Abstract
Weak transcranial direct current stimulation (tDCS) of the human motor cortex results in excitability shifts which occur during and after stimulation. These excitability shifts are polarity-specific with anodal tDCS enhancing excitability, and cathodal reducing it. To explore the origin of this excitability modulation in more detail, we measured the input–output curve and motor thresholds as global parameters of cortico-spinal excitability, and determined intracortical inhibition and facilitation, as well as facilitatory indirect wave (I-wave) interactions. Measurements were performed during short-term tDCS, which elicits no after-effects, and during other tDCS protocols which do elicit short- and long-lasting after-effects. Resting and active motor thresholds remained stable during and after tDCS. The slope of the input–output curve was increased by anodal tDCS and decreased by cathodal tDCS. Anodal tDCS of the primary motor cortex reduced intracortical inhibition and enhanced facilitation after tDCS but not during tDCS. Cathodal tDCS reduced facilitation during, and additionally increased inhibition after its administration. During tDCS, I-wave facilitation was not influenced but, for the after-effects, anodal tDCS increased I-wave facilitation, while cathodal tDCS had only minor effects. These results suggest that the effect of tDCS on cortico-spinal excitability during a short period of stimulation (which does not induce after-effects) primarily depends on subthreshold resting membrane potential changes, which are able to modulate the input-output curve, but not motor thresholds. In contrast, the after-effects of tDCS are due to shifts in intracortical inhibition and facilitation, and at least partly also to facilitatory I-wave interaction, which is controlled by synaptic activity.
The transcranial application of weak direct currents (transcranial direct current stimulation, tDCS) to the human primary motor cortex is capable of eliciting cortical excitability changes. The nature of these modulations depends on tDCS polarity. Anodal tDCS increases excitability, whereas cathodal tDCS diminishes it (Nitsche & Paulus, 2000). The respective changes evolve during tDCS, and remain for up to 1 h after it ceases, provided it lasts sufficiently long. These effects are probably located intracortically; the excitability of the cortico-spinal tract remains unchanged (Nitsche & Paulus, 2000, 2001; Nitsche et al. 2003a). As suggested by recent pharmacological studies, the effects during a short-lasting tDCS, which elicits no after-effects, depend on the activity of sodium and calcium channels but not on efficacy changes of N-methyl-d-aspartate (NMDA) and gamma-amino-butyric acid (GABA) receptors, and thus are probably generated solely by polarity-specific shifts of the resting membrane potential. Conversely, the formation of after-effects critically depends on membrane potential changes, but has been demonstrated to involve also modulations of NMDA receptor efficacy (Liebetanz et al. 2002; Nitsche et al. 2003b, 2004a, b). However, apart from these pharmacological studies, not much is yet known about the intracortical neuronal systems modulated by tDCS.
Here our aim has been to learn more about the origin of the excitability changes during tDCS as well as the after-effects. Therefore, we applied several transcranial magnetic stimulation (TMS) protocols known to test specific cortical neuronal systems in the human motor cortex, by revealing changes during tDCS as well as short- and long-lasting after-effects of tDCS on motor-cortex excitability.
Active and resting motor thresholds (MT) and input–output curves (I–O curves) resemble global measures of cortico-spinal excitability (Abbruzzese & Trompetto, 2002; Chen, 2000). MTs are defined as the minimum TMS intensity resulting in motor evoked potentials (MEP), the resting MT during muscle relaxation and active MT during moderate voluntary contraction. They probably reflect neuronal membrane excitability, as they are increased under voltage-gated sodium channel blocker medication (Ziemann et al. 1996), but are not shifted by drugs modulating GABAergic or glutamatergic transmission (Ziemann et al. 1996, 1998a). Because within the MT protocol, TMS-intensity is at threshold level by definition, here the excitability of a central core region in the cortical muscle representation field is monitored. Compared to MTs, the input–output curve (I–O curve) serves as an index of excitability of larger neuronal populations. Here, MEP amplitudes elicited by increasing TMS intensities are recorded. The resulting slope due to increased TMS intensity reflects the recruitment of larger neuronal populations. Similar to MTs, the I–O curve depends on neuronal membrane excitability, because its slope is decreased by sodium and calcium channel blockers. However, synaptic mechanisms are involved additionally, as it is modulated by drugs influencing the GABAergic and the adrenergic system (Boroojerdi et al. 1999) and may be modulated by the glutamatergic system additionally (Di Lazzaro et al. 2003).
Motor-cortex inhibition and facilitation were studied using a double-stimulation protocol (Kujirai et al. 1993). Here, a subthreshold TMS stimulus is followed by a suprathreshold test stimulus. The resulting inhibition or facilitation of the MEP amplitude elicited by the test stimulus is determined by the interstimulus interval (ISI), is of intracortical origin and reflects excitability of inhibitory and excitatory interneurones. As inhibition is enhanced and facilitation diminished by GABAergic and antiglutamatergic substances, but not influenced by ion channel-blockers (Chen et al. 1997, Liepert et al. 1997; Ziemann et al. 1996, 1998a), they may reflect primarily the activity of the glutamatergic and GABAergic systems in the motor cortex. Motor cortex indirect-waves (I-waves) are cortico-spinal waves generated by motor cortex stimulation, which evolve after the first or direct cortico-spinal volley (direct or d-wave) and are also probably under control of intracortical neuronal circuits (Ziemann & Rothwell, 2000). These were explored using another TMS double-stimulation protocol (Ziemann et al. 1998b). Here a suprathreshold TMS test stimulus is followed by a second subthreshold stimulus. The resulting increase of the MEP amplitude is specific for certain ISIs and probably reflects cortical interactions between the circuits responsible for the generation of indirect cortico-spinal waves. As I-wave facilitation is reduced by GABAergic drugs, but not by ion channel bockers (Ziemann et al. 1998c), it may reflect the activity of the GABAergic system in the motor cortex. Because I-waves are diminished by ketamine, an NMDA receptor-antagonist, if administered in high dosages, the glutamatergic system may also be involved in the regulation of I-wave activity (Ghaly et al. 2001).
Methods
Subjects
Twelve to 20 healthy subjects were included in each experiment (for details see Table 1). Only subjects who did not take any medication or herbal drugs, including non-prescription drugs, participated. All gave informed written consent and were paid for participating. The investigation was approved by the Ethics Committee of the University of Göttingen, and conformed to the Declaration of Helsinki.
Table 1.
