Abstract
Hydrogen peroxide (H2O2) is a reactive oxygen species, responsible for cytotoxic damage through the formation of hydroxyl radicals. Dopamine (DA) neurones of the substantia nigra pars compacta (SNc) are highly sensitive to metabolic stress, and they typically respond to energy deprivation with membrane hyperpolarization, mainly through opening of ATP-dependent K+ channels. Accordingly, H2O2 (3 mm) induced a tolbutamide-sensitive outward current in DA neurones. Conversely, in a hypoxic medium, H2O2 reverted membrane hyperpolarization, which is associated with oxygen deprivation in DA neurones, restored their action potential firing, and reduced the hypoxia-mediated outward current in a concentration-dependent manner, between 0.1 and 3 mm (IC50 0.6 ± 0.1 mm). Notably, H2O2 did not counteract membrane hyperpolarization associated with hypoglycaemia, moreover, when catalase was inhibited with 3-amino-1,2,4-triazole (3-AT; 30 mm), H2O2 did not reduce hypoxia-mediated outward current. The counteracting action of H2O2 on hypoxia-mediated effects was further confirmed by single-unit extracellular recordings of presumed DA neurones in acute midbrain slices preparations, using a planar multi-electrode array device. Whilst a prolonged period of hypoxia (40 min) caused firing suppression, which did not recover after perfusion in normoxic conditions, the presence of H2O2 (3 mm) during this prolonged hypoxic period rescued most of the neurones from irreversible firing inhibition. Accordingly, morphological studies showed that H2O2 counteracts the cytochrome c release provoked by prolonged hypoxic treatment. Taken together, our data suggest that H2O2 prevents the metabolic stress of DA neurones induced by hypoxia by serving as a supplementary source of molecular oxygen, through its degradation by catalase.
Dopamine (DA) neurones of the substantia nigra pars compacta (SNc) are highly sensitive to metabolic stress. When exposed to a hypoxic medium, they respond with a slow hyperpolarization, mainly due to opening of ATP-dependent potassium channels (KATP), which probably represents a safety mechanism to preserve energy consumption (Mercuri et al. 1994a). If the hypoxic insult is prolonged, this early hyperpolarization is replaced by a profound and irreversible depolarization, due to opening of cationic conductances, precluding cell death (Mercuri et al. 1994a).
The production of reactive oxygen species (ROS) is an additional harmful side-effect of hypoxia/reoxygenation (Traystman et al. 1991; Zhang et al. 2002), such that oxidants like the superoxide anion (O2−), peroxynitrite (ONOO−) and hydrogen peroxide (H2O2) are synthesized by cells during ischaemia-reperfusion, and are thought to be prime mediators of neuronal destruction. Indeed, also in the dopaminergic system, reactive oxygen radicals associated with cellular respiration can cause neurodegeneration (Ebadi et al. 1996; Cohen et al. 1997; Farooqui & Horrocks, 1998; Olanow & Tatton, 1999). However, it should be noted that free radicals have been associated with cellular protection in other systems (Duranteau et al. 1998; Das et al. 1999). Moreover, there is emerging evidence that under normal physiological conditions, ROS can serve as cellular messengers (Topper et al. 1996; Wung et al. 1999), regulate signalling pathways (Klann & Thiels, 1999), modulate synaptic transmission (Pellmar, 1987; Chen et al. 2001) and synaptic plasticity (Auerbach & Segal, 1997; Klann & Thiels, 1999). With regard to H2O2, it appears that it is not only a damaging factor for neurones (Topper et al. 1996; Auerbach & Segal, 1997; Klann & Thiels, 1999; Wung et al. 1999), but mediates Ca2+-dependent plastic changes (Yermolaieva et al. 2000) and modulates DA release (Chen et al. 2002).
H2O2 is one of the ROS generated in conditions of metabolic stress (Russell & Jackson, 1994; Zulueta & Sawhney, 1997; Jovanovic et al. 2001; Wilhelm et al. 2003). It is mainly produced in the mitochondria, where partial reduction of molecular oxygen in the electron transport chain results in formation of the superoxide anion (Boveris & Chance, 1973). In addition, there are a number of H2O2-producing enzymes, such as monoamine oxidase and amino acid oxidase (Graham et al. 1978; Chen et al. 2001). On the other hand, two forms of superoxide dismutase (SOD; cytosolic copper/zinc-containing SOD, and mitochondrial manganese-containing SOD) catalyse the dismutation of the superoxide radical to H2O2 (Fridovich, 1989). H2O2, in turn, can lead to the formation of hydroxyl radicals via the Fenton reaction (Halliwell, 1999), and thus to cytotoxic damage.
The intracellular concentration of H2O2 is determined by a balance of formation and conversion into H2O by catalase and glutathione peroxidase (GPx) (Halliwell B, 1999), and cellular metabolism may be impaired when H2O2 is not neutralized by these downstream enzymatic pathways. For instance, free H2O2 can affect glucose metabolism and alter KATP channel function (Krippeit-Drews et al. 1999; Maechler et al. 1999). However, H2O2 elimination through catalase results in production of H2O and O2, thus H2O2 provides an alternative source for O2 that may supposedly be protective in hypoxic conditions (Topper et al. 1996; Auerbach & Segal, 1997; Klann & Thiels, 1999; Wung et al. 1999). Indeed, previous electrophysiological observations in the hippocampus have shown a recovery of the synaptic function in hypoxia by H2O2 (Fowler, 1997). Moreover, in slices of the spinal cord, H2O2 may act as a supplementary source of O2 (Walton & Fulton, 1983).
