Abstract
Cerebral blood flow (CBF) is typically reduced during stable non-rapid eye movement (non-REM) sleep compared with the waking level. It is not known when in the sleep cycle these changes occur. However, spontaneous fluctuations in alpha and theta rhythm during sleep onset are associated with marked changes in cardio-respiratory control. The aim of this study was to test the hypothesis that changes in CBF would occur during sleep onset and would be related to changes in cortical activity. Middle cerebral artery velocity (MCAV) was measured using transcranial Doppler ultrasound, as an index of CBF, in 10 healthy subjects. Sleep state, ventilation, end tidal carbon dioxide (PET,CO2), arterial oxygen saturation (SaO2), mean arterial blood pressure (MABP) and cardiac R-R interval (RR) were monitored simultaneously. Immediately following the transition from alpha to theta rhythm (the transition from wake to sleep), ventilation V˙E decreased by 13.4% and tidal volume (VT) by 12.2% (P < 0.01); PET,CO2 increased by 1.9% (P < 0.01); respiratory frequency (fR) and SaO2 did not change significantly. MCAV increased by 9.7% (P < 0.01); MABP decreased by 3.2% (P < 0.01) but RR did not change significantly. Immediately following the transition from theta to alpha rhythm (spontaneous awakening), V˙E increased by 13.3% (P < 0.01); VT increased by 11.4% (P < 0.01); PET,CO2 decreased by 1.9%(P < 0.01); MCAV decreased by 11.1% (P < 0.01) and MABP decreased by 7.5%; fR, SaO2 and RR did not change significantly. These changes in MCAV during sleep onset cannot be attributed to changes in ventilation or MABP. We speculate that the changes in cerebral vascular tone during sleep onset are mediated neurally, by regulatory mechanisms linked to the changes in cortical state, and that these mechanisms are different from those regulating the longer-term reduction in CBF associated with stable non-REM sleep.
A number of studies have described the regulation of cerebral blood flow (CBF) during stable non-rapid eye movement (non-REM) sleep in healthy humans; in this state CBF is typically reduced compared with wakefulness (Townsend et al. 1973; Sakai et al. 1980; Madsen et al. 1991; Droste et al. 1993; Hajak et al. 1994; Kuboyama et al. 1997). This reduction is believed to be mainly linked to a decrease in the metabolic demand of brain tissue in non-REM sleep (Madsen et al. 1991). Ventilation falls during non-REM sleep with consequent hypercapnia; this paradoxically should elicit cerebral vasodilatation and increase CBF. However, the cerebral vascular response to hypercapnia is decreased during non-REM sleep (Meadows et al. 2003); this reduction in cerebrovascular reactivity would limit the hypercapnia-induced vasodilatation allowing the observed reduction in CBF to occur during this state.
Less consistent changes in CBF have been reported during sleep onset and light sleep. One study reported that CBF velocity, measured using Doppler ultrasound, was maintained at the same level during stage I sleep as wakefulness and then decreased progressively from stage II to IV non-REM sleep (Hajak et al. 1994). Others have reported transient increases in CBF velocity during stage II sleep (Kuboyama et al. 1997). A study using positron emission tomography determined regional CBF changes during stage I sleep and demonstrated relative flow increases in the occipital lobes (Kjaer et al. 2002). These observations suggest that the regulation of CBF during sleep onset differs from that of established sleep; however, none of these studies related the changes in CBF to the rapid changes in cortical activity that occur during this period.
Sleep onset can be considered to begin with the first occurrence of slowing of the EEG, the appearance of relatively low voltage mixed frequency EEG containing a predominance of theta activity and the absence of rapid eye movements. It ends at the beginning of stable stage II sleep (Rechtschaffen & Kales, 1968). During this phase the EEG contains spontaneous fluctuations in alpha and theta rhythms, including alpha–theta transitions, and theta–alpha transitions, which can be considered as transitions from wakefulness to sleep and spontaneous awakening, respectively (Colrain et al. 1987; Trinder et al. 1992; Burgess et al. 1999). These fluctuations in cortical state that occur during this phase are associated with marked changes in cardio-respiratory control. In particular, ventilation decreases with the alpha–theta transition and increases with the theta–alpha transition (Colrain et al. 1987; Trinder et al. 1992; Burgess et al. 1999).
The aim of the present study was to test the hypotheses that the changes in CBF would occur during sleep onset in healthy humans and that these changes would be related to changes in cortical activity, specifically that CBF would decrease at the alpha–theta transition and increase at the theta–alpha transition. To describe the changes in CBF we measured middle cerebral artery velocity during sleep onset using transcranial Doppler ultrasound (TCD).
Methods
This study was carried out with local ethical approval (Royal Brompton and Harefield Hospital Ethics Committee) and conformed to the Declaration of Helsinki. All volunteers gave written, informed consent.
Inclusion criteria
Since marked physiological differences exist between the elderly and young populations (Browne et al. 2001, 2003), we chose an age cut-off limit of 50 years. It has also been reported that hypercapnic cerebral vascular reactivity is significantly higher in premenopausal females than males (Kastrup et al. 1997); therefore we only studied males. In doing this we intended to limit any age- and gender-related physiological variation in our observations. Subjects who snored or had any significant medical history such as respiratory, cardiac and neurological disorders or were currently taking any medication or had recently undergone surgery were excluded.