Details of the different stimulation protocols and subject characteristics of the experiments
| Subjects | |||||
|---|---|---|---|---|---|
| Experiment* | n | Sex (M/F) | Age (years) | MT%† | Test pulse amplitude (mV) |
| Resting MT | |||||
| Intra-tDCS-effects | 20 | 10 F/10 M | 27 ± 4 | n.a. | n.a. |
| Long-lasting after-effects | 12 | 5 F/7 M | 27 ± 4 | n.a. | n.a. |
| Active MT | |||||
| Intra-tDCS-effects | 18 | 10 F/8 M | 28 ± 5 | n.a. | n.a. |
| Long-lasting after-effects | 12 | 5 F/7 M | 27 ± 4 | n.a. | n.a. |
| Recruitment | |||||
| Intra-tDCS-effects | 12 | 5 F/7 M | 26 ± 5 | ||
| Resting MT, anodal tDCS | 55 ± 6 | n.a. | |||
| Resting MT, cathodal tDCS | 56 ± 7 | n.a. | |||
| Resting MT, no tDCS | 56 ± 7 | n.a. | |||
| Short-lasting after-effects | 13 | 5 F/8 M | 25 ± 1 | ||
| Resting MT, anodal tDCS | 48 ± 11 | n.a. | |||
| Resting MT, cathodal tDCS | 48 ± 11 | n.a. | |||
| Resting MT, before anodal tDCS | 48 ± 11 | n.a. | |||
| Resting MT, before cathodal tDCS | 48 ± 11 | n.a. | |||
| Long-lasting after-effects | 12 | 5 F; 7 M | 27 ± 4 | ||
| Resting MT, anodal tDCS | 61 ± 7 | n.a. | |||
| Resting MT, cathodal tDCS | 60 ± 8 | n.a. | |||
| Resting MT, before anodal tDCS | 61 ± 7 | n.a. | |||
| Resting MT, before cathodal tDCS | 60 ± 8 | n.a. | |||
| Double stimulation | |||||
| Intra-tDCS-effects | 20 | 12 F; 8 M | 27 ± 4 | ||
| Active MT, anodal tDCS | 56 ± 5 | 0.831 ± 0.155 | |||
| Active MT, cathodal tDCS | 56 ± 5 | 0.791 ± 0.197 | |||
| Active MT, no tDCS | 56 ± 5 | 0.823 ± 0.287 | |||
| Short-lasting after-effects | 14 | 7 F; 7 M | 25 ± 1 | ||
| Active MT, anodal tDCS | 45 ± 7 | 1.007 ± 0.020 | |||
| Active MT, cathodal tDCS | 45 ± 7 | 1.006 ± 0.017 | |||
| Active MT, before anodal tDCS | 45 ± 7 | 1.002 ± 0.018 | |||
| Active MT, before cathodal tDCS | 45 ± 7 | 1.013 ± 0.025 | |||
| Long-lasting after-effects | 12 | 5 F; 7 M | 27 ± 4 | ||
| Active MT, anodal tDCS | 46 ± 5 | 0.999 ± 0.138 | |||
| Active MT, cathodal tDCS | 44 ± 5 | 0.916 ± 0.183 | |||
| Active MT, before anodal tDCS | 46 ± 5 | 0.956 ± 0.144 | |||
| Active MT, before cathodal tDCS | 44 ± 5 | 0.957 ± 0.123 | |||
| I-waves | |||||
| Intra-tDCS-effects | 16 | 9 F, 7 M | 26 ± 5 | ||
| Resting MT, anodal tDCS | 55 ± 8 | 0.904 ± 0.158 | |||
| Resting MT, cathodal tDCS | 57 ± 10 | 0.956 ± 0.249 | |||
| Resting MT, no tDCS | 55 ± 8 | 0.889 ± 0.143 | |||
| Short-lasting after-effects | 13 | 5 F; 8 M | 25 ± 1 | ||
| Resting MT, anodal tDCS | 63 ± 4 | 1.008 ± 0.003 | |||
| Resting MT, cathodal tDCS | 63 ± 4 | 1.002 ± 0.008 | |||
| Resting MT, before anodal tDCS | 63 ± 4 | 1.004 ± 0.006 | |||
| Resting MT, before cathodal tDCS | 63 ± 4 | 1.006 ± 0.004 | |||
| Long-lasting after-effects | 12 | 5 F; 7 M | 27 ± 4 | ||
| Resting MT, anodal tDCS | 61 ± 7 | 0.958 ± 0.060 | |||
| Resting MT, cathodal tDCS | 59 ± 8 | 0.973 ± 0.102 | |||
| Resting MT, before anodal tDCS | 61 ± 7 | 0.952 ± 0.059 | |||
| Resting MT, before cathodal tDCS | 59 ± 8 | 0.957 ± 0.073 | |||
Data are presented ±s.d.
Experiment refers to the protocols applied for each tDCS condition (intra-tDCS, short- and long-lasting after-effects). There were 12–20 subjects in each experiment; age and gender distribution were comparable between experiments.
Percentage of maximum stimulator output. MT and single test-pulse amplitude means did not differ significantly between the respective conditions (Student's t test, P > 0.05). F, female; M, male; n.a., not applicable.
tDCS of the motor cortex
Direct currents were applied through a pair of saline-soaked surface sponge electrodes (35 cm2) and delivered by a specially developed battery-driven constant current stimulator (Schneider Electronic, Gleichen, Germany) with a maximum output of 2 mA. The motor-cortex electrode was fixed over the area representing the right abductor digiti minimi muscle (ADM) as identified by TMS, and the other electrode contralaterally above the right orbit. In the different experiments, the tDCS current (1.0 mA) was applied continuously for 4 s (for intra-tDCS measurements), 7 min (for short-lasting after-effects), and (for long-lasting after-effects) 9 min cathodal tDCS or 13 min anodal tDCS, because these tDCS conditions had resulted in the desired excitability changes in previous experiments (Nitsche & Paulus, 2000, 2001; Nitsche et al. 2003a). Constant current flow was controlled by a voltmeter. Nearly all subjects were aware of the current flow as an itching sensation with both polarities and at both electrodes, and/or by perceiving light flashes when the current was turned on and off.
Measurement of motor-system excitability by TMS
Cortical MEPs were recorded from the right ADM following determination of its motor-cortical representational field by single pulse TMS (duration 300 μs). These pulses were induced using a Magstim 200 magnetic stimulator (Magstim Company, Whiteland, Dyfed, UK) and a figure of-eight magnetic coil (diameter of one winding, 70 mm; peak magnetic field, 2.2 Tesla). The coil was held tangentially to the skull, with the handle pointing backwards and laterally at 45° from midline. The optimum position was defined as the site where TMS resulted consistently in the largest MEP. Surface electromyography (EMG) was recorded from the right ADM by use of Ag–AgCl electrodes in a belly tendon montage. The signals were amplified and filtered with a time constant of 10 ms and a low-pass filter of 2.5 kHz. Signals were then digitized at an analog-to-digital rate of 5 kHz, and further relayed into a laboratory computer using the Neuroscan software collection (Neuroscan Inc., Herndon, VA, USA) and conventional averaging software.
Motor threshold determination
The resting MT was defined as the minimum TMS intensity which elicited a peak-to-peak MEP-amplitude of 50 μV or more in resting muscle, in at least 3 out of 6 measurements. The active MT was the minimum intensity eliciting a MEP of a superior size compared to moderate spontaneous muscular background activity (∼ 15% of the maximum muscle strength) in at least 3 out of 6 trials.
