Abstract
The purpose of the present investigation was to determine how fasted-state protein synthesis was affected, acutely, by resistance training. Eight men (24.8 ± 1.7 years, body mass index = 23.2 ± 1.0 kg m−2; means ± s.e.m.) undertook an 8 week programme of unilateral resistance exercise training (3 sessions week−1, progression from two to four sets; intensity was 80% of the subjects' single repetition maximum (1RM): knee extension and leg press). Following training, subjects underwent two primed constant infusions of l-[ring-13C6]phenylalanine to determine mixed and myofibrillar muscle protein synthesis (MPS) at rest and 12 h after an acute bout of resistance exercise at the same exercise intensity – each leg 80% of 1RM. Biopsies (vastus lateralis) were taken to measure incorporation of labelled phenylalanine into mixed and myofibrillar skeletal muscle proteins and yield fractional MPS. Training resulted in significant dynamic strength gains that were greater (P < 0.001) in the trained leg. Hypertrophy of type IIa and IIx fibres (P < 0.05) was observed following training. After training, resting mixed MPS rate was elevated (+48%; P < 0.05). Acutely, resistance exercise stimulated mixed MPS only in the untrained leg (P < 0.05). Myofibrillar MPS was unchanged at rest following training (P= 0.61). Myofibrillar MPS increased after resistance exercise (P < 0.05), but was not different between the trained and untrained legs (P= 0.36). We observed divergent changes in resting mixed versus myofibrillar protein synthesis with training. In addition, resistance training modified the acute response of MPS to resistance exercise by dampening the increased synthesis of non-myofibrillar proteins while maintaining the synthesis of myofibrillar proteins.
Resistance exercise has been shown to stimulate muscle protein synthesis (MPS) in an absolute sense and also compared with muscle protein breakdown (MPB; (Biolo et al. 1995; Phillips et al. 1997, 1999). Changes in MPB after resistance exercise are of a much lesser magnitude compared with the changes in MPS (Phillips et al. 1997, 1999). In addition, MPS is more responsive to feeding than MPB (Biolo et al. 1995, 1997; Borsheim et al. 2002; Tipton et al. 1999). The responsive nature of MPS to both resistance exercise and feeding is an indication that changes in MPS are predominant in determining muscle protein accrual. Ingestion of amino acids in the post-exercise period further stimulates MPS leading to a synergistic addition of the independent effects of amino acid ingestion and resistance exercise (Biolo et al. 1995, 1997; Tipton et al. 1999; Borsheim et al. 2002), which ultimately results in muscle fibre hypertrophy.
Resistance training modifies the acute response of MPS to resistance exercise at the same relative intensity (80% of single repetition maximum (1RM)) by attenuating the rise in MPS in trained compared with untrained individuals (Phillips et al. 1999). In addition, a longitudinal study revealed that the acute exercise-induced rise in MPS was attenuated in the fed state; hence, the diminished response of MPS with training is not specific to the fasted state and appears to be a response to the exercise stimulus itself (Phillips et al. 2002). In these studies (Phillips et al. 1999, 2002) the response of mixed muscle proteins was examined. There has been good correspondence between observed mixed MPS rates and the synthetic rates of individual myofibrillar proteins such as actin and myosin (Balagopal et al. 1997a, b; Hasten et al. 2000). However, we propose that part of the reason we observed a diminished response of MPS in trained persons (Phillips et al. 1999, 2002) was that quantitatively important changes may be occurring in myofibrillar MPS that may be diluted by smaller changes in sarcoplasmic or mitochondrial proteins, when mixed protein responses are measured. That is, resistance training was modifying how each protein subfraction responded to the stimulus of exercise. This may be particularly true with chronic interventions (i.e. resistance training) in which changes in gene abundances (Balagopal et al. 2001), protein synthetic machinery, and signalling pathways are more likely to occur (Rennie & Wackerhage, 2003).
The purpose of this study was to examine the response of mixed and myofibrillar MPS to an acute bout of resistance exercise in an untrained and trained state. We examined the myofibrillar fraction from the mixed muscle with the hypothesis that resistance exercise training was more likely to affect the response of this fraction versus other cellular protein subfractions. To minimize intersubject variability, we utilized a unilateral resistance exercise training model in which a resistance-trained leg could be simultaneously compared with a contralateral ‘sedentary’ (untrained) leg. Since feeding and exercise are synergistic in their effects on MPS, they are likely signalling rises in MPS via different signalling pathways (Rennie & Wackerhage, 2003); hence, to isolate the intrinsic effect of the exercise stimulus, studies were performed in the fasted state. Our hypothesis, given the close correspondence between the synthesis of mixed muscle and that of other protein subfractions (Louis et al. 2003; Moore et al. 2005), was that changes in myofibrillar MPS would be similar in magnitude and direction to those seen in mixed MPS in the untrained leg, but that training would alter this relationship and stimulate muscle myofibrillar protein synthesis.