Measurements
Each subject participated in the study at the sleep laboratory in the Royal Brompton Hospital on one occasion. All were asked not to consume alcohol or caffeine on the day of the study. Each had their height and weight recorded, underwent spirometry, and completed an Epworth sleepiness questionnaire.
The breathing circuit consisted of facemask (B&D, Electromedical) connected to a pneumotachograph (model 4700A, Hans Rudolph). The end-tidal partial pressure of carbon dioxide (PET,CO2) was measured by using rapidly responding gas analysers (CD-3A carbon dioxide analyser, AEI Technologies) via a thin catheter placed at the opening of the nostril. A ‘dry-line’ (PK Morgan) was fitted to the sampling tube to minimize moisture build-up. The arterial oxygen saturation (SaO2) was continuously monitored with a pulse oximeter (N-200E oximeter, Nellcor) via a finger probe. A two-lead electrocardiogram was used to monitor cardiac activity (Lifetrack, HME). A continuous measure of arterial blood pressure was determined using a photoplethysomnography cuff placed on a finger (Finapres BP monitor 2300, Ohmeda).
A 2 MHz pulsed Doppler ultrasound system (TC22, SciMed) was used to measure the left middle cerebral artery velocity (MCAV). The location of MCA was identified by an insonation pathway through the left temporal window just above the zygomatic arch by use of standard techniques, which have been previously described (Aaslid et al. 1982; Poulin et al. 1996; Meadows et al. 2003). A headband was used to hold the ultrasound probe, ensuring optimal insonation position and angle for the duration of the experiment. The signal from the Doppler probe, essentially, provides a continuous measurement of MCAV throughout the cardiac cycle. The continuous MCAV signal was then sampled, along with all other physiological data, at a frequency of 100 Hz using a computerized data-acquisition system (Micro 1401, Spike 2, Cambridge Electronics Design). For each cardiac cycle, the mean value for the velocity associated with the maximum frequency of the Doppler shift was calculated. Any sleep–wake transition periods (see later) in which artifact was present in the Doppler signal or when waveforms were not clearly outlined by the set envelope were discarded (Meadows et al. 2003). MCAV is a reliable index of cerebral blood flow as long as the cross-sectional area of the MCA remains constant. However, changes in MCA may result from changes in intraluminal pressure or from changes in MCA tone. To account for this, Poulin & Robbins (1996) have used the power of the reflected Doppler signal as an index of MCA cross-sectional area; the product of the power and the intensity weighted mean velocity is an index of MCA blood flow. In the present study, such data were available in seven subjects and are presented to support the interpretations based on MCAV alone.
Sleep study
Electroencephalograms (EEG: C3A2, C4A1, O1A2), electrooculograms (EOG: F7A1, F8A1) and electromyograms (EMG: submental) were recorded (model 12, Grass Instrument) using the International 10-20 system of electrode placement. Epoch-based sleep staging was performed using standard criteria (Rechtschaffen & Kales, 1968).
Protocol
Each subject was allowed to go to bed at their normal bedtime in a dark and quiet room. Initially, the subjects were requested to remain awake for at least 10 min before falling to sleep so that baseline data could be collected during wakefulness. In order to obtain multiple fluctuations in alpha and theta rhythm, subjects were woken using an auditory stimulus each time they entered stable stage II sleep. If the subjects did not respond to the auditory stimulus the investigator immediately awakened them by either calling their name or turning on the lights; the subjects were then allowed to resume sleep. The process was repeated for four cycles or until approximately 4 h of data had been collected. Data associated with the induced arousals in stage II were not included in the subsequent analyses and are not considered further.
Data analysis
Respiratory variables including expiratory tidal volume (VT), respiratory frequency (fR), PET,CO2 and SaO2 were analysed on a breath-by-breath basis. Expired ventilation V˙E was calculated breath by breath. Cardiovascular variables including MCAV, mean arterial blood pressure (MABP), and cardiac R-R interval (RR) were initially derived on a beat-by-beat basis, and then transformed to a second-by-second basis by linear interpolation.
EEG transition analysis
During the sleep onset period, periods of alpha activity (8–12 Hz) are interspersed with periods of theta activity (3–7 Hz), each of varying length; these periods have no discernible pattern except that theta activity predominates as the sleep onset period progresses. To reliably determine changes in cardiovascular and respiratory variables associated with the alpha and theta transitions, average transitions were constructed in each individual with variables time-aligned to the point of either the alpha–theta or theta–alpha transition. To do this, alpha and theta activities were determined by two investigators, blinded to all other data except that necessary to determine respiratory timing. Data sections containing artefact were excluded from analysis. Data analysis was performed following the approach of others (Colrain et al. 1987). Each breath was assigned as either ‘alpha’ or ‘theta’ according to the predominance of alpha or theta activity. An alpha–theta transition was defined as a train of contiguous ‘alpha breaths’ (minimum 2, maximum 5) followed immediately by contiguous ‘theta breaths’ (minimum 2, maximum 5). For each individual a single transition was constructed by averaging the respiratory variables from all individual trains on a breath-by-breath basis; the averaging was time-aligned to the point of transition from alpha breaths to theta breaths. The final composite was therefore 10 breaths long (5 breaths before to 5 breaths after the transition); no correction was made for breath-to-breath variability in respiratory frequency. The cardiovascular variables were averaged similarly but on a second-by-second basis (20 s before and 20 s after). Theta–alpha transitions were defined and analysed in a comparable way. With this approach, all individuals contributed one alpha–theta and one theta–alpha transition to the subsequent group analysis.
