Abstract
P/C-type inactivation of Kv channels is thought to involve conformational changes in the outer pore of the channel, culminating in a partial constriction of the selectivity filter. Recent studies have identified a number of phenotypic differences in the inactivation properties of different Kv channels, including different sensitivities to elevation of extracellular K+ concentration, and different state dependencies of inactivation. We have demonstrated that an alternatively spliced short form of Kv1.5, resulting in disruption of the T1 domain, exhibits a shift in the state dependence of inactivation in this channel, and in the current study we have examined this further to contrast the properties of inactivation from open versus closed states. In a TEA+-sensitive mutant of Kv1.5 (Kv1.5 R487T), 10 mm extracellular TEA+ inhibits inactivation in both full-length and T1-deleted channels, but does not inhibit closed-state inactivation in T1-deleted channel forms. Similarly, substitution of K+ and Na+ with Cs+ ions in the recording medium inhibits inactivation of both full-length and T1-deleted channel forms, but fails to inhibit closed-state inactivation of T1-deleted channels. Collectively, these data distinguish between open-state and closed-state inactivation, and suggest the presence of multiple possible mechanisms of inactivation coexisting in Kv1 channels.
Recent studies have demonstrated that different Kv channel types can exhibit a broad spectrum of non-N-type inactivation phenotypes, with differences in sensitivity to extracellular K+ and TEA+, and differences in state dependence (Klemic et al. 1998, 2001; Jerng et al. 1999). Classical P/C-type inactivation of Shaker and its mammalian Kv1 homologues is generally not voltage dependent at potentials that saturate channel open probability, and is inhibited by extracellular TEA+ or elevation of extracellular K+ (Grissmer & Cahalan, 1989; Choi et al. 1991; Hoshi et al. 1991; Lopez-Barneo et al. 1993). In contrast, inactivation of Kv2.1 channels is accelerated by high concentrations of extracellular K+, and exhibits an apparent voltage dependence, such that inactivation is favoured at voltages near the V1/2 of activation, and reduced at higher voltages (VanDongen et al. 1990; Klemic et al. 1998; Immke et al. 1999). This voltage dependence results in a characteristic upturned inactivation–voltage relationship that has led to the term ‘U-type inactivation’, and has been attributed to inactivation that occurs preferentially from partially activated closed states (Klemic et al. 1998, 2001; Kurata et al. 2001). Other channels, including the rapidly inactivating mammalian Kv4 family, also exhibit prominent closed-state inactivation that is accelerated by elevated extracellular K+ concentrations (Jerng et al. 1999; Bahring et al. 2001). There appears to be considerable diversity among non-N-type inactivation mechanisms of Kv channels (Rasmusson et al. 1998).
We have previously demonstrated that disruption of the T1 domain of the Shaker homologue Kv1.5 dramatically alters the inactivation phenotype of this channel (Kurata et al. 2001, 2002). Full-length Kv1.5 channels exhibit a voltage-independent inactivation mechanism, with properties similar to a classical mechanism of P/C-type inactivation (Fedida et al. 1999; Kurata et al. 2001). However, a truncated form of Kv1.5 (Kv1.5ΔN209), that has been detected in murine and human cardiomyocytes (Tamkun et al. 1991; Attali et al. 1993; Fedida et al. 1993), exhibits a significant voltage dependence of inactivation that is very similar to that observed in Kv2.1 (Kurata et al. 2001, 2002). Kv1.5ΔN209 also exhibits significant voltage dependence of recovery from inactivation, and rate-dependent cumulative inactivation properties that differ from the full-length channel, indicating that N-terminal truncation alters the state dependence of inactivation of Kv1.5 (Klemic et al. 1998; Kurata et al. 2001, 2002). Most importantly, N-terminal truncations of Kv1.5 remain the only known mutations to confer a U-shaped inactivation–voltage relationship in Kv channels. Therefore, from a biophysical perspective, comparisons of the inactivation properties of full-length Kv1.5 and truncated forms of the channel may provide novel insights into the properties of channel inactivation taking place at different stages in the activation pathway. Furthermore, despite the fact that truncated isoforms of Kv1.5 may arise by alternative splicing in cardiomyocytes (Attali et al. 1993), and reports of T1-deleted forms of Kv1.1 generated by post-translational proteolysis (Strang et al. 2001), there is little understanding of how the unique gating properties of truncated Kv1.5 channels may alter or contribute to the cardiac action potential.
In this study, we set out to compare the properties of inactivation of full-length Kv1.5 channels with the closed-state inactivation process that takes place in Kv1.5ΔN209. To this end, we have characterized the modulation of inactivation and recovery from inactivation of full-length Kv1.5 and Kv1.5ΔN209 channels using various experimental conditions that modulate inactivation in one or both channels. Our experiments identify several conditions that appear to selectively inhibit the inactivation mechanism present in full-length Kv1.5, and do not affect the closed-state inactivation process present in Kv1.5ΔN209 channels. Collectively, the results suggest that inactivation of Kv1.5ΔN209 channels is composed of two distinct processes: an inactivation mechanism similar to full-length Kv1.5, and a closed-state inactivation mechanism that is absent or minimal in full-length Kv1.5 channels and exhibits different regulation by permeant cations, extracellular TEA+, and voltage.
Methods
Cell culture
All cells were grown in Minimal Essential Medium (MEM) with 10% fetal bovine serum, at 37°C in an air–5% CO2 incubator. Unless otherwise noted HEK-293 cell lines stably expressing full-length human Kv1.5 or the short form of human Kv1.5, Kv1.5ΔN209, were used in all experiments. The Kv1.5ΔN209 short form was generated by removal of the NcoI–NcoI fragment of Kv1.5 (Kurata et al. 2001). HEK-293 cells were stably transfected with full-length Kv1.5 or Kv1.5ΔN209 cDNAs using LipofectACE reagent (Canadian Life Technologies, Bramalea, ON, USA).
Measurements of full-length and truncated Kv1.5 R487T mutant channels were carried out in transiently transfected HEK 293 cells. One day before transfection, cells were plated on glass coverslips in 35 mm Petri dishes with 20–30% confluence. On the day of transfection, cells were washed once with MEM with 10% fetal bovine serum. In order to identify the transfected cells efficiently, channel DNA was cotransfected with a vector encoding GFP. Channel DNA was mixed with pGFP (1: 1 ratio, 1 μg each) and 2 μl of LipofectAMINE 2000 (Canadian Life Technologies) in 100 μl of serum-free media, then added to the dishes containing HEK 293 cells. Cells were allowed to grow overnight before recording, and positive transfectants were identified at the time of recording using an epifluorescence attachment on the patch clamp recording apparatus.
Solutions
For recording in K+ conditions, patch pipettes contained (mm): NaCl, 5; KCl, 135; Na2ATP, 4; GTP, 0.1; MgCl2, 1; EGTA, 5; Hepes, 10; adjusted to pH 7.2 with KOH. The low extracellular K+ bath solution contained (mm): NaCl, 135; KCl, 5; Hepes, 10; sodium acetate, 2.8; MgCl2, 1; CaCl2, 1; adjusted to pH 7.4 with NaOH. The high extracellular K+ bath solution contained (mm): KCl, 135; Hepes, 10; dextrose, 5; MgCl2, 1; CaCl2, 1; adjusted to pH 7.4 with KOH. For recordings in Cs+ or Rb+ solutions, patch pipettes contained (mm): CsCl or RbCl, 135; MgCl2, 1; EGTA, 10; Hepes, 5; adjusted to pH 7.2 with CsOH or RbOH. Bath solutions contained (mm): CsCl or RbCl, 135; Hepes, 10; dextrose, 10; MgCl2, 1; CaCl2, 1; and was adjusted to pH 7.4 with CsOH or RbOH. All chemicals were from Sigma Aldrich Chemical Co. (Mississauga, Ont., Canada).
