 1) repolarization of cardiac APD of mouse ventricular myocytes. These findings strongly suggest a novel role of Kv4.2/4.3 channels in cell volume regulation and may also provide an alternative mechanism for the link between electrical remodelling and structural remodelling of the heart under pathological conditions such as myocardial hypertrophy, heart failure, hypoxia, ischaemia and reperfusion.
Molecular mechanisms responsible for volume regulation of Ito,fast in mouse heart
The molecular basis of voltage-dependent outward K+ currents in native cardiac myocytes is very complicated and may vary in different species and in different regions and cell types of the same heart (Wang et al. 1999; Nerbonne & Guo, 2002; Zicha et al. 2003; Decher et al. 2004). At least five outward K+ currents (Ito,fast, Ito,slow, IK,slow1, IK,slow2 and Iss) concomitantly exist in native adult mouse heart, and it is difficult to efficiently separate and accurately assess each component (Xu et al. 1999b; Guo et al. 2000; London et al. 2001; Zhou et al. 2003). Ito,fast is present in all left ventricular apex cells and in most left ventricular septum cells, whereas Ito,slow is identified only in left ventricular septum cells (Xu et al. 1999b; Guo et al. 2000). IK,slow, and Iss exhibit their molecular properties different from either Ito,fast or Ito,slow. It is believed that Ito,slow may be encoded by Kv1.4 α-subunit (Guo et al. 2000), while IK,slow may be encoded by Kv1.5 (IK,slow1) and Kv2.1 (IK,slow2) (Xu et al. 1999b; Zhou et al. 2003). In the present study we used a combination of voltage protocol and pharmacological tools as previously described to selectively separate Ito,fast from other K+ currents in mouse cardiac myocytes (Brouillette et al. 2004). We found that Ito,fast, but not Iss was regulated by changes in cell volume.
Both Kv4.2 and Kv4.3 have been suggested to be major molecular subunits in the formation of Ito,fast in cardiac myocytes of many species (Kaab et al. 1998; Wang et al. 1999; Nerbonne & Guo, 2002). Functional channels underlying mouse ventricular Ito,fast may be a heteromultimer of Kv4.2/4.3 α-subunits assembling with auxiliary subunits (e.g. Kv channel-interacting protein 2 (KChIP2)) (Guo et al. 2002), it is still, however, difficult to reproduce the exact functional channel for endogenous Ito,fast in the heterologous expression system by simply expressing these genes together since no information is currently available regarding the exact composition of these subunits for the functional channels in terms of the numbers for each subunits and the isoforms of KChIP2. On the other hand, in most species studied to date, Ito is encoded by either Kv4.2 or Kv4.3, not always by a combination of the two. The recent identification by Sanguinetti's laboratory of new isoforms of KChIP2 in the heart clearly showed that the functional diversity of the Ito is far more complicated than people have thought (Decher et al. 2004). Obviously, it would be extremely difficult to study a particular KChIP2 isoform and its combination with Kv4.2 or Kv4.3 or both. Nevertheless, our results on both Kv4.2 and Kv4.3 channels suggest that the regulation of the channels by cell volume may be essentially through modulation of the α-subunits because the properties of the regulation of these expressed Kv4.2/4.3 channels in NIH/3T3 cells are almost identical to those of their native functional correlate Ito,fast, in terms of voltage-dependent activation and inactivation, recovery from inactivation, and sensitivity to osmotic stress, PKC and phosphatase. Our results also further support the notion that Kv4.2 and Kv4.3 are major components responsible for Ito,fast in native mouse ventricular myocytes.