Input–output curve
The I–O curve was determined using TMS intensities of 100, 110, 130, and 150% relative to the resting MT (15 stimuli per block, with the order of the blocks randomised).
Intracortical inhibition and facilitation
Intracortical inhibition and facilitation were measured using a TMS double-stimulation protocol including 2, 3, 5, 10 and 15 ms ISIs, the first three ISIs revealing inhibitory, and the last two ISIs facilitatory effects (Kujirai et al. 1993). In this protocol the subthreshold conditioning stimulus precedes the test stimulus. The pairs of stimuli were organized in blocks, in which each ISI was represented once, and there was one additional single test pulse. The blocks were repeated 15 times and the order of the different pulses was pseudorandomised between blocks. The intensity of the conditioning pulse was 70% of the active MT. This relatively weak TMS intensity was chosen to prevent ceiling or floor effects. Too strong inhibitory or facilitatory efficacy of the conditioning TMS stimulus could have diminished the possible influence of tDCS on these parameters. The single test-pulse TMS intensity was adjusted to achieve a baseline MEP of ∼ 1 mV, and re-adjusted during the respective tDCS protocol, if needed, to compensate for effects of global excitability changes on test-pulse amplitude. This was done because it has been shown that different test-pulse amplitudes induce different levels of intracortical inhibition (Stefan et al. 2002; Chen, 2004)
I-wave facilitation
I-wave facilitation was investigated using a double-stimulation protocol, in which the TMS test stimulus precedes the conditioning stimulus (Ziemann et al. 1998b). The ISIs were between 0.5 and 5.1 ms, and tested at 0.2 ms intervals. The pairs of stimuli were organized in five blocks. Each block included five double pulses and a single test pulse, and each block was repeated 15 times. The intensity of the conditioning pulse was 70% of the resting MT to avoid ceiling effects. The single test-pulse TMS intensity was adjusted to achieve a baseline MEP of ∼ 1 mV, and re-adjusted during the respective stimulation protocol if needed, to compensate for effects of global excitability changes on test-pulse amplitude.
Experimental procedures
Each experiment was conducted in a repeated-measures design. Subjects were seated in a reclining chair. First, the left motor-cortex area representing the right ADM was identified using TMS (coil position which lead to the largest MEPs of ADM). Then one tDCS electrode, referred to in the following as providing either cathodal or anodal stimulation (depending on the polarity used), was fixed at this position. The other electrode was placed contralaterally on the forehead above the orbita.
Intra-tDCS excitability changes
To determine intra-tDCS excitability changes, a series (0.1 Hz) of TMS-evoked MEPs immediately before the end of a 4-s-long tDCS (anodal or cathodal tDCS) and another MEP series, without preceding tDCS, were recorded in accordance with the above-mentioned protocols (I–O curve, inhibition/facilitation, and I-waves), resulting in a total of three sessions (anodal tDCS, cathodal tDCS, and no-tDCS sessions) for each protocol. For the double-stimulation protocols, the TMS intensity needed to evoke baseline MEP amplitudes of ∼ 1 mV magnitude in the no-tDCS and tDCS conditions were determined beforehand. The protocols were applied in randomised order inter-individually (except the threshold-determination, which had to be performed first to determine the stimulation intensities for the remaining protocols). To avoid interfering effects from sleepiness, the duration of each session was limited to ∼ 2 h. However, current and non-current conditions of one protocol were measured in one session, with the exception of the I-wave measures, which had to be performed on two subsequent days because of the multitude of stimuli.
Short-lasting after-effects
With regard to the short-lasting after-effects, first, a baseline of TMS-evoked MEPs was recorded at 0.25 Hz according to one of the above-mentioned protocols. Afterwards the DC current was switched on. Anodal and cathodal tDCS were performed for 7 min; these tDCS durations are known to modify cortical excitability for ∼ 5–10 min after the end of stimulation (Nitsche & Paulus, 2001; Nitsche et al. 2003a). After turning off the current, MEPs of the same protocol were recorded for 5 min at 0.25 Hz. Thus the resulting four conditions for each protocol were: before and after anodal tDCS, and before and after cathodal tDCS. For the double-stimulation protocols, the TMS intensity needed to elicit single-pulse MEP amplitudes of 1 mV magnitude was re-determined immediately after the end of tDCS and adjusted, if necessary. Anodal and cathodal tDCS were separated by at least 1 h. This interval between the sessions was chosen because it has been shown that tDCS of 7 min duration induces after-effects lasting for ∼ 5–10 min after tDCS (Nitsche & Paulus, 2001; Nitsche et al. 2003a), and thus after 1 h they should have disappeared. A maximum of two tDCS sessions was carried out per person per day.
Long-lasting after-effects
The evaluation of the long-lasting after-effects was performed in a similar way to experiment 2, except for the longer duration tDCS. Anodal tDCS was performed for 13 min, and cathodal tDCS for 9 min. We chose these different tDCS durations, because 9 min cathodal and 13 min anodal tDCS have been shown to modify cortical excitability for ∼ 1 h after the end of stimulation (Nitsche & Paulus, 2001; Nitsche et al. 2003a). After turning off the current, MEPs were recorded for up to 40 min at 0.25 Hz. The order of the different protocols was inter-individually randomised. Anodal and cathodal stimulation were separated by at least 1 week.
To keep subjects in as consistent a state of alertness as possible during the experiments, they were allowed to listen to low-volume music and were addressed by the experimenter if they showed signs of tiredness. Hereby the subjects were kept awake but relaxed, especially during the pause between the respective experimental blocks. None of the blocks lasted longer than 10 min continuously.
Calculations and statistics
To compare MTs, the inter-individual means of the TMS intensity at active and resting MTs were calculated for the tDCS and no-tDCS conditions (intra-tDCS effects) and the before- and after-tDCS conditions (long-lasting after-effects) for anodal and cathodal tDCS separately. For both stimulation conditions, values without or before tDCS were compared with those during or after tDCS using Student's t tests (paired samples, two-tailed, level of significance 0.05).
For the remaining conditions, first, intra-individual MEP amplitude means were calculated for each TMS stimulation condition (TMS intensity with regard to I–O curve, ISI with regard to intracortical inhibition/facilitation and I-wave facilitation), separately for before/after (short- and long-lasting after-effects) or with/without (intra-tDCS effects) anodal and cathodal tDCS values. For the double-stimulation protocols, the resulting means were standardized to the respective single-pulse condition. Then inter-individual means for each condition were calculated. Repeated-measures analyses of variance (ANOVAs, independent variables ISI/stimulation intensity and DC (with/without or before/after), dependent variable MEP amplitude) were calculated. In Table 2 the results of ‘overall’ ANOVAs, including anodal/cathodal tDCS as well as the no-tDCS (intra-tDCS effects) and both baseline measures (before anodal and cathodal tDCS) are reported. These calculations were performed to compare not only the baseline-post tDCS values of one polarity, but also the baseline values of both polarities to control for differences in subsequent post hoc testing. Table S1 (Supplemental material) presents the results of ANOVAs comparing anodal and cathodal tDCS separately with the respective baseline/without tDCS. MEP amplitude differences between tDCS conditions (with and without tDCS; before or after tDCS) for single ISI, or single stimulus intensities as well as between the respective baseline values were tested by post hoc Fisher's LSD tests (P < 0.05).
