Abstract
Large, reproducible interindividual differences exist in resting sympathetic nerve activity among normotensive humans with similar arterial pressures, resulting in a lack of correlation between muscle sympathetic nerve activity (MSNA) and arterial pressure among individuals. Although it is known that the arterial pressure is the main short-term determinant of MSNA in humans via the arterial baroreflex, the lack of correlation among individuals suggests that the level of arterial pressure is not the only important input in regulation of MSNA in humans. We studied the relationship between cardiac output (CO) and baroreflex control of sympathetic activity by measuring MSNA (peroneal microneurography), arterial pressure (arterial catheter), CO (acetylene uptake technique) and heart rate (HR; electrocardiogram) in 17 healthy young men during 20 min of supine rest. Across individuals, MSNA did not correlate with mean or diastolic blood pressure (r < 0.01 for both), but displayed a significant negative correlation with CO (r = −0.71, P= 0.001). To assess whether CO is related to arterial baroreflex control of MSNA, we constructed a baroreflex threshold diagram for each individual by plotting the percentage occurrence of a sympathetic burst against diastolic pressure. The mid-point of the diagram (T50) at which 50% of cardiac cycles are associated with bursts, was inversely related to CO (r=−0.75, P < 0.001) and stroke volume (SV) (r = −0.57, P = 0.015). We conclude that dynamic inputs from CO and SV are important in regulation of baroreflex control of MSNA in healthy, normotensive humans. This results in a balance between CO and sympathetically mediated vasoconstriction that may contribute importantly to normal regulation of arterial pressure in humans.
Sympathetic nerve activity is of major importance for the control of peripheral vascular resistance in humans, but its relationship to arterial blood pressure is complex. In a given individual, on a beat-to-beat basis, arterial pressure has a large influence via the arterial baroreflex, so that even minor changes in arterial pressure elicit opposing reflex changes in sympathetic nerve traffic. However, among individuals, a lack of correlation between muscle sympathetic nerve activity (MSNA) and arterial pressure has been noted (Sundlöf & Wallin, 1978; Skarphedinsson et al. 1997). This is related to the fact that, although MSNA at rest is remarkably reproducible in an individual over time (Sundlöf & Wallin, 1977; Fagius & Wallin, 1993), there are large interindividual differences among humans with similar arterial pressures in the normotensive range (Sundlöf & Wallin, 1978; Skarphedinsson et al. 1997). Evidence from studies of noradrenaline spillover in the heart and kidney suggest that similar large interindividual differences are present in sympathetic activity to other vascular beds as well (Wallin et al. 1992; 1996).
Taken together, these observations suggest that, in the long term, baroreflex control of MSNA is modulated by factors in addition to the level of arterial pressure alone. One possibility is that there is a balance between cardiac output (CO) and sympathetically mediated vasoconstriction. As mean arterial pressure is the product of CO and total peripheral vascular resistance (TPR), and MSNA contributes significantly to TPR, an inhibitory influence of CO on MSNA would explain the lack of relationship between MSNA and arterial pressure among normotensives humans.
Interindividual differences in MSNA, as they relate to blood pressure and cardiac output, have not been studied previously. Our goal in the present experiments was to assess these relationships in normotensive subjects. We assessed baroreflex control of MSNA by constructing baroreflex threshold diagrams of the relationship between diastolic blood pressure (DBP) and the occurrence of sympathetic bursts (Sundlöf & Wallin, 1978; Kienbaum et al. 2001). We then investigated whether the mid-point (the T50 value) of this baroreflex function curve was related to CO, stroke volume (SV) and heart rate (HR).
The specific hypotheses tested were: (i) that resting MSNA, expressed as bursts per minute or bursts per 100 heart beats (hb), is inversely related to CO; and (ii) that arterial baroreflex control of MSNA shows a systematic relationship to CO in resting humans.
Methods
Subjects
The protocol for this study was approved by the Institutional Review Board of the Mayo Foundation, and the study was performed in accordance with the Declaration of Helsinki. Eighteen healthy young men (mean age ±s.e.m. 26.9 ± 1.2 years; height 1.78 ± 0.03 m; body weight 77.6 ± 2.5 kg) volunteered to participate and gave written informed consent. (In one experiment, technical difficulties led to an inability to measure cardiac output; thus data involving CO, SV and TPR are reported for n = 17 subjects). The subjects were non-smokers with no history of cardiovascular or other chronic diseases. They were asked not to consume anything except water in the 2 h before the experiment, and not to consume caffeine on the day of the experiment, or alcohol within 24 h of the experiment.
