Abstract
Asphyxia, which occurs during obstructive sleep apnoeic events, alters the baroreceptor reflex and this may lead to hypertension. We have recently reported that breathing an asphyxic gas resets the baroreceptor–vascular resistance reflex towards higher pressures. The present study was designed to determine whether this effect was caused by the reduced oxygen tension, which affects mainly peripheral chemoreceptors, or by the increased carbon dioxide, which acts mainly on central chemoreceptors. We studied 11 healthy volunteer subjects aged between 20 and 55 years old (6 male). The stimulus to the carotid baroreceptors was changed using graded pressures of −40 to +60 mmHg applied to a neck chamber. Responses of vascular resistance were assessed in the forearm from changes in blood pressure (Finapres) divided by brachial blood flow velocity (Doppler) and cardiac responses from the changes in RR interval and heart rate. Stimulus–response curves were defined during (i) air breathing, (ii) hypoxia (12% O2 in N2), and (iii) hypercapnia (5% CO2 in 95% O2). Responses during air breathing were assessed both prior to and after either hypoxia or hypercapnia. We applied a sigmoid function or third order polynomial to the curves and determined the maximal differential (equivalent to peak sensitivity) and the corresponding carotid sinus pressure (equivalent to ‘set point’). Hypoxia resulted in an increase in heart rate but no significant change in mean blood pressure or vascular resistance. However, there was an increase in vascular resistance in the post-stimulus period. Hypoxia had no significant effect on baroreflex sensitivity or ‘set point’ for the control of RR interval, heart rate or mean arterial pressure. Peak sensitivity of the vascular resistance response to baroreceptor stimulation was significantly reduced from −2.5 ± 0.4 units to −1.4 ± 0.1 units (P < 0.05) and this was restored in the post-stimulus period to −2.6 ± 0.5 units. There was no effect on ‘set point’. Hypercapnia, on the other hand, resulted in a decrease in heart rate, which remained reduced in the post-stimulus period and significantly increased mean blood pressure. Baseline vascular resistance was significantly increased and then further increased in the post-control period. Like hypoxia, hypercapnia had no effect on baroreflex control of RR interval, heart rate or mean arterial pressure. There was, also no significant change in the sensitivity of the vascular resistance responses, however, ‘set point’ was significantly increased from 74.7 ± 4 to 87.0 ± 2 mmHg (P < 0.02). This was not completely restored to pre-stimulus control levels in the post-stimulus control period (82.2 ± 3 mmHg). These results suggest that the hypoxic component of asphyxia reduces baroreceptor–vascular resistance reflex sensitivity, whilst the hypercapnic component is responsible for increasing blood pressure and reflex ‘set point’. Hypercapnia appears to have a lasting effect after the removal of the stimulus. Thus the effect of both peripheral and central chemoreceptors on baroreflex function may contribute to promoting hypertension in patients with obstructive sleep apnoea.
Obstructive sleep apnoea (OSA) is characterized by cessation of breathing due to airway obstruction during sleep. This leads to a reduction in arterial oxygen content and an increase in arterial carbon dioxide content. Patients with OSA are at increased risk of developing arterial hypertension (Partinen & Telakivi, 1992; Parati et al. 1997; Bixler et al. 2000; Grote et al. 2000; Lavie et al. 2000; Nieto et al. 2000) and this risk is independent of common risk factors such as obesity (Peppard et al. 2000). We have recently reported that breathing an asphyxic gas mixture results in a resetting of the vascular resistance component of the carotid baroreflex causing it to function around higher pressures (Cooper et al. 2004). This may provide a mechanism by which obstructive sleep apnoea leads to hypertension. Asphyxia is the combination of hypoxia and hypercapnia and these have different effects, with hypoxia acting mainly through peripheral chemoreceptors and hypercapnia acting mainly through central chemoreceptors. It is therefore possible that these two stimuli have differing effects on the baroreflex. The aim of the present study, therefore, was to examine the effects on the baroreceptor reflex of the two components of asphyxia applied individually. Knowledge of these separate effects may provide information on the extent to which the different chemoreceptors are responsible.
Methods
Subjects
We recruited 11 healthy volunteer control subjects aged 20–55 years (25.4 ± 3.1 years). All subjects were apparently healthy with no symptoms of cardiovascular or respiratory disease and no history of snoring or excessive daytime sleepiness. They were not taking any cardiovascular acting medications. None was hypertensive, as defined by a systolic pressure greater than 140 mmHg or diastolic pressure greater than 95 mmHg (mean pressure ranged from 71 to 101 mmHg). All subjects were asked to refrain from drinking caffeine-containing drinks on the days of the experiment. All subjects gave informed written consent and the study was approved by the Leeds Teaching Hospitals Research Ethics Committee. All procedures conformed to the Declaration of Helsinki.
Measurements
The methods used were similar to those previously described (Cooper et al. 2004). Subjects sat comfortably and breathed through a mouthpiece connected to a three-way valve. ECG was recorded using three bipolar leads and a Hewlett Packard recorder (78325C, Boebringen, Germany). Brachial arterial pressure was determined using a standard sphygmomanometer taking diastolic pressure as Korotkof phase 5. Finger arterial pressure was recorded continuously during each experiment using a finger photoplethysmographic device (Finapres, Ohmeda 2300, Wisconsin, USA). Finger estimates were verified using the brachial values. Forearm blood velocity was determined using a pulse wave Doppler system (T2-Dop, DWL Elektronische System GmbH, Sipplingen, Germany) with a 4 mHz probe positioned over the brachial artery at or near the antecubital fossa. The probe was positioned so that the strongest signal with the smallest angle of insonation was attained and then firmly clamped in position and extreme care was taken to ensure that the angle with the brachial artery remained constant during the entirety of each experiment. End tidal CO2 and O2 were sampled continuously through expiratory ports on the mouthpiece connected to a CO2 analyser (Instrumentation Laboratories, IL200, Lexington, MA, USA) and an O2 analyser (Servomex 570A, Crowborough, UK). Arterial oxygen saturation was estimated using finger pulse oximetry (Datax Ohmeda 3800, Louisville, CO, USA). Mouth pressure was recorded through a catheter connected to a mouthpiece and a pressure transducer (Statham P23Gb). All variables were recorded on a direct writing electrostatic recorder (Gould, Ballainvilliers, France, model ES1000) and on a personal computer via a data acquisition program (Windaq, Dataq Instruments, Akron, OH, USA).