The goal of the present report was to assess a possible protective role of H2O2 in DA neurones of the rat SNc exposed to a hypoxic insult. To this aim, in slice preparations, we used electrophysiological and morphological techniques to investigate DA neurone responses to short or prolonged exposure to an O2-deprived medium.
Methods
Brain slices preparation and electrophysiology
Wistar rats (21–24 days old) were anaesthetized with halothane and killed by decapitation. All experiments followed international guidelines on the ethical use of animals from the European Communities Council Directive of 24 November 1986 (86/609/EEC). The brain was rapidly removed from the skull, and horizontal midbrain slices (240–300 μm) were cut in cold artificial cerebrospinal fluid (ACSF) using a vibratome, and left to recover at 33°C for at least 20–30 min. Slices were placed in a recording chamber and submerged in a continuously flowing (3.5 ml min−1, 33.5°C) ACSF. ACSF composition was (mm): NaCl 126, KCl 2.5, MgCl2 1.2, CaCl2 2.4, NaH2PO4 1.2, NaHCO3 19, glucose 10 (Mercuri et al. 1995).
Hypoxic or hypoglycaemic solutions were obtained by saturating the standard ACSF with a gas mixture of 95% N2 and 5% CO2, or by omitting glucose from standard ACSF, respectively.
Intracellular and patch-clamp recordings were obtained from neurones of the SNc. DA neurones were identified on the basis of their electrophysiological properties, thus by the presence of regular spontaneous firing activity, prominent time-dependent hyperpolarization-activated current (Ih) in response to hyperpolarizing voltage steps and hyperpolarization by DA (10–30 μm) (Grace & Onn, 1989; Mercuri et al. 1995). Only neurones meeting these criteria were studied.
Intracellular recordings
The recording electrodes prepared from 1.5 mm borosilicate capillaries (Clark Electromedical Instruments, UK) were pulled with a P-97 Flaming/Brown puller (Sutter Instruments Co., CA, USA) and filled with a 2 m KCl-containing solution. The electrodes had a tip resistance of 50–80 MΩ. Membrane voltage and current signals were recorded using an Axoclamp-2 A amplifier (Axon Instruments, Union City, CA USA). Under single-electrode voltage clamp (−60 mV holding potential, Vhold), the switching frequency was 3–4 Hz, and a duty cycle of 30% was used. The headstage voltage was continuously monitored on a separate oscilloscope to ensure sufficient decay of the electrode transient. The signals were digitized by use of an A/D converter (Digidata 1200; Axon Instruments) and saved in a computer with Axotape software (Axon Instruments) for off-line analysis.
Patch-clamp recordings
Slices were transferred to a submerged recording chamber on the stage of an upright microscope (Axioscop FS; Zeiss, Göttingen, Germany), equipped for infrared video microscopy (Hamamatsu, Tokyo, Japan), allowing a direct visualization of the recorded neurones. Whole-cell voltage-clamp recordings (Vhold−60 mV) were obtained using an amplifier (Axopatch 200B, Axon Instruments) from visually and electrophysiologically identified DA neurones using patch pipettes (3–4 MΩ) made from 1.5 mm borosilicate glass (WPI, Sarasota, FL, USA) and pulled with a PP 83 Narishige puller (Tokyo, Japan). Membrane currents were digitized at 5 kHz through a Digidata 1200B A/D converter, acquired and analysed using pClamp software (Axon Instruments). Pipettes were filled with a standard internal solution containing (mm): potassium gluconate 145, CaCl2 0.1, MgCl2 2, Hepes 10, EGTA 0.75, MgATP 2, Na3GTP 0.3; or potassium methylsulphate 145, KCl 8, Hepes 10, MgATP 2, Na3GTP, 0.3 (pH 7.35 with KOH). Access resistance was monitored at regular intervals.
Multielectrode recordings
Extracellular signals were acquired on a planar multi-electrode array using the Panasonic MED64 System (Multi Electrode Systems, Whitestone, NY, USA). Horizontal midbrain slices (300 μm) of the ventral midbrain were placed over an 8 × 8 array of planar microelectrodes, each 20 μm × 20 μm in size, with an interpolar distance of 100 μm (MED-P2105; Matsushita Electric Industrial Co., Ltd, Osaka, Japan). Slices were positioned over the multi-electrode array under visual control through a stereomicroscope (Wild M650, Switzerland) in such a way that the area closed to the medial terminal nucleus of the accessory optic tract covered most of the electrodes (see Fig. 5A). Signals were low-cut filtered at 100 Hz and digitized at 100 kHz with a 6071E Data Acquisition Card (National Instruments, Austin, TX, USA) using Performer 2.0 software (Tensor Biosciences, Irvine, CA, USA).