Immunoblotting and antibodies
Canine heart tissue was kindly supplied by Dr David Van Wagoner of the Cleveland Clinic. All surgical procedures and experimental protocols were approved by the Cleveland Clinic Foundation Institutional Animal Care and Use Committee (Cleveland, OH, USA). Approximately 0.2 g of frozen heart was minced on dry ice and homogenized immediately in 5 ml lysis buffer (0.025 m phosphate buffer, pH 7.2 with, 10% glycerol and 0.5% IGEPAL (Sigma), 1 mm iodoacetamide, 0.2 trypsin inhibitor units ml−1 aprotinin, 1 mm PMSF) using an Ultra-Turrax T25 homogenizer (IKA Laboratechnik, Germany). Nuclei and cell debris were pelleted at 1000 g for 10 min and the supernatant was run on SDS-PAGE gels for Western analysis. All homogenization and fractionations were carried out at 4°C. Blots were probed with a polyclonal anti-Kv1.5 antibody generated in our laboratory, a rabbit antibody against the C-terminus of hKv1.5 (aa 537–553 EQGTQSQGPGLDRGVQR; 1 : 10 000). This antibody was chosen for its unique sequence region at the C-terminus that is not shared with other Kv channels. It shares 12 of 17 residues with canine Kv1.5 and detected canine Kv1.5 under a number of experimental conditions (Fedida et al. 2003). Detection was by HRP-labelled goat anti-rabbit antibody (Jackson Immuno Research, PA, USA) and Renaissance Western blot Chemiluminescence Reagent Plus (NEN).
Electrophysiological procedures
Coverslips containing cells were removed from the incubator before experiments and placed in a superfusion chamber (volume 250 μl) containing the control bath solution at 22–23°C, and perfused with bathing solution throughout the experiments. Whole-cell current recording and data analysis were done using an Axopatch 200A amplifier and pCLAMP 8 software (Axon Instruments). Patch electrodes were fabricated using thin-walled borosilicate glass (World Precision Instruments, FL, USA). Electrodes had resistances of 1–3 MΩ when filled with control filling solution. Capacity compensation and 80% series resistance compensation were used in all whole-cell recordings. The mean capacitance and series resistance from 82 cells studied was 16.2 ± 0.6 pF and 2.4 ± 0.1 MΩ (before series resistance compensation). No leak subtraction was used when recording currents, and zero current levels are denoted by the dashed lines in the current tracings. Data were sampled at 10–20 kHz and filtered at 5–10 kHz. Membrane potentials have not been corrected for small junctional potentials between bath and pipette solutions. Throughout the text data are shown as mean ±s.e.m.
Data analysis
The voltage dependence of inactivation of Kv1.5 and Kv1.5ΔN209 were fitted with Boltzmann functions of the form y= 1/(1 + exp[(V − V1/2)/k]) + C, respectively, where V1/2 represents the voltage at which 50% inactivation occurred, V is the membrane potential, and k is the slope factor that reflects the steepness of the voltage dependence. C represents the fraction of non-inactivating channels at potentials where inactivation was most complete. Slopes of the upturn of inactivation–voltage relationships were determined by linear regression, and are listed in the text with 95% confidence intervals for the slope. Throughout the text, data are presented as mean ±s.e.m., and tests for significance between two groups were carried out using Student's t test or the Mann-Whitney U test.
Results
N-terminal deletions of Kv1.5 allow additional pathways of inactivation
We have previously reported that deletion of residues in the N-terminus of Kv1.5, particularly within the T1 domain, can dramatically alter the inactivation properties of the channel (Kurata et al. 2001, 2002). RNA of splice variants encoding an N-terminally truncated form of Kv1.5 (Kv1.5ΔN209) have been isolated from cardiac tissue of several species, suggesting that Kv1.5ΔN209 may be present in vivo at certain developmental stages, although protein expression of truncated channel isoforms has not yet been demonstrated (Tamkun et al. 1991; Attali et al. 1993; Fedida et al. 1993). In recent studies we have used an antibody (Fig. 1A) directed against the C-terminus of Kv1.5 to establish the presence of Kv1.5 in canine and mouse heart (Fedida et al. 2003; Brunet et al. 2004) Following on from this work, we have also detected multiple immunoreactive bands, suggestive of multiple forms of Kv1.5 in lysates of canine atrial tissue from several different animals (Fig. 1B). The predicted size of full-length canine Kv1.5 is 71 kDa, although in Western blots we find that it normally runs at a slightly higher molecular weight of ∼83 kDa, suggesting an additional 12 kDa arising from glycosylation. The predicted size of a truncated form corresponding to Kv1.5ΔN209 is ∼44 kDa, and similar post-translational processing of Kv1.5ΔN209 and full-length Kv1.5 could account for the ∼58 kDa band observed consistently in Western blots (Fig. 1B).
Figure 1. Multiple isoforms of Kv1.5 in canine cardiac tissue.
A, C-terminal epitope recognized by the antibody used for Kv1.5 detection. B, Western blot of lysates of canine atrial tissue from six separate animals. A Lowry assay was run in triplicate for each sample and 15 μg per sample were loaded in each lane. The C-terminal Kv1.5 antibody detects species at 83 and 58 kDa.
The inactivation properties of the long and short (Kv1.5ΔN209) forms of Kv1.5 thought to exist in myocardium are illustrated in Fig. 2, to highlight the major differences between these two forms of the Kv1.5 channel. From a holding potential of −80 mV, cells were pulsed for 5 s to voltages between −70 and +70 mV in 10 mV increments (P2), followed by a test pulse to +60 mV (P3). We included a brief control pulse to +60 mV (P1) to ensure that the interpulse interval was sufficient to allow channel recovery, and to ensure that no significant channel rundown occurred during the protocol. The extent of inactivation that occurred during P2 was quantified by normalizing the peak current in P3 to the peak current in P1. The P3/P1 ratio reflects the number of channels that remain available following the P2 pulse.
Figure 2. Multiple pathways of inactivation in Kv1.5.
A, inactivation–voltage relationships were determined by whole-cell patch clamp recordings from HEK 293 cells stably expressing Kv1.5 or Kv1.5ΔN209. From a holding potential of −80 mV, cells were depolarized briefly to +60 mV (P1), followed by a 2 s rest at −80 mV. Cells were then depolarized for 5 s between −70 and +70 mV in 10 mV steps (P2), followed by a 500 ms test pulse to +60 mV (P3). B, traces to +60 mV (left) or 0 mV (right) in cells expressing full-length Kv1.5 or Kv1.5ΔN209 have been normalized to their respective peak currents, to directly compare the time course of inactivation. C, as an index of inactivation during P2, peak current during the test pulse (P3) was normalized to peak current during the control pulse (P1).