Statistical analysis
All variables were expressed as the mean ±s.d. except where otherwise stated. For each variable, significant differences between the pre-transition baseline (mean of breaths −5 to −2 or −20 to −5 s) and the post-transition period were tested using a one-way repeated measures analysis of variance (ANOVA). When the overall effect was significant, post hoc pairwise comparisons (Dunnett's) were conducted between the pre-transition baseline and each post-transition time point. Statistical analysis was performed using SPSS version 11.5 (SPSS Inc., Chicago); statistical significance was determined at P < 0.05.
Results
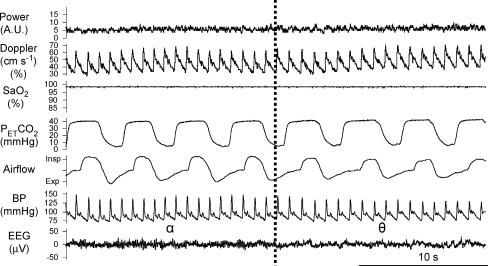
Of 13 male volunteers studied, three were excluded from the analysis: notable snoring was present in one and insufficient data were obtained from the other two. For the remaining 10, the mean age, body mass index, forced expiratory volume in one second/forced vital capacity (FEV1/FVC), and Epworth Sleepiness Scale of subjects were 27.5 ± 6.0 years, 22.8 ± 2.3 kg m−2, 84.3 ± 8.3%, and 4.8 ± 2.8, respectively. We obtained 14 (median, range: 7–32) alpha–theta and 11 (5–32) theta–alpha transitions per subject. Figure 1 shows a typical alpha–theta transition recorded in one individual; a small increase in the continuous recording of the MCAV can be seen, which is concomitant with reductions in airflow and blood pressure.
Figure 1. Original record of alpha–theta transition in one individual.
The vertical dotted line is the time point of transition. The continuous recording of the MCAV increases with reduction in airflow and BP during the alpha–theta transition. Power, Doppler power signal; Doppler, continuous recording of the MCAV; SaO2, arterial oxygen saturation; PET,CO2, partial pressure of carbon dioxide; BP, arterial blood pressure; EEG, electroencephalogram (lead O1A2).
Changes in respiratory variables associated with the alpha–theta transition
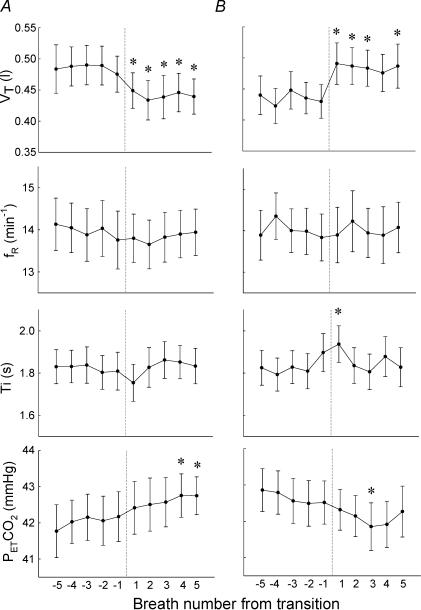
During the alpha–theta transition, V˙E in post-transition (theta activity) reduced significantly (P < 0.01, Table 1) compared with pre-transition (alpha activity). This was associated with a rapid reduction in VT (Fig. 2A), the reduction being significant at 2, 3 and 5 breaths post-transition compared with the pre-transition baseline (P < 0.01). The changes in inspiratory time (Ti) and fR were not significant, and when combined with these findings, the reduction in ventilation was mainly due to change in VT during the alpha–theta transition (Table 1; Fig. 2A).
Table 1.
Changes in cardio-respiratory variables before and after transition
| Alpha to theta transition | Theta to alpha transition | |||||
|---|---|---|---|---|---|---|
| Pre-transition mean | Post-transition max/min | Time from 0 to max/min | Pre-transition mean | Post-transition max/min | Time from 0 to max/min | |
| V˙E | 6.7 ± 1.0 | 5.8 ± 1.0 | breath 2 | 6.0 ± 0.9 | 6.8 ± 1.2 | breath 2 |
| VT(l) | 0.49 ± 0.10 | 0.43 ± 0.11 | breath 2 | 0.44 ± 0.09 | 0.49 ± 0.11 | breath 1 |
| fR(min−1) | 14.0 ± 2.0 | N.S. | — | 14.1 ± 1.7 | N.S. | — |
| Ti (s) | 1.82 ± 0.25 | N.S. | — | 1.81 ± 0.25 | 1.94 ± 0.28 | breath 1 |
| PET,CO2 (mmHg) | 42.0 ± 2.1 | 42.8 ± 1.9 | breath 4 | 42.7 ± 1.9 | 41.9 ± 2.1 | breath 4 |
| SaO2 (%) | 97 ± 1 | N.S. | — | 97 ± 1 | N.S. | — |
| MCAV (cm s−1) | 52.8 ± 13.1 | 57.9 ± 14.8 | 9 s | 55.7 ± 14.3 | 49.5 ± 12.3 | 10 s |
| MABP (mmHg) | 95 ± 13 | 92 ± 13 | 5 s | 93 ± 13 | 100 ± 14 | 6 s |
| RR (s) | 1.05 ± 0.14 | N.S. | — | 1.07 ± 0.12 | N.S. | — |
Pre-transition mean: average of −5 to −2 breaths for respiratory data, average of −20 to −5 s for cardiovascular data. Post-transition max/min: if significantly different from pre-transition mean (P < 0.01). Data were expressed as mean ± s.d. (n = 10), V˙E, expiratory ventilation;, VT, tidal volume; fR, respiratory frequency; Ti, inspiratory time; PET,CO2, partial pressure of end tidal carbon dioxide; SaO2, arterial oxygen saturation; MCAV, middle cerebral artery velocity; MABP,: mean arterial blood pressure; RR, cardiac R-R interval.