Numerous previous studies have clearly demonstrated that cell swelling induces protein dephosphorylation whereas cell shrinkage causes protein phosphorylation in a variety of cell systems, including epithelial (Haas et al. 1995) and endothelial (Klein et al. 1993) cells, erythrocytes (Lytle, 1998), Ehrlich mouse ascites tumour cells (Larsen et al. 1994), cardiac myocytes (Duan et al. 1995, 1999a; Hall et al. 1995) and vascular smooth muscle cells (Zhong et al. 2002). For example, phosphorylation/dephosphorylation processes mediated by PKC and phosphatases may be critically involved in the osmotic regulation of volume-regulated Cl− currents encoded by genes of the CIC chloride channel family (ClC-3) (ICl,vol) (Duan et al. 1995, 1999a; Zhong et al. 2002; Baumgarten & Clemo, 2003) or ClC-2 (ICl,ir) (Rutledge et al. 2001). In this study we found that the osmotic regulation of endogenous Ito,fast and its molecular correlates Kv4.2 and Kv4.3 channels is also coupled to activities of PKC and phosphatases. To date, at least seven isoforms have been identified in mammalian cardiac myocytes, including α, β, δ, ɛ, η, ι and ζ. Among Ca2+-independent, novel PKCs, δ, ɛ, and η are expressed in the heart (Schreiber et al. 2001). Both Ca2+-sensitive (PKCα) and -insensitive (PKCδ and PKCɛ) PKC isozymes are abundantly expressed in NIH/3T3 cells (Szallasi et al. 1994). Similar to our previous observations, the effect of cell swelling on Ito,fast was also studied under experimental conditions where [Ca2+]i is strongly buffered by EGTA (10 mm) to separate the Ca2+-independent Ito,fast from other Ca2+-dependent currents such as ICl,Ca. Therefore, our results indicate a prominent role for Ca2+-independent signalling pathways, such as Ca2+-independent PKC isoforms, in the mechanism of cell volume regulation of Ito,fast in the mouse heart. It has been found that in both fetal and adult mouse ventricles the majority of PKC activity was Ca2+ independent (Schreiber et al. 2001). Immunoprecipitation assays indicated that PKCδ and PKCɛ were responsible for the majority of the Ca2+-independent activity in mouse ventricles (Schreiber et al. 2001). Therefore, both isoforms may be involved in the regulation of Ito,fast in mouse heart. Previous studies by Zhong et al. clearly demonstrated that hypotonic cell swelling is accompanied by translocation of PKCε from the vicinity of the membrane to cytoplasmic and perinuclear locations, which may be responsible for the cell volume regulation of the volume-regulated Cl− channels in the vascular smooth muscle cells (Zhong et al. 2002). PKCε has been found to directly modulate Ito. The transmural differences in the subcellular expression of PKCε also parallel the transmural gradient of Ito (Thorneloe et al. 2001b). The basal level of ion channel activity under isosmotic conditions may be determined by the balance between basal activities of protein kinases (e.g. PKC) and phosphatases (e.g. PP1/2A), as is the case in ICl,vol or ClC-3 channels (Duan et al. 1995, 1999a; Zhong et al. 2002; Baumgarten & Clemo, 2003). These results provide further evidence that protein kinase(s)/phosphatase(s)-mediated protein phosphorylation and dephosphorylation may be a common signal transduction pathway for the cellular response to cell volume changes that directly control the function of proteins such as ion channels.
It is very important to know how changes in cell volume regulate channel activity in terms of voltage-dependent gating properties and/or conformational changes of the channel proteins after phosphorylation or dephosphorylation. Po et al. (2001) reported that PKC phosphorylation shifts steady-state inactivation of human Kv4.3L while others have reported that PKC inhibits rat Kv4.2 and Kv4.3 and native Ito in rat cardiac myocytes without change in their steady-state inactivation properties (Nakamura et al. 1997; Shimoni & Liu, 2003). We hypothesized that hyposmotic challenge may activate Ito,fast (or Kv4.2/4.3 channels) by affecting its voltage-dependent gating properties through dephosphorylation of the channel protein due to: inactivation of one or more PKC isoforms or other protein kinases; or activation of phosphotases. In this study therefore we carefully studied the effects of osmotic shock on the voltage-dependent gating properties of both endogenous Ito,fast in native ventricular myocytes and Kv4.2 and Kv4.3 channels expressed in NIH/3T3 cells. The results indicate that the activation of the Kv4.2/4.3 channels and endogenous Ito,fast by hyposmotic cell swelling was voltage independent and no changes in the activation, inactivation and recovery from inactivation were observed. Our results are consistent with those of Nakamura et al. (1997) and Shimoni and Liu (2003) but different from those of Po et al. (2001). The possible explanations for the discrepancy between these studies may be due to different variants of Kv4.3 (e.g. Kv4.3L versus Kv4.3S) between human and rat and/or different expression systems. At this point, it is still not known how osmotic challenge-induced phosphorylation or dephosphorylation of the Kv4.2/4.3 channels causes closing or opening of the channels, respectively. Direct measurement of changes in the activity of kinases and/or phosphatases and biochemical evidence for phosphorylation of Kv4.2 or Kv4.3 channels during alterations of cell volume are needed to further validate whether hyposmotic challenge and PDBu treatment act on the same site. In the case of ClC-3 channels, it seems that phosphorylation/dephosphorylation of the N-terminus is the key link between cell swelling and channel opening (Duan et al. 1999a). It is therefore reasonable to propose mutation experiments on the consensus PKC phosphorylation sites to test directly whether phosphorylation and dephosphorylation are linked to cell volume regulation, as in the case of ClC-3 channels (Duan et al. 1999a). There are, however, numerous consent PKC phosphorylation sites in rat long form Kv4.3 (Kv4.3L) and Kv4.2, respectively. It would be an extremely difficult task to identify which amino acid would need to be mutated to yield meaningful phenotypes. It should also be pointed out that it is possible that phosphorylation and dephosphorylation of the channel protein may cause conformational changes to prevent further regulation by changes in cell volume, which may not necessarily be linked to the signal transduction pathways of osmotic stress. Therefore, the mutation experiments were not performed in this study.