The inactivation–voltage relationships of Kv1.5 and Kv1.5ΔN209 differ in many respects. Deletion of the N-terminus of Kv1.5 results in much more profound inactivation at intermediate depolarizations, a ∼10 mV hyperpolarizing shift of the V1/2 of inactivation, and a U-shaped voltage dependence of inactivation. Between voltages of −20 and +60 mV, the upturn of the inactivation–voltage relationship can be approximated by a linear fit with a slope of 2.0 ± 0.1% per 10 mV. We have suggested in previous work that this altered inactivation phenotype results from enhanced inactivation from partially activated closed states in Kv1.5ΔN209, and that deletion of the N-terminus does little to disrupt the slow inactivation process normally observed in full-length Kv1.5 channels (Kurata et al. 2001). Rather, the Kv1.5ΔN209 truncation appears to permit additional pathways of closed-state inactivation that do not contribute significantly to inactivation of full-length Kv1.5. This feature is reflected in the normalized traces in Fig. 2B: when pulsed to +60 mV the time course of inactivation is very comparable between full-length Kv1.5 and Kv1.5ΔN209; however, at intermediate depolarizations, more inactivation is clearly apparent in Kv1.5ΔN209. This is also apparent in the inactivation–voltage relationships in Fig. 2C, because at depolarized potentials where inactivation from closed states is least favourable, the extent of inactivation in Kv1.5ΔN209 approaches that of the full-length Kv1.5 channel. An important aspect of these data is that inactivation of the short form of Kv1.5 is most prominent (and most different from the full-length channel) between −20 and 0 mV, which are highly relevant potentials for the plateau of the action potential, and thus the role of this current in heart.
Despite these recent advances in our understanding of state-dependent inactivation in various Kv channels, it remains unclear whether closed-state inactivation is a fundamentally different process from P/C-type inactivation, a process generally considered to be strongly coupled to channel opening. To describe some experimental data, models in which closed- and open-inactivated states can interconvert appear to be adequate (Scheme 1; Kurata et al. 2001). However, other experimental work has implied that a distinct mechanism of closed-state inactivation (U-type) can coexist with P/C-type inactivation in Shaker (Scheme II; Klemic et al. 2001). In this study, we present a number of experiments to investigate the biophysical properties of the closed-state inactivation mechanism revealed by deletion of the Kv1.5 N-terminus, and to determine whether this process is equivalent to the P/C-type inactivation mechanism observed in full-length Kv1.5 channels.
 |
Scheme 1 |
 |
Scheme 2 |
Enhanced closed-state inactivation in Kv1.5ΔN209
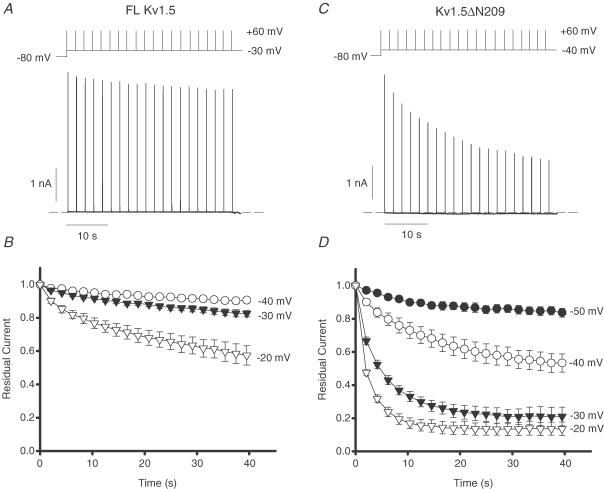
Careful examination of the inactivation properties of full-length Kv1.5 and Kv1.5ΔN209 at voltages insufficient to significantly activate channels also emphasizes the altered state-dependence described for Kv1.5ΔN209. From our prior studies we have established the activation relationships for full-length Kv1.5 and Kv1.5ΔN209 at 10 and 5 mV intervals (Kurata et al. 2001, 2002), such that the activation V1/2 values of Kv1.5 and Kv1.5ΔN209 are −10.8 ± 0.8 and −20.3 ± 1.7 mV, respectively (Kurata et al. 2002; Fig. 1). Cells stably expressing either full-length Kv1.5 or Kv1.5ΔN209 were held at voltages between −50 and −20 mV, and pulsed briefly (15 ms) to +60 mV at 2 s intervals to examine the onset of inactivation at these intermediate voltages (Fig. 3A and C). At all voltages above −50 mV, Kv1.5ΔN209 exhibited significantly more inactivation than full-length Kv1.5 (Fig. 3B and D). Importantly, the activation V1/2 of Kv1.5ΔN209 is shifted by −10 mV relative to full-length Kv1.5 (Kurata et al. 2001). The mean activation of full-length Kv1.5 was only significant at −20 mV (0.094 ± 0.016) and not significant at more negative potentials, whereas the mean activation of Kv1.5ΔN209 was significant at −30 mV (0.078 ± 0.06) and −20 mV (0.54 ± 0.10), with discernible activation only at −35 mV (0.03). However, even when this difference is taken into account, considerably more inactivation was observed in Kv1.5ΔN209. For instance, a membrane voltage of −30 mV elicited very little inactivation (18 ± 2%) over 40 s in full-length Kv1.5 channels, and only −20 mV, a potential which activated significant numbers of channels, resulted in obvious inactivation, suggesting that this might be open-state dependent (Fig. 3C). In contrast, pulses from −40 mV in Kv1.5ΔN209, a potential at which no significant activation occurred, resulted in 47 ± 5% inactivation of Kv1.5ΔN209 (Fig. 3D), which is significantly greater than that found at −30 mV in full-length Kv1.5 (P < 0.05, Fig. 3). These data illustrate the importance of open-state inactivation in full-length Kv1.5 and closed-state inactivation in Kv1.5ΔN209 channels.
Figure 3. Closed-state inactivation of full-length Kv1.5 and Kv1.5ΔN209.
Cells stably expressing either full-length Kv1.5 or Kv1.5ΔN209 were held at voltages between −50 and −20 mV, and were pulsed for 15 ms to +60 mV at 2 s intervals to monitor the extent of channel inactivation (5 mm K+i/135 mm K+o ionic conditions). Sample data in A illustrate inactivation of full-length Kv1.5 with a holding potential of −30 mV, and C illustrates inactivation of Kv1.5ΔN209 with a holding potential of −40 mV. Mean data at multiple voltages for full-length Kv1.5 and Kv1.5ΔN209 are illustrated in B and D, respectively.
Differential modulation of inactivation by extracellular K+ ions
To demonstrate a P/C-type mechanism of inactivation in Kv1.5, and to compare the regulation of inactivation by extracellular cations in Kv1.5 and Kv1.5ΔN209, we have examined the effects of elevation of extracellular K+ on inactivation in both channels (Fig. 4). This experiment was prompted by reports that other K+ channels with a U-shaped inactivation–voltage relationship, including Kv2.1 and Kv3.1, often exhibit more pronounced inactivation in the presence of high extracellular K+ (Klemic et al. 1998, 2001), in contrast with previous studies of Shaker and other channels exhibiting a P/C-type inactivation mechanism (Lopez-Barneo et al. 1993; Molina et al. 1997). Inactivation was measured in both full-length Kv1.5 and Kv1.5ΔN209, in two different extracellular K+ concentrations (5 and 135 mm), at two different conditioning voltages (+10 and +60 mV in full-length Kv1.5; −20 and +60 mV in Kv1.5ΔN209). The ‘intermediate voltages’ (+10 and −20 mV) used in Fig. 4 were selected based on the position of the ‘foot’ of the inactivation–voltage relationships for full-length Kv1.5 and Kv1.5ΔN209 (Fig. 2C). To quantify inactivation, cells were given a 100 ms control pulse to 60 mV (P1), rested for 2 s at −80 mV, stepped to one of the two conditioning voltages for 5 s (P2), followed by a test pulse to +60 mV (P3). Sample data are shown normalized to the P1 test pulse, to correct for differences in current magnitude due to driving force changes in different extracellular K+ conditions. Therefore, the magnitude of current in the P3 test pulse reflects the extent of inactivation during the conditioning pulse (P2). Because the effects of extracellular K+ in Kv1.5 are quite small, experiments were conducted with a paired design, with summarized data presented in the bar graphs in Fig. 4C and F. The bar graphs show the mean difference in fractional inactivation observed in 135 mm K+ and 5 mm K+ in full-length Kv1.5 (filled bars) and Kv1.5ΔN209 (open bars). In these graphs a positive number indicates less inactivation in 135 mmversus 5 mm K+.