Methods
Participants
All subjects (n = 8) were male and were recruited locally via posters and announcements. Subjects were screened for known health risks and conditions. All subjects were non-smokers, were not taking any medication, and were not involved in any structured form of physical activity. No subject had engaged in resistance exercise of any form for a period of at least 18 months prior to entering the study. All subjects gave written informed consent prior to participating in the study, which was approved by the local ethics committees of McMaster University and Hamilton Health Sciences. Subjects were on average 24.8 ± 1.7 years of age and had a body mass index of 23.2 ± 1.0 kg m2.
General experimental protocol
Subjects resistance trained for 8 weeks, thrice weekly. Subjects' legs were randomized to be trained based on 1RM isotonic strength in a counterbalanced fashion, such that equal numbers trained their stronger and weaker leg. Training included knee extension and leg press. The volume of exercise per training session progressed from two sets per exercise in week 1, to three sets per exercise in week 2, and finally to four sets per exercise in weeks 3 to 8. All exercises were performed at 80% of the subject's single repetition maximum (1RM) and consisted of 8–10 repetitions per set, with the exception of weeks 5 to 8, in which the fourth set was performed to failure. Maximal isotonic strength was determined in the trained leg every two weeks, to maintain the training intensity. Maximal isotonic strength in the non-trained leg was only measured before and after training.
For determination of muscle fibre size (see below), skeletal muscle biopsies were taken from the vastus lateralis of the trained leg before and after 8 weeks of training, and mounted in optimal cutting temperature (OCT) medium (Tissue Tech, Sakura Finetechnical, Japan). We took a biopsy from the untrained leg after, but not before, training. Our rationale for only sampling tissue from the untrained after the contralateral leg had been resistance trained was to confirm that the untrained limb did not differ phenotypically (i.e. fibre size, fibre distribution, myosin heavy chain (MHC) composition) from the trained leg before it underwent resistance training.
After 72 h had elapsed following their last resistance training session, subjects received a primed constant infusion of l-[ring-13C6]phenylalanine, with muscle biopsies taken from both the trained and untrained legs to estimate the resting rates of mixed and myofibrillar MPS. Subjects then engaged in a further week (four sessions) of training prior to receiving a second infusion of the same isotope 12 h after the last resistance training session.
Infusion protocols
At ∼06.00 h after an overnight fast (subjects were advised to eat an identical light meal at ∼21.00 h the evening prior to both infusions) subjects were infused with l-[ring-13C6]phenylalanine 10 days apart to measure resting MPS, which was always carried out first, and MPS after an acute bout of resistance exercise in both the trained and untrained legs. The acute bout of resistance exercise that preceded the second infusion consisted of four sets (10 repetitions per set) each of knee extension and leg press for each leg at 80% of each leg's 1RM. The result was that the trained leg performed a volume of exercise (repetitions × load) that was 70 ± 4% greater for leg press and 85 ± 6% greater for knee extensions versus the untrained leg (P < 0.001). Thus, the acute effect of resistance exercise in the untrained and the trained leg, at the same relative intensity, was simultaneously assessed.
All isotopes were filtered through a 0.2 µm filter before infusion. A primed (2 µmol kg−1) constant (0.05 µmol min−1 kg−1) infusion of l-[ring-13C6]phenylalanine was initiated, and muscle and blood samples were taken. Following 2 h of infusion, a muscle biopsy of the vastus lateralis was taken under local anaesthesia (2% xylocaine) from both the trained and untrained leg (∼3–6 min between biopsies) using our custom suction-modified 5 mm Bergstrom needle, as previously described (Phillips et al. 2002; Stewart et al. 2003). Following a further 4 h of infusion, a second biopsy was taken. Blood samples were taken at rest (0 h) and at 2 h, 4 h, and 6 h thereafter.
Blood
Whole blood was drawn from the catheter and placed into heparinized tubes. Plasma was separated by centrifugation and stored at −50°C until further analysis. Plasma insulin concentration was determined using standard radio-immunoassay insulin kits (Diagnostic Products Corporation, Los Angeles, CA, USA).
Muscle
A portion of a biopsy taken both before and after training in the trained leg as well as after training in the untrained leg was wiped free of blood and cleaned of any connective tissue prior to being embedded in OCT medium (Tissue Tech, Sakura Finetechnical, Japan), with its fibres orientated perpendicular to horizontal, and frozen immediately in isopentane cooled by liquid nitrogen. A second portion of each biopsy was blotted free of blood and frozen in liquid nitrogen within 15 s of excision. Samples were stored at −86°C until further analysis.
Embedded tissue was sectioned and assayed for the determination of muscle fibre types by histochemical analysis as previously described (Stewart et al. 2003). Cross sections of each muscle sample were also sectioned and processed for analysis with gel electrophoresis, as outlined previously (Stewart et al. 2003), to determine relative MHC content. We found good correspondence between histochemistry values for percentage area and the relative percentage of MHC determined by electrophoresis for each fibre type (r= 0.89, P < 0.001, not shown).