Measurements
All studies were performed in a General Clinical Research Center laboratory at the Mayo Clinic, where ambient temperature was controlled between 22 and 24°C. Upon arrival to the laboratory, subjects rested quietly in the supine position during instrumentation. A 5-cm, 20-gauge arterial catheter was placed in a radial or brachial artery, using aseptic technique after local anaesthesia with 2% lignocaine. This catheter was connected to a pressure transducer placed at heart level and used for measurement of arterial pressure. A 3-lead ECG was used for continuous monitoring of HR.
CO was measured using the open-circuit acetylene uptake technique, as previously described (Johnson et al. 2000). This technique has been validated against direct Fick measurements of cardiac outputs for a range of cardiac output values (Johnson et al. 2000). The instrumentation period included a practice measurement of CO to familiarize the subject with the procedure. This practice value was not included in the CO data presented in the results.
Multiunit MSNA was recorded with a tungsten microelectrode in the peroneal nerve, posterior to the fibular head, as described by Sundlöf & Wallin (1977). The recorded signal was amplified 80 000-fold, band-pass filtered (700–2000 Hz), rectified and integrated (resistance-capacitance integrator circuit, time-constant 0.1 s) using a nerve-traffic analyser.
Protocol
We continuously recorded arterial pressure, ECG and integrated MSNA throughout a period of 20 min of supine rest. CO was measured in duplicate during the last 5–7 min of the recording period.
Data analysis
Data were sampled at 240 Hz and stored on a personal computer for offline analysis. Mean arterial pressure (MAP) was calculated as the time integral over the pressure pulse. MSNA, HR, MAP and diastolic blood pressure (DBP) were taken as 4-min averages during the 4-min period immediately preceding the first CO measurement. CO is reported as the average of the two measurements for each individual. SV was calculated as CO/HR; TPR was calculated as MAP/CO.
Sympathetic bursts in the integrated neurogram were identified using a custom-manufactured automated analysis program; burst identification was then corrected by visual inspection by a single investigator. The program then compensated for baroreflex latency, and associated each sympathetic burst with the appropriate cardiac cycle. Baroreflex control of MSNA was analysed as previously reported by Sundlöf & Wallin (1978) and Kienbaum et al. (2001). Briefly, for each subject an automated ‘threshold analysis’ was performed, in which the percentage occurrence of a sympathetic burst was plotted against diastolic pressure (Kienbaum et al. 2001). This relationship, referred to as a threshold curve, is approximately linear and varies among individuals. For each threshold curve a linear slope and a T50 value can be identified. The T50 value represents the diastolic pressure at which 50% of cardiac cycles are associated with sympathetic bursts, and the slope provides a measure of the variability of the threshold for occurrence of bursts around this T50 value.
Statistics
Group average data are expressed as mean ±s.e.m. To assess the relationship of MSNA and T50 values with cardiovascular variables, linear regression analysis was used. P < 0.05 was accepted as statistically significant.