Figure 4. Effects of extracellular K+ on inactivation kinetics in Kv1.5 and Kv1.5ΔN209.
Inactivation during 5 s pulses was quantified as described in Fig. 2. HEK 293 cells stably expressing (A and D) Kv1.5 or (B and E) Kv1.5ΔN209 were pulsed to a P2 voltage of either 60 mV (lower panels) or a voltage at the foot of the inactivation–voltage relationship (upper panels, +10 mV for Kv1.5 and −20 mV for Kv1.5ΔN209, determined from the inactivation–voltage relationships in Fig. 2C). The extent of inactivation was determined by the ratio of the peak currents in P3 and P1. C and F, mean data of differences in the P3/P1 ratio in 5 mm K+o versus 135 mm K+o in both Kv1.5 and Kv1.5ΔN209.
In this experiment, elevation of extracellular K+ resulted in a small but consistent inhibition of Kv1.5 inactivation, by between 3% and 4% at both +10 mV and +60 mV (Fig. 4A and D, filled bars in Fig. 4C and F). Although this is a small effect, it is consistent with a P/C-type mechanism of inactivation in full-length Kv1.5, as suggested in previous studies of Shaker and homologues (Lopez-Barneo et al. 1993; Rasmusson et al. 1995; Fedida et al. 1999). In contrast, Kv1.5ΔN209 exhibited an opposite sensitivity to K+ (Fig. 4B and E, open bars in Fig. 4C and F), with 8–10% more inactivation observed in 135 mm extracellular K+ compared with the 5 mm extracellular K+ condition. The effects of extracellular K+ on inactivation of Kv1.5ΔN209 were present at both voltages examined (−20 and +60 mV), and slighty greater at −20 mV (10.3 ± 2.1%) than at +60 mV (9.1 ± 1.5%). Recording in 135 mm extracellular K+ did not significantly alter the magnitude of the upturn of the inactivation–voltage relationship in Kv1.5ΔN209, which could be approximated with a linear fit with a slope of 1.9 ± 0.1% per 10 mV (data not shown). These effects of K+o are relatively minor compared with the effects observed in other channels such as Kv3.1, where elevation of extracellular K+ accelerates inactivation almost 3-fold (Klemic et al. 2001). Nevertheless, a paradoxical regulation of inactivation by extracellular K+ appears to be shared among several channels that exhibit a U-shaped inactivation–voltage relationship (Klemic et al. 1998, 2001).
Modulation of Kv1.5ΔN209 inactivation properties by permeant ions and external TEA+
Because the effects of K+ on Kv1.5 inactivation are quite small, we have examined the effects of other permeant or blocking ions known to inhibit P/C-type inactivation in Shaker and its mammalian homologues. With this approach, we have also identified several experimental manipulations that appear to selectively inhibit ‘open-state’ inactivation, without interfering with the inactivation process present in truncated forms of Kv1.5, and these are discussed in the sections that follow. To introduce our reasoning in these experiments, it is useful to consider in advance the expected effects of inhibition of open state versus closed state inactivation on the properties of the inactivation–voltage relationship. Experimental conditions that inhibit P/C-type inactivation from the open state generally do so with very weak voltage dependence. An example is the inhibition of P/C-type inactivation by extracellular TEA+ (see below), and the net result is expected to be an upward shift of the plateau of the inactivation–voltage relationship. In Kv1.5ΔN209, we have suggested that macroscopic inactivation results from a combination of inactivation from the open state and closed states. In this case, manipulations that inhibit closed-state inactivation would have strong effects on channel inactivation at intermediate voltages (where closed-state inactivation is maximal), but weaker effects at high voltages (where closed-state inactivation contributes minimally to the overall time course of inactivation). Therefore, the overall result of conditions that inhibit closed-state inactivation in Kv1.5ΔN209 would be a reduction in the slope of the ‘upturn’ of the inactivation–voltage relationship (i.e. a reduction in the magnitude of the ‘upturn’ relative to the peak non-inactivated current).
In full-length Kv1.5 channels, Rb+ substitution for K+ mildly inhibits inactivation during the 5 s pulses used in our experiments, resulting in an upward shift of the plateau of the inactivation–voltage relationship by roughly 10% (Fig. 5A and C). In Kv1.5ΔN209 channels, Rb+ also inhibits inactivation (Fig. 5B); however, the slope of the upturn of the inactivation–voltage relationship is clearly blunted relative to the data collected with K+ as the permeating ion (Fig. 5D). In Rb+ recording conditions, the slope of the upturn of the inactivation–voltage relationship was described with a linear fit with a slope of 1.0 ± 0.1% per 10 mV, significantly smaller than the slope in 5/135 mm K+ or 135/135 mm K+ conditions (P < 0.05). This result suggests that Rb+ is able to inhibit inactivation from both open and closed states in Kv1.5ΔN209.
Figure 5. Rb+ substitution attenuates both open-state and closed-state dependent inactivation.
A and B, HEK 293 cells expressing either full-length Kv1.5 or Kv1.5ΔN209 were pulsed from a holding potential of −80 mV to +60 mV for 6 s, to observe the time course of inactivation. C and D, inactivation–voltage relationships for Kv1.5 and Kv1.5ΔN209 were determined as described in Fig. 2; however, K+ was replaced with Rb+ in both the pipette and extracellular solutions. In D, the inactivation–voltage relationships for full-length Kv1.5 have been superimposed in grey, for comparison.
The effects of Rb+ substitution contrast significantly with the effects of extracellular TEA+ on the time course of inactivation. A feature used to distinguish P/C-type inactivation in early studies of Shaker channels is inhibition by extracellular TEA+ ions. The WT Kv1.5 channel pore is actually extremely insensitive to extracellular TEA+, due to the presence of an arginine residue at position 487, equivalent to residue T449 in Shaker (MacKinnon & Yellen, 1990; Yellen et al. 1991; Lopez-Barneo et al. 1993; Fedida et al. 1999). We introduced the R487T mutation into Kv1.5 and Kv1.5ΔN209 to confer sensitivity to extracellular TEA+. Studies in Shaker suggest that this mutation allows extracellular TEA+ to inhibit P/C-type inactivation, whereas mutations with a higher affinity for TEA+ (e.g. Kv1.5 R487Y) show little inhibition of inactivation by extracellular TEA+ (Molina et al. 1997). Kv1.5 R487T channels are weakly blocked by extracellular TEA+, exhibiting roughly 50% blockade at a TEA+o concentration of 10 mm. In Kv1.5 R487T/ΔN209 channels, the inactivation–voltage relationship resembles that seen in Kv1.5ΔN209 channels (compare Figs 2C and 6B). As in full-length Kv1.5 R487T channels, application of 10 mm extracellular TEA+ clearly inhibits the extent of inactivation observed during 5 s depolarizations (Fig. 6A), but interestingly does not reduce the upturn of the inactivation–voltage relationship (Fig. 6B). The upturn of the Kv1.5 R487T/ΔN209 inactivation–voltage relationship was described by linear fits with slopes of 1.9 ± 0.1% per 10 mV in control conditions and a slightly steeper 2.3 ± 0.1% per 10 mV in 10 mm extracellular TEA+ (no statistical difference). This feature of the inactivation–voltage relationship indicates that extracellular TEA+ does not inhibit the closed-state inactivation mechanism present in Kv1.5ΔN209, suggesting that the closed-state inactivation mechanism revealed in short forms of Kv1.5 is distinct from the P/C-type inactivation mechanism present in full-length Kv1.5 or Kv1.5 R487T channels.
Figure 6. Extracellular TEA+ does not affect closed-state inactivation.