Muscle samples reserved for analysis of isotopic abundance, both intracellular free pool and protein-bound enrichment, were lyophilized to dryness. Once dry, samples were powdered and extracted by incubation in ice-cold 0.6 m perchloric acid (PCA) while on ice. PCA extracts from muscle were frozen for later analysis of intracellular phenylalanine enrichment by gas chromatography-mass spectrometry. The pellet that remained after PCA extraction of the intracellular amino acids was then frozen in liquid nitrogen and subsequently dried under vacuum. The dried pellet was then hydrolysed in 2 ml of 6 m HCl at 100°C for 24 h. The acid hydrolysate was run over a small ‘clean up’ column (100–200 mesh Dowex resin, H+-50W-AX8; Sigma, St Louis, MO, USA) and the tertiary-butyl dimethylsilyl (t-BDMS) derivative of phenylalanine was prepared, as previously detailed (Phillips et al. 1997, 1999).
Another portion of the muscle biopsy (∼30 mg wet weight) was homogenized in a high-salt buffer (mm: 20 Hepes, 2 EGTA, 50 NaF, 50 KCl, 0.2 EDTA, 50 β-glycerophosphate, 0.1 µm PMSF). The resulting homogenate was prepared as described elsewhere (Bohéet al. 2001) to yield a clean myofibrillar protein fraction, which was lyophilized to dryness, hydrolysed and analysed as per the mixed protein pellet (see above).
Gas chromatography-mass spectrometry analysis
All samples were analysed using an Agilent (Mississaugua, ON, Canada) 6890 GC oven with a 5973N mass spectrometer in electron-impact ionization mode and monitoring of ions as described (Phillips et al. 2002). Protein-bound amino acid enrichment was determined as described (Phillips et al. 1997, 1999), using a procedure originally described by Patterson et al. (1997). To obtain the greatest accuracy of the response, we did not overload the column with sample. Furthemore, we analysed all samples for one subject within a single run in a given day (triplicate injections of each sample took ∼8 h) to avoid day-to-day instrument response error (Patterson et al. 1998). We also used a series of standard curves incorporating a range of m+ 0 (m/z 234) ion abundances, to which samples were matched based on their m + 0 ion abundance, to account for any concentration dependency in the determination of the 240/238 ion ratio (Patterson et al. 1998). By matching samples to standard curves based on a similar ion abundance of the baseline (m/z 234) ion, we observed excellent agreement with tracer to tracee ratios obtained by calculation according to a method described (Patterson et al. 1998) (r= 0.98, P < 0.0001, data not shown).
Calculations
Muscle protein fractional synthetic rate (FSR, in % h−1) was calculated according to the precursor-product equation as described (Phillips et al. 1997, 1999, 2002). Briefly, we used intracellular phenyalanine enrichment as the precursor for incorporation into both mixed and myofibrillar protein fractions according to the precursor product equation:
Where Emp is the protein-bound enrichment in muscle protein (i.e. mixed or myofibrillar); Epp is the enrichment in the precursor pool, which in this case was intracellular free phenyalanine; t is the time (1 and 2) of each muscle biopsy. We used a primed constant infusion to avoid stimulating MPS by using a flooding dose of amino acids (Smith et al. 1998). We chose to use intracellular free phenylalanine as a surrogate for the true precursor for protein synthesis, namely phenylalanine-charged tRNA, since in the fasted state intracellular enrichment appears to be a reasonable approximation of true precursor enrichment (Caso et al. 2002).
Statistics
Data were analysed using a one-way analysis of variance (ANOVA) for fibre type parameters. A two-way repeated measures ANOVA was used to analyse strength gains due to training (pre- versus post-training) and differences between legs, as well as changes in mixed and myofibrillar MPS. Where significant main effects were observed, a Tukey post hoc test was used to isolate the differences. Correlations were determined using a Pearson product correlation coefficient. Significance was set at P < 0.05. All data are reported as means ± standard error (s.e.m.).
Results
Strength
Maximal isotonic force generation increased progressively in the trained leg as a result of training (P < 0.001, Table 1). Isotonic force-generating capacity also increased significantly in the untrained leg, but the change was larger in the trained leg (P < 0.001, Table 1).
Table 1.
Maximal isotonic force in the trained (T) and untrained (UT) legs
| Pre-training | Week 3 | Week 5 | Week 7 | Post-training | |
|---|---|---|---|---|---|
| T-KE | 40.3 (3.3)a | 46.9 (3.0)a | 58.8 (4.1)b | 67.5 (3.4)b | 74.7 (3.3)c,* |
| UT-KE | 38.4 (2.9)a | — | — | — | 42.6 (2.1)b |
| T-LP | 133.8 (13.9)a | 147.5 (15.5)a | 178.8 (15.7)b | 192.6 (15.1)b | 226.3 (14.9)c,* |
| UT-LP | 127.5 (12.5)a | — | — | — | 135.0 (9.8)b |
Values are means (s.e.m.) in kg. T – trained leg; UT – untrained leg; KE – knee extension; LP – leg press. Values with different superscripts are significantly different from other values in that leg (P < 0.05);
significantly different from UT (P < 0.001).