Results
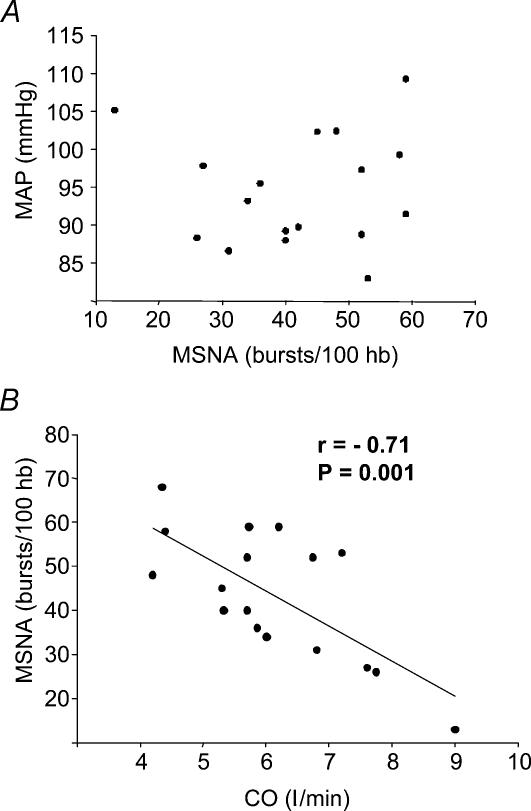
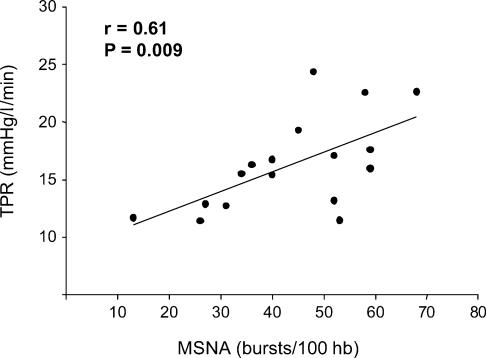
Group average data for resting haemodynamic and neural variables were as follows: MAP 95 ± 2 mmHg; CO 6.1 ± 0.4 l min−1; HR 57 ± 2 bursts min−1; SV 108 ± 6 ml; MSNA 24 ± 2 bursts min−1, and 44 ± 3 bursts (100 hb)−1. Resting MSNA did not correlate with MAP (Fig. 1A) or DBP (r= 0.01 for both). However, as shown in Fig. 1B, MSNA displayed a significant inverse correlation with CO (r=−0.71, P= 0.001). The values shown in Fig. 1 represent MSNA expressed as bursts (100 hb)−1; the relationships were similar when MSNA was expressed as bursts min−1 (for MAP, r= 0.00; for CO, r=−0.46; P= 0.06). Additionally, TPR was significantly correlated with MSNA (r= 0.61, P= 0.009), as shown in Fig. 2.
Figure 1. Regression analysis of MSNA with MAP and CO.
A, lack of correlation between mean arterial pressure (MAP) and MSNA across all subjects. B, linear regression analysis showing significant inverse correlation between CO and MSNA.
Figure 2. Linear regression plot showing significant correlation between MSNA and TPR.
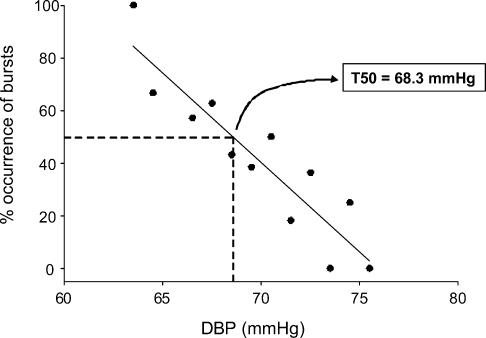
The relationship between CO and MSNA suggests that CO influences the way the arterial baroreflex controls MSNA. Therefore, our next goal was to assess whether CO or some component thereof (HR or SV) was related to baroreflex control of MSNA. Figure 3 shows a representative example of a baroreflex threshold curve in one individual. The T50 calculation, demonstrated in the figure, shows the diastolic pressure at which 50% of cardiac cycles were associated with sympathetic bursts.
Figure 3. Representative example of a threshold curve in one individual.
Lower diastolic pressures were associated with a higher percentage occurrence of sympathetic bursts, and vice-versa. The T50 value is the diastolic pressure at which 50% of cardiac cycles are associated with sympathetic bursts (in this case, 68.3 mmHg).