Table 2.
Results of the ANOVAs
| Study | d.f. | F-value | P-value |
|---|---|---|---|
| I–O curve | |||
| Intra DC (4-s tDCS) | |||
| DC | 2 | 17.763 | < 0.001* |
| TMS intensity | 3 | 52.101 | < 0.001* |
| DC × TMS intensity | 6 | 5.053 | < 0.001* |
| 7 min tDCS | |||
| DC | 3 | 22.706 | < 0.001* |
| TMS intensity | 3 | 25.733 | < 0.001* |
| DC × TMS intensity | 9 | 9.987 | < 0.001* |
| 9 or 13 min tDCS | |||
| DC | 3 | 4.725 | 0.008* |
| TMS intensity | 3 | 82.583 | < 0.001* |
| DC × TMS intensity | 9 | 2.953 | 0.004* |
| Double stimulation | |||
| Intra-tDCS (4-s tDCS) | |||
| DC | 2 | 6.947 | 0.003* |
| ISI | 4 | 23.408 | < 0.001* |
| DC × ISI | 8 | 1.975 | 0.053 |
| 7 min tDCS | |||
| DC | 3 | 25.334 | < 0.001* |
| ISI | 4 | 40.763 | < 0.001* |
| DC × ISI | 12 | 0.556 | 0.874 |
| 9 or 13 min tDCS | |||
| DC | 3 | 3.841 | < 0.001* |
| ISI | 4 | 11.802 | 0.018* |
| DC × ISI | 12 | 2.149 | 0.018* |
| I-waves | |||
| Intra-tDCS (4-s tDCS) | |||
| DC | 2 | 0.023 | 0.977 |
| ISI | 23 | 4.846 | < 0.001* |
| DC × ISI | 46 | 1.095 | 0.312 |
| 7 min tDCS | |||
| DC | 3 | 15.178 | < 0.001* |
| ISI | 23 | 28.880 | < 0.001* |
| DC × ISI | 69 | 1.420 | 0.015* |
| 9 or 13 min tDCS | |||
| DC | 3 | 4.179 | 0.013* |
| ISI | 23 | 10.314 | < 0.001* |
| DC × ISI | 69 | 0.843 | 0.813 |
Repeated-measures ANOVAs were calculated for the intra-tDCS and the after-effect parts of the study with regard to the I–O curve, intracortical inhibition and facilitation and I-wave protocols. Intra-tDCS refers to the 4 s tDCS protocol, which elicits no after-effects, with TMS applied during tDCS. 7 min tDCS refers to the tDCS protocol eliciting short-lasting after-effects (∼ 10 min duration), and 9 or 13 min tDCS to the tDCS protocol eliciting long-lasting after-effects (∼ 1 h duration). Degrees of freedom (d.f) differ for intra-tDCS and after-effect protocols. For the intra-tDCS-effects, there were three sessions (anodal tDCS, cathodal tDCS and no-tDCS), while for the after-effects, there were four conditions, due to the measure of the respective TMS protocols before and after anodal and cathodal tDCS, thus resulting in ‘before-anodal’, ‘before-cathodal’ and ‘after-anodal’ as well as ‘after-cathodal’ values. As can be seen from the results of the ANOVAs, tDCS modulated the I–O curve and intracortical inhibition/facilitation during tDCS as well as for the after-effects of tDCS, while the modulating effects of tDCS on I-waves were restricted to the after-effects.
P < 0.05.
We controlled for MT and single test-pulse MEP amplitude differences between the tDCS and no-tDCS conditions (intra-tDCS effects) or before vs. after tDCS (after-effects) conditions in each protocol (I–O curve, inhibition/facilitation, I-waves) by applying Student's t tests (paired samples, two-tailed, level of significance 0.05). For the short-lasting after-effects, we additionally used post hoc Student's t tests (paired samples, two-tailed, P < 0.05) to compare the TMS intensities to achieve test pulse-values of 1 mV and the test-pulse amplitudes between the first and second tDCS sessions of the intracortical inhibition/facilitation protocol (baseline measures), to exclude any interference between the two sessions. Moreover, we calculated correlations for the short-lasting after-effects of the I–O curve, intracortical inhibition/facilitation and I-wave protocols to determine whether the intra-individual sensitivity to anodal tDCS vs. cathodal tDCS is similar (Table S2, supplemental material).
Results
Motor thresholds in the tDCS- and non-current conditions (intra-tDCS) and the respective baseline versus post-tDCS (long-lasting after-effects of tDCS) conditions did not differ in active and resting motor threshold, as revealed by the results of the respective t-tests (P > 0.05). For the intra-tDCS measures, the resting MT (±s.d.) was 54.9 ± 5.9% of maximum stimulator output in the no-tDCS condition, 54.7 ± 5.8% in the anodal tDCS condition, and 55.0 ± 5.6% in the cathodal condition. The mean intra-tDCS AMT was 44.7 ± 8.2% in the no-tDCS condition, 44.5 ± 8.6% in the anodal tDCS condition, and 44.5 ± 8.3% in the cathodal condition. For the long-lasting after-effects, the resting MT was 61.5 ± 7.7% in the anodal tDCS condition and 61.3 ± 7.6% in the cathodal condition before and after tDCS, whereas the active MT was 46.0 ± 4.8% in the anodal and 44.4 ± 5.0% in the cathodal condition before and after tDCS.
Input–output curve
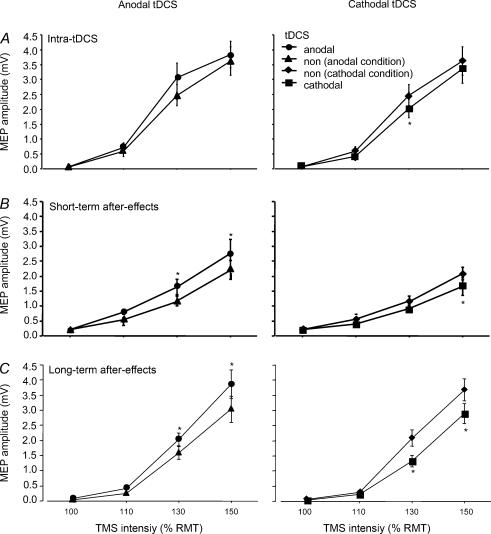
As the ANOVAs show (Table 2), the main effects of TMS intensity, tDCS and the interaction between both factors were significant for the intra-tDCS condition (4 s tDCS, MEP elicited during tDCS) as well as for the short-lasting (7 min tDCS, MEP elicited after the end of tDCS) and long-lasting (9 min cathodal, 13 min anodal tDCS, MEP elicited after the end of tDCS) after-effects of tDCS. As shown in Fig. 1, anodal tDCS increased the slope of the I–O curve, whereas cathodal stimulation diminished it in each tDCS condition. However, in the intra-tDCS condition anodal tDCS resulted only in an insignificant tendency for enhanced excitability.