Figure 2. Changes in respiratory variables during sleep onset.
A, alpha–theta transition; B, theta–alpha transition. VT, tidal volume; fR, respiratory frequency; Ti, inspiratory time; PET,CO2, end tidal carbon dioxide. Variables were plotted breath by breath and expressed as the mean ± s.e.m. (n = 10). *P < 0.01 compared with the pre-transition mean (one-way repeated measures ANOVA with post hoc multiple comparisons).
PET,CO2 in post-transition was significantly elevated compared with pre-transition (P < 0.01, Table 1). Figure 2A indicates that PET,CO2 increased steadily, the increase being significant 4–5 breaths post-transition (P < 0.01). SaO2 did not change significantly (Table 1).
Changes in cardiovascular variables associated with the alpha–theta transition
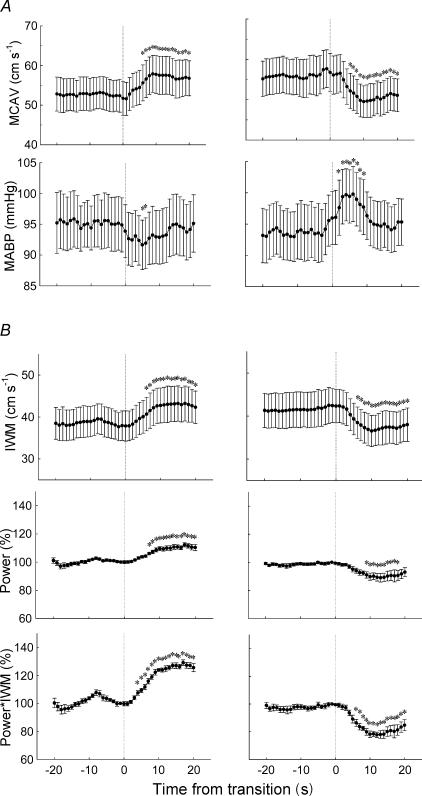
In the post-transition period, MCAV was significantly elevated compared with pre-transition (P < 0.01); this was associated with a reduction in MABP (P < 0.01); RR was unchanged (Table 1). The increase in MCAV occurred abruptly just after the transition, peaking at 9 s and remaining elevated for several seconds, the increase being significant 6–20 s post-transition (P < 0.01, Fig. 3A). The reduction in MABP began at the transition with the nadir occurring 5 s post-transition; it then slowly recovered to the baseline level within 15 s post-transition, reductions being significant 5 and 6 s post-transition (P < 0.01, Fig. 3A).
Figure 3. Changes in cardiovascular variables during sleep onset.
A, simultaneous changes in MCAV and MABP (n = 10). B, simultaneous changes in IWM, power, and product of power and IWM (n = 7). Left, alpha–theta transition; right, theta–alpha transition. Variables were replotted second by second using interpolation and expressed as the mean ± s.e.m.*P < 0.01 compared with the pre-transition mean (one-way repeated measures ANOVA with post hoc multiple comparisons). MCAV, middle cerebral artery velocity; MABP, mean arterial blood pressure; IWM, intensity-weighted mean velocity; power, Doppler power signal normalized by the value of −1 s; power*IWM, product of power and IWM normalized by the value at −1 s.
Consistent with the increase in MCAV following the transition, the intensity weighted mean (IWM), the power and the product (IWM*power) all increased significantly following the transition (Fig. 3B).
Changes in respiratory variables associated with the theta–alpha transition
There was rapid increase in ventilation just after the theta–alpha transition (Table 1). This increase was related mainly to increase in VT, with no change in fR; there was a small transient increase in Ti The increase in VT was significant at 1, 2, 3 and 5 breaths post-transition compared with the pre-transition baseline (P < 0.01). PET,CO2 decreased significantly at breath 3 post-transition (P < 0.01; Fig. 2B).
Changes in cardiovascular variables associated with the theta–alpha transition
MCAV reduced abruptly after the theta–alpha transition, being significant 6–20 s post-transition (P < 0.01; Table 1, Fig. 3B). Consistent with the decrease in MCAV following the transition, the IWM, the power and IWM*power all decreased significantly following the transition (Fig. 3B).