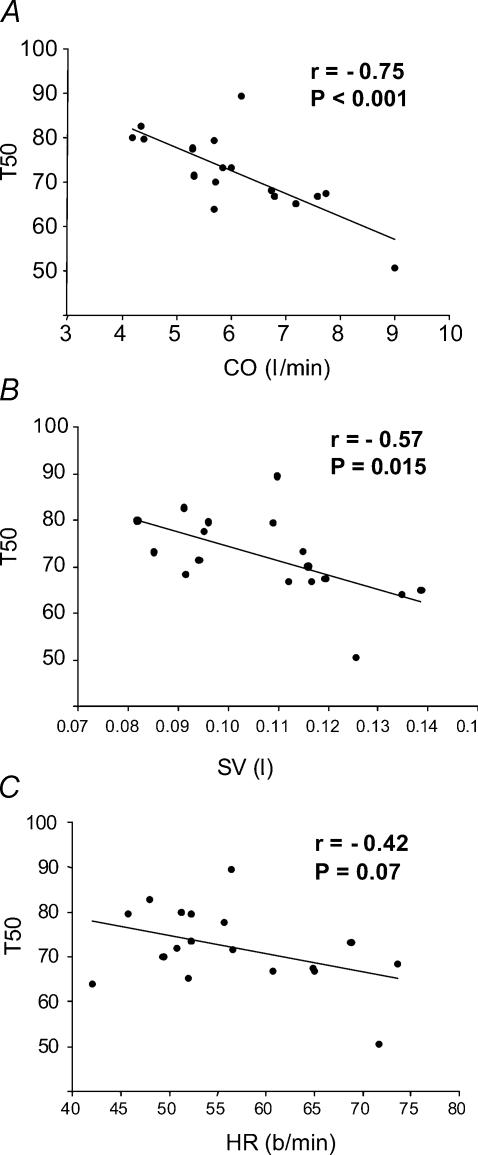
Figure 4A shows regression analysis of T50 values plotted as a function of CO for all subjects. There was a significant relationship between the two variables (r=−0.75, P < 0.001). Figure 4B and C shows corresponding relationships for SV (r=−0.57, P= 0.015) and HR (r=−0.42, P= 0.07), respectively. As shown in Fig. 5, there was also a significant correlation between our index of baroreflex control of MSNA (i.e. T50) and resting sympathetic activity (r= 0.72, P < 0.001).
Figure 4. Linear regression plots of the relationships between T50 and CO, SV and HR.
A, linear regression plot of the relationship between T50 and CO showing significant inverse correlation. B, the linear regression plot for T50versus SV also shows a significant inverse correlation, suggesting that SV may contribute to the mechanism by which CO influences baroreflex control of MSNA. C, the relationship between T50 and HR was weaker and non-significant, suggesting that HR may be less important with regard to the CO–baroreflex interaction.
Figure 5. Linear regression analysis of MSNA as a function of T50.
Baroreflex control of MSNA, as represented by the T50 value, was predictive of MSNA at rest among individuals.
The relationships observed for CO and MSNA were not related to variations in body size among our subjects. Relationships between CO and indices of body size (e.g. surface area and body weight) were weak and non-significant (r≤ 0.38, P > 0.10), and MSNA was not related to body size (r= 0.17). Furthermore, correcting for body size by presenting CO data in terms of cardiac index (CI = CO/body surface area) did not alter the relationships observed (for example, the relation between CI and MSNA had a r-value of 0.67).
Discussion
The most striking new findings of the present study are twofold. First, MSNA was inversely related to CO, such that individuals with high resting CO had low MSNA, and vice versa. Second, baroreflex control of MSNA (the T50 value of the threshold curve) was inversely related to CO and SV, suggesting that these latter variables have inhibitory influences on baroreflex control of MSNA at rest in healthy subjects.
In conducting the present study, our hypotheses were formed based on the previous observations that sympathetic vasoconstrictor nerve traffic exhibits no systematic relationship with resting arterial pressure among individuals (Sundlöf & Wallin, 1978). Our present results confirm the lack of correlation, and suggest that a balance between MSNA and CO at rest may help maintain arterial pressure relatively constant in spite of interindividual differences in sympathetic traffic. MAP can be expressed as the product of CO and TPR. There is evidence that resting MSNA reflects the levels of resting activity in cardiac and renal sympathetic nerves as well (Wallin et al. 1992, 1996). This, together with the present finding of a significant correlation between MSNA and TPR (Fig. 2), supports the idea that, under resting conditions, MSNA is a good indicator of ‘net’ peripheral vasoconstrictor nerve traffic. As SNA is a major controller of TPR, it is then logical that an inverse relationship between MSNA and CO results in mean arterial pressures which appear independent of MSNA.
In the present study, we used the midpoint (T50) of the baroreflex threshold curve (discussed below), a control characteristic of baroreflex control of MSNA, to assess whether baroreflex control of MSNA was influenced by CO. Our findings of significant inverse relationships of T50 with both CO and SV suggest important roles for these dynamic inputs in baroreflex control of MSNA in humans. These results have several mechanistic implications, as discussed in the following paragraphs.