In the intact heart the mechanism of channel activation by cell swelling may be more complicated than in the isolated individual cells described here. For instance, there are many aspects of heterogeneity across the left ventricular wall. In addition to the regional differences in the expression of Kv currents or the Kv subunits (Kv4.2, Kv4.3, KChIP2, Kv1.5, Kv2.1) encoding these currents in adult male and female mouse ventricles (Brunet et al. 2004), the transmural gradient in PKC isozymes (e.g. PKCɛ) across the ventricular wall has been described in rat heart (Thorneloe et al. 2001a; Shimoni & Liu, 2003).The electrophysiological heterogeneity in the transmural myocardium of the murine left ventricle may become even more complex due to significant remodelling under pathological conditions (Thorneloe et al. 2001a; Shimoni & Liu, 2003; Zicha et al. 2003). It is thus very possible that under some pathological conditions like hypertrophy and ischaemia, the regulation of Ito,fast may be significantly remodelled along with differential subcellular expression and activation of PKC isozymes in the heart. In fact, the transmural heterogeneity in Ito has been suggested to contribute to arrhythmogenesis and change in contractile states during the pathological progression of hypertrophy and heart failure (Oudit et al. 2001; Li et al. 2002), myocardial ischaemia and infarction (Kaprielian et al. 2002) and Brugada syndrome (Di Diego et al. 2002). Therefore, whether the mechanisms for the regulation of Kv4.2/4.3 channels described in this study are applicable in the intact heart and what the consequences of the volume regulation of these channels are under physiological or pathological situations need to be further investigated at an integrated level.
Significance
Although changes in Kv4.2/4.3 channels have been correlated to electrical remodelling in many heart diseases including myocardial ischaemia and infarction (Huang et al. 2000) and cardiac hypertrophy and failure (Sah et al. 2003), possible involvement of Kv4.2/4.3 channels in the initiation and development of heart disease remains to be elucidated. Our results provide the first evidence that cardiac Kv4.2/4.3 channels can be regulated by cell volume and strongly suggest that Ito,fast may be an important pathological substrate associated with osmotic challenges and cell volume changes. These findings are important because the osmotic-stress regulation of Ito,fast may represent a novel mechanism for many important cellular functions, including cell proliferation and apoptosis (Wright & Rees, 1998; Lang et al. 2000; Kocic et al. 2001; Baumgarten & Clemo, 2003; Remillard & Yuan, 2004) which are involved not only in cardiac cells but also in many other cell types in the context of health and disease. Therefore, these data may provide new insight into our understanding of the role of Kv4.2/4.3 in adaptive remodelling in response to physiological and pathophysiological stresses associated with osmotic challenges and cell volume perturbations.
Acknowledgments
We are very grateful to Drs Susumu Ohya and Yuji Imaizumi (Department of Molecular & Cellular Pharmacology, Nagoya City University, Japan) for the generous gift of Kv4.2 and Kv4.3 cDNA. This study was supported by NIH (HL63914) and NCRR (P20 RR15581). G.-L.W. is a postdoctoral fellow of the American Heart Association, Western States Affiliate.