MABP increased transiently, being significantly increased 3–6 s post-transition (P < 0.01, Fig. 3B). SaO2 and RR did not change significantly during the theta–alpha transitions (Table 1).
Discussion
This is the first study to describe the changes in CBF related to the changes in cortical states during the sleep onset period. With the alpha–theta transition (transition to sleep), CBF velocity increased coincident with a reduction in ventilation and blood pressure. Conversely with the theta–alpha transition (spontaneous awakening), CBF velocity decreased coincident with an increase in ventilation and blood pressure, the change in CBF velocity being more abrupt than the change in velocity associated with the alpha–theta transition.
Brain blood flow during sleep onset
CBF is typically lower during non-REM sleep than wakefulness and reduces progressively as sleep stages become deeper (Hajak et al. 1994; Kuboyama et al. 1997). Associated with that change, our group previously reported cerebral vascular responses to hypoxia and hypercapnia during stage III–IV non-REM sleep are reduced compared with wakefulness (Meadows et al. 2003, 2004). Thus, we hypothesized CBF velocity might decrease at the transition from wakefulness to sleep, coupled with the changes in EEG from alpha to theta activity. Similarly, we anticipated an increase in CBF velocity with spontaneous awakening from sleep, coupled with the transition from theta to alpha. Contrary to our hypothesis, the present study showed CBF velocity increased at the alpha–theta transition and decreased at the theta–alpha transition. Therefore, we speculate that the regulation of brain blood flow during sleep onset has a different mechanism from the long-term reduction in cerebral blood flow associated with stable non-REM sleep.
We are confident that the alpha–theta transitions and the theta–alpha transitions were correctly defined in this study. The investigators were very experienced in EEG analysis; in addition, the EEG changes were coincident with changes in respiratory variables which have previously been reported at sleep onset and awakening (Colrain et al. 1987; Trinder et al. 1992). The reductions in ventilation at the alpha–theta transition are attributable to reductions in the activity of both upper airway dilator muscles and respiratory pump muscles (Kay et al. 1994; Worsnop et al. 1998), which can be associated with a reduction in central respiratory drive due to the loss of wakefulness stimuli (Morrell et al. 1993, 1995, 1996). At the theta–alpha transition (awakening from sleep), the increase in ventilation is a consequence of the return of the wakefulness stimuli or due to state-dependent changes in the hypercapnic response (Corfield et al. 1995). It is noteworthy that, in the present study, inspiratory time was not significantly altered following the alpha–theta transition but increased transiently following the theta–alpha transition. Thus the predominant state-dependent effect on respiration, in this context, appears related to changes in central respiratory ‘drive’ and not ‘timing’.
It is possible that the observed changes in MCAV that follow the alpha–theta transition may be secondary to the increase in PET,CO2 that is produced by the reduction in ventilation at the transition However, the MCAV increase was relatively rapid compared with the rise in PET,CO2. In addition, the magnitude of the increase in MCAV is relatively large compared with the increase in PET,CO2 (Table 1) and would be inconsistent with the known sensitivity to CO2 either awake or asleep (Meadows et al. 2003). Finally, the time course of the cerebral vascular response to CO2 is slow compared with the timescale of events here (Poulin et al. 1996). It is therefore unlikely that the changes in MCAV at the alpha–theta transition are secondary to the changes in PET,CO2. Similarly the decrease in MCAV following the theta–alpha transition are unlikely to be attributable to the fall in PET,CO2.
We made no direct measurements of cerebrovascular tone; however, the increase in CBF following the alpha–theta transition occurred despite a fall in arterial blood pressure and the decrease in CBF following the theta–alpha transition occurred despite a rise in blood pressure. Assuming that central venous pressure (and/or intracranial pressure) remain unchanged during the transitions, it can be concluded that the alpha–theta transition is directly associated with cerebral vasodilatation and the theta–alpha transition with cerebral vasoconstriction. As these changes in cerebrovascular tone do not appear to be due to the indirect effect of cardiovascular or respiratory factors, they may be directly related to changes in cortical state reflected in the changes in alpha and theta rhythms. The mechanism for such fluctuations in cerebrovascular tone during the sleep onset period is not known.
The most notable effects of awakening from sleep on the cardiovascular system are increases in heart rate, blood pressure and peripheral vasoconstriction (Morgan et al. 1996). Recent studies suggest that the cardiovascular responses at arousal from sleep are reflex-like and elicited by the act of arousing, and are different from the responses that occur during normal wakefulness (Horner et al. 1997; Trinder et al. 2003). Those responses are not thought to be homeostatic because they induce changes in the cardiovascular system that are beyond those required to return to a stable wakefulness level and exceed physiological need. This phenomenon may be a protective function against potential threat present immediately upon awakening (Davies et al. 1993). Arousal from sleep is associated with acute sympathetic activity and decreases in parasympathetic activity in dogs (Horner et al. 1995). However, the role of the sympathetic nervous system in the regulation of CBF in humans is debatable. Although some studies have shown that sympathetic activation reduces CBF and cerebral vascular reactivity to CO2 (Jordan et al. 2000), the established view is that the sympathetic nervous system plays a minor role in the regulation of CBF under physiological conditions (LeMarbre et al. 2003). Nevertheless, it is possible that sympathetic activation may play some part in the acute increase in cerebral vascular tone associated with the sleep–wake transition. This speculation is further supported by observations that dynamic changes in CBF may be under autonomic neural control in both humans (Zhang et al. 2002) and other mammals (Sercombe et al. 1979; Busija et al. 1980). As cerebral vessels are richly innervated, it is also possible that alternative neural pathways may exist which mediate the changes in cerebral vascular tone during this period.