Muscle fibre and MHC characteristics
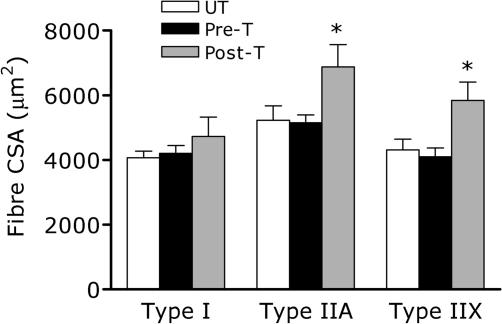
Fibre sizes were not significantly different between the trained leg pre-training and the untrained leg, post-training of the trained leg (Fig. 1). Eight weeks of thrice weekly resistance training resulted in a significant increase in fibre area of the IIa and IIx fibres only (Fig. 1). Training was without significant effect on any of the other fibre properties including: % fibre type, % area, and MHC distribution (Table 2).
Figure 1. Histologically determined muscle fibre cross sectional area (CSA).
UT, untrained; Pre-T, pre-training in the trained (T) leg; Post-T, post-training in the trained leg. *Significantly different from Pre (P < 0.05). Values are means ± s.e.m. (n = 8).
Table 2.
Percentage muscle fibre composition and area by histochemistry and percentage myosin heavy chain (MHC) composition from electrophoretic analysis
| Type I | Type IIa | Type IIx | |
|---|---|---|---|
| % | |||
| UT | 36.8 (2.5) | 35.7 (5.6) | 27.7 (3.6) |
| Pre-T | 35.1 (4.1) | 39.5 (3.0) | 27.1 (3.3) |
| Post-T | 37.4 (2.7) | 44.3 (2.9) | 23.4 (2.7) |
| % area | |||
| UT | 30.7 (4.5) | 41.5 (4.8) | 27.8 (4.3) |
| Pre-T | 33.4 (5.1) | 39.5 (3.5) | 27.1 (2.6) |
| Post-T | 30.3 (3.3) | 45.3 (3.0) | 24.4 (2.5) |
| % MHC | |||
| UT | 32.7 (4.5) | 42.7 (4.8) | 24.6 (4.3) |
| Pre-T | 34.1 (5.1) | 40.7 (3.5) | 25.2 (2.6) |
| Post-T | 32.6 (3.3) | 46.2 (3.0) | 21.2 (2.5) |
Values are means (s.e.m.), n = 8 per cell %, histochemically determined percentage of fibres by composition; % area, percentage of total fibre area occupied by fibre type; % MHC, percentage of total myosin heavy chain for by fibre type; UT, untrained; Pre-T, pre-training in trained leg; Post-T, post-training in trained leg.
Blood
Insulin concentrations remained unchanged during the infusion and were between 4 and 8 U ml−1 throughout. Phenylalanine enrichment in blood was constant from 2 h to 6 h in both the resting and post-exercise protocol, with no differences between the two protocols. Mean tracer (t) to tracee (T) ratio was between 0.071 and 0.093 throughout each protocol (mean t T−1= 0.081 ± 0.005).
Muscle protein fractional synthetic rate (FSR)
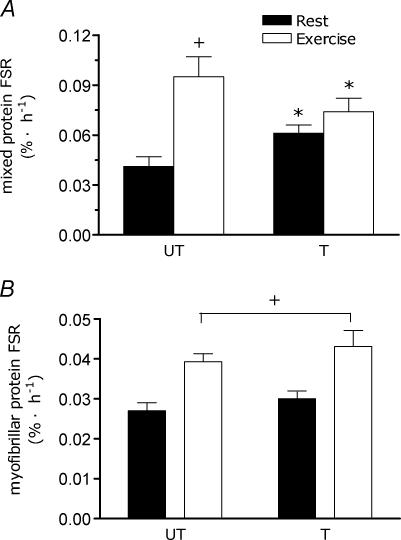
Stable intracellular enrichments were found in the free amino pool in every subject (mean coefficient of variation for biopsies at 2 h and 6 h varied between 1% and 7%, mean = 4.1 ± 0.7%) regardless of the protocol. Mean intracellular free t T−1 ratio was 0.077 ± 0.004. Mixed muscle protein FSR in the trained leg was elevated at rest after training compared with the untrained leg (untrained rest = 0.041 ± 0.006, trained rest = 0.061 ± 0.005, all in % h−1; P < 0.05, Fig. 2A). Acutely, resistance exercise resulted in an increase in mixed protein FSR in the untrained leg (+ 132%, P < 0.01) but not in the trained leg (+ 21%; Fig. 2A). Myofibrillar protein FSR at rest was similar between the trained and the untrained legs (untrained rest = 0.027 ± 0.002, trained rest = 0.030 ± 0.002, all in % h−1; P= 0.61, Fig. 2B). Acutely, resistance exercise resulted in a stimulation of myofibrillar protein FSR in both the trained (+ 44%) and untrained (+ 42%) legs (Fig. 2B), with no significant differences between conditions (P= 0.59).