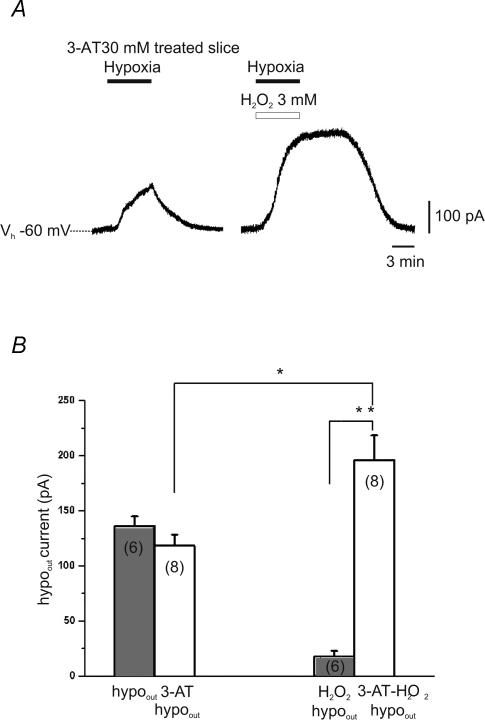
Figure 5. The block of catalase prevents H2O2 inhibition of hypoout.
A, patch-clamp voltage-clamp recording of hypoout in the continuous presence of the catalase inhibitor 3-amino-1,2,4-triazole (3-AT; 30 mm). When the hypoxic insult was repeated in the same DA neurone in the presence of H2O2 (3 mm), hypoout was not reduced, rather an increase of hypoout amplitude was observed. B, bars indicate the mean amplitude of hypoout in control conditions (grey bars) and in presence of 3-AT (30 mm; white bars). *P < 0.05 and **P < 0.001, Student's t test. The numbers of observations are in parentheses.
The frequency of the fast transients corresponding to spontaneous action potential firing was calculated off-line with Performer 2.0 software (Tensor Biosciences) using an amplitude threshold adjusted by visual inspection in each individual active channel. The activity was measured over a 15 s recording period repeated every 60 s. After a control period of 15–20 min, slices were exposed for 40 min to hypoxic ACSF. Recovery from hypoxia was then followed for a period of 40–50 min
Histology and confocal microscopy
Slice preparation was identical to that described for electrophysiological recordings. After dissection, slices were transferred in a holding chamber and left to recover for 30 min (33°C) in standard ACSF medium saturated with an O2/CO2 gas mixture. They were then divided into three groups: the first group (control) consisted of slices maintained for 30 min in standard ACFS saturated with O2/CO2 mixture; the second group (hypoxia) was placed in a chamber containing the ACFS saturated with a N2/CO2 gas mixture for 30 min; the third group (hypoxia in H2O2) was placed in a chamber containing H2O2 (3 mm) in ACSF saturated with N2/CO2 for 30 min. After treatment, slices from all three groups were left to recover for 30 min in standard oxygenated ACFS, and then fixed in 4% paraformaldehyde/phosphate buffer (PB) for 5 h, and after three washings in PB, they were transferred to 30% sucrose/PB at 4°C until they sank. Then, slices were cut into 40-μm-thick horizontal sections using a freezing microtome and directly mounted on slides. Three sections from each slice were used for quantitative evaluation.
Since considerable data indicate that hypoxia-induced ATP depletion provokes release of cytochrome c from mitochondria (Sims & Anderson, 2002), and cytochrome c immunohistochemistry is often used to assess cell death in experimental models (Fujimura et al. 2000; Galeffi et al. 2000), we employed this technique to assess cell damage in the three experimental groups. DA cells were identified by tyrosine hydroxylase (TH) immunoreactivity. Double cytochrome c and TH immunofluorescence was carried out on sections mounted on slides. Sections were incubated overnight in a mixture of the following primary antibodies: goat antityrosine hydroxylase (1:500; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and mouse anticytochrome c (1:100; Promega, Madison, WI, USA). Antibody solutions were prepared in PB and 0.3% Triton X-100, and each incubation step was followed by three washes in PB. After overnight incubation with the cocktail of primary antibodies, sections were incubated for 2 h at room temperature in a mixture of secondary antibodies including Cy3-conjugated donkey antigoat IgG, and Cy2-conjugated donkey antimouse IgG (1:100; Jackson Immunoresearch Laboratories, West Grove, PA, USA). Sections were then air-dried and coverslipped with Gel/Mount (Biomeda, Foster City, CA, USA). Images were acquired through a confocal laser scanning microscope (CLSM; Zeiss, LSM 510) equipped with an argon laser emitting at 488 nm, and a helium–neon laser emitting at 543 nm. Plates were generated adjusting the contrast and brightness of digital images (Corel Draw, 9).