Figure 1. tDCS shifts the slope of the input–output curve polarity-specifically.
The MEP amplitudes (means ±s.e.m.) at 100, 110, 130 and 150% of resting MT (RMT) are shown for the intra-tDCS conditions (A) and the short- (B) and long-lasting (C) after-effects. During tDCS, cathodal stimulation diminishes the MEP-amplitude relative to no-tDCS values, whereas anodal tDCS tends to enhance it. Due to the experimental protocol, the no-tDCS (non) curves used for comparisons in the anodal and cathodal intra-tDCS conditions are identical (applies also to the following figures). For the after-effects, the direction of the current-induced MEP amplitude changes is similar to the effects during stimulation, but the anodal tDCS-elicited effects are more clear-cut here. Here, no-tDCS (non) values represent the ‘before-tDCS’ baselines and are different for the anodal and cathodal conditions (applies also to the following figures). *P < 0.05, Fisher's LSD test, comparing the respective tDCS and no-tDCS values.
Intracortical inhibition and facilitation
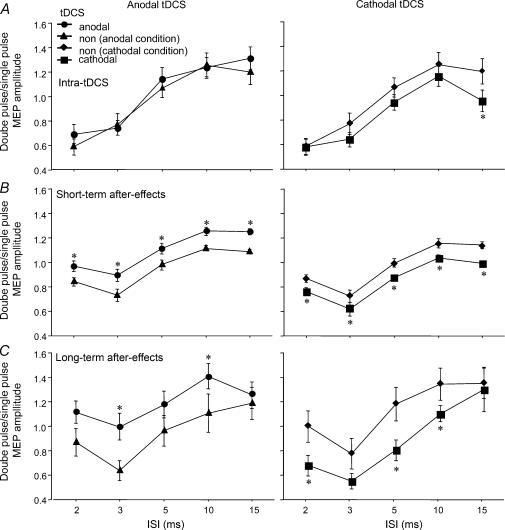
For the intra-tDCS condition, the ANOVA showed significant main effects of tDCS and ISI. The interaction between the two factors was not significant (Table 2). Here anodal tDCS did not shift cortical inhibition or facilitation, while cathodal tDCS reduced facilitation (Fig. 2). Similarly, the ANOVAs conducted for the short- and long-lasting after-effects of tDCS revealed significant main effects of tDCS and ISI. For the long-lasting after-effects, moreover, the interaction between the two factors was significant. With regard to the short-lasting after-effects of tDCS, anodal tDCS reduced inhibition and enhanced facilitation, while cathodal tDCS had the reverse effect. Baseline test-pulse amplitudes and TMS intensity (as a percentage of resting MT) to achieve those amplitudes did not differ between the first and second measurements, when anodal tDCS was performed first and cathodal tDCS thereafter (TMS intensity, with anodal tDCS 47%, and with cathodal tDCS 46%, P= 0.834; test-pulse amplitude with anodal tDCS 1.002 mV, and with cathodal tDCS 1.016 mV, P= 0.235) and the reverse arrangement (TMS intensity with anodal tDCS 42%, and with cathodal tDCS 43%, P= 0.978; test-pulse amplitude with anodal tDCS 1.005 mV, and with cathodal tDCS 1.005 mV, P= 0.859). For long-lasting after-effects, anodal tDCS reduced inhibition at ISIs of 3 ms, wheras cathodal tDCS enhanced inhibition significantly at ISIs 2 and 5 ms. However, non-significant trends towards enhanced inhibition in the cathodal 3 ms condition as well as in the anodal 2 and 5 ms conditions can also be seen. Anodal tDCS enhanced facilitation at ISI 10 ms, while cathodal tDCS reduced facilitation in this condition. In contrast to the short-lasting after-effects, for the long-lasting after-effects the ISI 15 ms condition was not influenced by anodal or cathodal tDCS (Fig. 2).
Figure 2. Intracortical inhibition and facilitation is modulated by tDCS.
The single-pulse standardized double-stimulation MEP amplitude ratios ±s.e.m. are depicted for ISIs revealing inhibitory (ISIs of 2, 3 and 5 ms) and facilitatory (ISIs of 10 and 15 ms) effects for the different tDCS protocols. A, during a short tDCS, which elicits no after-effects, anodal stimulation does not shift inhibition and facilitation relative to the no-tDCS (non) values. Cathodal stimulation reduces facilitation for the ISI of 15 ms. B, however, during the short-lasting after-effects, cathodal stimulation reduces the amplitude of all ISIs tested, whereas anodal tDCS results in reversed effects, thus reducing inhibition and increasing facilitation. C, for the long-lasting after-effects, the principal effect is identical, but not all ISIs are modulated here by tDCS significantly. *P < 0.05, Fisher's LSD test, comparing the respective tDCS and no-tDCS values.
I-wave facilitation
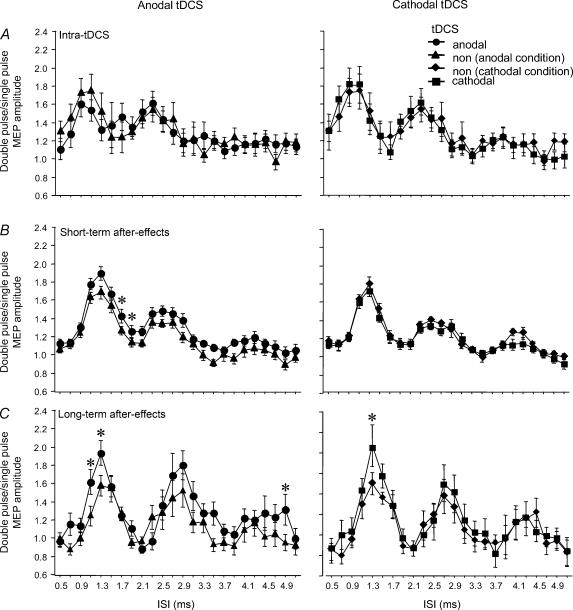
For the intra-tDCS condition, the ANOVA shows a significant main effect for ISI. The tDCS and interaction between ISI and tDCS were not significant. With the tDCS protocol producing short-lasting after-effects, the ANOVA reveals significant main effects for ISI and DC and a significant interaction between both factors. Anodal tDCS increased MEP amplitudes between the first and second peak significantly. Cathodal tDCS was without significant effects. For the long-lasting after-effects of tDCS, the main effects of ISI and DC stimulation were significant. Both anodal and cathodal tDCS increased the amplitude of the first I-wave, whereas for the later I-waves only anodal stimulation had an effect; however, that effect was restricted to a single ISI (Fig. 3).