Figure 2. Muscle fractional synthetic rate (FSR) at rest and following acute resistance exercise in the untrained (UT) and trained (T) limb.
A, mixed muscle protein FSR (% h−1) in the UT and T legs at rest and following an acute bout of resistance exercise. *Significantly different (P < 0.01) from the same value in the UT state; †significantly different from rest in the same leg (P < 0.05). B, myofibrillar protein FSR (% h−1) in the UT and T legs at rest and following an acute bout of resistance exercise. +Significantly different from rest (main effect for exercise; P < 0.05). Note difference in axes scales between panels A and B. Values are means ± s.e.m. (n = 8).
Discussion
The main findings of this study were that eight weeks of unilateral resistance exercise, which resulted in significant muscle fibre hypertrophy, increased resting mixed, but not myofibrillar MPS. An acute bout of resistance exercise at the same relative intensity in each limb, resulting in a greater amount of work performed by the trained limb, resulted in marked stimulation of mixed MPS only in the untrained limb. Conversely, myofibrillar MPS showed an acute increase, of similar magnitude, in response to resistance exercise in both the trained and untrained legs.
Previously, we have observed a higher, albeit not statistically significant, mixed MPS rate at rest in trained versus untrained persons (Phillips et al. 1999). When examined longitudinally (i.e. pre- and post-training), we observed elevated mixed MPS at rest in the fed state (Phillips et al. 2002). Similar results have been seen with resistance training in rats (Farrell et al. 1999a). The mechanisms for this training-induced elevation of resting mixed MPS are unclear. In the present study, as previously (Phillips et al. 1999, 2002), we waited until 72 h after the last workout to make the measurement; hence, the elevated resting mixed MPS in the present investigation is unlikely to have been a residual effect of the last exercise bout, considering that mixed MPS almost returns to baseline 48 h after exercise (Phillips et al. 1997). What is evident is that the resting elevation in mixed MPS does not consist of an elevation in myofibrillar MPS. It appears instead that elevated rates of synthesis of mixed proteins are from other protein subfractions (i.e. collagen, sarcoplasmic, and/or mitochondrial proteins). We propose that the increased resting MPS is not contributing significantly to protein accrual, which is occurring for the most part in the first 24 h following resistance exercise (Phillips et al. 1997), since MPB is also elevated with training (Phillips et al. 1999). Hence, the result of this elevated resting FSR is simply an overall increased rate of turnover of proteins other than myofibrillar proteins at rest, following resistance training. This may reflect an increased clearance (MPB) of potentially damaged proteins, with a concerted increase in protein renewal (MPS). Evidence that increased contractile activity promotes an increase in MPB is indirectly supported by findings of elevated proteolytic activity in chronic low-frequency stimulation models (Sultan et al. 2000, 2001).
Hypertrophy involves the accumulation of myofibrillar proteins, but expansion of the cellular protein fractions containing sarcoplasmic constituents (i.e. sarcoplasmic reticulum) also occurs with resistance exercise (Klitgaard et al. 1989). Moreover, results from acute isotopically determined turnover studies show that resistance exercise robustly stimulated synthesis of sarcoplasmic proteins (Louis et al. 2003). We hypothesized that, of all protein subfractions, the synthetic rate of the myofibrillar fraction would be likely be robustly affected by resistance exercise training. Hence, we focused on training-induced changes not only in mixed but also in myofibrillar MPS. Our working hypothesis, given the close correspondence between mixed and other protein subfractions (Louis et al. 2003; Moore et al. 2005) as well as individual protein synthetic rates (Balagopal et al. 1997a, b; Hasten et al. 2000), was that changes in myofibrillar MPS would be similar to those seen in mixed MPS. Contrary to our hypothesis, we observed that changes in myofibrillar MPS did not show the same pattern of change after resistance training as those in the untrained state. Specifically, when mixed MPS was elevated at rest after resistance training, myofibrillar MPS remained unchanged (Fig. 2). In addition, despite no change in mixed MPS in the trained leg after a greater amount of resistive work, the acute exercise-induced increase in myofibrillar MPS was of similar magnitude in the trained and untrained legs (Fig. 2B). The root of the reduced response of mixed MPS to acute resistance exercise in the trained state (Farrell et al. 1999a; Phillips et al. 1999, 2002); present observations) is not known, but may have involved changes in mRNA abundance (Balagopal et al. 2001), changes in peptide chain initiation factor abundance, altered signalling pathway activation, or a combination of these factors.