A, cells expressing Kv1.5ΔN209 were pulsed from a holding potential of −80 mV to +60 mV, to observe the time course of inactivation in control conditions, or in the presence of 10 mm extracellular TEA+. B, inactivation–voltage relationships for Kv1.5ΔN209 R487T were determined as described in Fig. 2, in the presence or absence of 10 mm extracellular TEA+.
These observations of the effects of extracellular TEA+ are reinforced by our characterization of the effects of Cs+ permeation on channel inactivation. Previous studies from our laboratory have suggested that substitution of K+ with Cs+ as the permeant ion strongly inhibits inactivation of full-length Kv1.5 channels (Fedida et al. 1999). This conclusion is also corroborated by the ability of Cs+ ions to potently prevent gating charge immobilization, and to prevent the development of slow Na+ tail currents associated with P/C-type inactivation in Kv1.5 (Chen et al. 1997; Fedida et al. 1999; Wang & Fedida, 2002). This finding is confirmed experimentally in Fig. 7A, showing inactivation of full-length Kv1.5 at +60 mV in symmetrical Cs+ (135/135 mm) recording conditions. It is evident that, compared with K+ conditions, the extent of P/C-type inactivation is significantly attenuated when Cs+ serves as the primary permeant ion. Inactivation–voltage relationships for full-length Kv1.5 determined in Cs+ or K+ recording conditions (using the same protocol described in Fig. 2A) suggest that this inhibition of inactivation in full-length Kv1.5 is constant at all depolarized voltages (Fig. 7C).
Figure 7. Cs+ substitution selectively attenuates open-state dependent inactivation.
A and B, cells stably expressing full-length Kv1.5 or Kv1.5ΔN209 were pulsed from a holding potential of −80 mV to +60 mV with either K+ or Cs+ as the permeant ion. Inactivation–voltage relationships for Kv1.5 (C) and Kv1.5ΔN209 (D) were determined as described in Fig. 1, with K+ or Cs+ in both the pipette and extracellular solutions. In D the inactivation–voltage relationships collected for full-length Kv1.5 are superimposed in grey, for comparison.
Figure 7B illustrates that the symmetrical Cs+ condition also inhibits inactivation of Kv1.5ΔN209 channels. As for full-length Kv1.5 channels, inactivation is weaker at all voltages examined when K+ is substituted with Cs+. As described above, if Cs+ substitution inhibited closed-state inactivation, we would expect to see a ‘blunted’, shallower upturn of the inactivation–voltage relationship. However, the upturn of the Kv1.5ΔN209 inactivation–voltage relationship appears unchanged when compared with recordings in K+ (Fig. 7D). In Cs+ conditions, the slope of the upturn of the inactivation–voltage relationship was described with a linear fit with a slope of 2.1 ± 0.1% per 10 mV, not statistically different from the slopes of 2.0 ± 0.1% per 10 mV in 5/135 mm K+ conditions, or 1.9 ± 0.1% per 10 mV in 135/135 mm K+ conditions (data shown only for the 5/135 mm K+ recording conditions, Fig. 7D). Therefore, this result suggests that the inactivation–voltage relationship for Kv1.5ΔN209 consists of two components: a Cs+-sensitive component similar to inactivation in full-length Kv1.5 (Fig. 7A and C), and a Cs+-insensitive component that accounts for the upturn of the inactivation–voltage relationship (Fig. 7B and D). Collectively, these data imply a distinction between inactivation from the open state versus closed states of the channel.
Modulation of recovery from inactivation by prepulse potential and extracellular K+
As described above, our experiments were initiated with the working hypothesis that truncated Kv1.5 channels inactivate by multiple mechanisms, with P/C-type inactivation favoured at depolarized potentials where channel open probability is maximal. In contrast, at intermediate voltages near the V1/2 for channel activation, channels show greater occupancy of partially activated closed states than at higher voltages, and closed-state inactivation becomes favourable. We hypothesized that if channels access distinct inactivated states at different voltages (i.e. via closed-state versus open-state inactivation), then the recovery kinetics may depend on the voltage at which channels are inactivated. Similar findings have been reported in an earlier study of recovery from inactivation in Shaker, in which inactivation of channels at strongly depolarized voltages resulted in different recovery kinetics than inactivation at voltages closer to the V1/2 of channel activation (Klemic et al. 2001). Recovery from inactivation was measured using a modification of the protocol shown in Fig. 2A. From a holding potential of −80 mV, cells were depolarized to +60 mV (P1, 20 ms), briefly repolarized to −80 mV, depolarized for 7 s to a variable potential to inactivate the channels (P2, 7 s), repolarized to a recovery voltage of −110 mV for a variable duration, and finally depolarized briefly to +60 mV (P3, 20 ms). The ratio of P3/P1 current magnitudes provides an index for the extent of recovery at various time intervals.
We examined the time course of recovery in full-length Kv1.5, after inactivating the channel at either +60 or +10 mV. Summary data are plotted in Fig. 8C, together with parameters of bi-exponential fits to the time course of recovery. In full-length Kv1.5, the rate of recovery from inactivation is relatively independent of the inactivating voltage, as are the relative contributions of the fast and slow components of the recovery time course. In Kv1.5ΔN209 channels, the time course of recovery was examined after inactivating prepulses to either +60 or −20 mV (chosen because at −20 mV the extent of closed-state inactivation is maximal). Inactivating prepulses of −20 mV result in different kinetics of recovery than when channels are inactivated at +60 mV (Fig. 8D). In particular, inactivation at −20 mV increases the amplitude of the fast component of recovery, and decreases the amplitude of the slow component of recovery when compared with the recovery kinetics observed following depolarizations to +60 mV. The net effect is the observation of a slightly more rapid rate of recovery when Kv1.5ΔN209 channels are inactivated at −20 mV versus+60 mV. This result suggests that at −20 mV, channels preferentially inactivate via a different mechanism (which recovers faster) than at +60 mV (which recovers more slowly).
Figure 8. Effects of prepulse potential on recovery from inactivation.
A and B, after a brief control pulse to +60 mV (P1), HEK 293 cells stably expressing full-length Kv1.5 or Kv1.5ΔN209 were depolarized to +60 mV or an intermediate voltage (+10 mV in full-length Kv1.5, −20 mV in Kv1.5ΔN209) for 7 s (P2), repolarized to −110 mV for a variable duration, followed by a brief test pulse (P3) to +60 mV. C and D, recovery from inactivation during the recovery period was parameterized with the ratio of P3/P1, and records from individual cells were fitted with double exponential equations. The fit parameters are included on the plots.
The effects of prepulse potential on the kinetics of recovery are mirrored by the effects of changing the concentration of extracellular K+ (Fig. 9, Table 1). As mentioned above, this examination of recovery kinetics in different extracellular K+ conditions was motivated by reports that closed-state inactivation in channels such as Kv2.1 exhibits paradoxical sensitivity to extracellular K+, such that increasing extracellular K+ results in acceleration of inactivation rather than the deceleration of inactivation observed in classical examples of P/C-type inactivation, and that elevation of extracellular K+ can accelerate closed-state inactivation in Shaker (Klemic et al. 1998). We observed that elevation of extracellular K+ generally results in an increase in the amplitude of the fast component of recovery from inactivation, and a decrease in the amplitude of the slow component in Kv1.5ΔN209 (Fig. 9, Table 1). Note that this effect is most significant at +60 mV, where the amount of closed-state inactivation is normally minimal. In contrast, the extracellular K+ condition has little effect on the amplitudes of the fast and slow components of recovery in the full-length Kv1.5 channel. These data are consistent with a model in which elevated extracellular K+ mimics the effects of inactivation at intermediate potentials in Kv1.5ΔN209, favouring an inactivation mechanism that exhibits more rapid recovery kinetics.