Qualitative and quantitative observations were limited to the SNc, which was identified by its shape, cellular density and proximity to the medial terminal nucleus of the accessory optic tract. Observations were carried out on five sections for each slice. All labelled neurones with the nucleus clearly identifiable were counted within the confines of a squared frame box (500 μm per side) placed in close proximity of the medial terminal nucleus of the accessory optic tract. Cell counting was performed on digital images acquired through the confocal microscope using a ×10 objective at a 0.7 zoom factor. Two digital images of the same optical section (one for each laser channel, green and red) were acquired and digitally merged in a third image, which was used for cell counting. Cellular labelling was analysed off-line through the CLSM proprietary image analysis program (Zeiss, LSM software 2.3) by zooming in on the cells and by serially excluding each channel (green and red) to positively identify each labelled cell as TH positive, cytochrome-c positive or cytochrome c/TH double labelled. All labelled neurones were then differently marked electronically according to the labelling characteristics to allow recording and storing of the quantitative data. Data were then pooled across section and averaged across slices.
Drugs
H2O2, 3-amino-1,2,4-triazole (3-AT) and dopamine (DA) hydrochloride were from Sigma-Aldrich (Milan, Italy). H2O2 was diluted daily from a 30% stock solution. The concentration of the stock solution was 8.8 m.
Data analysis
For intracellular and patch-clamp recordings, numerical data were expressed as means ±s.e.m. Student's t test for paired and independent observations was used to compare data. P < 0.05 was considered significant. To estimate the IC50 and maximal response, the concentration–response curve was fitted with a least-squares regression using a logistic equation: y=ax/(x+b), where a is maximal effect, x is drug concentration, and b is the concentration attaining half-maximal effect.
For multi-electrode recordings, the cumulative plots of the post-hypoxic firing frequency shown in Fig. 5D were obtained by normalizing the firing rate in each channel to its own control level, measured before the hypoxic challenge. The normalized firing frequencies at 40 min after reoxygenation in all active channels of each slice were then expressed in a cumulative probability form (bin size 0.05). Such cumulative distributions were then averaged across slices exposed to hypoxic ACSF alone or in the presence of H2O2.
For histological experiments, the following data were considered for each condition: total number of TH-positive neurones, total number of cytochrome-c-positive neurones, and percentage of TH-positive neurones that were also cytochrome-c-positive. Means ±s.d. for each experimental condition were calculated, and group differences were statistically analysed by two-way ANOVA. Post hoc comparison was carried out by Tukey's post hoc test (StatView 5.0 SAS, Cary, NC, USA). Significance was set at P < 0.001.
Results
H2O2 counteracts hypoxia-induced hyperpolarization of DA neurones
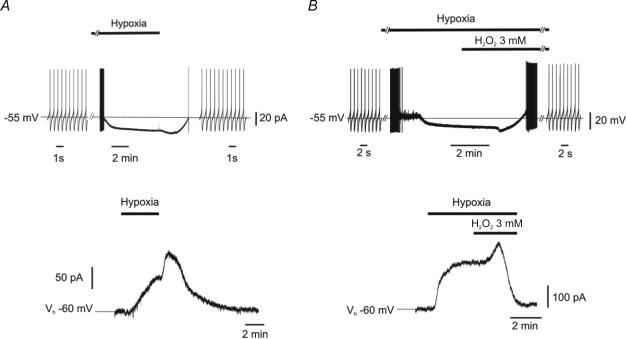
As previously observed (Mercuri et al. 1994a, b), a brief period (5–10 min) of hypoxia produced a reversible hyperpolarization (20 ± 2 mV) in DA neurones of the SNc (Fig. 1A, upper trace; n = 11). Under voltage-clamp conditions (Vhold−60 mV), hypoxia caused an outward current (hypoout) that started after ∼1 min of N2/CO2 superfusion, and reached a plateau within 2–3 min (161.2 ± 13.9 pA; n = 8; Fig. 1A, lower trace). Upon reoxygenation, a further post-hypoxic hyperpolarization (7.3 ± 2.8 mV)/outward current (47.0 ± 12.0 pA) was observed, followed by recovery of action potential firing or holding current (Fig. 1A). We then conducted similar experiments by applying H2O2 (3 mm) while neurones were exposed to hypoxic ACSF (Fig. 1B). Hypoxic ACSF abolished action potential firing of DA cells. However, when H2O2 (3 mm) was added to the hypoxic medium, an initial transient hyperpolarization was observed (8.0 ± 3.0 mV), followed by a rapid (3.0 ± 1.0 min) recovery of action potential firing (Fig. 1B, upper trace; n = 6). Likewise, in experiments performed in voltage clamp (Vhold−60 mV), H2O2 (3 mm) abolished hypoout (Fig. 1B, lower trace; n = 6) and this effect was preceded by a transient outward current (50.0 ± 10.0 pA).
Figure 1. H2O2 opposes hypoxia-induced inhibition in dopamine neurones.
Traces were obtained from separate dopamine (DA) neurones recorded with sharp electrodes in current-clamp (upper traces) or voltage clamp (lower traces) mode. A, in control conditions, hypoxia caused firing discharge inhibition (upper trace), associated with membrane hyperpolarization. When normoxic conditions were restored, a further transient hyperpolarization was observed, followed by a slow recovery of the membrane potential and the firing activity. Accordingly, the outward current induced by hypoxia (lower trace) was reverted upon reoxygenation and preceded by a transient post-hypoxic outward current. B, similar experiments were conducted with H2O2 (3 mm) while DA neurones were exposed to the hypoxic medium. Shortly after perfusion with H2O2, a transient hyperpolarization was observed, followed by complete recovery of action potential firing (upper trace). Similarly, the outward current induced by hypoxia (lower trace) was reverted by H2O2 (3 mm) and preceded by a transient outward current.