Figure 3. I-wave facilitation is modulated by tDCS.
Single pulse-standardized double-stimulation MEP amplitude ratios ±s.e.m. are depicted for I-wave facilitation within the different tDCS protocols. A, during a short tDCS, which elicits no after-effects, it does not shift I-wave facilitation relative to the no-tDCS (non) values. B, for the short-lasting after-effects, cathodal tDCS does not modulate I-wave peaks, whereas anodal tDCS results in a separation of I-wave peak-values. C, for the long-lasting after-effects, anodal and cathodal tDCS increase the first I-wave peaks, while only anodal tDCS increases the fourth I-wave peak. *P < 0.05, Fisher's LSD test, comparing the respective tDCS and no-tDCS values.
Motor thresholds did not differ significantly under any of the tDCS conditions applied (i.e. tDCS, no-tDCS, before tDCS, and after tDCS); the single test-pulse MEP amplitudes were identical for all protocols in all conditions (Table 1). For the short-lasting after effects, a trend towards a negative correlation between intra-individual MEP amplitude changes elicited by anodal and cathodal tDCS was observed for the I–O curve and intracortical facilitation/inhibition protocol, but which was significant only for one TMS intensity in the I–O curve protocol. Thus for these protocols, subjects who react well to anodal tDCS seem to do so for cathodal tDCS. In contrast, for the I-wave protocol correlations were mixed (correlation values and significances are displayed in Table S2 in the Supplemental material).
Discussion
In this study we explored the modulation of different TMS protocols known to involve cortico-spinal (I–O curves and MTs) or intracortical neuronal systems (inhibition/facilitation, and I-wave facilitation) using tDCS in order to learn more about the mechanisms important for the overall excitability changes induced by the transcranial application of direct currents to the human motor cortex.
No effect of tDCS on MTs
Active and resting MTs were not modulated during tDCS nor as part of the after-effects of DC stimulation. Nevertheless, animal experiments have demonstrated a polarity-dependent de- or hyperpolarizing effect on neuronal membranes during DC stimulation (Purpura & McMurtry, 1965), and pharmacological studies suggest a similar mechanism in humans, because ion channel blockers have been shown to diminish tDCS-induced excitability shifts (Nitsche et al. 2003b). Thus, one might have expected a modulation of MTs, which are thought to depend on membrane polarization (Chen, 2000), at least during tDCS. However, as the modulatory activity of tDCS seems to be restricted to intracortical neurones (Nitsche & Paulus, 2001; Nitsche et al. 2003a), and MT may depend on cortical as well as cortico-spinal neurone polarization, the effects of tDCS may not have sufficed to induce a relevant MT shift. With regard to the after-effects, which seem to be based on changes of NMDA receptor efficacy, but not on membrane polarization (which would not outlast the stimulation itself (Nitsche et al. 2003b)) the results meet expectations, as MTs are not influenced by pharmacological NMDA receptor modulation (Ziemann et al. 1998a).
tDCS modulates the I–O curve polarity-dependently
For the I–O curve, the results essentially confirm those achieved with single pulse TMS stimulation so far (Nitsche & Paulus, 2000, 2001; Nitsche et al. 2003a); that is, depending on tDCS polarity, tDCS modulated cortico-spinal excitability, and thus the population of neurones activated by a given TMS intensity: anodal tDCS increased the slope of the I–O curve and cathodal tDCS diminished it.
During tDCS (intra-tDCS effects), the anodal stimulation-elicited effects are far less prominent than the after-effects of tDCS, while the intra- and after-tDCS effects are more similar in the cathodal tDCS condition. This is paralleled by similar differences in previous single-pulse experiments (Nitsche & Paulus, 2000) and may be due to the different physiological mechanisms involved (membrane polarization vs. receptor efficacy modification). From animal experiments, it is known that it is easier to induce excitability-diminishing neuroplastic modifications than to enhance excitability (Malenka & Bear, 2004). Thus, it may be that cathodal intra-tDCS effects already include some kind of synaptic modification, whereas anodal tDCS intra-tDCS effects do not, and that this may have caused the differences. Alternatively, the differences might be caused by a kind of membrane excitability-ceiling effect, which for the intra-tDCS effects prevents any further prominent enhancement of excitability by anodal tDCS, due to a relatively high baseline of motor-cortex excitability. This possible explanation is supported by the fact that anodal tDCS tends to enhance excitability only when tested with moderate TMS intensities within the intra-tDCS condition.
Why the I–O curves, but not MTs, are modulated by tDCS needs an explanation, because both protocols measure cortico-spinal excitability. Here, it is important that, by measuring the I–O curve, a somewhat larger neuronal population is activated as compared to MT testing (Chen, 2000). Thus, the sensitivity of the I–O curve in detecting cortical excitability changes may be superior, especially because the tDCS electrodes cover a relatively large cortical area. As GABAergic, adrenergic and glutamatergic mechanisms may contribute to the slope of the I–O curve (Chen et al. 2000; Di Lazzaro et al. 2003), but not to MT, an effect of tDCS on these pharmacologically defined systems could have also caused the different effects. However, GABAergic and adrenergic mechanisms have been demonstrated not to be involved directly in the intra-tDCS excitability changes nor in the induction of after-effects (Nitsche et al. 2004b, c).
Intracortical inhibition and facilitation are modified by tDCS
Intracortical inhibition and facilitation were prominently modulated by tDCS, especially with regard to the after-effects: For the short-lasting after-effects (7 min tDCS), inhibition was diminished and facilitation increased by anodal tDCS, whereas the effect of cathodal tDCS was the reverse. This result fits well with the fact that the after-effects of tDCS as well as intracortical inhibition and facilitation are at least partly controlled by NMDA receptor activity (Nitsche et al. 2003a; Ziemann et al. 1998a).
Essentially, the results are identical for the long-lasting after-effects (9 or 13 min tDCS). However, here the effects were not significant for single ISIs and were absent for the 15-ms ISI under the anodal and cathodal tDCS conditions. This may be due to group differences between the experiments, but could also be caused by different time courses of the stability of the excitability shifts for single ISIs: While the TMS protocol applied for testing inhibition and facilitation for the short-lasting after-effects of tDCS was applied immediately after tDCS in each case, it was applied for the long-lasting after-effects up to 35 min after DC stimulation in some subjects.