Figure 9. Effects of prepulse potential and extracellular K+ on recovery from inactivation.
Recovery from inactivation was measured under various extracellular K+ conditions, and with various prepulse voltages in cells expressing full-length Kv1.5 (A, B) or Kv1.5ΔN209 (C, D), with the same protocol described in Fig. 8. The time course of recovery was fitted with a bi-exponential equation. Fit parameters shown as insets in each panel represent the relative weights of the fast and slow recovery components. Detailed fit parameters are presented in Table 1.
Table 1.
Effects of extracellular K+ on recovery from inactivation in Kv1.5 and Kv1.5ΔN209
| Full-length Kv1.5 | Kv1.5ΔN209 | |||||||
|---|---|---|---|---|---|---|---|---|
| Inactivation voltage | +10 mV | +60 mV | −20 mV | −60 mV | ||||
| [k+]o | 5 | 135 | 5 | 135 | 5 | 135 | 5 | 135 |
| τfast | 0.15 ± 0.02 | 0.18 ± 0.05 | 0.17 ± 0.03 | 0.13 ± 0.02 | 0.085 ± 0.007* | 0.09 ± 0.02 | 0.10 ± 0.05 | 0.17 ± 0.06 |
| τslow | 2.6 ± 0.2 | 2.0 ± 0.5 | 3.19 ± 0.4 | 3.0 ± 0.5 | 1.14 ± 0.02* | 0.69 ± 0.06* | 1.8 ± 0.3* | 3.6 ± 1.2 |
| Fast component (%) | 17 ± 1 | 22 ± 2 | 15 ± 4 | 15 ± 1 | 25 ± 3* | 33 ± 6 | 5.3 ± 0.9* | 28 ± 11 |
| Slow component (%) | 30 ± 1 | 26 ± 1 | 37 ± 3 | 32 ± 1 | 42 ± 2* | 47 ± 8 | 56 ± 4* | 47 ± 5 |
| Non-inactivated (%) | 53 ± 1 | 53 ± 1 | 49 ± 6 | 54 ± 2 | 32 ± 1* | 20 ± 2* | 39 ± 3 | 24 ± 5* |
| Weight (fast) | 36 ± 1 | 45 ± 2 | 28 ± 2 | 32 ± 3 | 37 ± 4 | 42 ± 7 | 9 ± 2* | 38 ± 14 |
| Weight (slow) | 64 ± 1 | 55 ± 2 | 72 ± 2 | 68 ± 2 | 63 ± 3 | 58 ± 9 | 91 ± 6* | 62 ± 7 |
Recovery from inactivation was examined using the protocol described in Fig. 8, with either 5 mm or 135 mm extracellular K+, and fitted with a bi-exponential equation.
Statistical difference between Kv1.5ΔN209 and full-length Kv1.5 in the same condition (P < 0.05). Amplitudes of each component of recovery are listed as the fraction of total current (s labelled fast component and slow component), and as the fraction of inactivated current (s labelled weight (fast) and weight (slow)).
Discussion
P/C- and U-type inactivation
Slow inactivation of mammalian Kv1 channels is thought to be voltage independent and coupled to channel opening, and this mechanism is generally referred to as C-type or P/C-type inactivation (Hoshi et al. 1991; Olcese et al. 1997; Klemic et al. 2001; Kehl et al. 2002). The voltage independence of this process is reflected in a flat inactivation–voltage relationship at voltages that saturate channel open probability (Hoshi et al. 1991), as shown in Fig. 2C by the open symbols. The characterization of short forms of Kv1.5 (Kurata et al. 2001) and Kv1.5 T1-deletion mutants (Kurata et al. 2002), together with recent studies in Shaker (Klemic et al. 2001), suggest that Shaker family K+ channels are able to exhibit at least two distinct inactivation phenotypes. As suggested by Klemic et al. (2001), we have referred to these here as P/C-type and U-type inactivation. We have previously demonstrated that disruption of the T1 domain of Kv1.5 channels dramatically alters their inactivation properties, imparting a U-shaped inactivation–voltage relationship similar to Kv2.1 channels (Kurata et al. 2001, 2002). To date, N-terminal truncations and T1 domain mutations of Kv1.5 remain the only ones identified that impart a U-shaped inactivation–voltage relationship to a Kv channel, and may provide unique insights into the mechanism(s) underlying closed-state inactivation of Kv channels (Kurata et al. 2002), as a direct comparison of the two inactivation mechanisms is permitted in a single channel. In particular, while Kv1.5ΔN209 inactivates significantly more than full-length Kv1.5 channels at intermediate voltages, the extent of inactivation closely approaches the full-length channel at high voltages (e.g. +60 mV or greater, Fig. 2C). For this reason, we have been able to suggest that N-terminal truncation of Kv1.5 leaves the P/C-type inactivation mechanism of the full-length channel largely unaltered, but adds additional pathways for channel inactivation that contribute significantly to the overall inactivation time course at intermediate depolarizations (Kurata et al. 2001). The question that we have tried to address here, using this unique property of Kv1.5, is whether this additional pathway of U-type inactivation is a variant of the P/C-type mechanism, or whether its properties are sufficiently discrete to require different structural mechanisms.
Separation of P/C- and U-type inactivation mechanisms
Functionally, these two inactivation phenotypes differ greatly. P/C-type inactivation occurs predominantly from the open state, is inhibited by extracellular K+ or TEA+, and exhibits relatively slow and weakly voltage-dependent recovery from inactivation (Hoshi et al. 1991; Rasmusson et al. 1995, 1998; Klemic et al. 2001). Channels exhibiting U-type inactivation properties, however, appear to exhibit preferential inactivation from partially activated closed states, rapid and strongly voltage-dependent recovery from inactivation, and in some channel types accelerated inactivation with elevation of extracellular K+ or TEA+ (Klemic et al. 1998, 2001). Our experiments have extended these results to other cations with somewhat surprising results. Both Rb+ and Cs+ slow Kv1.5 inactivation during sustained depolarizations at positive potentials, when compared with K+ as the permeating ion (Figs 5A and 7A; (Fedida et al. 1999), and this mechanism appears to be preserved in Kv1.5ΔN209 (Figs 5B and 7B). The inactivation mechanism in full-length Kv1.5 accounts for the Cs+-sensitive component of inactivation in Kv1.5ΔN209. Shortening of the N-terminus allows inactivation via an additional Cs+-insensitive pathway, accounting for the deeper inactivation and indistinguishable upturn of the inactivation–voltage relationship in Kv1.5ΔN209 whether K+ or Cs+ is the permeant ion (Fig. 7D). Thus, our results provide some confirmation/validation of our earlier interpretation of Kv1.5ΔN209 gating, where we suggested that the excessive inactivation observed at intermediate depolarizations was the result of accelerated inactivation from intermediate closed states in the activation pathway (Kurata et al. 2001).
We have previously suggested that Cs+ traps Kv1.5 channels in the P-type inactivated state and prevents the structural transition of channels into deeply inactivated P/C-type states (Wang & Fedida, 2001). Interestingly, our data suggest that the inactivation process from closed states underlying the Kv1.5ΔN209 phenotype is inaccessible to Cs+ modulation. The importance of this experiment is that closed-state inactivation in Kv1.5ΔN209 is demonstrated to be distinct from the P/C-type inactivation process, as suggested in Shaker (Klemic et al. 2001). Rb+, on the other hand, appears to diminish all components of Kv1.5ΔN209 inactivation (Fig. 5), and this may reflect its known action on channel open probability (Demo & Yellen, 1992). Prolonged occupancy of Rb+ in the selectivity filter will inhibit/delay the conformational changes underlying P/C-type inactivation. Furthermore, by increasing the stability of the open state, the closed-state inactivation responsible for the upturn of the inactivation–voltage relationship will be indirectly diminished, as Rb+-occupied channels will dwell less often in closed states.