CO is a function of HR and SV, and the relationship between T50 and SV (Fig. 4B) suggests that SV is a key factor. Recent work from Levine and colleagues supports an important role for stroke volume in control of MSNA (Levine et al. 2002; Fu et al. 2005). Their data suggest that stroke volume, and not diastolic pressure, is the major determinant of MSNA responses during perturbations such as head-up tilt, in which SV is decreased, but diastolic pressure can be unchanged or even increase. Studies such as these led the authors to propose that SV may be a more appropriate independent variable for analysis of MSNA responses than is diastolic pressure during simulated orthostasis (Fu et al. 2005). At the very least, their data and the data from the present study are supportive of an important modulatory role for stroke volume in arterial baroreflex regulation of MSNA in humans.
In considering the present data, it is important that arterial baroreceptors respond to mechanical distortion or deformation, and not to arterial pressure per se (Angell James, 1971). In this context, stroke volume directly influences other dynamic variables like pulse pressure and the rate of change of pressure (dP/dt), which alter the mechanical deformation of the baroreceptive areas. Increased stroke volume, for example, leads to increased pulse pressure, which has been shown to sensitize baroreceptor afferent activity in animal models (Kirchheim, 1976; Chapleau & Abboud 1987, 1989), an effect that would increase reflex sympathoinhibition. Similar influences of stroke volume and/or cardiac output may be important in explaining the influences of these variables that we observed in the present study. This suggests the interesting possibility that what appears to be a lack of relationship between arterial pressure and MSNA among normotensive humans may be the representation of individual points on several different baroreflex curves that vary based on dynamic inputs like blood flow (CO) and stroke volume (pulse pressure, dP/dt).
In subjects with high CO and SV, the average filling of the heart should be greater than in subjects with low CO and SV. This greater distension would increase afferent input from cardiopulmonary receptors (Johnson et al. 1974; Weisbrod et al. 2004), which would inhibit efferent sympathetic activity (Koike et al. 1975; Victor & Mark, 1985; Charkoudian et al. 2004). An argument against this possibility may be the marked reduction of MSNA that occurs after cardiac transplantation (Kaye et al. 1993; Rundqvist et al. 1996, 1997); cardiac afferents are denervated in a transplanted heart. Related possibilities are that reflex inhibition of MSNA could occur via increased intermittent stretch on arterial baroreceptor populations with increased SV (Lacolley et al. 1992; Taylor et al. 1995), or via increased baroreceptor afferent activity associated with increased flow-mediated shear stress at higher levels of CO (Hajduczok et al. 1988). Several other factors involving interactions among control of blood volume, cardiac contractility, and mechanisms of adrenergic vasoconstriction may have implications for the interpretation of the present data, but can not be directly addressed within the context of the present analysis.
Our use of the T50 value as an important control characteristic of the arterial baroreflex is based on the fact that the threshold for outflow of sympathetic impulses (bursts) is not constant even at rest. Presumably, brainstem nuclei involved in central control of autonomic function (such as the nucleus tractus solitarius) receive a number of peripheral afferent inputs, as well as input from higher brain centres (e.g. emotionally induced), the relative strengths of which are graded and vary over time. The net effect of the variations is to induce an instability of the baroreflex threshold, i.e. cardiac cycles with identical blood pressure values are not consistently associated with efferent sympathetic activity. The T50 value provides information about the average setting of the baroreflex over the actual range of blood pressures, and the slope of the diagram (not used in the present study) is a measure of the variability of the threshold. In principle, the T50 value can be regarded as analogous to the EC50 value (effective concentration needed to elicit a 50% response) in assessment of responsiveness to pharmacological substances. As shown in Fig. 5, a lower T50 (as seen in individuals with higher CO) results in less sympathetic activity.