During tDCS (intra-tDCS effects), anodal tDCS did not shift intracortical inhibition or facilitation, while cathodal tDCS, diminished facilitation. The lack of effect under the anodal condition is in line with the assumption that such a short period of stimulation does not induce changes of NMDA receptor efficacy (Nitsche et al. 2003a), and that pure membrane potential changes do not shift intracortical excitability (Ziemann et al. 1996). However, cathodal tDCS did change intracortical excitability here. This may be caused by a slight modulation of NMDA receptor activity even for this short period of stimulation, which would be more easily detectable for cathodal tDCS because of the generally more stable effects of this condition as compared with anodal tDCS.
Minor effects of tDCS on I-wave facilitation
During tDCS (intra-tDCS effects), no effect of tDCS was evident for the I-wave facilitation condition. Within the short-lasting after-effects, anodal tDCS increased the MEP amplitudes of the troughs between the first and second peak, while cathodal tDCS was again without effect. For the long-lasting after-effects, both anodal and cathodal tDCS increased the amplitude of the first I-wave peak, while only anodal tDCS increased the amplitude of one later peak.
The lack of a tDCS effect on I-wave facilitation by tDCS protocols which do not elicit after-effects (intra-tDCS condition) fits well with the assumption that the excitability modulation generated by tDCS in this case depends primarily on membrane polarization, while I-wave facilitation depends on GABAergic and, to a smaller extent, on glutamatergic mechanisms. The increase of the I-wave peak and trough-amplitudes after anodal tDCS, which elicits after-effects, are probably caused by synaptic modifications generated by tDCS. Thus, the increase of the I-wave trough-amplitudes within the short-lasting after-effects of tDCS is probably due to a dispersion of the I-wave peaks and not to an I-wave-independent increase of all TMS double-stimulation amplitudes as compared to the single stimuli, as for the short and long ISIs the pre- and post-tDCS amplitudes did not differ. However, the effects of tDCS on I-wave facilitation in general were relatively minor compared to those on intracortical inhibition and facilitation and, with one exception, were restricted to the anodal tDCS condition, probably due to the more prominent dependency of I-wave facilitation on GABAergic mechanisms, which seem not to be influenced by tDCS to a great extent, at least on the intracortical level (Nitsche et al. 2004b).
The facilitatory effects of both anodal and cathodal tDCS on the first I-wave within the long-lasting after-effects is surprising. Anodal stimulation may have caused an enhanced I-wave peak-amplitude by increasing cortical facilitation. For the cathodal tDCS condition, this result could have been caused by a deactivating effect of cathodal tDCS on inhibitory interneurones, controlling the first I-wave peak, which might vary in the time course. Which inhibitory neuronal populations are involved cannot be derived directly from our results. However, the principle mechanism of an inhibitory effect of cathodal tDCS – even on inhibitory interneurones – seems possible, as was recently suggested as an explanation for the reduction of transcallosal inhibition by cathodal tDCS (Lang et al. 2004). Alternatively, it cannot be ruled out that cathodal tDCS increases the excitability of subpopulations of excitatory interneurones, which influence the MEP amplitude within the first I-wave, but are of minor influence with regard to the overall excitability diminution elicited by cathodal tDCS, which is expressed in the reduction of single pulse MEP amplitudes.
General remarks
Taken together, the results of this study suggest that the net cortico-spinal excitability modulation induced during tDCS, which elicits no after-effects, critically depends on membrane polarization, but not so much on synaptic modifications. This is demonstrated by the trend towards an increased slope of the I–O curve achieved by anodal tDCS and the reduced slope brought about by cathodal DC stimulation, and also by there being little or no effect of tDCS on intracortical inhibition, facilitation and I-wave facilitation.
However, for the after-effects of tDCS, the shift of the latter parameters suggests a prominent involvement of intracortical synaptic mechanisms in the resulting excitability modulations. Here, anodal tDCS increased not only the slope of the I–O curve, but also increased facilitation, diminished inhibition, and to some extent increased I-wave peaks, whereas cathodal tDCS resulted in the reverse effects, with the exception of I-wave facilitation. Taking into account pharmacological studies, these effects can probably be explained by tDCS-generated modifications of NMDA receptor efficacy (Liebetanz et al. 2002; Nitsche et al. 2003b), as the I–O curve, intracortical inhibition and facilitation as well as I-wave facilitation are thought to be at least partly controlled by these receptors. While other receptors and ion channels may contribute in general to cortical excitability, as measured by these protocols, it is not very likely that they participate in the after-effects on cortical excitability produced by tDCS, as ion channel activity modifications alone would not be stable long enough after the cessation of tDCS to be relevant for the induction of long-lasting after-effects, because of the short duration of recurrent excitation. Moreover, these did not modify the tDCS-induced excitability diminutions in former studies (Nitsche et al. 2003b). Adrenergic mechanisms have been found to be involved in the stabilization, but not the induction, of after-effects of tDCS (Nitsche et al. 2004c). GABAergic mechanisms may have a certain influence on the formation of the anodal stimulation-induced lasting excitability elevations, but not on the diminutions (Nitsche et al. 2004b). Moreover, if one assumes a direct GABAergic participation in the formation of the anodal tDCS-generated excitability enhancement, it should be a reduction of GABAergic activity. Currently available data from pharmacological studies are not in full accordance, because they report an abolition of the intracortical effects of anodal tDCS after administration of the activity-dependent GABA-agonist lorazepam (Nitsche et al. 2004b).
In a recently conducted study, no effect of long-lasting after-effects of tDCS on intracortical inhibition/facilitation was described (Siebner et al. 2004). However, it is difficult to compare this study with ours, because the specific protocols applied differ in some aspects: The ISIs tested were not identical, which may be relevant, because not all ISIs are modulated by tDCS to the same extent. Moreover, TMS test pulse-amplitude, which is important for the amount of inhibition and facilitation which can be achieved (Stefan et al. 2002; Chen, 2004), was not adjusted in the former experiment, and the higher intensity of the conditioning pulse applied in that study may have led to ceiling or bottom-effects. These factors may have contributed to the absence of effects which were reported there. This is underscored by another study, in which the after-effects of anodal stimulation on intracortical inhibition and facilitation were tested with a protocol more comparable to the one used in our study, and which showed results similar to the ones described here (Hummel et al. 2005).
As the results of this study favour a time-dependent modulation of different cortical systems beyond the general modification of intracortical excitability by tDCS, future studies should address this question. Moreover, studying the modulation of other electrophysiological mechanisms, such as long-latency inhibition, later I-wave activity and electroencephalographic activity, could more greatly help to clarify the mode of action of tDCS.
Acknowledgments
This work was partially supported by the Deutsche Forschungsgemeinschaft, grant PA 419/9-1, and partly by the Bundesministerium für Bildung und Forschung.
Supplemental material
The online version of this paper can be accessed at: 10.1113/jphysiol.2005.092429
jp.physoc.org/cgi/content/full/jphysiol.2005.092429/DC1 and contains supplemental material consisting of two tables:
Table S1. Results of the ANOVAs separately comparing anodal/cathodal tDCS with no-tDCS/baseline values.