Carotid baroreceptor tests
Changes in the stimulus to carotid sinus baroreceptors were effected by changing the extramural carotid pressures by applying suction or pressure to the neck overlying the sinuses. Negative pressures were applied using a lead chamber similar to that described by Eckberg et al. (1975). The neoprene lined chamber was moulded to fit to the subject from the lower border of the mandible to the upper border of the chest and to the posterior neck muscles. To apply positive pressures we used paired chambers as described by Kelly et al. (1993, 1996). These were smaller chambers made from thermoplastic and were of a range of sizes to fit various shapes of neck. The open ends of the devices were lined with a thin latex membrane to transmit the pressure to the carotid bifurcation regions, without air leaking. Pressure in the chamber(s) was recorded using a catheter and a Statham P23Gb transducer. The chamber(s) were connected via a solenoid valve to a 10 litre reservoir, the pressure in which was controlled by a vacuum/pressure source (Henry NV300, Numatic, Beaminster, UK). Tests of baroreceptor responses were performed by setting the pressure in the reservoir to the required level and opening the solenoid valve while the neck chamber(s) were held in place. Baroreceptor stimulus–response relationships were determined by changing the pressure in the neck chamber(s) in the following sequence: −40, −20, −10, +10, +20, +40, +60 mmHg and then in reverse order. For vascular responses, each pressure was maintained for 20 s and responses determined as the maximum changes in pressure/flow from the mean values determined during the 10 s periods prior to stimulation. Pressure was restored to atmospheric between each step. For cardiac responses, neck pressure was applied during the last 5 s of a 10 s held expiration and responses determined as the maximum change in RR interval compared to the average of the three RR intervals before the onset of neck pressure.
Hypoxia and hypercapnia
Subjects breathed through a mouthpiece connected to a two-way valve. Either hypoxia or hyperoxic hypercapnia was applied by connecting the inspiratory port to a Douglas bag containing either 12% O2 in N2 or 5% CO2 in 95% O2.
Experimental procedure
Subjects were instructed to abstain from caffeine containing beverages from the evening before the tests. Tests were carried out on two separate occasions in random order, but always at the same time of day. The subjects were seated and the various monitoring devices attached. After the baseline assessments, either hypoxia or hyperoxic hypercapnia was applied.
Carotid baroreceptor stimulus–response relationships were determined as described, firstly, 10 min after breathing room air, then 10 min after breathing the hypoxic or hypercapnic mixtures. Finally, they were repeated after a further 10 min of breathing room air.
Data analysis
Vascular resistance was calculated as mean arterial pressure divided by mean brachial blood flow velocity. We analysed the average values of vascular resistance taken over each respiratory cycle (determined from the CO2 trace). Each value of vascular resistance during stimulation was calculated as the maximum percentage changes averaged over a complete respiratory cycle compared with the average values from the two respiratory cycles before the onset of stimulation. Values obtained during each intervention were compared with the two control tests undertaken before and after each intervention. Responses of mean arterial pressure were also calculated from the same beats used to calculate vascular resistance. We also determined the maximum change in RR interval and heart rate from the pre-stimulus three-beat average. Transmission of pressure from the collar to the carotid sinus was assumed to be 100% and carotid sinus transmural pressure was calculated as mean arterial pressure minus collar pressure.
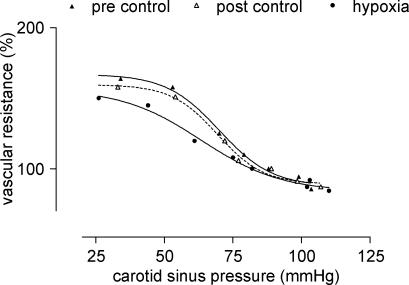
Pressure–response curves were plotted and fitted with either a sigmoid function or a third-order polynomial, depending on which curve best fitted the data (GraphPad Prism v3.0, GraphPad Software Inc., San Diego, CA, USA). An example from one subject is given in Fig. 1. The differentials of these curves were then calculated. The maximal differentials, which correspond to the maximum slopes of the pressure–response curves, were taken as the measures of baroreflex sensitivity. The carotid sinus pressures corresponding to the maximal differentials were termed ‘set-points’ and these values were used to determine possible resetting of the baroreflex. Each subject served as his or her own control on each experimental day. Absolute values of changes were not reported since, although conditions such as probe positioning were kept constant during each experimental day, they may not have been the same on different days. Values obtained during pre- and post-control and stimulus periods were compared using Dunnett's or Dunn's repeated measures ANOVA as appropriate. All values, unless otherwise stated, are presented as means ±s.e.m.
Figure 1. Example of a pressure–response curve from one subject.
Results
Effects of hypoxia
End-tidal partial pressures of oxygen and carbon dioxide
Values of end-tidal partial pressures of oxygen and carbon dioxide (PETO2 and PETCO2) and pulse oxygen saturation during baseline and whilst breathing the hypoxic gas mixture are given in Table 1. Breathing the hypoxic gas significantly decreased PETO2 from 110.6 ± 1.7 (pre-control) to 58.7 ± 2.5 mmHg (P < 0.00001). There was a small decrease in PETCO2, but this did not quite reach statistical significance (P= 0.05). Finger pulse oxygen saturation was also significantly decreased from 98.3 ± 0.3 to 88.8 ± 1.1% (P < 0.00001).
Table 1.
Effect hypoxia on partial pressure of end-tidal oxygen and carbon dioxide and pulse oxygen saturation
| Pre-control | Hypoxia | Post-control | |
|---|---|---|---|
| PO2 (mmHg) | 111.5 ± 2.1*** | 58.7 ± 2.5 | 109.8 ± 1.8*** |
| PCO2 (mmHg) | 41.5 ± 1.5 | 39.1 ± 1.9 | 41.0 ± 1.9* |
| Sp,O2 (%) | 98.3 ± 0.3*** | 88.8 ± 1.1 | 98.3 ± 0.2*** |
Compared to hypoxia
P < 0.02
P < 0.0001.
Resting values of vascular resistance, heart rate and blood pressure
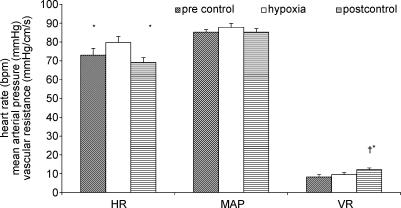
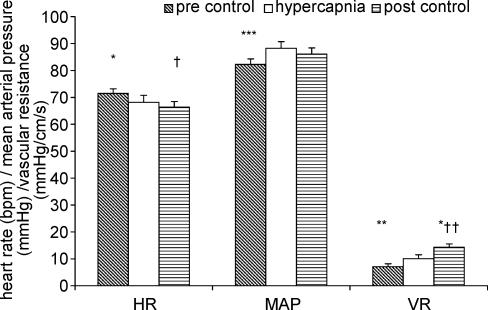
The effects of the hypoxia on vascular resistance, heart rate and blood pressure are displayed in Fig. 2. Hypoxia resulted in a significant increase in heart rate from 72.9 ± 3.6 to 79.7 ± 3.2 b.p.m. (P < 0.01), which returned to baseline levels (69.1 ± 8.1 b.p.m.) in the post-stimulus control period. There was no significant effect on mean arterial pressure. Vascular resistance did not change significantly from the initial control levels in response to hypoxia. However in the control period after the stimulus there was a significant increase in vascular resistance in comparison to both pre-stimulus control and hypoxia.