H2O2 does not oppose the effects of hypoglycaemia
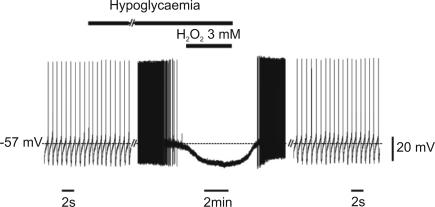
Metabolic stress associated with perfusion in a glucose-free ACSF has been reported to reversibly hyperpolarize DA neurones through mechanisms of action similar to those of hypoxia (Roeper et al. 1990; Marinelli et al. 2001). Therefore, we tested whether H2O2 may also counteract the effects of hypoglycaemia. As shown in Fig. 2, perfusion in hypoglycaemic medium blocked the firing activity of DA neurones and hyperpolarized the membrane potential (8.2 ± 1.3 mV; n = 3). However, in contrast to what we found during hypoxia, no recovery was observed following perfusion of H2O2 (3 mm, 5–10 min) in hypoglycaemic ACSF (n = 3), while a full recovery was observed after washout in normoglycaemic conditions.
Figure 2. H2O2 does not oppose hypoglycaemia-induced inhibition in DA neurones.
Trace record from a DA neurone recorded with a sharp electrode in current-clamp mode. Perfusion in a medium lacking glucose caused membrane hyperpolarization and firing inhibition. This effect was insensitive to H2O2 (3 mm), while a complete recovery was observed following washout in normoglycaemic conditions.
Opposing effects of H2O2 in normoxic and hypoxic conditions
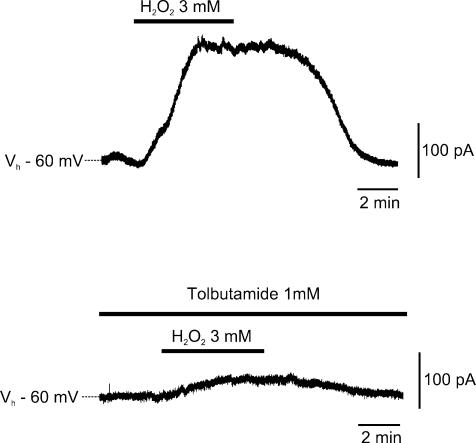
The action of H2O2 on the DA neurones was further explored in normoxic conditions. Unexpectedly, while H2O2 (3 mm) counteracted an outward current, when applied in a hypoxic medium (Fig. 1), it caused an outward current (176 ± 31 pA; n = 4) when applied in normoxic ACSF. This current was reversible at H2O2 washout, and was largely dependent on the opening of KATP conductances since it was sensitive to tolbutamide (1 mm; Fig. 3).
Figure 3. H2O2 opens KATP conductance in normoxic conditions.
Sharp-electrode voltage-clamp recording from a DA cell recorded in standard oxygenated artificial cerebrospinal fluid (ACSF). H2O2 (3 mm) induced a reversible outward current (upper trace). In the same cell, the KATP channel antagonist tolbutamide (1 mm) strongly inhibited the outward current produced by H2O2 (lower trace).
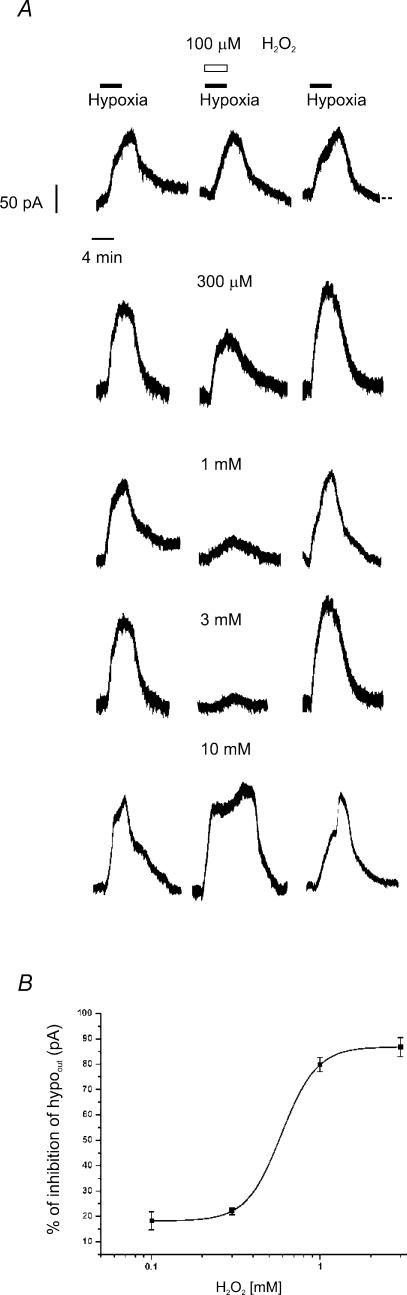
This opposite effect of H2O2 was further confirmed in experiments showing a reduced hypoout when DA neurones were exposed to the H2O2-containing hypoxic ACSF. The experimental protocol consisted of a control hypoxic challenge (5–10 min), followed by a second exposure to hypoxic ACSF containing increasing concentrations (0.1–10 mm) of H2O2. By comparing the maximal amplitude of hypoout in control hypoxic ACSF and in H2O2-containing hypoxic ACSF, we found a dose-dependent reversible reduction of hypoout at concentrations of H2O2 ranging from 0.1 to 3 mm (P < 0.05, n = 5, for each tested concentration; IC50= 0.6 ± 0.1 mm; Fig. 4A and B).