References
- Barry DM, Xu H, Schuessler RB, Nerbonne JM. Functional knockout of the transient outward current, long-QT syndrome, and cardiac remodeling in mice expressing a dominant-negative Kv4 alpha subunit. Circ Res. 1998;83:560–567. doi: 10.1161/01.res.83.5.560. [DOI] [PubMed] [Google Scholar]
- Baumgarten CM, Clemo HF. Swelling-activated chloride channels in cardiac physiology and pathophysiology. Prog Biophys Mol Biol. 2003;82:25–42. doi: 10.1016/s0079-6107(03)00003-8. [DOI] [PubMed] [Google Scholar]
- Befroy DE, Powell T, Radda GK, Clarke K. Osmotic shock: modulation of contractile function, pHi, and ischemic damage in perfused guinea pig heart. Am J Physiol. 1999;276:H1236–H1244. doi: 10.1152/ajpheart.1999.276.4.H1236. [DOI] [PubMed] [Google Scholar]
- Brouillette J, Clark RB, Giles WR, Fiset C. Functional properties of K+ currents in adult mouse ventricular myocytes. J Physiol. 2004;559:777–798. doi: 10.1113/jphysiol.2004.063446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet S, Aimond F, Li H, Guo W, Eldstrom J, Fedida D, Yamada KA, Nerbonne JM. Heterogeneous expression of repolarizing, voltage-gated K+ currents in adult mouse ventricles. J Physiol. 2004;559:103–120. doi: 10.1113/jphysiol.2004.063347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caballero R, Gomez R, Moreno I, Nunez L, Gonzalez T, Arias C, Guizy M, Valenzuela C, Tamargo J, Delpon E. Interaction of angiotensin II with the angiotensin type 2 receptor inhibits the cardiac transient outward potassium current. Cardiovasc Res. 2004;62:86–95. doi: 10.1016/j.cardiores.2003.12.029. [DOI] [PubMed] [Google Scholar]
- Cohen P. Signal integration at the level of protein kinases, protein phosphatases and their substrates. Trends Biochem Sci. 1992;17:408–413. doi: 10.1016/0968-0004(92)90010-7. [DOI] [PubMed] [Google Scholar]
- Decher N, Barth AS, Gonzalez T, Steinmeyer K, Sanguinetti MC. Novel KChIP2 isoforms increase functional diversity of transient outward potassium currents. J Physiol. 2004;557:761–772. doi: 10.1113/jphysiol.2004.066720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Diego JM, Cordeiro JM, Goodrow RJ, Fish JM, Zygmunt AC, Perez GJ, Scornik FS, Antzelevitch C. Ionic and cellular basis for the predominance of the Brugada syndrome phenotype in males. Circulation. 2002;106:2004–2011. doi: 10.1161/01.cir.0000032002.22105.7a. [DOI] [PubMed] [Google Scholar]
- Dixon JE, Shi W, Wang HS, McDonald C, Yu H, Wymore RS, Cohen IS, McKinnon D. Role of the Kv4.3 K+ channel in ventricular muscle. A molecular correlate for the transient outward current. Circ Res. 1996;79:659–668. doi: 10.1161/01.res.79.4.659. [DOI] [PubMed] [Google Scholar]
- Dorn GW, Force T. Protein kinase cascades in the regulation of cardiac hypertrophy. J Clin Invest. 2005;115:527–537. doi: 10.1172/JCI24178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan D, Cowley S, Horowitz B, Hume JR. A serine residue in Clc-3 links phosphorylation-dephosphorylation to chloride channel regulation by cell. J Gen Physiol. 1999a;113:57–70. doi: 10.1085/jgp.113.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan D, Fermini B, Nattel S. Potassium channel blocking properties of propafenone in rabbit atrial myocytes. J Pharmacol Exp Ther. 1993;264:1113–1123. [PubMed] [Google Scholar]
- Duan D, Fermini B, Nattel S. Alpha-adrenergic control of volume-regulated Cl− currents in rabbit atrial myocytes. Characterization of a novel ionic regulatory mechanism. Circ Res. 1995;77:379–393. doi: 10.1161/01.res.77.2.379. [DOI] [PubMed] [Google Scholar]
- Duan D, Hume JR. NO and the regulation of VSOACs. J Physiol. 2000;528:2. doi: 10.1111/j.1469-7793.2000.t01-1-00002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan D, Hume JR, Nattel S. Evidence that outwardly rectifying Cl− channels underlie volume-regulated Cl− currents in heart. Circ Res. 1997a;80:103–113. doi: 10.1161/01.res.80.1.103. [DOI] [PubMed] [Google Scholar]