Our observations suggest that CO and SV contribute an important dynamic component to baroreflex control of MSNA. Over the long-term, this allows both major contributors to MAP (CO and TPR) to effectively balance each other, at least in normotensive individuals. On a beat-by-beat basis, the dynamic influence of pulse pressure is a major reason for the cardiac rhythmicity of afferent input from the baroreceptors to central autonomic nuclei, and for the marked cardiac rhythmicity of efferent MSNA (Sundlöf & Wallin, 1977). Thus, the central neural regulation of baroreflex-controlled vasoconstrictor activity is performed on a ‘per-heartbeat’ basis. This probably explains why the relationships we observed between MSNA and systemic haemodynamic variables were always stronger when MSNA was expressed as bursts (100 hb)−1 rather than bursts min−1. For example, the relationship between CO and bursts min−1 had an r-value of 0.46, whereas that of CO and bursts (100 hb)−1 had an r= 0.71.
Interestingly, we also observed a stronger relationship between MSNA and TPR when MSNA was expressed as bursts (100 hb)−1 (r= 0.61) compared to bursts min−1 (r= 0.35). This may be counter-intuitive, as one would expect vasoconstriction, and TPR, to be related to total activity per unit time, not to heart rate. However, the exact relationships among nerve activity, noradrenaline kinetics, and resultant vasoconstriction are poorly understood. It may be that these results point to an important influence of heart rate on the vasoconstrictor influence of a given set of sympathetic bursts. For example, as muscle sympathetic bursts are cardiac cycle-dependent, a given burst will be likely to have a longer duration (and more noradrenaline release) during longer cardiac cycles (slower heart rates) than in shorter cardiac cycles (faster heart rates). This is an interesting question deserving of further study.
Although the present findings contribute to the understanding of how interindividual differences in MSNA, CO and SV are related to each other, our results provide no information as to why they arise. As noted above, the interindividual differences in resting MSNA are remarkably reproducible over time, making it unlikely that short-term influences of environmental changes have sustained effects on these values during steady-state rest. Some evidence suggests that interindividual differences in MSNA are of genetic origin (Wallin et al. 1993); it seems likely that this also applies to the corresponding variability in CO. This possibility would agree with recent findings of genetic polymorphisms in the β2-adrenergic receptor that may contribute to differences in control of peripheral blood flow and blood pressure among humans (Jindra et al. 2002; Castellano et al. 2003; Garovic et al. 2003).
The present findings raise the interesting possibility that pathophysiological conditions, in which blood pressure is not regulated appropriately, result from an imbalance in the factors discussed here which usually keep blood pressure at normal levels. It has been known for many years that arterial pressure must be the result of a balance between control of peripheral vascular resistance and central haemodynamics. Our study provides important new evidence that dynamic inputs from cardiac output and stroke volume may be important modulators of baroreflex control of sympathetic activity in humans. Thus, a pathological imbalance in the variables discussed here, such as an inappropriately elevated level of sympathetic nerve activity at a normal level of cardiac output, or vice versa, could be the basis for some disorders of arterial pressure regulation. Data from Narkiewicz & Somers (1999) may be consistent with this idea: they showed that in a subgroup of men with higher arterial pressures and heart rates (within the normal range), higher MSNA was associated with higher arterial pressure.
In summary, the results of the present study demonstrate a strong inverse relationship between CO and MSNA at rest, and are consistent with important modulatory influences of CO and SV on arterial baroreflex control of MSNA. We propose that these influences explain in part the apparent lack of relationship between MSNA and arterial pressure among normotensive humans. These findings suggest that the balance of CO and sympathetically mediated vasoconstriction is an important integrated mechanism underlying normal arterial pressure regulation, and may also have implications for mechanisms of pathophysiology.
Acknowledgments
We are grateful to the subjects for their enthusiastic participation, and to Shelly Roberts, Diane Wick, Karen Krucker, Ruth Kraft and Tomas Karlsson for excellent assistance in these studies and analysis of data. These studies were supported by NIH HL73884, NS32352, RR00585 and Swedish Medical Research Council Grant 12170.