Figure 4. H2O2 inhibition of the outward current induced by hypoxia (hypoout) is dose dependent.
A, patch-clamp voltage-clamp recordings of hypoout. Left traces, control hypoout; middle traces, hypoout obtained with concomitant perfusion of rising concentrations of H2O2 (0.1–10 mm); right traces, hypoout recorded 15 min after H2O2 washout. Note that H2O2 reversibly inhibited hypoout in a concentration-dependent manner, from 0.1 to 3.0 mm, while at 10 mm no reduction of hypoout was observed. B, dose–response curve of hypoout inhibition by H2O2 (0.1–3.0 mm). Each point represents the mean ±s.e.m. of n = 5 cells. Data points were fitted with a least-squares regression using a logistic equation.
Interestingly, maximal effects were obtained at 3 mm H2O2, but higher concentrations of H2O2 (10 mm) did not reduce hypoout (136.8 ± 12.1 versus 122.6 ± 17.1 pA in control and 10 mm H2O2, respectively; n = 3; P > 0.3 Student's paired t test; Fig. 4A).
Catalase is involved in H2O2-mediated reduction of hypoout
We then investigated whether the effects of H2O2 were secondary to intracellular O2 generation, through the catalase pathway (Llinas & Sugimori, 1980; Walton & Fulton, 1983; Halliwell, 1992; Fowler, 1997). To this aim, we evaluated the effects of H2O2 on hypoout in the continuous presence of the irreversible catalase inhibitor 3-AT (30 mm).
Slices were preincubated with 3-AT (30 mm) for ∼50 min, and were continuously perfused with this inhibitor during recordings. Under these conditions, exposure to hypoxic ACSF still resulted in a sustained outward current (Fig. 5A). No difference was found between hypoout in presence of 3-AT compared with that observed in control conditions (136.2 ± 8.7 pA, n = 6, versus 118.3 ± 10.0 pA, n = 8, in controls and in 3-AT, respectively; P > 0.2 Student's unpaired t test; Fig. 5B). However, when slices pretreated in 3-AT (30 mm) were exposed to the hypoxic medium containing H2O2 (3 mm), hypoout was not reduced, but rather it increased to 195.9 ± 22.4 pA (n = 8; P < 0.05 Student's paired t test; Fig. 4A and B). Thus, hypoout induced in H2O2 alone was significantly smaller than that observed in H2O2 and 3-AT (17.9 ± 5.0 versus 195.9 ± 22.4 pA; P < 0.001, Student's unpaired t test; Fig. 5B).
H2O2 prevents hypoxia-induced irreversible inhibition of DA neurone firing
The reduction by H2O2 of DA neurone hyperpolarization in hypoxic medium could be indicative of a positive effect of this peroxide, such that the presence of H2O2 alleviates the metabolic stress associated with O2 deprivation. However, if we consider membrane hyperpolarization as a safety mechanism to preserve energy consumption, H2O2-mediated reduction of hypoout could, in fact, be harmful for DA neurones exposed to a hypoxic medium.
In order to discriminate whether H2O2 may effectively be beneficial for DA neurones during early phases of metabolic stress, we evaluated the effects of prolonged perfusion with hypoxic ACSF. It is known that when DA neurones are exposed to a hypoxic medium for more than 25–30 min, the membrane hyperpolarization is replaced by an irreversible depolarization, probably reflecting neuronal death (Mercuri et al. 1994a). Therefore, we addressed the possibility that H2O2 may prevent this irreversible loss of electrical activity by O2 deprivation. To this aim, we recorded the spontaneous action potential firing of DA neurones in acute slice preparations using a multi-electrode device.