Figure 2. Effects of hypoxia on baseline heart rate (HR), mean arterial pressure (MAP) and vascular resistance (VR).
Compared to hypoxia *P < 0.005, compared to pre-control †P < 0.05.
Effects on baroreceptor function
The effects of hypoxia on baroreflex control of RR interval, heart rate and mean arterial pressure are given in Table 2. There was no significant effect on either baroreflex sensitivity or baroreflex ‘set point’ for any of these responses.
Table 2.
Effect of hypoxia on baroreflex sensitivity and ‘set point’ for mean pressure (MAP), RR interval and heart rate (HR) responses
| Pre-control | Hypoxia | Post-control | |
|---|---|---|---|
| MAP sensitivity | −0.54 ± 0.10 | −0.56 ± 0.05 | −0.62 ± 0.08 |
| MAP ‘set point’ | 88.0 ± 3.6 | 84.4 ± 3.1 | 82.3 ± 3.0 |
| RR sensitivity | 7.30 ± 2.40 | 6.7 ± 1.4 | 6.40 ± 1.10 |
| RR ‘set point’ | 92.8 ± 5.4 | 91.9 ± 4.0 | 89.2 ± 3.6 |
| HR sensitivity | 0.30 ± 0.04 | 0.44 ± 0.08 | 0.37 ± 0.07 |
| HR ‘set point’ | 92.2 ± 5.0 | 87.5 ± 4.0 | 91.9 ± 4.1 |
There was no significant difference between any of the variables.
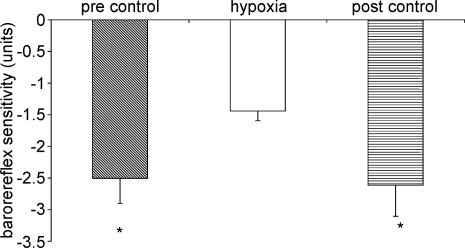
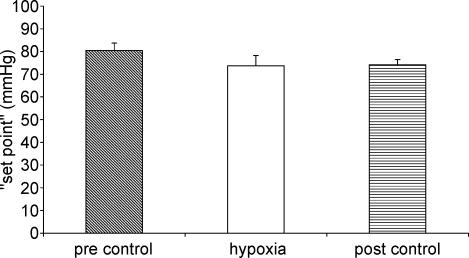
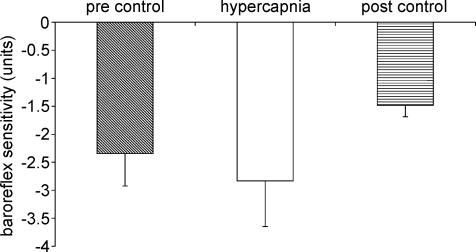
The effect of hypoxia on baroreflex control of vascular resistance is shown in Figs 3 and 4. Hypoxia resulted in a significant decrease in baroreflex sensitivity compared to both pre-stimulus and post-stimulus control periods (P < 0.05) (Fig. 3). ‘Set point’, however, was not affected by hypoxia (Fig. 4).
Figure 3. Effect of hypoxia on baroreflex sensitivity of vascular responses.
Compared to hypoxia *P < 0.05.
Figure 4. Effect of hypoxia on baroreflex ‘set point’ of vascular responses.
Effects of hypercapnia
End-tidal partial pressures of oxygen and carbon dioxide
The effects of hypercapnia on end-tidal gases and pulse oxygen saturation are given in Table 3. Hyperoxic hypercapnia produced the expected increases in both end-tidal PCO2 (from 41.9 ± 1.6 to 54.5 ± 1.8 mmHg; P < 0.00001) and PO2 (from 113.1 ± 1.6 to 641.7 ± 9.2 mmHg; P < 0.00001). Pulse oxygen saturation also increased significantly.
Table 3.
Effect hypercapnia on partial pressure of end-tidal oxygen and carbon dioxide and pulse oxygen saturation
| Pre-control | Hypercapnia | Post-control | |
|---|---|---|---|
| PO2 (mmHg) | 113.1 ± 1.6*** | 641.7 ± 9.2 | 117.7 ± 2.5†*** |
| PCO2 (mmHg) | 41.9 ± 1.6*** | 54.5 ± 1.8 | 43.9 ± 1.8†*** |
| Sp,O2 (%) | 98.0 ± 0.2*** | 99.8 ± 0.1 | 98.7 ± 0.2†* |
Compared to hypoxia
P < 0.005
P < 0.0001
compared to pre-control
P < 0.05.
Resting values of vascular resistance, heart rate and blood pressure
The effects of hypercapnia on vascular resistance, heart rate and blood pressure are displayed in Fig. 5. Hypercapnia caused a slight decrease in heart rate from 71.4 ± 1.7 b.p.m. to 68.2 ± 2.6 b.p.m. (P < 0.05), and this remained reduced in the post-stimulus control period (P < 0.05). There was an increase in arterial pressure (from 82.5 ± 2.2 to 88.3 ± 2.4 mmHg, P < 0.005). Mean arterial pressure in the post-stimulus control period was not significantly different from either pre-stimulus control or hypercapnia. Vascular resistance was significantly increased by 53.6 ± 20.2% (P < 0.01) in response to hypercapnia. In the post-stimulus control period there was a further significant increase in vascular resistance (28.6 ± 7.9%, P < 0.05).
Figure 5. Effect of hypercapnia on baseline heart rate (HR), mean arterial pressure (MAP) and vascular resistance (VR).
Compared to hypercapnia *P < 0.05, **P < 0.01, ***P < 0.005, compared to pre control +P < 0.05, ++P < 0.001.
Effects on baroreceptor function
The effects of hypercapnia on baroreflex control of RR interval, heart rate and mean arterial pressure responses are given in Table 4. There was no significant effect on either baroreflex sensitivity or baroreflex ‘set point’ for any of these responses.
Table 4.