- Duan D, Liu LL, Bozeat N, Huang ZM, Xiang SY, Wang GL, Ye L, Hume JR. Functional role of anion channels in cardiac diseases. Acta Pharmacol Sin. 2005;26:265–278. doi: 10.1111/j.1745-7254.2005.00061.x. [DOI] [PubMed] [Google Scholar]
- Duan D, Winter C, Cowley S, Hume JR, Horowitz B. Molecular identification of a volume-regulated chloride channel. Nature. 1997b;390:417–421. doi: 10.1038/37151. [DOI] [PubMed] [Google Scholar]
- Duan D, Ye L, Britton F, Horowitz B, Hume JR. A novel anionic inward rectifier in native cardiac myocytes. Circ Res. 2000;86:E63–E71. [PubMed] [Google Scholar]
- Duan D, Ye L, Britton F, Miller LJ, Yamazaki J, Horowitz B, Hume JR. Purinoceptor-coupled Cl− channels in mouse heart: a novel, alternative pathway for CFTR regulation. J Physiol. 1999b;521:43–56. doi: 10.1111/j.1469-7793.1999.00043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellershaw DC, Greenwood IA, Large WA. Dual modulation of swelling-activated chloride current by NO and NO donors in rabbit portal vein myocytes. J Physiol. 2000;528:15–24. doi: 10.1111/j.1469-7793.2000.00015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellershaw DC, Greenwood IA, Large WA. Modulation of volume-sensitive chloride current by noradrenaline in rabbit portal vein myocytes. J Physiol. 2002;542:537–547. doi: 10.1113/jphysiol.2002.018770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey N, Katus HA, Olson EN, Hill JA. Hypertrophy of the heart: a new therapeutic target? Circulation. 2004;109:1580–1589. doi: 10.1161/01.CIR.0000120390.68287.BB. [DOI] [PubMed] [Google Scholar]
- Guo W, Li H, Aimond F, Johns DC, Rhodes KJ, Trimmer JS, Nerbonne JM. Role of heteromultimers in the generation of myocardial transient outward K+ currents. Circ Res. 2002;90:586–593. doi: 10.1161/01.res.0000012664.05949.e0. [DOI] [PubMed] [Google Scholar]
- Guo W, Li H, London B, Nerbonne JM. Functional consequences of elimination of i(to,f) and i(to,s): early afterdepolarizations, atrioventricular block, and ventricular arrhythmias in mice lacking Kv1.4 and expressing a dominant-negative Kv4 alpha subunit. Circ Res. 2000;87:73–79. doi: 10.1161/01.res.87.1.73. [DOI] [PubMed] [Google Scholar]
- Haas M, McBrayer D, Lytle C. [Cl−]i-dependent phosphorylation of the Na-K-Cl cotransport protein of dog tracheal epithelial cells. J Biol Chem. 1995;270:28955–28961. doi: 10.1074/jbc.270.48.28955. [DOI] [PubMed] [Google Scholar]
- Hall SK, Zhang J, Lieberman M. Cyclic AMP prevents activation of a swelling-induced chloride-sensitive conductance in chick heart cells. J Physiol. 1995;488:359–369. doi: 10.1113/jphysiol.1995.sp020972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill OP, Huguenard JR, Prince DA. Patch-clamp studies of voltage-gated currents in identified neurons of the rat cerebral cortex. Cereb Cortex. 1991;1:48–61. doi: 10.1093/cercor/1.1.48. [DOI] [PubMed] [Google Scholar]
- Hatano N, Ohya S, Muraki K, Giles W, Imaizumi Y. Dihydropyridine Ca2+ channel antagonists and agonists block Kv4.2, Kv4.3 and Kv1.4 K+ channels expressed in HEK293 cells. Br J Pharmacol. 2003;139:533–544. doi: 10.1038/sj.bjp.0705281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzig S, Neumann J. Effects of serine/threonine protein phosphatases on ion channels in excitable membranes. Physiol Rev. 2000;80:173–210. doi: 10.1152/physrev.2000.80.1.173. [DOI] [PubMed] [Google Scholar]
- Hiraoka M, Kawano S, Hirano Y, Furukawa T. Role of cardiac chloride currents in changes in action potential characteristics and arrhythmias. Cardiovasc Res. 1998;40:23–33. doi: 10.1016/s0008-6363(98)00173-4. [DOI] [PubMed] [Google Scholar]
- Hoppe UC, Marban E, Johns DC. Molecular dissection of cardiac repolarization by in vivo Kv4.3 gene transfer. J Clin Invest. 2000;105:1077–1084. doi: 10.1172/JCI8757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B, Qin D, El Sherif N. Early down-regulation of K+ channel genes and currents in the postinfarction heart. J Cardiovasc Electrophysiol. 2000;11:1252–1261. doi: 10.1046/j.1540-8167.2000.01252.x. [DOI] [PubMed] [Google Scholar]