References
- Angell James JE. The effects of changes of extramural, ‘intrathoracic’, pressure on aortic arch baroreceptors. J Physiol. 1971;214:89–103. doi: 10.1113/jphysiol.1971.sp009420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano M, Rossi F, Giacche M, Perani C, Rivadossi F, Muiesan ML, Salvetti M, Beschi M, Rizzoni D, Agabiti-Rosei E. Beta2-adrenergic receptor gene polymorphism, age, and cardiovascular phenotypes. Hypertension. 2003;41:361–367. doi: 10.1161/01.hyp.0000052831.85600.79. [DOI] [PubMed] [Google Scholar]
- Chapleau MW, Abboud FM. Contrasting effects of static and pulsatile pressure on carotid baroreceptor activity in dogs. Circ Res. 1987;61:648–658. doi: 10.1161/01.res.61.5.648. [DOI] [PubMed] [Google Scholar]
- Chapleau MW, Abboud FM. Determinants of sensitization of carotid baroreceptors by pulsatile pressure in dogs. Circ Res. 1989;65:566–577. doi: 10.1161/01.res.65.3.566. [DOI] [PubMed] [Google Scholar]
- Charkoudian N, Martin EA, Dinenno FA, Eisenach JH, Dietz NM, Joyner MJ. Influence of increased central venous pressure on baroreflex control of sympathetic activity in humans. Am J Physiol Heart Circ Physiol. 2004;287:H1658–H1662. doi: 10.1152/ajpheart.00265.2004. [DOI] [PubMed] [Google Scholar]
- Fagius J, Wallin BG. Long-term variability and reproducibility of resting human muscle nerve sympathetic activity at rest, as reassessed after a decade. Clin Auton Res. 1993;3:201–205. doi: 10.1007/BF01826234. [DOI] [PubMed] [Google Scholar]
- Fu Q, Witkowski S, Okazaki K, Levine BD. Effects of gender and hypovolemia on sympathetic neural responses to orthostatic stress. Am J Physiol Regul Integr Comp Physiol. 2005;289:R109–R116. doi: 10.1152/ajpregu.00013.2005. [DOI] [PubMed] [Google Scholar]
- Garovic VD, Joyner MJ, Dietz NM, Boerwinkle E, Turner ST. Beta2-adrenergic receptor polymorphism and nitric oxide-dependent forearm blood flow responses to isoproterenol in humans. J Physiol. 2003;546:583–589. doi: 10.1113/jphysiol.2002.031138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajduczok G, Chapleau MW, Abboud FM. Rheoreceptors in the carotid sinus of dog. Proc Natl Acad Sci U S A. 1988;85:7399–7403. doi: 10.1073/pnas.85.19.7399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jindra A, Horky K, Peleska J, Jachymova M, Bultas J, Umnerova V, Heller S, Hlubocka Z. Association analysis of Arg16Gly polymorphism of the beta2-adrenergic receptor gene in offspring from hypertensive and normotensive families. Blood Press. 2002;11:213–217. doi: 10.1080/08037050213761. [DOI] [PubMed] [Google Scholar]
- Johnson BD, Beck KC, Proctor DN, Miller J, Dietz NM, Joyner MJ. Cardiac output during exercise by the open circuit acetylene washin method: comparison with direct Fick.PG -1650-8. J Appl Physiol. 2000;88:1650–1658. doi: 10.1152/jappl.2000.88.5.1650. [DOI] [PubMed] [Google Scholar]
- Johnson JM, Rowell LB, Niederberger M, Eisman MM. Human splanchnic and forearm vasoconstrictor responses to reductions of right atrial and aortic pressures. Circ Res. 1974;34:515–524. doi: 10.1161/01.res.34.4.515. [DOI] [PubMed] [Google Scholar]
- Kaye D, Thompson J, Jennings G, Esler M. Cyclosporine therapy after cardiac transplantation causes hypertension and renal vasoconstriction without sympathetic activation. Circulation. 1993;88:1101–1109. doi: 10.1161/01.cir.88.3.1101. [DOI] [PubMed] [Google Scholar]
- Kienbaum P, Karlssonn T, Sverrisdottir YB, Elam M, Wallin BG. Two sites for modulation of human sympathetic activity by arterial baroreceptors? J Physiol. 2001;531:861–869. doi: 10.1111/j.1469-7793.2001.0861h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchheim HR. Systemic arterial baroreceptor reflexes. Physiol Rev. 1976;56:100–177. doi: 10.1152/physrev.1976.56.1.100. [DOI] [PubMed] [Google Scholar]