Effect of hypercapnia on baroreflex sensitivity and ‘set point’ for mean pressure (MAP), RR interval and heart rate (HR) responses
| Pre-control | Hypercapnia | Post-control | |
|---|---|---|---|
| MAP sensitivity | −0.61 ± 0.11 | −0.49 ± 0.07 | −0.67 ± 0.11 |
| MAP ‘set point’ | 85.2 ± 2.7 | 85.3 ± 3.7 | 88.2 ± 4.2 |
| RR sensitivity | 5.52 ± 1.07 | 7.38 ± 1.01 | 6.34 ± 1.54 |
| RR ‘set point’ | 84.1 ± 4.3 | 83.7 ± 2.9 | 88.4 ± 3.4 |
| HR sensitivity | 0.34 ± 0.06 | 0.44 ± 0.06 | 0.38 ± 0.09 |
| HR ‘set point’ | 84.0 ± 4.3 | 84.3 ± 2.6 | 88.0 ± 3.9 |
There was no significant difference between any of the variables.
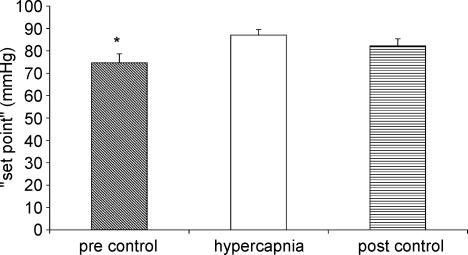
The effects of hypercapnia on baroreflex control of the vascular resistance are shown in Figs 6 and 7. Hypercapnia did not significantly alter baroreflex sensitivity (Fig. 6). There was a tendency for baroreflex sensitivity to decrease in the post-stimulus control period but this was not significant. Hypercapnia resulted in a significant increase in ‘set point’ (Fig. 7). This was not completely reset to pre-stimulus control levels in the post-stimulus control period.
Figure 6. Effect of hypercapnia on baroreflex sensitivity of vascular responses.
Figure 7. Effect of hypercapnia on baroreflex ‘set point’ of vascular responses.
Compared to hypercapnia *P < 0.05.
Discussion
We have recently published data showing that asphyxia (hypoxic hypercapnia) increases the ‘set point’ of the baroreflex control of vascular resistance without altering reflex sensitivity (Cooper et al. 2004). The present study aimed to examine further the interactions between baroreceptors and chemoreceptors to assess the relative importances of the hypoxic and the hypercapnic stimuli.
The principal findings of this study are as follows.
(1) Hypoxia reduces the sensitivity of baroreflex control of the vascular resistance without altering the reflex ‘set point’ and has no significant effect on baseline vascular resistance or mean arterial pressure.
(2) Hyperoxic hypercapnia has no effect on baroreflex sensitivity but significantly increases the reflex ‘set point’, the baseline vascular resistance and the mean arterial pressure. The effects of hypercapnia on vascular resistance and heart rate are sustained into the post-stimulus control period when the hypercapnic stimulus is removed.
Peripheral chemoreceptors are located in the carotid and aortic bodies and are responsive to changes in PO2, PCO2 and pH (West, 1995). Central chemoreceptors are located principally in the ventrolateral medulla and respond to changes in H+ concentration. Thus they are sensitive to changes in PCO2 (Mitchell, 1969). Hypoxia, therefore, would be expected to exert its effects mainly through the peripheral chemoreceptors. There is evidence that, due to interactions with the ventilatory response, hypoxia may have more complex effects and this is seen particularly on heart rate responses. If carotid chemoreceptors are selectively stimulated (ventilation controlled) heart rate decreases (Daly & Jones, 1998). However, as seen here, generalized hypoxia leads to tachycardia. This may be a secondary reflex effect from the increased respiration because, when ventilation is controlled during carotid chemoreceptor stimulation, heart rate decreases (Greenwood et al. 1976; Daly & Jones, 1998). Hypercapnia, on the other hand, particularly hyperoxic hypercapnia as used here with oxygen saturation being close to 100%, would have a minimal influence on the oxygen sensing chemoreceptors and the principal effect would be expected to be on the central chemoreceptors although again the responses in uncontrolled conditions are likely to be modified by the resulting respiratory response.
Our study differs from other previous related human studies in that we not only examined the effects of hypoxia and hypercapnia on the baroreflex control of heart rate, but also the control of mean arterial pressure and vascular resistance in healthy human subjects. It is this effect on blood pressure and vascular resistance which is of particular importance in the control of blood pressure and any changes in this are likely to lead to hypertension.
Effects of hypoxia
Stimulation of peripheral chemoreceptors by hypoxia has been shown to evoke increases in sympathetic outflow to the heart and also to renal, splanchnic and skeletal muscle vascular beds (Marshall, 1994). However, the effects on the heart may be more complex. Stimulation of vascularly isolated carotid chemoreceptors, during controlled ventilation has been shown to have negative chronotropic and inotropic effects which are sympathetically mediated, since responses were still present after vagotomy (James & Daly 1969; Greenwood et al. 1976). On the other hand, stimulation of isolated aortic chemoreceptors has the opposite effect of increasing heart rate and contractility (Hainsworth et al. 1978). In the present study generalized hypoxia, which stimulates both carotid and aortic baroreceptors, resulted in tachycardia. Estimation of ventilation rate in our experiments, however, showed no significant increase during the hypoxic stimulus so, unless only the depth of ventilation increased, this cannot explain the increase in heart rate. Previous work has also shown the effects of hypoxia on blood pressure in humans to be variable. Xie et al. (2001) and Halliwill & Minson (2002) reported that hypoxia causes significant increases in blood pressure. Another study (Ziegler et al. 1995), however, showed a vasodilatory response with a decrease in blood pressure. In the present study seven out of 11 subjects showed increases in blood pressure, two showed decreases and two showed no change. This resulted in an overall increase which was not quite significant (P = 0.05). We also found there was a considerable variation in response of baseline vascular resistance to hypoxia. Overall there was a trend for an increase in vascular resistance but this was not statistically significant. This variation may at least partly be explained by the contrasting systemic and local effects of hypoxia on skeletal muscle. The local effect of hypoxia on skeletal muscle is to cause vasodilatation, yet there is a systemic increase in sympathetic nerve activity which would have a vasoconstrictor effect (Tamisier et al. 2004).
These contrasting local and systemic effects of hypoxia may provide a possible mechanism for the observed reduction in baroreflex sensitivity during hypoxia. Some studies have suggested that hypoxia attenuates the vasoconstrictor response to sympathetic nerve stimulation (Hansen et al. 2000) and this could explain the reduced responses to baroreflex stimulation. Against this, however, are studies that have suggested that sympathetic vasoconstriction is well preserved during hypoxia (Weisbrod et al. 2001; Dinenno, 2003). It was suggested by Weisbrod et al. (2001) that during hypoxia, endogenous vasodilator mechanisms counter the vasoconstrictor effect of increased sympathetic activity. Further studies have suggested that the hypoxia induced vasodilatation leads to a compensatory rise in sympathetic tone through engagement of the arterial baroreflex, which persists after removal of the hypoxia (Tamisier et al. 2004). Indeed we did find an increase in vascular resistance in the post-stimulus control period, although we did not see any significant vasodilatation during hypoxia.