Limitations of the study
First, MCAV is a reliable index of cerebral blood flow as long as the cross-sectional area of the MCA remains constant. Others have used the Doppler power as an index of cross-sectional area of the MCA, and showed that calibre of the MCA did not change under moderate hypercapnia (Poulin & Robbins, 1996). Using this approach, our data indicate that some changes in MCA diameter do occur during the sleep–wake transition period, but that these changes in diameter will change MCA blood flow in ways, at least for the present study, that are qualitatively consistent with changes in MCAV, i.e. MCAV, MCA diameter and MCA blood flow all increase following the alpha–theta transition and all decrease following the theta–alpha transition. From this, we must also conclude that factors regulating cerebral vascular tone during the sleep onset period may affect both larger conduit and smaller resistance vessels.
Second, each breath was categorized as an alpha or theta breath according to whether alpha or theta activity was predominant. With this definition, the relative location of any transition relative to another may vary by up to one breath (Colrain et al. 1987; Trinder et al. 1992). However, this error is minimized by averaging data obtained from multiple transitions. In addition, for the statistical analysis, we compared the overall effect between the states, by omitting one breath immediately prior to the transition. As the cardiovascular data were aligned to the EEG transitions with a resolution of 1 s, the changes in MCAV can be more precisely related to the transition than can the respiratory variables.
Third, although alpha–theta and theta–alpha transitions typically exist in the period of sleep onset, they can be observed in other sleep stages, such as stage II and REM sleep. Trinder and co-workers showed the changes in cardio-respiratory variables during alpha–theta and theta–alpha transition are greater in stage II sleep than in stage I sleep (Trinder et al. 1992). They also showed the duration of the pre-transition period affects the magnitude of the changes during alpha–theta and theta–alpha transition. We analysed various length of transitions and excluded stage II sleep. Thus, any extrapolation of our data to this state may underestimate the potential changes in CBF. Likewise, the study of any changes in MCAV in REM sleep was beyond the scope of this study.
Finally, we only studied the first 4 h of the night. CBF during non-REM sleep decreases throughout the night and is at the lowest level in the morning (Hajak et al. 1994). Cerebral vascular reactivity to CO2 is lower in the morning than in the evening (Ameriso et al. 1994). Therefore if sleep–wake fluctuations occur in the early hours of the morning changes in CBF may be diminished.
Clinical implications
During sleep onset, the spontaneous fluctuations in alpha and theta rhythm are related to instability in breathing in normal subjects (Skatrud & Dempsey, 1983; Khoo et al. 1991) as well as patients with sleep-disordered breathing. The increase in CBF velocity observed in the present study may be interpreted as a benefit, to maintain an adequate perfusion against acute hypoxia, hypercapnia and systemic hypotension at the transition from wake to sleep. However, if the changes in MCAV reflect the changes in the posterior circulation supplying the medulla, then the changes in CBF during sleep onset may cause changes in central chemoreceptor stimulation which may promote breathing instability. It is not known if medullary metabolism changes during these transitions but such changes might further modulate any perfusion-related effects on breathing stability.
In summary, we found marked changes in cerebral blood flow velocity during the period of sleep onset: increases with the transition from wakefulness to sleep and decreases with awakening from sleep. These changes contrast with the longer-term reduction in cerebral blood flow associated with stable non-REM sleep. We speculate these changes in perfusion reflect changes in cerebral vascular tone, during the sleep onset period, that are mediated neurally, by regulatory mechanisms linked to the changes in cortical state. Assuming that the cardio-respiratory response at the theta–alpha transition during sleep onset is similar to the arousal from stage II, slow-wave (stages III/IV) or REM sleep, these reductions in CBF with arousal would indicate a significant inability of the cerebrovascular circulation to respond to the cardio-respiratory changes induced by conditions such as sleep-disordered breathing.
Acknowledgments
We would like to thank Dr John Trinder for helpful background discussion and advice about data analysis related to the transition from alpha to theta and theta to alpha. This study was supported by The Wellcome Trust.