- Koike H, Mark AL, Heistad DD, Schmid PG. Influence of cardiopulmonary vagal afferent activity on carotid chemoreceptor and baroreceptor reflexes in the dog. Circ Res. 1975;37:422–429. doi: 10.1161/01.res.37.4.422. [DOI] [PubMed] [Google Scholar]
- Lacolley PJ, Pannier BM, Slama MA, Cuche JL, Hoeks AP, Laurent S, London GM, Safar ME. Carotid arterial haemodynamics after mild degrees of lower-body negative pressure in man. Clin Sci (Lond) 1992;83:535–540. doi: 10.1042/cs0830535. [DOI] [PubMed] [Google Scholar]
- Levine BD, Pawelczyk JA, Ertl AC, Cox JF, Zuckerman JH, Diedrich A, Biaggioni I, Ray CA, Smith ML, Iwase S, Saito M, Sugiyama Y, Mano T, Zhang R, Iwasaki K, Lane LD, Buckey JC, Jr, Cooke WH, Baisch FJ, Eckberg DL, Blomqvist CG. Human muscle sympathetic neural and haemodynamic responses to tilt following spaceflight. J Physiol. 2002;538:331–340. doi: 10.1113/jphysiol.2001.012575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narkiewicz K, Somers VK. Interactive effect of heart rate and muscle sympathetic nerve activity on blood pressure. Circulation. 1999;100:2514–2518. doi: 10.1161/01.cir.100.25.2514. [DOI] [PubMed] [Google Scholar]
- Rundqvist B, Casale R, Bergmann-Sverrisdottir Y, Friberg P, Mortara A, Elam M. Rapid fall in sympathetic nerve hyperactivity in patients with heart failure after cardiac transplantation. J Card Fail. 1997;3:21–26. doi: 10.1016/s1071-9164(97)90005-1. [DOI] [PubMed] [Google Scholar]
- Rundqvist B, Elam M, Eisenhofer G, Friberg P. Normalization of total body and regional sympathetic hyperactivity in heart failure after heart transplantation. J Heart Lung Transplant. 1996;15:516–526. [PubMed] [Google Scholar]
- Skarphedinsson JO, Elam M, Jungersten L, Wallin BG. Sympathetic nerve traffic correlates with the release of nitric oxide in humans: implications for blood pressure control. J Physiol. 1997;501:671–675. doi: 10.1111/j.1469-7793.1997.671bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundlöf G, Wallin BG. The variability of muscle nerve sympathetic activity in resting recumbent man. J Physiol. 1977;272:383–397. doi: 10.1113/jphysiol.1977.sp012050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundlöf G, Wallin BG. Human muscle nerve sympathetic activity at rest. Relationship to blood pressure and age. J Physiol. 1978;274:621–637. doi: 10.1113/jphysiol.1978.sp012170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JA, Halliwill JR, Brown TE, Hayano J, Eckberg DL. ‘Non-hypotensive’ hypovolaemia reduces ascending aortic dimensions in humans. J Physiol. 1995;483:289–298. doi: 10.1113/jphysiol.1995.sp020585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor RG, Mark AL. Interaction of cardiopulmonary and carotid baroreflex control of vascular resistance in humans. J Clin Invest. 1985;76:1592–1598. doi: 10.1172/JCI112142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallin BG, Esler M, Dorward P, Eisenhofer G, Ferrier C, Westerman R, Jennings G. Simultaneous measurements of cardiac noradrenaline spillover and sympathetic outflow to skeletal muscle in humans. J Physiol. 1992;453:45–58. doi: 10.1113/jphysiol.1992.sp019217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallin BG, Kunimoto MM, Sellgren J. Possible genetic influence on the strength of human muscle nerve sympathetic activity at rest. Hypertension. 1993;22:282–284. doi: 10.1161/01.hyp.22.3.282. [DOI] [PubMed] [Google Scholar]
- Wallin BG, Thompson JM, Jennings GL, Esler MD. Renal noradrenaline spillover correlates with muscle sympathetic activity in humans. J Physiol. 1996;491:881–887. doi: 10.1113/jphysiol.1996.sp021265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisbrod CJ, Arnolda LF, McKitrick DJ, O'Driscoll G, Potter K, Green DJ. Vasomotor responses to decreased venous return: effects of cardiac deafferentation in humans. J Physiol. 2004;560:919–927. doi: 10.1113/jphysiol.2004.069732. [DOI] [PMC free article] [PubMed] [Google Scholar]