Our finding of no resetting of the baroreflex but a reduction in reflex sensitivity is in contrast to previous studies (Halliwill & Minson, 2002; Halliwill et al. 2003). A possible explanation for this is that our study examined vascular resistance responses, which would be affected by both the vasodilator and vasoconstrictor effects described above. Previous studies, however, have examined muscle sympathetic nerve activity (MSNA), which would only measure the vasoconstrictor effects and these would be expected to increase. Thus we measured a more overall response rather than just one part of it. Another explanation for differing results may also be due to technical factors. In our study we used the neck chamber technique to stimulate and unload carotid baroreceptors. We believe this technique to be advantageous since it is non-invasive and application of neck suction or neck pressure has no direct effect on the vasculature unlike the use of vasoactive drugs (Goldman & Saum, 1984; Bell et al. 1986; Munch et al. 1987) as used by previous studies (Halliwill & Minson, 2002). The neck chamber technique also has the advantage that the reflex can be investigated over a wider range of pressures allowing us to establish a sigmoid relationship for the reflex (Fig. 1), unlike the limited linear relationship described in previous studies. This enables ‘set point’ to be more clearly defined. The disadvantage of our technique is that it only stimulates carotid baroreceptors and the resultant responses will be subsequently buffered by other baroreceptors such as aortic and coronary receptors. In an attempt to minimize this buffering effect we recorded the responses at the point at which they were maximal. This was seen approximately 10–15 s after the application of the stimulus (Gulli et al. 2005).
Effects of hypercapnia
There have been previous studies in anaesthetized dogs in which hypercapnia was confined to the cephalic circulation and these showed evidence of increased sympathetic activity and increased responses to baroreceptor stimulation (Hainsworth et al. 1984; Ford et al. 1985; Soladoye et al. 1985). The present study, in conscious humans, also showed evidence of an increase in sympathetic activity, as seen from the increases in blood pressure and vascular resistance, but this study showed that baroreceptor sensitivity did not change. This apparent discrepancy may be explained by the fact that in the present study we actually determined the peak reflex sensitivity rather than the overall response. In the present study also, even though the sensitivity was unaffected, we found greater responses to changes in carotid sinus pressure when PCO2 was high.
Work on Sprague-Dawley rats has shown that hyperoxic hypercapnia causes an increase in mean arterial pressure, a decrease in heart rate, an increase in splanchnic sympathetic nerve activity (sSNA) but little effect on the baroreflex sensitivity of sSNA (Makeham et al. 2004). These results also agree with the present study in that we found an increase in mean pressure, a decrease in heart rate, an increase in vascular resistance but no change in the sensitivity of baroreflex control of the vascular resistance in response to hyperoxic hypercapnia.
In the present study, as well as an increase in mean arterial pressure we found that hyperoxic hypercapnia resulted in an increased ‘set point’ in the baroreflex control of vascular resistance. Since hyperoxic conditions have been shown to reduce the responsiveness of peripheral chemoreceptors to hypercapnia (Lahiri & DeLaney, 1975), it is not unreasonable to assume that this effect is predominantly due to interaction with the central chemoreceptors.
The reduced heart rate and increased mean arterial pressure which occurred during hypercapnia did not return to baseline levels in the post-stimulus control period. In fact heart rate was further reduced and significantly lower than during the initial control period. Vascular resistance was also significantly greater in the post-stimulus control period compared to the pre-stimulus control period. Thus 10 min of breathing room air was not sufficient to reverse the effects of hypercapnia. The after-effects on baroreflex control of vascular resistance were also interesting. Baroreflex sensitivity showed an insignificant tendency to increase during hypercapnia, yet it tended to be reduced below pre-stimulus control levels in the post-stimulus control period. Hypercapnia increased ‘set point’ and although it was reduced in the post-stimulus control period it did not return to baseline levels.
These results suggest that there is a selective sympathetic activation to the vasculature which persists after the stimulus is removed. This is consistent with the results of Xie et al. (2000) who found muscle sympathetic nerve activity remained elevated 20 min after the removal of an asphyxic stimulus. The reduction in heart rate during hypercapnia may be attributable to the direct cardiac depressant activity of hypercapnia (Marshall, 1986) and it appears that this also persists after the removal of the stimulus.
Interactions between chemoreceptor and baroreceptor reflexes
There is little doubt that considerable interaction occurs between peripheral chemoreceptor and arterial baroreceptor reflexes. Somers et al. (1991) reported a selective interaction between peripheral, but not central chemoreceptors and arterial baroreceptors. They found that baroreflex activation inhibited the sympathetic response to hypoxia, whilst the response to hypercapnia was preserved. In our study we examined the interaction in the opposite direction, i.e. we looked at the effect of chemoreceptor stimulation on baroreflex responses. We also found that hypoxia, but not hypercapnia, altered the baroreflex sensitivity. Thus, it appears that the interaction occurs in both directions – baroreflex activation inhibits peripheral chemoreflex responses and peripheral chemoreflex activation has an inhibitory effect on arterial baroreflex responses. One possible explanation for this selective interaction may be that it is due to the close proximity of nerve endings within the central nervous system. Both peripheral chemoreceptor and arterial baroreceptor afferent fibres terminate in the nucleus tractus solitarii of the medulla (Miura & Reis, 1972) and it is possible that interneuronal connections may facilitate interactions between these reflexes (Miura & Reis, 1972; Somers et al. 1991).
Relevance to physiology and pathophysiology
There are a number of physiological and pathophysiological conditions where baroreflex responses to hypoxia and hypercapnia may be of relevance. Examples are the hypoxia experienced during high altitude or hypoxia with or without hypercapnia in disease states such as chronic obstructive pulmonary disease (COPD). In severe COPD PaO2 may be less than 60 mmHg and PaCO2 greater than 45 mmHg (Bartels et al. 2000). A reduction in baroreflex sensitivity and increased sympathetic modulation has been demonstrated in patients with COPD (Patakas et al. 1982; Bartels et al. 2000). This is improved by supplemental oxygen therapy (Bartels et al. 2000, 2004) and exercise training (Costes et al. 2004). The reduced baroreflex sensitivity is thought to be due to hypoxia causing increased pulmonary artery pressure leading to chronic stimulation of pulmonary stretch receptors (Costes et al. 2004), resulting in vasoconstriction (Hainsworth, 1991). A reduction in baroreflex sensitivity is associated with increased risk of cardiovascular morbidity and mortality and cardiac arrhythmias and stroke are common in advanced COPD (Costes et al. 2004).