References
- Ameriso SF, Mohler JG, Suarez M, Fisher M. Morning reduction of cerebral vasomotor reactivity. Neurology. 1994;44:1907–1909. doi: 10.1212/wnl.44.10.1907. [DOI] [PubMed] [Google Scholar]
- Aaslid R, Markwalder TM, Nornes H. Noninvasive transcranial Doppler ultrasound recording of flow velocity in basal cerebral arteries. J Neurosurg. 1982;57:769–774. doi: 10.3171/jns.1982.57.6.0769. [DOI] [PubMed] [Google Scholar]
- Browne HA, Adams L, Simonds AK, Morrell MJ. Impact of age on breathing and resistive pressure in people with and without sleep apnea. J Appl Physiol. 2001;90:1074–1082. doi: 10.1152/jappl.2001.90.3.1074. [DOI] [PubMed] [Google Scholar]
- Browne HAK, Adams L, Simonds AK, Morrell MJ. Ageing does not influence the sleep-related decrease in the hypercapnic ventilatory response. European J Respiratory Physiol. 2003;21:523–529. doi: 10.1183/09031936.03.00039002. [DOI] [PubMed] [Google Scholar]
- Burgess HJ, Kleiman J, Trinder J. Cardiac activity during sleep onset. Psychophysiology. 1999;36:298–306. doi: 10.1017/s0048577299980198. [DOI] [PubMed] [Google Scholar]
- Busija DW, Heistad DD, Marcus ML. Effects of sympathetic nerves on cerebral vessels during acute, moderate increases in arterial pressure in dogs and cats. Circ Res. 1980;46:696–702. doi: 10.1161/01.res.46.5.696. [DOI] [PubMed] [Google Scholar]
- Colrain IM, Trinder J, Fraser G, Wilson GV. Ventilation during sleep onset. J Appl Physiol. 1987;63:2067–2074. doi: 10.1152/jappl.1987.63.5.2067. [DOI] [PubMed] [Google Scholar]
- Corfield DR, Morrell MJ, Guz A. The nature of breathing during hypocapnia in awake man. Respir Physiol. 1995;101:145–159. doi: 10.1016/0034-5687(95)00026-a. [DOI] [PubMed] [Google Scholar]
- Davies RJ, Belt PJ, Roberts SJ, Ali NJ, Stradling JR. Arterial blood pressure responses to graded transient arousal from sleep in normal humans. J Appl Physiol. 1993;74:1123–1130. doi: 10.1152/jappl.1993.74.3.1123. [DOI] [PubMed] [Google Scholar]
- Droste DW, Berger W, Schuler E, Krauss JK. Middle cerebral artery blood flow velocity in healthy persons during wakefulness and sleep: a transcranial Doppler study. Sleep. 1993;16:603–609. [PubMed] [Google Scholar]
- Hajak G, Klingelhofer J, Schulz-Varszegi M, Matzander G, Sander D, Conrad B, Ruther E. Relationship between cerebral blood flow velocities and cerebral electrical activity in sleep. Sleep. 1994;17:11–19. doi: 10.1093/sleep/17.1.11. [DOI] [PubMed] [Google Scholar]
- Horner RL, Brooks D, Kozar LF, Tse S, Phillipson EA. Immediate effects of arousal from sleep on cardiac autonomic outflow in the absence of breathing in dogs. J Appl Physiol. 1995;79:151–162. doi: 10.1152/jappl.1995.79.1.151. [DOI] [PubMed] [Google Scholar]
- Horner RL, Sanford LD, Pack AI, Morrison AR. Activation of a distinct arousal state immediately after spontaneous awakening from sleep. Brain Res. 1997;778:127–134. doi: 10.1016/s0006-8993(97)01045-7. [DOI] [PubMed] [Google Scholar]
- Jordan J, Shannon JR, Diedrich A, Black B, Costa F, Robertson D, Biaggioni I. Interaction of carbon dioxide and sympathetic nervous system activity in the regulation of cerebral perfusion in humans. Hypertension. 2000;36:383–388. doi: 10.1161/01.hyp.36.3.383. [DOI] [PubMed] [Google Scholar]
- Kastrup A, Thomas C, Hartmann C, Schabet M. Sex dependency of cerebrovascular CO2 reactivity in normal subjects. Stroke. 1997;28:2353–2356. doi: 10.1161/01.str.28.12.2353. [DOI] [PubMed] [Google Scholar]
- Kay A, Trinder J, Bowes G, Kim Y. Changes in airway resistance during sleep onset. J Appl Physiol. 1994;76:1600–1607. doi: 10.1152/jappl.1994.76.4.1600. [DOI] [PubMed] [Google Scholar]
- Khoo MC, Gottschalk A, Pack AI. Sleep-induced periodic breathing and apnea: a theoretical study. J Appl Physiol. 1991;70:2014–2024. doi: 10.1152/jappl.1991.70.5.2014. [DOI] [PubMed] [Google Scholar]
- Kjaer TW, Law I, Wiltschiotz G, Paulson OB, Madsen PL. Regional cerebral blood flow during light sleep – a H(2)(15)O-PET study. J Sleep Res. 2002;11:201–207. doi: 10.1046/j.1365-2869.2002.00303.x. [DOI] [PubMed] [Google Scholar]
- Kuboyama T, Hori A, Sato T, Mikami T, Yamaki T, Ueda S. Changes in cerebral blood flow velocity in healthy young men during overnight sleep and while awake. Electroencephalogr Clin Neurophysiol. 1997;102:125–131. doi: 10.1016/s0921-884x(96)95054-7. [DOI] [PubMed] [Google Scholar]