As with Cs+, addition of extracellular TEA+ was able to significantly inhibit P/C-type inactivation in both full-length Kv1.5 R487T and Kv1.5 R487T/ΔN209, but the extent of the upturn of the inactivation–voltage relationship remained essentially unaltered under these experimental conditions (Fig. 6). A simple interpretation of these observations is that Cs+ and extracellular TEA+ ions selectively inhibit the P/C-type inactivation mechanism observed in full-length Kv1.5 channels, and do not affect the closed-state inactivation mechanism revealed by truncation of the Kv1.5 N-terminus. Since it is well understood that extracellular TEA+ has specific actions at the outer pore of the potassium channel that compete with the ability of the channel to adopt the locally constricted state recognized as P/C-type inactivation (Yellen et al. 1991; Ikeda & Korn, 1995; Molina et al. 1997; Loots & Isacoff, 2000) the results suggest that the ‘U-type’ closed-state inactivation process does not involve the same conformational changes of the channel as those responsible for P/C-type inactivation. Unfortunately, the conformational changes associated with ‘U-type’ or closed-state inactivation remain poorly understood. Structure–function studies in Kv1.5 have shown that N-terminal intracellular domains, and particularly the highly conserved T1 domain, are somehow involved in allowing the channel to access these closed-inactivated states (Kurata et al. 2002). Other studies in Kv2.1 and its auxiliary α-subunits have suggested an important role for proline residues in the inner cavity in regulating state-dependent inactivation (Kerschensteiner et al. 2003). Clearly, further experiments are required to understand the role of intracellular domains, and the structural basis for differences in regulation by extracellular cations, in the closed-state inactivation mechanisms of Kv channels.
Recovery from inactivation
Although less direct, the effects of prepulse voltage and extracellular K+ concentration on the biexpoenential recovery kinetics of Kv1.5 and Kv1.5ΔN209 provide further evidence for the presence of multiple distinct pathways of inactivation. In general, multiple experimental manipulations that enhanced closed-state inactivation of Kv1.5ΔN209 increased the amplitude of the fast component of recovery from inactivation in this channel. Closed-stated inactivation was maximized by conditioning channels at an intermediate voltage (−20 mV, Fig. 8), which optimizes channel occupancy in partially activated closed states. Increasing extracellular K+ also promoted inactivation of Kv1.5ΔN209, and this presumably arose from an effect on a ‘U-type’ or closed-state inactivation process specific to Kv1.5ΔN209, since extracellular K+ exerts an opposite effect on inactivation of the full-length Kv1.5 channel. Consistent with this, elevation of extracellular K+ appears to promote the closed-state inactivation processes in Kv2.1 and Kv3.1 (Klemic et al. 1998, 2001). Both of these experimental manipulations (high extracellular K+ and conditioning at intermediate voltages) enhanced the amplitude of the fast component of recovery in Kv1.5ΔN209, suggesting that the closed-state inactivation process maximized in these conditions recovers with rapid kinetics. Since the kinetics of recovery (particularly the balance of fast and slow components) appear to be influenced by the conditions during the inactivating pulse, the different inactivation processes leading to multiple components of recovery are probably mutually exclusive rather than interconvertable.
A significant difficulty in interpreting the kinetics of recovery from inactivation is that there is also a bi-exponential time course of recovery in full-length Kv1.5 channels (Figs 8 and 9, and Table 1). While we are unsure of the fundamental mechanisms governing the time course of recovery, bi-exponential recovery kinetics have been frequently reported in other Kv channels including Shaker (Levy & Deutsch, 1996; Klemic et al. 2001). The study of Klemic et al. (2001) interpreted the multi-exponential recovery kinetics as a reflection of distinct recovery kinetics from two different mechanisms of inactivation (U-type and P/C-type). We have adopted similar reasoning in our interpretation of recovery kinetics in full-length Kv1.5 and Kv1.5ΔN209. That is, there is clearly a rapid component to recovery from inactivation in these channels, and we have interpreted changes in the amplitude of this component to reflect changes in the extent of an alternative (closed-state) mechanism of inactivation in Kv1.5ΔN209. Important, however, is the observation that full-length Kv1.5 channels clearly exhibit a rapid component to recovery from inactivation, but show little evidence of any significant closed-state inactivation (Fig. 3). They do not exhibit a U-shaped inactivation–voltage relationship (Fig. 2), or excessive cumulative inactivation during trains of repetitive depolarizations (Kurata et al. 2001). Thus, it is unclear whether the rapid component of recovery from inactivation can be tied to a distinct inactivation process in full-length Kv1.5, as has been suggested for Shaker (Klemic et al. 2001). The kinetics of recovery from inactivation of the full-length Kv1.5 channel do appear to be slightly altered by extracellular K+ or prepulse voltage (though not to the same extent as Kv1.5ΔN209; Figs 8 and 9), and this could reflect contributions of multiple inactivation processes in full-length Kv1.5 (e.g. P/C-type and a minor contribution of U-type inactivation) that are differentially regulated by voltage and permeant ions. This raises the possibility that the significant effects of N-terminal deletions on Kv1.5 inactivation arise by specifically enhancing (or altering the state dependence of) an inactivation mechanism that is normally present in full-length Kv1.5 channels but overshadowed by P/C-type inactivation.
Conclusion
N-terminal truncation of Kv1.5 exerts significant effects on the inactivation properties of the channel that can be explained by acceleration of inactivation from partially activated closed states. We have demonstrated that the closed-state inactivation process revealed in N-terminally deleted forms of Kv1.5 is insensitive to cations (Cs+ and extracellular TEA+) that inhibit inactivation of the full-length channel. Our data also suggest that experimental conditions favouring closed-state inactivation mechanisms alter the kinetics of recovery from inactivation. The differential regulation of closed-state inactivation by cations and voltage suggests that multiple distinct mechanisms of inactivation can co-exist in truncated forms of Kv1.5.
Acknowledgments
This work was supported by grants from the Heart and Stroke Foundations of British Columbia and Yukon, and the CIHR to D.F. H.K. was supported by predoctoral fellowships from the CIHR and Michael Smith Foundation for Health Research. We thank Anu Khurana for assistance with cell culture and David Van Wagoner for the supply of canine atrial tissue.