- Hume JR, Duan D, Collier ML, Yamazaki J, Horowitz B. Anion transport in heart. Physiol Rev. 2000;80:31–81. doi: 10.1152/physrev.2000.80.1.31. [DOI] [PubMed] [Google Scholar]
- Hunter T. Protein kinases and phosphatases: the yin and yang of protein phosphorylation and signaling. Cell. 1995;80:225–236. doi: 10.1016/0092-8674(95)90405-0. [DOI] [PubMed] [Google Scholar]
- Ishihara H, Martin BL, Brautigan DL, Karaki H, Ozaki H, Kato Y, Fusetani N, Watabe S, Hashimoto K, Uemura D. Calyculin A and okadaic acid: inhibitors of protein phosphatase activity. Biochem Biophys Res Commun. 1989;159:871–877. doi: 10.1016/0006-291x(89)92189-x. [DOI] [PubMed] [Google Scholar]
- Jennings ML, Schulz RK. Okadaic acid inhibition of KCl cotransport. Evidence that protein dephosphorylation is necessary for activation of transport by either cell swelling or N-ethylmaleimide. J Gen Physiol. 1991;97:799–817. doi: 10.1085/jgp.97.4.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns DC, Nuss HB, Marban E. Suppression of neuronal and cardiac transient outward currents by viral gene transfer of dominant-negative Kv4.2 constructs. J Biol Chem. 1997;272:31598–31603. doi: 10.1074/jbc.272.50.31598. [DOI] [PubMed] [Google Scholar]
- Kaab S, Dixon J, Duc J, Ashen D, Nabauer M, Beuckelmann DJ, Steinbeck G, McKinnon D, Tomaselli GF. Molecular basis of transient outward potassium current downregulation in human heart failure: a decrease in Kv4.3 mRNA correlates with a reduction in current density. Circulation. 1998;98:1383–1393. doi: 10.1161/01.cir.98.14.1383. [DOI] [PubMed] [Google Scholar]
- Kaprielian R, Sah R, Nguyen T, Wickenden AD, Backx PH. Myocardial infarction in rat eliminates regional heterogeneity of AP profiles, Ito K+ currents, and [Ca2+]i transients. Am J Physiol Heart Circ Physiol. 2002;283:H1157–H1168. doi: 10.1152/ajpheart.00518.2001. [DOI] [PubMed] [Google Scholar]
- Kassiri Z, Zobel C, Nguyen TT, Molkentin JD, Backx PH. Reduction of Ito causes hypertrophy in neonatal rat ventricular myocytes. Circ Res. 2002;90:578–585. doi: 10.1161/01.res.0000012223.86441.a1. [DOI] [PubMed] [Google Scholar]
- Klein JD, Perry PB, O'Neill WC. Regulation by cell vlume of Na+–K+–2Cl− cotransport in vascular endothelial cells: role of protein phosphorylation. J Membr Biol. 1993;132:243–252. doi: 10.1007/BF00235741. [DOI] [PubMed] [Google Scholar]
- Kocic I, Hirano Y, Hiraoka M. Ionic basis for membrane potential changes induced by hypoosmotic stress in guinea-pig ventricular myocytes. Cardiovasc Res. 2001;51:59–70. doi: 10.1016/s0008-6363(01)00279-6. [DOI] [PubMed] [Google Scholar]
- Lang F, Busch GL, Ritter M, Volkl H, Waldegger S, Gulbins E, Haussinger D. Functional significance of cell volume regulatory mechanisms. Physiol Rev. 1998;78:247–306. doi: 10.1152/physrev.1998.78.1.247. [DOI] [PubMed] [Google Scholar]
- Lang F, Ritter M, Gamper N, Huber S, Fillon S, Tanneur V, Lepple-Wienhues A, Szabo I, Gulbins E. Cell volume in the regulation of cell proliferation and apoptotic cell death. Cell Physiol Biochem. 2000;10:417–428. doi: 10.1159/000016367. [DOI] [PubMed] [Google Scholar]
- Larsen AK, Jensen BS, Hoffmann EK. Activation of protein kinase C during cell volume regulation in Ehrlich mouse ascites tumor cells. Biochim Biophys Acta. 1994;1222:477–482. doi: 10.1016/0167-4889(94)90057-4. [DOI] [PubMed] [Google Scholar]
- Li GR, Lau CP, Ducharme A, Tardif JC, Nattel S. Transmural action potential and ionic current remodeling in ventricles of failing canine hearts. Am J Physiol Heart Circ Physiol. 2002;283:H1031–H1041. doi: 10.1152/ajpheart.00105.2002. [DOI] [PubMed] [Google Scholar]
- London B, Guo W, Pan X, Lee JS, Shusterman V, Rocco CJ, Logothetis DA, Nerbonne JM, Hill JA. Targeted replacement of Kv1.5 in the mouse leads to loss of the 4-aminopyridine-sensitive component of IK,slow and resistance to drug-induced qt prolongation. Circ Res. 2001;88:940–946. doi: 10.1161/hh0901.090929. [DOI] [PubMed] [Google Scholar]