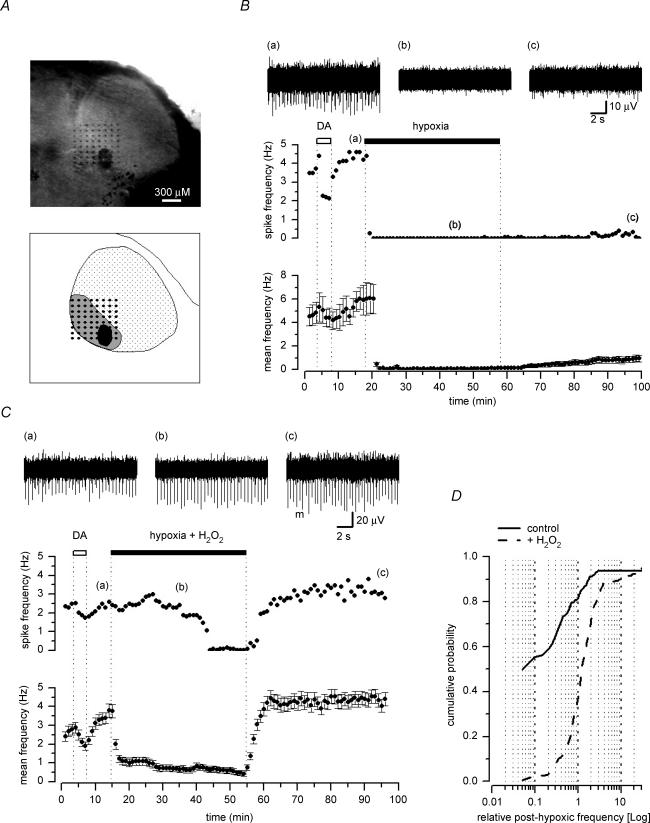
Midbrain slices were placed over a planar 8 × 8 multi-electrode array, so that most of the electrodes covered an area corresponding to the SNc. This area could be identified as a crescent-shaped region around the medial terminal nucleus of the accessory optic tract (Fig. 6A). Fast transients corresponding to spontaneous action potential firing were detected in 496 recording channels and from a total of 16 slices (31.0 ± 3.6 active channels per slice). Spikes occurred at a mean frequency of 5.4 ± 0.3 Hz (n = 496), but were found to differ in shape and amplitude, probably reflecting spontaneous action potentials arising from more than one neurone detected by a single planar electrode. We did not perform any spike-sorting discrimination of single- and multi-unit responses; however, we used a pharmacological tool to discriminate the source of this activity. It is well known that in the SNc, DA neurones are selectively inhibited by DA. Therefore, we briefly exposed the slices to DA (10 μm) prior to any experimental protocol. We observed that in the vast majority of the active channels, the overall spontaneous firing rate was reduced by DA (Fig. 6B and C), hence indicating that the recorded activity could largely be ascribed to spontaneous action potentials generated by DA neurones.
Figure 6. Irreversible inhibition of DA neurones firing by long hypoxic exposure is prevented by H2O2.
A, photograph of an horizontal midbrain slice containing the substantia nigra (top), with a schematic representation (bottom) of the region comprising the pars reticulata (dotted area) and the pars compacta (grey area) of the substantia nigra around the medial terminal nucleus of the accessory optic tract (black area). The slice was placed over an array of 8 × 8 planar electrodes, covering most of the pars compacta. B and C, plots of the spontaneous firing frequency against time, measured on a selected active channel (top) and of the mean (±s.e.m.) spike frequency (bottom) of all active channels in the same 8 × 8 array (n = 30 in B, and n = 44 in C). Perfusion with DA (10 μm) reversibly inhibited the spike frequency detected on the selected recording channel and on the averaged activity in both B and C. Exposure to a hypoxic medium for 40 min completely abolished the activity in all active channels in B.Conversely, when the hypoxic medium contained 3 mm H2O2 (C), the spontaneous firing activity was largely maintained and the averaged activity was reduced, but not abolished. Upon reoxygenation, the firing activity remained depressed in the selected channel of B, although some recovery could be detected on the averaged activity; in C, the spontaneous firing completely recovered in both the selected channel and the averaged activity. On top are shown corresponding traces of the single selected channel in the plot, acquired at the times indicated by the corresponding numbers. D, averaged cumulative plots of the relative firing frequency (bin size 0.05) from each single active channel, recorded 40 min after reoxygenation following exposure to hypoxic ACSF (continuous line; 7 slices) or hypoxic ACSF in 3 mm H2O2 (dashed line; 9 slices). The firing rate was normalized in each active channel to its own basal level measured before the hypoxic challenge.
In slices exposed to hypoxic ACSF, all the spontaneous firing was abolished within 2–3 min of perfusion (Fig. 6B). After 40 min in hypoxic medium, we washed in normoxic ACSF, but the large majority of neurones did not recover their action potential firing, even after 40–50 min of reoxygenation (Fig. 6B). The cumulative plot in Fig. 6D (see Methods) was obtained from all slices exposed to hypoxia in normal ACSF (n = 7, total number of active channels = 170). It shows that no recovery (relative frequency = 0.1) was observed in 55.3% of the active channels, while in only 25.9% of the active channels a recovery higher than 50% (i.e. relative frequency = 0.5) was obtained, with occasional cases of increased firing rate following reoxygenation.
When slices (n = 9, total number of active channels = 326) were exposed to hypoxic ACSF with H2O2 (3 mm), the spontaneous firing was normally reduced, but rarely was completely abolished (Fig. 5C). More importantly, in most cases, a recovery of action potentials firing rate was observed after 40–50 min perfusion in oxygenated ACSF. As shown in the cumulative plot of Fig. 6D, in only 2.3% of the active channels no recovery (relative frequency = 0.1) was observed, while in 86.4% of the active channels the firing rate recovered by at least 50% (i.e. relative frequency = 0.5).