Table S2. Correlations of the effects of anodal/cathodal tDCS for different TMS protocols (short-lasting after-effects).
This material can also be found as part of the full-text HTML version available from www.blackwell-synergy.com
References
- Abbruzzese G, Trompetto C. Clinical and research methods for evaluating cortical excitability. J Clin Neurophysiol. 2002;19:307–321. doi: 10.1097/00004691-200208000-00005. [DOI] [PubMed] [Google Scholar]
- Boroojerdi B, Battaglia F, Muellenbacher W, Cohen LG. Evaluation of the effects of CNS-active drugs on cortical excitability in intact humans. Neurology. 1999;52(Suppl. 2):A457. [Google Scholar]
- Chen R. Studies of human motor physiology with transcranial magnetic stimulation. Muscle Nerve Suppl. 2000;9:S26–S32. doi: 10.1002/1097-4598(2000)999:9<::aid-mus6>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Chen R. Interaction between excitatory and inhibitory cirxuits in the human motor cortex. Exp Brain Res. 2004;154:1–10. doi: 10.1007/s00221-003-1684-1. [DOI] [PubMed] [Google Scholar]
- Chen R, Samii D, Canos M, Wassermann EM, Hallett M. Effects of phenytoin on cortical excitability in humans. Neurology. 1997;49:881–883. doi: 10.1212/wnl.49.3.881. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Profice P, Pennisi MA, Pilato F, Zito G, Dileone M, Nicoletti R, Pasqualetti P, Tonali PA. Ketamine increases human motor cortex excitability to transcranial magnetic stimulation. J Physiol. 2003;547:485–496. doi: 10.1113/jphysiol.2002.030486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaly RF, Ham JH, Lee JJ. High-dose ketamine hydrochloride maintains somatosensory and magnetic motor evoked potentials in primates. Neurol Res. 2001;23:881–886. doi: 10.1179/016164101101199342. [DOI] [PubMed] [Google Scholar]
- Hummel F, Celnik P, Giraux P, Floel A, Wu WH, Gerloff C, Cohen LG. Effects of non-invasive cortical stimulation on skilled motor function in chronic stroke. Brain. 2005;128:490–499. doi: 10.1093/brain/awh369. [DOI] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD. Corticocortical inhibition in human motor cortex. J Physiol. 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang N, Nitsche MA, Paulus W, Rothwell JC, Lemon R. Effects of transcranial DC stimulation over the human motor cortex on corticospinal and transcallosal excitability. Exp Brain Res. 2004;156:439–443. doi: 10.1007/s00221-003-1800-2. [DOI] [PubMed] [Google Scholar]
- Liebetanz D, Nitsche MA, Tergau F, Paulus W. Pharmacological approach to synaptic and membrane mechanisms of DC-induced neuroplasticity in man. Brain. 2002;125:2238–2247. doi: 10.1093/brain/awf238. [DOI] [PubMed] [Google Scholar]
- Liepert J, Schwenkreis P, Tegenthoff M, Malin JP. The glutamate antagonist riluzole suppresses intracortical facilitation. J Neural Transm. 1997;104:1207–1214. doi: 10.1007/BF01294721. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: An embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Fricke K, Henschke U, Schlitterlau A, Liebetanz D, Lang N, Henning S, Tergau F, Paulus W. Pharmacological modulation of cortical excitability shifts induced by transcranial DC stimulation. J Physiol. 2003b;553:293–301. doi: 10.1113/jphysiol.2003.049916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Grundey J, Liebetanz D, Lang N, Tergau F, Paulus W. Catecholaminergic consolidation of motor cortex plasticity in humans. Cereb Cortex. 2004c;14:1240–1245. doi: 10.1093/cercor/bhh085. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Jaussi W, Liebetanz D, Lang N, Tergau F, Paulus W. Consolidation of externally induced human motor cortical neuroplasticity by d-cycloserine. Neuropsychopharmacology. 2004a;29:1573–1578. doi: 10.1038/sj.npp.1300517. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Liebetanz D, Schlitterlau A, Henschke U, Fricke K, Lang N, Henning S, Frommann K, Paulus W, Tergau F. GABAergic modulation of DC-stimulation-induced motor cortex excitability shifts in the human. Eur J Neurosci. 2004b;19:2720–2726. doi: 10.1111/j.0953-816X.2004.03398.x. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Nitsche MS, Klein CC, Tergau F, Rothwell JC, Paulus W. Level of action of cathodal DC polarisation induced inhibition of the human motor cortex. Clin Neurophysiol. 2003a;114:600–604. doi: 10.1016/s1388-2457(02)00412-1. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 2000;527:633–639. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology. 2001;57:1899–1901. doi: 10.1212/wnl.57.10.1899. [DOI] [PubMed] [Google Scholar]
- Purpura DP, McMurtry JG. Intracellular activities and evoked potential changes during polarization of motor cortex. J Neurophysiol. 1965;28:166–185. doi: 10.1152/jn.1965.28.1.166. [DOI] [PubMed] [Google Scholar]
- Siebner HR, Lang N, Rizzo V, Nitsche MA, Paulus W, Lemon RN, Rothwell JC. Pre-conditioning of low-frequency repetitive transcranial magnetic stimulation with transcranial DC stimulation: evidence for homeostatic plasticity in human motor cortex. J Neurosci. 2004;24:3379–3385. doi: 10.1523/JNEUROSCI.5316-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan K, Kunesch E, Benecke R, Cohen LG, Classen J. Mechanisms of enhancement of human motor cortex excitability induced by interventional paired associative stimulation. J Physiol. 2002;543:699–708. doi: 10.1113/jphysiol.2002.023317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Chen R, Cohen LG, Hallett M. Dextromethorphan decreases the excitability of the human motor cortex. Neurology. 1998a;51:1320–1324. doi: 10.1212/wnl.51.5.1320. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Lonnecker S, Steinhoff BJ, Paulus W. Effects of antiepileptic drugs on motor cortex excitability in humans: a transcranial magnetic stimulation study. Ann Neurol. 1996;40:367–378. doi: 10.1002/ana.410400306. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Rothwell JC. I-waves in motor cortex. J Clin Neurophysiol. 2000;17:397–405. doi: 10.1097/00004691-200007000-00005. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Tergau F, Wassermann EM, Wischer S, Hildebrandt J, Paulus W. Demonstration of facilitatory I wave interaction in the human motor cortex by paired transcranial magnetic stimulation. J Physiol. 1998b;511:181–190. doi: 10.1111/j.1469-7793.1998.181bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann U, Tergau F, Wischer S, Hildebrandt J, Paulus W. Pharmacological control of facilitatory I-wave interaction in the human motor cortex. A paired transcranial magnetic stimulation study. Electroencephalogr Clin Neurophysiol. 1998c;109:321–330. doi: 10.1016/s0924-980x(98)00023-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.