- Lytle C. A volume-sensitive protein kinase regulates the Na-K-2Cl cotransporter in duck red blood cells. Am J Physiol. 1998;274:C1002–C1010. doi: 10.1152/ajpcell.1998.274.4.C1002. [DOI] [PubMed] [Google Scholar]
- Nakamura TY, Coetzee WA, Vega-Saenz d. M, Artman M, Rudy B. Modulation of Kv4 channels, key components of rat ventricular transient outward K+ current, by PKC. Am J Physiol. 1997;273:H1775–H1786. doi: 10.1152/ajpheart.1997.273.4.H1775. [DOI] [PubMed] [Google Scholar]
- Nerbonne JM, Guo W. Heterogeneous expression of voltage-gated potassium channels in the heart: roles in normal excitation and arrhythmias. J Cardiovasc Electrophysiol. 2002;13:406–409. doi: 10.1046/j.1540-8167.2002.00406.x. [DOI] [PubMed] [Google Scholar]
- Ohya S, Tanaka M, Oku T, Asai Y, Watanabe M, Giles WR, Imaizumi Y. Molecular cloning and tissue distribution of an alternatively spliced variant of an A-type K+ channel alpha-subunit, Kv4.3 in the rat. FEBS Lett. 1997;420:47–53. doi: 10.1016/s0014-5793(97)01483-x. [DOI] [PubMed] [Google Scholar]
- Oudit GY, Kassiri Z, Sah R, Ramirez RJ, Zobel C, Backx PH. The molecular physiology of the cardiac transient outward potassium current (Ito) in normal and diseased myocardium. J Mol Cell Cardiol. 2001;33:851–872. doi: 10.1006/jmcc.2001.1376. [DOI] [PubMed] [Google Scholar]
- Po SS, Wu RC, Juang GJ, Kong W, Tomaselli GF. Mechanism of alpha-adrenergic regulation of expressed hKv4.3 currents. Am J Physiol Heart Circ Physiol. 2001;281:H2518–H2527. doi: 10.1152/ajpheart.2001.281.6.H2518. [DOI] [PubMed] [Google Scholar]
- Priebe L, Beuckelmann DJ. Cell swelling causes the action potential duration to shorten in guinea-pig ventricular myocytes by activating IK,ATP. Pflugers Arch. 1998;436:894–898. doi: 10.1007/pl00008087. [DOI] [PubMed] [Google Scholar]
- Rees SA, Vandenberg JI, Wright AR, Yoshida A, Powell T. Cell swelling has differential effects on the rapid and slow components of delayed rectifier potassium current in guinea pig cardiac myocytes. J Gen Physiol. 1995;106:1151–1170. doi: 10.1085/jgp.106.6.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remillard CV, Yuan JX. Activation of K+ channels: an essential pathway in programmed cell death. Am J Physiol Lung Cell Mol Physiol. 2004;286:L49–L67. doi: 10.1152/ajplung.00041.2003. [DOI] [PubMed] [Google Scholar]
- Rutledge E, Bianchi L, Christensen M, Boehmer C, Morrison R, Broslat A, Beld AM, George AL, Greenstein D, Strange K. CLH-3, a ClC-2 anion channel ortholog activated during meiotic maturation in C. elegans oocytes. Curr Biol. 2001;11:161–170. doi: 10.1016/s0960-9822(01)00051-3. [DOI] [PubMed] [Google Scholar]
- Sah R, Ramirez RJ, Oudit GY, Gidrewicz D, Trivieri MG, Zobel C, Backx PH. Regulation of cardiac excitation–contraction coupling by action potential repolarization: role of the transient outward potassium current (Ito) J Physiol. 2003;546:5–18. doi: 10.1113/jphysiol.2002.026468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber KL, Paquet L, Allen BG, Rindt H. Protein kinase C isoform expression and activity in the mouse heart. Am J Physiol Heart Circ Physiol. 2001;281:H2062–H2071. doi: 10.1152/ajpheart.2001.281.5.H2062. [DOI] [PubMed] [Google Scholar]
- Shimoni Y, Liu XF. Role of PKC in autocrine regulation of rat ventricular K+ currents by angiotensin and endothelin. Am J Physiol Heart Circ Physiol. 2003;284:H1168–H1181. doi: 10.1152/ajpheart.00748.2002. [DOI] [PubMed] [Google Scholar]
- Steenbergen C, Hill ML, Jennings RB. Volume regulation and plasma membrane injury in aerobic, anaerobic, and ischemic myocardium in vitro. Effects of osmotic cell swelling on plasma membrane integrity. Circ Res. 1985;57:864–875. doi: 10.1161/01.res.57.6.864. [DOI] [PubMed] [Google Scholar]
- Szallasi Z, Smith CB, Pettit GR, Blumberg PM. Differential regulation of protein kinase C isozymes by bryostatin 1 and phorbol 12-myristate 13-acetate in NIH 3T3 fibroblasts. J Biol Chem. 1994;269:2118–2124. [PubMed] [Google Scholar]