References
- Attali B, Lesage F, Ziliani P, Guillemare E, Honoré E, Waldmann R, Hugnot J-P, Mattéi M-G, Lazdunski M, Barhanin J. Multiple mRNA isoforms encoding the mouse cardiac Kv1.5 delayed rectifier K+ channel. J Biol Chem. 1993;268:24283–24289. [PubMed] [Google Scholar]
- Bahring R, Boland LM, Varghese A, Gebauer M, Pongs O. Kinetic analysis of open- and closed-state inactivation transitions in human Kv4.2 A-type potassium channels. J Physiol. 2001;535:65–81. doi: 10.1111/j.1469-7793.2001.00065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet S, Aimond F, Li H, Guo W, Eldstrom J, Fedida D, Yamada KA, Nerbonne JM. Heterogeneous expression of repolarizing, voltage-gated K+ currents in adult mouse ventricles. J Physiol. 2004;559:103–120. doi: 10.1113/jphysiol.2004.063347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen FSP, Steele D, Fedida D. Allosteric effects of permeating cations on gating currents during K+ channel deactivation. J General Physiol. 1997;110:87–100. doi: 10.1085/jgp.110.2.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi KL, Aldrich RW, Yellen G. Tetraethylammonium blockade distinguishes two inactivation mechanisms in voltage-activated K+ channels. Proc Natl Acad Sci U S A. 1991;88:5092–5095. doi: 10.1073/pnas.88.12.5092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demo SD, Yellen G. Ion effects on gating of the Ca2+-activated K+ channel correlates with occupancy of the pore. Biophys J. 1992;61:639–648. doi: 10.1016/S0006-3495(92)81869-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedida D, Eldstrom J, Hesketh JC, Lamorgese M, Castel L, Steele DF, Van Wagoner DR. Kv1.5 is an important component of repolarizing K+ current in canine atrial myocytes. Circ Res. 2003;93:744–751. doi: 10.1161/01.RES.0000096362.60730.AE. [DOI] [PubMed] [Google Scholar]
- Fedida D, Maruoka ND, Lin S. Modulation of slow inactivation in human cardiac Kv1.5 channels by extra and intra-cellular permeant cations. J Physiol. 1999;515:315–329. doi: 10.1111/j.1469-7793.1999.315ac.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedida D, Wible B, Wang Z, Fermini B, Faust F, Nattel S, Brown AM. Identity of a novel delayed rectifier current from human heart with a cloned K+ channel current. Circ Res. 1993;73:210–216. doi: 10.1161/01.res.73.1.210. [DOI] [PubMed] [Google Scholar]
- Grissmer S, Cahalan M. TEA prevents inactivation while blocking open K+ channels in human T lymphocytes. Biophys J. 1989;55:203–206. doi: 10.1016/S0006-3495(89)82793-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi T, Zagotta WN, Aldrich RW. Two types of inactivation in Shaker K+ channels: effects of alterations in the carboxy-terminal region. Neuron. 1991;7:547–556. doi: 10.1016/0896-6273(91)90367-9. [DOI] [PubMed] [Google Scholar]
- Ikeda SR, Korn SJ. Influence of permeating ions on potassium channel block by external tetraethylammonium. J Physiol. 1995;486:267–272. doi: 10.1113/jphysiol.1995.sp020809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Immke D, Wood M, Kiss L, Korn SJ. Potassium-dependent changes in the conformation of the Kv2.1 potassium channel pore. J General Physiol. 1999;113:819–836. doi: 10.1085/jgp.113.6.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerng HH, Shahidullah M, Covarrubias M. Inactivation gating of Kv4 potassium channels – Molecular interactions involving the inner vestibule of the pore. J General Physiol. 1999;113:641–659. doi: 10.1085/jgp.113.5.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehl SJ, Eduljee C, Kwan DCH, Zhang S, Fedida D. Molecular determinants of the inhibition of human Kv1.5 potassium currents by external protons and Zn2+ J Physiol. 2002;541:9–24. doi: 10.1113/jphysiol.2001.014456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerschensteiner D, Monje F, Stocker M. Structural determinants of the regulation of the voltage-gated potassium channel Kv2.1 by the modulatory alpha-subunit Kv9.3. J. Biol. Chem. 2003;278:18154–18161. doi: 10.1074/jbc.M213117200. [DOI] [PubMed] [Google Scholar]
- Klemic KG, Kirsch GE, Jones SW. U-type inactivation of Kv3.1 and Shaker potassium channels. Biophys J. 2001;81:814–826. doi: 10.1016/S0006-3495(01)75743-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemic KG, Shieh CC, Kirsch GE, Jones SW. Inactivation of Kv2.1 potassium channels. Biophys J. 1998;74:1779–1789. doi: 10.1016/S0006-3495(98)77888-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurata HT, Soon GS, Eldstrom JR, Lu GWK, Steele DF, Fedida D. Amino-terminal determinants of U-type inactivation of voltage-gated K+ channels. J Biol Chem. 2002;277:29045–29053. doi: 10.1074/jbc.M111470200. [DOI] [PubMed] [Google Scholar]
- Kurata HT, Soon GS, Fedida D. Altered state dependence of C-type inactivation in the long and short forms of human Kv1.5. J General Physiol. 2001;118:315–332. doi: 10.1085/jgp.118.3.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy DI, Deotsch C. A voltage-dependent role for K+ in recovery from c-type inactivation. Biophys. J. 1996;71:3157–3166. doi: 10.1016/S0006-3495(96)79509-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loots E, Isacoff EY. Molecular coupling of S4 to a K+ channel's slow inactivation gate. J General Physiol. 2000;116:623–636. doi: 10.1085/jgp.116.5.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Barneo J, Hoshi T, Heinemann SH, Aldrich RW. Effects of external cations and mutations in the pore region on C-type inactivation of Shaker potassium channels. Recept Channels. 1993;1:61–71. [PubMed] [Google Scholar]
- MacKinnon R, Yellen G. Mutations affecting TEA blockade and ion permeation in voltage-activated K+ channels. Science. 1990;250:276–279. doi: 10.1126/science.2218530. [DOI] [PubMed] [Google Scholar]
- Molina A, Castellano AG, Lopez-Barneo J. Pore mutations in Shaker K+ channels distinguish between the sites of tetraethylammonium blockade and C-type inactivation. J Physiol. 1997;499:361–367. doi: 10.1113/jphysiol.1997.sp021933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olcese R, Latorre R, Toro L, Bezanilla F, Stefani E. Correlation between charge movement and ionic current during slow inactivation in Shaker K+ channels. J General Physiol. 1997;110:579–589. doi: 10.1085/jgp.110.5.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmusson RL, Morales MJ, Castellino RC, Zhang Y, Campbell DL, Strauss HC. C-type inactivation controls recovery in a fast inactivating cardiac K+ channel (Kv1.4) expressed in Xenopus oocytes. J Physiol. 1995;489:709–721. doi: 10.1113/jphysiol.1995.sp021085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmusson RL, Morales MJ, Wang S, Liu S, Campbell DL, Brahmajothi MV, Strauss HC. Inactivation of voltage-gated cardiac K+ channels. Circ Res. 1998;82:739–750. doi: 10.1161/01.res.82.7.739. [DOI] [PubMed] [Google Scholar]
- Strang C, Cushman SJ, DeRubeis D, Peterson D, Pfaffinger PJ. A central role for the T1 domain in voltage-gated potassium channel formation and function. J Biol Chem. 2001;276:28493–28502. doi: 10.1074/jbc.M010540200. [DOI] [PubMed] [Google Scholar]
- Tamkun MM, Knoth KM, Walbridge JA, Kroemer H, Roden DM, Glover DH. Molecular cloning and characterization of two voltage-gated K+ channel cDNAs from human ventricle. FASEB J. 1991;5:331–337. doi: 10.1096/fasebj.5.3.2001794. [DOI] [PubMed] [Google Scholar]
- VanDongen AMJ, Frech G, Drewe JA, Joho RH, Brown AM. Alteration and restoration of K+ channel function by deletions at the N- and C-termini. Neuron. 1990;5:433–443. doi: 10.1016/0896-6273(90)90082-q. [DOI] [PubMed] [Google Scholar]
- Wang Z, Fedida D. Gating charge immobilization caused by the transition between inactivated states in the Kv1.5 channel. Biophys J. 2001;81:2614–2627. doi: 10.1016/S0006-3495(01)75905-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Fedida D. Uncoupling of gating charge movement and closure of the ion pore during recovery from inactivation in the Kv1.5 channel. J General Physiol. 2002;120:249–260. doi: 10.1085/jgp.20028591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellen G, Jurman ME, Abramson T, MacKinnon R. Mutations affecting internal TEA blockade identify the probable pore-forming region of a K+ channel. Science. 1991;251:939–942. doi: 10.1126/science.2000494. [DOI] [PubMed] [Google Scholar]