With training, the elevated rate of mixed MPS at rest appears to consist of increased synthesis of non-myofibrillar proteins, since we did not find an elevation in resting myofibrillar MPS after training. We saw no change in mixed MPS acutely after training, but this was not mirrored by a similar response in myofibrillar MPS, which increased to a similar extent as that seen in the untrained leg. In contrast, the untrained muscle when exposed to resistance exercise responded with a much greater synthetic response, which must have comprised a substantial rise in non-myofibrillar as well as myofibrillar protein synthesis. We speculate that in the untrained state, resistance exercise, as a relatively novel stimulus, when performed induces a large disturbance of homeostatic balance, which stimulates a non-specific rise in the synthesis of all muscle proteins. However, resistance training induces adaptations that dampen the perturbation from homeostasis induced by resistance exercise, even with work being set at the same relative intensity (i.e. greater total work performed in the trained state). The result is that the signal that stimulates an overall rise in mixed MPS in the untrained state is ‘refined’ after resistance training and is preferentially directed towards the synthesis of myofibrillar proteins. This could be due, but not mutually exclusive to, changes in mRNA abundance, training-induced adaptations in protein content and/or sensitivity of the signalling pathways that activate exercise-induced MPS. For example, a number of reports have now shown that feeding and/or insulin activate, in humans, components of the mammalian target of rapamycin (mTOR) pathway, often via stimulation of the protein kinase B (akt) pathway (Liu et al. 2001, 2002, 2003; Cuthbertson et al. 2005). In rodents, resistance exercise appears to act predominantly on the eukaryotic initiation factor (eIF) 2b, to promote GDP exchange to enhance tRNA binding, with less effect on the mTOR pathway and ribosomal assembly (Farrell et al. 1999b, 2000). Hence, given that the effects of resistance exercise and feeding are synergistic (Biolo et al. 1995, 1997; Tipton et al. 1999; Borsheim et al. 2002), it is tempting to speculate that each stimulus independently activates the protein synthetic apparatus via a distinct pathway, or at least a distinct arm of the same pathway. Alternatively, each stimulus could additively activate the same pathway (i.e. mTOR), with the idea that greater activation would lead to greater protein synthesis. Our speculation on these issues clearly indicates a need for further experiments in this area.
The unilateral resistance training model we utilized resulted in hypertrophy in the trained limb (Fig. 1). A muscle biopsy from the untrained limb taken at the same time as the post-training biopsy from the trained limb indicated muscle fibres no different in size from the pre-trained biopsy in the trained limb. Our interpretation of this observation is that the untrained limb was phenotypically (i.e. fibre size –Fig. 1; and fibre distribution –Table 2) no different from the pre-trained biopsy of the trained leg prior to training. We admit that our observation does not preclude that muscle fibres in the untrained limb did not have some other underlying genotypic differences; however, from a gross morphological and fibre type standpoint, the fibres appear no different. It also unlikely that some systemic changes in hormone concentrations affected the untrained limb, since unilateral resistance exercise does not induce changes in anabolic hormones such as growth hormone or testosterone, and neither does it affect cortisol (S.B. Wilkinson and S. Phillips, unpublished observations). Hence, we propose that the untrained limb is an appropriate control for the trained state limb. We stand by this statement, despite relatively minor strength increases (Table 1), which are almost certainly entirely due to neurally based mechanisms and not fibre size (i.e. muscle cross-sectional area) changes (Sale, 1992).
We acknowledge that the results we present here are specific to the fasted state and that if tested in the fed state, subjects would likely show a different pattern of response. For example, the attenuated exercise-induced response of mixed MPS in the trained state could be due to a limitation in amino acid supply. We elected to study the responses in the fasted state first to examine, in isolation, the essential response of exercise per se prior to studying the fed-state responses. We also realize that since we have studied only one time point post-exercise, we have no information on the time course of these responses.
Acknowledgments
We thank the subjects for their time and effort and Ms. G. Scime for her assistance during data collection and portions of the analysis. This work was supported by a grant from the Canadian National Sciences and Engineering Research Council (NSERC) to S.M.P. S.M.P. is supported by a Canadian Institutes for Health Research New Investigator Award and by the Premier's Research Excellence Award (PREA – Ontario). P.L.K. was partially supported by funding from PREA – Ontario during this project.