Acute exposure to high altitude has also been associated with a reduction in baroreflex sensitivity (Sagawa et al. 1997; Roche et al. 2002; Sevre et al. 2002) without resetting of the reflex (Sagawa et al. 1997).
In the present study hypoxia resulted in a mean oxygen saturation of approximately 89%. Similar levels of desaturation are often seen during OSA (Chaudhary et al. 1998), although they may fall below 70% in patients with severe OSA (Aldrich, 1999). The changes in PETCO2 and PETO2 in the present study were also similar to those which occur during a 60 s breath-hold (Shephard, 1994). However, there was no period of apnoea during the present study and this may have affected the responses. This is discussed in the relevance to OSA section below. Hypoxia has been shown to cause a more profound reduction in baroreflex sensitivity in patients with OSA compared to control subjects (Ziegler et al. 1995; Klawe et al. 1999).
Comparison with our previous study
In our previous study we found that asphyxia increased ‘set point’ but did not alter sensitivity. In the present study we found that hypoxia decreased sensitivity whereas hypercapnia increased ‘set point’. The question may be asked, therefore, why asphyxia did not also result in a decrease in sensitivity. The results of the present study actually showed that hypercapnia resulted in an increase in sensitivity although this was not significant, and it is possible that during asphyxia this increase in sensitivity negates the hypoxia-induced small reduction in sensitivity so that overall there is no change.
Relevance of these results to OSA
A limitation of this study when extrapolating the results to obstructive sleep apnoea is that all the responses with the exception of the cardiac responses were measured during spontaneous breathing and not during apnoea. Furthermore ventilation rate was not controlled. However, we do not believe that this would discredit our results. Apnoea eliminates the inhibitory influence of pulmonary stretch receptors and potentiates the sympathetic response to both hypoxia and hypercapnia (Somers et al. 1995; Kara et al. 2003). Thus if our subjects had been apnoeic the responses are likely to have been greater than those seen here. However, for the assessment of both mean pressure and vascular resistance subjects would have had to be apnoeic for 30 s (10 s control and 20 s stimulus). This would have been extremely difficult to do during hypoxic and particularly hypercapnic conditions and would also have elicited further uncontrolled changes in PO2 and PCO2. Voluntary control of breathing whilst eliminating the effects of breathing frequency and tidal volume on the responses, can in itself through cortical influences affect the responses by reducing parasympathetic influence on cardiovascular regulation (Patwardhan et al. 1995). Cooke et al. (1998) reported that fixed rate breathing may decrease the response to chemoreceptor stimulation with CO2. Also previous authors have reported that ‘during hypercapnia even highly motivated subjects cannot override the stimulus to breathe and maintain constant ventilation’ (Trzebski et al. 1995). Thus apnoea, controlled breathing or spontaneous breathing would all have had varying influences on the results, so we decided to use spontaneous breathing, which would be least distressing to our subjects. Another point to consider is that in OSA apnoea is not truly apnoea in terms of cessation of respiratory activity. Respiratory effort continues against a closed airway, and thus inspiratory reflexes in the lungs continue to be stimulated and this stimulation may even be increased by the increased intrathoracic pressure generated by the obstructive apnoeic event.
The other major limitation when applying the results of the current study to obstructive sleep apnoea and hypertension is that the subjects in the current study were normotensive, non-apnoeic, healthy volunteers. The sympathetic response to apnoea in mild untreated hypertensives has been found to be much greater than matched normotensives (Somers et al. 1988) and an enhanced peripheral chemoreceptor sensitivity has be shown in patients with obstructive sleep apnoea (Narkiewicz et al. 1998). Also our subjects were not obese, unlike the majority of patients with OSA. Obesity in itself is not thought to be the cause of the enhanced peripheral chemoreflex response, but has been shown to result in an exaggerated central chemoreflex response in non-apnoeic subjects (Narkiewicz et al. 1999). Thus it is possible that greater responses may be expected in hypertensive, apnoeic, obese subjects.
Conclusion
The results of the present study suggest that the hypercapnic component of asphyxia may be of greater importance than hypoxia in causing sympathetically induced increases in mean arterial pressure and vascular resistance. Hypoxia, however, also causes a smaller increase in mean pressure and may be the cause of the decreased sensitivity of baroreflex control of vascular resistance previously reported during asphyxia (Cooper et al. 2004). Thus it appears that interaction of both peripheral and central chemoreceptors on baroreflex function during asphyxic conditions may contribute to the observed decreased baroreflex sensitivity and increased ‘set point’. This alteration in baroreflex function if sustained during normoxic, isocapnic wakefulness could promote hypertension in patients with obstructive sleep apnoea.
Acknowledgments
This work was supported by a grant from the British Heart Foundation (PG2001090).
References
- Aldrich MS. Sleep Medicine. New York: Oxford University Press; 1999. Obstructive sleep apnoea syndrome; pp. 202–230. [Google Scholar]
- Bartels MN, Gonzalez JM, Kim W, De Meersman RE. Oxygen supplementation and cardiac-autonomic modulation in COPD. Chest. 2000;118:691–696. doi: 10.1378/chest.118.3.691. [DOI] [PubMed] [Google Scholar]
- Bartels MN, Jelic S, Basner RC, Ngai P, Gonzalez JM, De Meersman RE. Supplemental oxygen increases arterial stiffness in chronic obstructive pulmonary disease. Respir Med. 2004;98:84–89. doi: 10.1016/j.rmed.2002.09.001. [DOI] [PubMed] [Google Scholar]