- LeMarbre G, Stauber S, Khayat RN, Puleo DS, Skatrud JB, Morgan BJ. Baroreflex-induced sympathetic activation does not alter cerebrovascular CO2 responsiveness in humans. J Physiol. 2003;551:609–616. doi: 10.1113/jphysiol.2003.046987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen PL, Schmidt JF, Wildschiodtz G, Friberg L, Holm S, Vorstrup S, Lassen NA. Cerebral O2 metabolism and cerebral blood flow in humans during deep and rapid-eye-movement sleep. J Appl Physiol. 1991;70:2597–2601. doi: 10.1152/jappl.1991.70.6.2597. [DOI] [PubMed] [Google Scholar]
- Meadows GE, Dunroy HM, Morrell MJ, Corfield DR. Hypercapnic cerebral vascular reactivity is decreased, in humans, during sleep compared with wakefulness. J Appl Physiol. 2003;94:2197–2202. doi: 10.1152/japplphysiol.00606.2002. [DOI] [PubMed] [Google Scholar]
- Meadows GE, O'Driscoll DM, Simonds AK, Morrell MJ, Corfield DR. Cerebral blood flow response to isocapnic hypoxia during slow-wave sleep and wakefulness. J Appl Physiol. 2004;97:1343–1348. doi: 10.1152/japplphysiol.01101.2003. [DOI] [PubMed] [Google Scholar]
- Morgan BJ, Crabtree DC, Puleo DS, Badr MS, Toiber F, Skatrud JB. Neurocirculatory consequences of abrupt change in sleep state in humans. J Appl Physiol. 1996;80:1627–1636. doi: 10.1152/jappl.1996.80.5.1627. [DOI] [PubMed] [Google Scholar]
- Morrell MJ, Harty HR, Adams L, Guz A. Changes in total pulmonary resistance and PCO2 between wakefulness and sleep in normal human subjects. J Appl Physiol. 1995;78:1339–1349. doi: 10.1152/jappl.1995.78.4.1339. [DOI] [PubMed] [Google Scholar]
- Morrell MJ, Harty HR, Adams L, Guz A. Breathing during wakefulness and NREM sleep in humans without an upper airway. J Appl Physiol. 1996;81:274–281. doi: 10.1152/jappl.1996.81.1.274. [DOI] [PubMed] [Google Scholar]
- Morrell MJ, Shea SA, Adams L, Guz A. Effects of inspiratory support upon breathing in humans during wakefulness and sleep. Respir Physiol. 1993;93:57–70. doi: 10.1016/0034-5687(93)90068-l. [DOI] [PubMed] [Google Scholar]
- Poulin MJ, Liang PJ, Robbins PA. Dynamics of the cerebral blood flow response to step changes in end-tidal PCO2 and PO2 in humans. J Appl Physiol. 1996;81:1084–1095. doi: 10.1152/jappl.1996.81.3.1084. [DOI] [PubMed] [Google Scholar]
- Poulin MJ, Robbins PA. Indexes of flow and cross-sectional area of the middle cerebral artery using doppler ultrasound during hypoxia and hypercapnia in humans. Stroke. 1996;27:2244–2250. doi: 10.1161/01.str.27.12.2244. [DOI] [PubMed] [Google Scholar]
- Rechtschaffen A, Kales A. Bethesda, MD: National Institutes of Health; 1968. A Manual of Standardized Terminology, Techniques, and Scoring System for Sleep Stages of Human Subjects. [Google Scholar]
- Sakai F, Meyer JS, Karacan I, Derman S, Yamamoto M. Normal human sleep: regional cerebral hemodynamics. Ann Neurol. 1980;7:471–478. doi: 10.1002/ana.410070514. [DOI] [PubMed] [Google Scholar]
- Sercombe R, Lacombe P, Aubineau P, Mamo H, Pinard E, Reynier-Rebuffel AM, Seylaz J. Is there an active mechanism limiting the influence of the sympathetic system on the cerebral vascular bed? Evidence for vasomotor escape from sympathetic stimulation in the rabbit. Brain Res. 1979;164:81–102. doi: 10.1016/0006-8993(79)90008-8. [DOI] [PubMed] [Google Scholar]
- Skatrud JB, Dempsey JA. Interaction of sleep state and chemical stimuli in sustaining rhythmic ventilation. J Appl Physiol. 1983;55:813–822. doi: 10.1152/jappl.1983.55.3.813. [DOI] [PubMed] [Google Scholar]
- Townsend RE, Prinz PN, Obrist WD. Human cerebral blood flow during sleep and waking. J Appl Physiol. 1973;35:620–625. doi: 10.1152/jappl.1973.35.5.620. [DOI] [PubMed] [Google Scholar]
- Trinder J, Allen N, Kleiman J, Kralevski V, Kleverlaan D, Anson K, Kim Y. On the nature of cardiovascular activation at an arousal from sleep. Sleep. 2003;26:543–551. [PubMed] [Google Scholar]
- Trinder J, Whitworth F, Kay A, Wilkin P. Respiratory instability during sleep onset. J Appl Physiol. 1992;73:2462–2469. doi: 10.1152/jappl.1992.73.6.2462. [DOI] [PubMed] [Google Scholar]
- Worsnop C, Kay A, Pierce R, Kim Y, Trinder J. Activity of respiratory pump and upper airway muscles during sleep onset. J Appl Physiol. 1998;85:908–920. doi: 10.1152/jappl.1998.85.3.908. [DOI] [PubMed] [Google Scholar]
- Zhang R, Zuckerman JH, Iwasaki K, Wilson TE, Crandall CG, Levine BD. Autonomic neural control of dynamic cerebral autoregulation in humans. Circulation. 2002;106:1814–1820. doi: 10.1161/01.cir.0000031798.07790.fe. [DOI] [PubMed] [Google Scholar]