Supplemental material
The online version of this paper can be accessed at: 10.1113/jphysiol.2005.093708
http://jp.physoc.org/cgi/content/full/jphysiol.2005.093708/DC1 and contains supplemental material which contains data from a unilateral resistance training study in which muscle biopsies were taken from both legs prior to and following training. This material can also be found as part of the full-text HTML version available from http://www.blackwell-synergy.com
References
- Balagopal P, Ljunovist Q, Nair KS. Skeletal muscle myosin heavy-chain synthesis rate in healthy humans. Am J Physiol. 1997a;272:E45–E50. doi: 10.1152/ajpendo.1997.272.1.E45. [DOI] [PubMed] [Google Scholar]
- Balagopal P, Rooyackers OE, Adey DB, Ades PA, Nair KS. Effects of aging on in vivo synthesis of skeletal muscle myosin heavy-chain and sarcoplasmic protein in humans. Am J Physiol. 1997b;273:E790–E800. doi: 10.1152/ajpendo.1997.273.4.E790. [DOI] [PubMed] [Google Scholar]
- Balagopal P, Schimke JC, Ades PA, Adey DB, Nair KS. Age effect on transcript levels and synthesis rate of muscle MHC and response to resistance exercise. Am J Physiol Endocrinol Metabolism. 2001;280:E203–E208. doi: 10.1152/ajpendo.2001.280.2.E203. [DOI] [PubMed] [Google Scholar]
- Biolo G, Maggi SP, Williams BD, Tipton KD, Wolfe RR. Increased rates of muscle protein turnover and amino acid transport after resistance exercise in humans. Am J Physiol. 1995;268(31):E514–E520. doi: 10.1152/ajpendo.1995.268.3.E514. [DOI] [PubMed] [Google Scholar]
- Biolo G, Tipton KD, Klein S, Wolfe RR. An abundant supply of amino acids enhances the metabolic effect of exercise on muscle protein. Am J Physiol. 1997;273:E122–E129. doi: 10.1152/ajpendo.1997.273.1.E122. [DOI] [PubMed] [Google Scholar]
- Bohé J, Low JF, Wolfe RR, Rennie MJ. Latency and duration of stimulation of human muscle protein synthesis during continuous infusion of amino acids. J Physiol. 2001;532:575–579. doi: 10.1111/j.1469-7793.2001.0575f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsheim E, Tipton KD, Wolf SE, Wolfe RR. Essential amino acids and muscle protein recovery from resistance exercise. Am J Physiol Endocrinol Metab. 2002;283:E648–E657. doi: 10.1152/ajpendo.00466.2001. [DOI] [PubMed] [Google Scholar]
- Caso G, Ford GC, Nair KS, Garlick PJ, McNurlan MA. Aminoacyl-tRNA enrichment after a flood of labeled phenylalanine: insulin effect on muscle protein synthesis. Am J Physiol Endocrinol Metab. 2002;282:E1029–E1038. doi: 10.1152/ajpendo.00215.2001. [DOI] [PubMed] [Google Scholar]
- Cuthbertson D, Smith K, Babraj J, Leese G, Waddell T, Atherton P, Wackerhage H, Taylor PM, Rennie MJ. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J. 2005;19:422–424. doi: 10.1096/fj.04-2640fje. [DOI] [PubMed] [Google Scholar]
- Farrell PA, Fedele MJ, Hernandez J, Fluckey JD, Miller JL, III, Lang CH, Vary TC, Kimball SR, Jefferson LS. Hypertrophy of skeletal muscle in diabetic rats in response to chronic resistance exercise. J Appl Physiol. 1999a;87:1075–1082. doi: 10.1152/jappl.1999.87.3.1075. [DOI] [PubMed] [Google Scholar]
- Farrell PA, Fedele MJ, Vary TC, Kimball SR, Lang CH, Jefferson LS. Regulation of protein synthesis after acute resistance exercise in diabetic rats. Am J Physiol. 1999b;276:E721–E727. doi: 10.1152/ajpendo.1999.276.4.E721. [DOI] [PubMed] [Google Scholar]
- Farrell PA, Hernandez JM, Fedele MJ, Vary TC, Kimball SR, Jefferson LS. Eukaryotic initiation factors and protein synthesis after resistance exercise in rats. J Appl Physiol. 2000;88:1036–1042. doi: 10.1152/jappl.2000.88.3.1036. [DOI] [PubMed] [Google Scholar]
- Hasten DL, Pak-Loduca J, Obert KA, Yarasheski KE. Resistance exercise acutely increases MHC and mixed muscle protein synthesis rates in 78–84 and 23–32 yr olds. Am J Physiol Endocrinol Metab. 2000;278:E620–E626. doi: 10.1152/ajpendo.2000.278.4.E620. [DOI] [PubMed] [Google Scholar]
- Klitgaard H, Ausoni S, Damiani E. Sarcoplasmic reticulum of human skeletal muscle: age-related changes and effect of training. Acta Physiol Scand. 1989;137:23–31. doi: 10.1111/j.1748-1716.1989.tb08717.x. [DOI] [PubMed] [Google Scholar]
- Liu Z, Jahn LA, Long W, Fryburg DA, Wei L, Barrett EJ. Branched chain amino acids activate messenger ribonucleic acid translation regulatory proteins in human skeletal muscle, and glucocorticoids blunt this action. J Clin Endocrinol Metab. 2001;86:2136–2143. doi: 10.1210/jcem.86.5.7481. [DOI] [PubMed] [Google Scholar]