- Bell LB, Seagard JL, Auperku EJ, Kampine JP. Mechanical effects of vasoactive drugs on carotid sinus. Am J Physiol. 1986;250:R1074–R1080. doi: 10.1152/ajpregu.1986.250.6.R1074. [DOI] [PubMed] [Google Scholar]
- Bixler EO, Vgontzas AN, Lin HM, Ten Have T, Leiby BE, Vela-Bueno A, Kales A. Association of hypertension and sleep-disordered breathing. Arch Intern Med. 2000;160:2289–2295. doi: 10.1001/archinte.160.15.2289. [DOI] [PubMed] [Google Scholar]
- Chaudhary B, Dasti S, Park Y, Brown T, Davis H, Akhtar B. Hour-to-hour variability of oxygen saturation in sleep apnea. Chest. 1998;113:719–722. doi: 10.1378/chest.113.3.719. [DOI] [PubMed] [Google Scholar]
- Cooke WH, Cox JF, Diedrich AM, Taylor JA, Beightol LA, Ames JE, Hoag JB, Seidel H, Eckberg DL. Controlled breathing protocols probe human autonomic cardiovascular rhythms. Am J Physiol. 1998;274:H709–H718. doi: 10.1152/ajpheart.1998.274.2.h709. [DOI] [PubMed] [Google Scholar]
- Cooper VL, Bowker CM, Pearson SB, Elliott MW, Hainsworth R. Effects of simulated obstructive sleep apnoea on the human carotid baroreceptor-vascular resistance reflex. J Physiol. 2004;557:1055–1065. doi: 10.1113/jphysiol.2004.062513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costes F, Roche F, Pichot V, Vergnon JM, Garet M, Barthelemy JC. Influence of exercise training on cardiac baroreflex sensitivity in patients with COPD. Eur Respir J. 2004;23:396–401. doi: 10.1183/09031936.04.00040304. [DOI] [PubMed] [Google Scholar]
- Daly M, De B, Jones JF. Respiratory modulation of carotid and aortic body reflex left ventricular inotropic responses in the cat. J Physiol. 1998;509:895–907. doi: 10.1111/j.1469-7793.1998.895bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinenno FA. Hypoxic regulation of blood flow in humans. Alpha-adrenergic receptors and functional sympatholysis in skeletal muscle. Adv Exp Med Biol. 2003;543:237–248. [PubMed] [Google Scholar]
- Eckberg DL, Cavanaugh MS, Mark AL, Abboud FM. A simplified neck suction device for activation of carotid baroreceptors. J Lab Clin Med. 1975;85:167–173. [PubMed] [Google Scholar]
- Ford R, Hainsworth R, Rankin AJ, Soladoye AO. Abdominal vascular responses to changes in carbon dioxide tension in the cephalic circulation of anaesthetized dogs. J Physiol. 1985;358:417–431. doi: 10.1113/jphysiol.1985.sp015559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman WF, Saum WR. A direct excitatory action of catecholamines on rat aortic baroreceptors in vitro. Circ Res. 1984;55:18–30. doi: 10.1161/01.res.55.1.18. [DOI] [PubMed] [Google Scholar]
- Greenwood PV, Hainsworth R, Karim F, Morrison GW, Sofola OA. The effect of stimulation of carotid chemoreceptors on the inotropic state of the left ventricle. J Physiol. 1976;266:47P–48P. [PubMed] [Google Scholar]
- Grote L, Hedner J, Peter JH. Sleep-related breathing disorder is an independent risk factor for uncontrolled hypertension. J Hypertens. 2000;18:679–685. doi: 10.1097/00004872-200018060-00004. [DOI] [PubMed] [Google Scholar]
- Gulli G, Cooper VL, Claydon VE, Hainsworth R. Prolonged latency in the baroreflex mediated vascular resistance response in subjects with postural related syncope. Clin Auton Res. 2005;15:207–212. doi: 10.1007/s10286-005-0273-8. [DOI] [PubMed] [Google Scholar]
- Hainsworth R. Reflexes from the heart. Physiol Rev. 1991;71:617–658. doi: 10.1152/physrev.1991.71.3.617. [DOI] [PubMed] [Google Scholar]
- Hainsworth R, Karim F, Sofola OA, Wood LM. Aortic chemoreceptors and the heart in dogs. J Physiol. 1978;284:167P–168P. [PubMed] [Google Scholar]
- Hainsworth R, McGregor KH, Rankin AJ, Soladoye AO. Cardiac inotropic responses from changes in carbon dioxide tension in the cephalic circulation of anaesthetized dogs. J Physiol. 1984;357:23–35. doi: 10.1113/jphysiol.1984.sp015486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwill JR, Minson CT. Effect of hypoxia on arterial baroreflex control of heart rate and muscle sympathetic nerve activity in humans. J Appl Physiol. 2002;93:857–864. doi: 10.1152/japplphysiol.01103.2001. [DOI] [PubMed] [Google Scholar]
- Halliwill JR, Morgan BJ, Charkoudian N. Peripheral chemoreflex and baroreflex interactions in cardiovascular regulation in humans. J Physiol. 2003;552:295–302. doi: 10.1113/jphysiol.2003.050708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen J, Snader M, Hald CF, Vitor RG, Thomas GD. Metabolic modulation of sympathetic vasoconstriction in human skeletal muscle: role of tissue hypoxia. J Physiol. 2000;527:387–396. doi: 10.1111/j.1469-7793.2000.00387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James JE, Daly M de B. Cardiovascular responses in apnoeic asphyxia: role of arterial chemoreceptors and the modification of their effects by a pulmonary vagal inflation reflex. J Physiol. 1969;201:87–104. doi: 10.1113/jphysiol.1969.sp008744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kara T, Narkiewicz K, Somers VK. Chemoreflexes – physiology and clinical implications. Acta Physiol Scand. 2003;177:377–384. doi: 10.1046/j.1365-201X.2003.01083.x. [DOI] [PubMed] [Google Scholar]
- Kelly AP, Croft JS, Hainsworth R. A paired neck chamber for applying localized stimuli to the carotid baroreceptors in humans. J Physiol. 1996;497:5P. [Google Scholar]
- Kelly AP, El-Bedawi KM, Hainsworth R. An improved neck chamber for the study of carotid baroreceptors in humans. J Physiol. 1993;467:142P. [Google Scholar]
- Klawe JJ, Tafil-Klawe M, Jorg-Hermann P, Smietanowski M. Cardiac response study towards activation and inactivation of the carotid baroreceptor in obstructive sleep apnea patients. Med Sci Monit. 1999;5:449–451. [Google Scholar]
- Lahiri S, DeLaney RG. Relationship between carotid chemoreceptor activity and ventilation in the cat. Respir Physiol. 1975;24:267–286. doi: 10.1016/0034-5687(75)90018-3. [DOI] [PubMed] [Google Scholar]
- Lavie P, Herer P, Hoffstein V. Obstructive sleep apnoea syndrome as a risk factor for hypertension: population study. BMJ. 2000;320:479–482. doi: 10.1136/bmj.320.7233.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makeham JM, Goodchild AK, Costin NS, Pilowsky PM. Hypercapnia selectively attenuates the somato-sympathetic reflex. Respir Physiol Neurobiol. 2004;140:133–143. doi: 10.1016/j.resp.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Marshall JM. Modulation of the centrally-evoked visceral alerting/defence response by changes in CSF pH at the ventral surface of the medulla oblongata by systemic hypercapnia. Pflugers Arch. 1986;407:46–54. doi: 10.1007/BF00580719. [DOI] [PubMed] [Google Scholar]