- Takimoto K, Li D, Hershman KM, Li P, Jackson EK, Levitan ES. Decreased expression of Kv4.2 and novel Kv4.3 K+ channel subunit mRNAs in ventricles of renovascular hypertensive rats. Circ Res. 1997;81:533–539. doi: 10.1161/01.res.81.4.533. [DOI] [PubMed] [Google Scholar]
- Thorneloe KS, Liu XF, Walsh MP, Shimoni Y. Transmural differences in rat ventricular protein kinase C epsilon correlate with its functional regulation of a transient cardiac K+ current. J Physiol. 2001a;533:145–154. doi: 10.1111/j.1469-7793.2001.0145b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorneloe KS, Liu XF, Walsh MP, Shimoni Y. Transmural differences in rat ventricular protein kinase C epsilon correlate with its functional regulation of a transient cardiac K+ current. J Physiol. 2001b;533:145–154. doi: 10.1111/j.1469-7793.2001.0145b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg JI, Bett GC, Powell T. Contribution of a swelling-activated chloride current to changes in the cardiac action potential. Am J Physiol. 1997;273:C541–C547. doi: 10.1152/ajpcell.1997.273.2.C541. [DOI] [PubMed] [Google Scholar]
- Vandenberg JI, Rees SA, Wright AR, Powell T. Cell swelling and ion transport pathways in cardiac myocytes. Cardiovasc Res. 1996;32:85–97. [PubMed] [Google Scholar]
- Wang Z, Feng J, Shi H, Pond A, Nerbonne JM, Nattel S. Potential molecular basis of different physiological properties of the transient outward K+ current in rabbit and human atrial myocytes. Circ Res. 1999;84:551–561. doi: 10.1161/01.res.84.5.551. [DOI] [PubMed] [Google Scholar]
- Wright AR, Rees SA. Cardiac cell volume: crystal clear or murky waters? A comparison with other cell types. Pharmacol Ther. 1998;80:89–121. doi: 10.1016/s0163-7258(98)00025-4. [DOI] [PubMed] [Google Scholar]
- Xu H, Barry DM, Li H, Brunet S, Guo W, Nerbonne JM. Attenuation of the slow component of delayed rectification, action potential prolongation, and triggered activity in mice expressing a dominant-negative Kv2 alpha subunit. Circ Res. 1999a;85:623–633. doi: 10.1161/01.res.85.7.623. [DOI] [PubMed] [Google Scholar]
- Xu H, Guo W, Nerbonne JM. Four kinetically distinct depolarization-activated K+ currents in adult mouse ventricular myocytes. J Gen Physiol. 1999b;113:661–678. doi: 10.1085/jgp.113.5.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Li H, Nerbonne JM. Elimination of the transient outward current and action potential prolongation in mouse atrial myocytes expressing a dominant negative Kv4 alpha subunit. J Physiol. 1999c;519:11–21. doi: 10.1111/j.1469-7793.1999.0011o.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Dong PH, Zhang Z, Ahmmed GU, Chiamvimonvat N. Presence of a calcium-activated chloride current in mouse ventricular myocytes. Am J Physiol Heart Circ Physiol. 2002;283:H302–H314. doi: 10.1152/ajpheart.00044.2002. [DOI] [PubMed] [Google Scholar]
- Yue L, Melnyk P, Gaspo R, Wang Z, Nattel S. Molecular mechanisms underlying ionic remodeling in a dog model of atrial fibrillation. Circ Res. 1999;84:776–784. doi: 10.1161/01.res.84.7.776. [DOI] [PubMed] [Google Scholar]
- Zhong J, Wang GX, Hatton WJ, Yamboliev IA, Walsh MP, Hume JR. Regulation of volume-sensitive outwardly rectifying anion channels in pulmonary arterial smooth muscle cells by PKC. Am J Physiol Cell Physiol. 2002;283:C1627–C1636. doi: 10.1152/ajpcell.00152.2001. [DOI] [PubMed] [Google Scholar]
- Zhou J, Kodirov S, Murata M, Buckett PD, Nerbonne JM, Koren G. Regional upregulation of Kv2.1-encoded current, IK,slow2, in Kv1DN mice is abolished by crossbreeding with Kv2DN mice. Am J Physiol Heart Circ Physiol. 2003;284:H491–H500. doi: 10.1152/ajpheart.00576.2002. [DOI] [PubMed] [Google Scholar]
- Zicha S, Moss I, Allen B, Varro A, Papp J, Dumaine R, Antzelevich C, Nattel S. Molecular basis of species-specific expression of repolarizing K+ currents in the heart. Am J Physiol Heart Circ Physiol. 2003;285:H1641–H1649. doi: 10.1152/ajpheart.00346.2003. [DOI] [PubMed] [Google Scholar]