- Liu Z, Jahn LA, Wei L, Long W, Barrett EJ. Amino acids stimulate translation initiation and protein synthesis through an Akt-independent pathway in human skeletal muscle. J Clin Endocrinol Metab. 2002;87:5553–5558. doi: 10.1210/jc.2002-020424. [DOI] [PubMed] [Google Scholar]
- Liu Z, Wu Y, Nicklas EW, Jahn LA, Price WJ, Barrett EJ. Unlike insulin, amino acids stimulate p70S6K but not GSK-3 or glycogen synthase in human skeletal muscle. Am J Physiol Endocrinol Metab. 2003;286:E523–E528. doi: 10.1152/ajpendo.00146.2003. [DOI] [PubMed] [Google Scholar]
- Louis M, Poortmans JR, Francaux M, Berre J, Boisseau N, Brassine E, Cuthbertson DJ, Smith K, Babraj JA, Waddell T, Rennie MJ. No effect of creatine supplementation on human myofibrillar and sarcoplasmic protein synthesis after resistance exercise. Am J Physiol Endocrinol Metab. 2003;285:E1089–E1094. doi: 10.1152/ajpendo.00195.2003. [DOI] [PubMed] [Google Scholar]
- Moore DR, Phillips SM, Babraj JA, Smith K, Rennie MJ. Myofibrillar and collagen protein synthesis in human skeletal muscle after maximal shortening and lengthening contractions. Am J Physiol Endocrinol Metab. 2005;288:E1153–E1159. doi: 10.1152/ajpendo.00387.2004. [DOI] [PubMed] [Google Scholar]
- Patterson BW, Zhang XJ, Chen Y, Klein S, Wolfe RR. Measurement of very low stable isotope enrichments by gas chromatography/mass spectrometry: application to measurement of muscle protein synthesis. Metabolism. 1997;46:943–948. doi: 10.1016/s0026-0495(97)90084-6. [DOI] [PubMed] [Google Scholar]
- Patterson BW, Zhao G, Klein S. Improved accuracy and precision of gas chromatography/mass spectrometry measurements for metabolic tracers. Metabolism. 1998;47:706–712. doi: 10.1016/s0026-0495(98)90035-x. [DOI] [PubMed] [Google Scholar]
- Phillips SM, Parise G, Roy BD, Tipton KD, Wolfe RR, Tamopolsky MA. Resistance-training-induced adaptations in skeletal muscle protein turnover in the fed state. Can J Physiol Pharmacol. 2002;80:1045–1053. doi: 10.1139/y02-134. [DOI] [PubMed] [Google Scholar]
- Phillips SM, Tipton KD, Aarsland A, Wolf SE, Wolfe RR. Mixed muscle protein synthesis and breakdown following resistance exercise in humans. Am J Physiol. 1997;273:E99–E107. doi: 10.1152/ajpendo.1997.273.1.E99. [DOI] [PubMed] [Google Scholar]
- Phillips SM, Tipton KD, Ferrando AA, Wolfe RR. Resistance training reduces the acute exercise-induced increase in muscle protein turnover. Am J Physiol. 1999;276:E118–E124. doi: 10.1152/ajpendo.1999.276.1.E118. [DOI] [PubMed] [Google Scholar]
- Rennie MJ, Wackerhage H. Connecting the dots for mechanochemical transduction in muscle. J Physiol. 2003;553:1. doi: 10.1113/jphysiol.2003.054197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sale DG. Neural adaptation to resistance training. Med Sci Sports Exerc. 1992;20:S135–S145. doi: 10.1249/00005768-198810001-00009. [DOI] [PubMed] [Google Scholar]
- Smith K, Reynolds N, Downie S, Patel A, Rennie MJ. Effects of flooding amino acids on incorporation of labeled amino acids into human muscle protein. Am J Physiol. 1998;275:E73–E78. doi: 10.1152/ajpendo.1998.275.1.E73. [DOI] [PubMed] [Google Scholar]
- Stewart BG, Tarnopolsky MA, Hicks AL, McCartney N, Mahoney DJ, Staron RS, Phillips SM. Treadmill training-induced adaptations in muscle phenotype in persons with incomplete spinal cord injury. Muscle Nerve. 2003;30:61–68. doi: 10.1002/mus.20048. [DOI] [PubMed] [Google Scholar]
- Sultan KR, Dittrich BT, Leisner E, Paul N, Pette D. Fiber type-specific expression of major proteolytic systems in fast- to slow-transforming rabbit muscle. Am J Physiol Cell Physiol. 2001;280:C239–C247. doi: 10.1152/ajpcell.2001.280.2.C239. [DOI] [PubMed] [Google Scholar]
- Sultan KR, Dittrich BT, Pette D. Calpain activity in fast, slow, transforming, and regenerating skeletal muscles of rat. Am J Physiol Cell Physiol. 2000;279:C639–C647. doi: 10.1152/ajpcell.2000.279.3.C639. [DOI] [PubMed] [Google Scholar]
- Tipton KD, Ferrando AA, Phillips SM, Doyle DJ, Wolfe RR. Postexercise net protein synthesis in human muscle from orally administered amino acids. Am J Physiol. 1999;276:E628–E634. doi: 10.1152/ajpendo.1999.276.4.E628. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.