- Marshall JM. Peripheral chemoreceptors and cardiovascular regulation. Physiol Rev. 1994;74:543–594. doi: 10.1152/physrev.1994.74.3.543. [DOI] [PubMed] [Google Scholar]
- Mitchell RA. Respiratory chemosensitivity in the medulla oblongata. J Physiol. 1969;202:3P–4P. [PubMed] [Google Scholar]
- Miura M, Reis DJ. The role of the solitary and paramedian reticular nuclei in mediating cardiovascular reflex responses from carotid baro- and chemoreceptors. J Physiol. 1972;223:525–548. doi: 10.1113/jphysiol.1972.sp009861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munch PA, Thoren PN, Brown AM. Dual effects of norepinephrine and mechanisms of baroreceptor stimulation. Circ Res. 1987;61:409–419. doi: 10.1161/01.res.61.3.409. [DOI] [PubMed] [Google Scholar]
- Narkiewicz K, Kato M, Pesek CA, Somers VK. Human obesity is characterized by a selective potentiation of central chemoreflex sensitivity. Hypertension. 1999;33:1153–1158. doi: 10.1161/01.hyp.33.5.1153. [DOI] [PubMed] [Google Scholar]
- Narkiewicz K, van de Bome PJH, Cooley RL, Dyken ME, Somers VK. Sympathetic activity in obsese subjects with and without obstructive sleep apnea. Circulation. 1998;98:772–776. doi: 10.1161/01.cir.98.8.772. [DOI] [PubMed] [Google Scholar]
- Nieto FJ, Young TB, Lind BK, Shahar E, Samet JM, Redline S, D'Agostino RB, Newman AB, Lebowitz MD, Pickering TG. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA. 2000;283:1829–1836. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- Parati G, Di Rienzo M, Bonsignore MR, Insalaco G, Marrone O, Castiglioni P, Bonsignore G, Mancia G. Autonomic cardiac regulation in obstructive sleep apnea syndrome: evidence from spontaneous baroreflex analysis during sleep. J Hypertens. 1997;15:1621–1626. doi: 10.1097/00004872-199715120-00063. [DOI] [PubMed] [Google Scholar]
- Partinen M, Telakivi T. Epidemiology of obstructive sleep apnea syndrome. Sleep. 1992;15(6 Suppl.):S1–S4. doi: 10.1093/sleep/15.suppl_6.s1. [DOI] [PubMed] [Google Scholar]
- Patakas D, Louridas G, Kakavelas E. Reduced baroreceptor sensitivity in patients with chronic obstructive pulmonary disease. Thorax. 1982;37:292–295. doi: 10.1136/thx.37.4.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patwardhan AR, Vallurupalli S, Evans JM, Bruce EN, Knapp CF. Override of spontaneous respiratory pattern generator reduces cardiovascular parasympathetic influence. J Appl Physiol. 1995;79:1048–1054. doi: 10.1152/jappl.1995.79.3.1048. [DOI] [PubMed] [Google Scholar]
- Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–1384. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- Roche F, Reynaud C, Garet M, Pichot V, Costes F, Barthelemy JC. Cardiac baroreflex control in humans during and immediately after brief exposure to simulated high altitude. Clin Physiol Funct Imaging. 2002;22:301–306. doi: 10.1046/j.1475-097x.2002.00434.x. [DOI] [PubMed] [Google Scholar]
- Sagawa S, Torii R, Nagaya K, Wada F, Endo Y, Shiraki K. Carotid baroreflex control of heart rate during acute exposure to simulated altitudes of 3,800 m and 4,300 m. Am J Physiol. 1997;273:R1219–R1223. doi: 10.1152/ajpregu.1997.273.4.R1219. [DOI] [PubMed] [Google Scholar]
- Sevre K, Bendz B, Rostrup M. Reduced baroreceptor reflex sensitivity and increased blood pressure variability at 2400 m simulated cabin altitude. Aviat Space Environ Med. 2002;73:632–634. [PubMed] [Google Scholar]
- Shephard JW. Cardiorespiratory changes in obstructive sleep apnea. In: Kryger MH, Roth T, Dement WC, editors. Principals and Practice of Sleep Medicine. Philadelphia, PA, USA: W.B. Saunders Company; 1994. pp. 657–666. [Google Scholar]
- Soladoye AO, Rankin AJ, Hainsworth R. Influence of carbon dioxide tension in the cephalic circulation on hindlimb vascular resistance in anaesthetized dogs. Q J Exp Physiol. 1985;70:527–538. doi: 10.1113/expphysiol.1985.sp002939. [DOI] [PubMed] [Google Scholar]
- Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96:1897–1904. doi: 10.1172/JCI118235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers VK, Mark AL, Abboud FM. Potentiation of sympathetic nerve responses to hypoxia in borderline hypertensive subjects. Hypertension. 1988;11:608–612. doi: 10.1161/01.hyp.11.6.608. [DOI] [PubMed] [Google Scholar]
- Somers VK, Mark AL, Abboud FM. Interaction of baroreceptor and chemoreceptor reflex control of sympathetic nerve activity in normal humans. J Clin Invest. 1991;87:1953–1957. doi: 10.1172/JCI115221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamisier R, Norman D, Anand A, Choi Y, Weiss JW. Evidence of sustained forearm vasodilatation after brief isocapnic hypoxia. J Appl Physiol. 2004;96:1782–1787. doi: 10.1152/japplphysiol.01241.2003. [DOI] [PubMed] [Google Scholar]
- Trzebski A, Smith ML, Beightol LA, Fritsch-Yelle JM, Rea RF, Eckberg DL. Modulation of human sympathetic periodicity by mild, brief hypoxia and hypercapnia. J Physiol Pharmacol. 1995;46:17–35. [PubMed] [Google Scholar]
- Weisbrod CJ, Minson CT, Joyner MJ, Halliwill JR. Effect of regional phentolamine on hypoxic vasodilatation in healthy humans. J Physiol. 2001;537:613–621. doi: 10.1111/j.1469-7793.2001.00613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West JB. Respiratory Physiology – The Essentials. 5th edn. Baltimore, MD, USA: Williams & Wilkins; 1995. Control of ventilation; pp. 117–132. [Google Scholar]
- Xie A, Skatrud JB, Crabtree DC, Puelo DS, Goodman BM, Morgan BJ. Neurocirculatory consequences of intermittent asphyxia in humans. J Appl Physiol. 2000;89:1333–1339. doi: 10.1152/jappl.2000.89.4.1333. [DOI] [PubMed] [Google Scholar]
- Xie A, Skatrud JB, Puleo DS, Morgan BJ. Exposure to hypoxia produces long-lasting sympathetic activation in humans. J Appl Physiol. 2001;91:1555–1562. doi: 10.1152/jappl.2001.91.4.1555. [DOI] [PubMed] [Google Scholar]
- Ziegler MW, Nelesen RA, Mills PJ, Ancoli-Israel S, Clausen JL, Watkins L, Dimsdale JE. The effect of hypoxia on baroreflexes and pressor sensitivity in sleep apnea and hypertension. Sleep. 1995;18:859–865. [PubMed] [Google Scholar]