H2O2 inhibits hypoxia-induced cytochrome c release
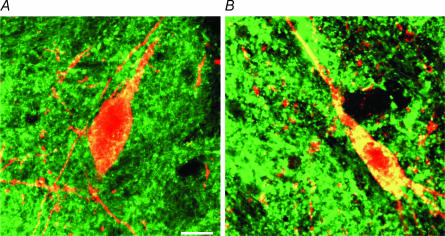
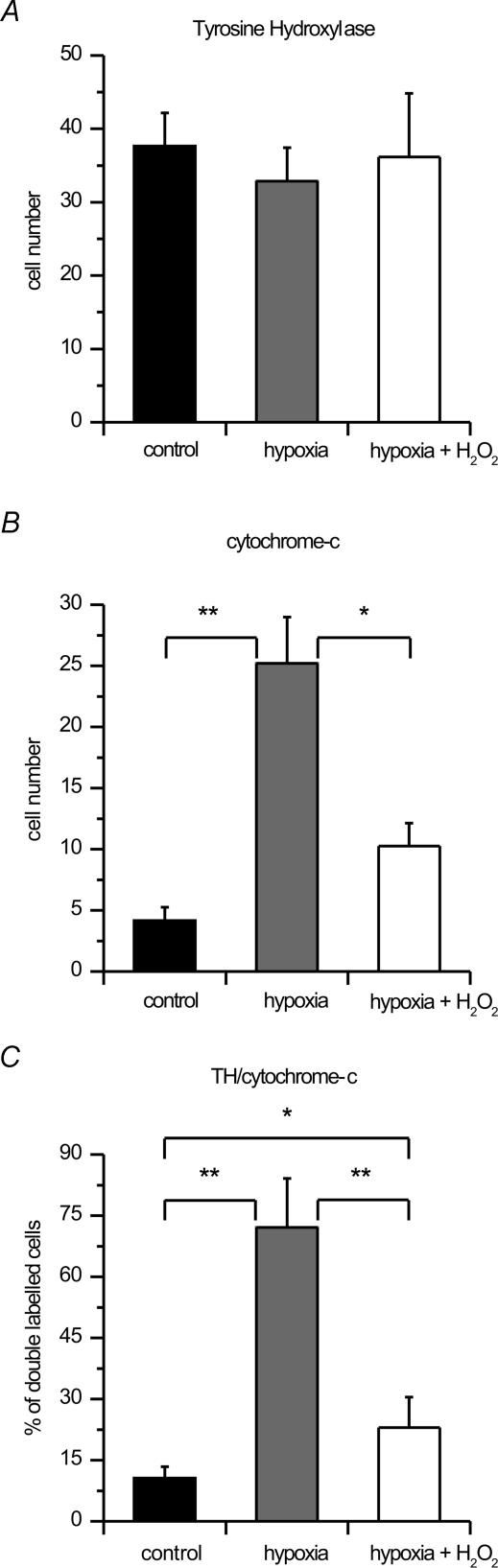
A more direct evaluation of the effects of H2O2 on DA neurones exposed to hypoxia was obtained by means of immunohistochemical techniques. We analysed TH and cytochrome c immunoreactivity in midbrain slices subdivided into three experimental groups (n = 5 in each group): control slices, slices exposed to hypoxic ACSF, and slices exposed to hypoxic ACSF in 3 mm H2O2 (see Methods). TH- and cytochrome-c-immunoreactive cells were present in all cases of the three experimental groups. In the control group, TH cell labelling was intense with a good preservation of cell morphology, while cytochrome c cell labelling was scarce and characterized by a distinct punctate cytosolic labelling. In contrast, in the hypoxic group we observed a small decrement of TH-positive cells and a pronounced clustered and fusion pattern of cytochrome c immunoreactivity, indicating release of cytochrome c in the cytosol (Fig. 7B). In the hypoxia and H2O2 group, the decrease of TH cellular density, as well as the release of cytochrome c in the cytosol was less evident (Fig. 7A). Quantitative analysis was carried out by counting all TH-positive cells, cells releasing cytochrome c and double-labelled cells (Fig. 8). The mean number of TH-positive cells presented a slight reduction in the two treated groups in comparison with the controls (Fig. 7A). Indeed, TH-positive cells were 37.7 ± 4.5 in controls, 32.9 ± 4.6 in the hypoxic group, and 36.2 ± 8.7 in hypoxic plus H2O2 (3 mm). Conversely, a marked difference was observed for cells releasing cytochrome c in the cytosol. The mean number of released cytochrome-c-positive cells was 4.2 ± 1.1 in control cases, 25.2 ± 3.8 after hypoxic treatment, and 10.3 ± 1.9 after hypoxia plus H2O2 treatment (Fig. 8B). Also, taking into account the percentage of cells that presented both TH and released cytochrome c labelling (double-labelled cells), clear differences were found among groups. In controls, 10.6 ± 2.8% of TH cells presented cytochrome c labelling, while a sixfold increase was present after hypoxia, with 72.1 ± 12.0% of TH cells presenting released cytochrome c positivity. H2O2 treatment during hypoxia significantly reduced this increase. Indeed, in hypoxia and H2O2, only 23.0 ± 7.5% of TH cells were double labelled with cytochrome c (Fig. 8C).
Figure 7. Confocal images of SNc DA cells.
Double immunofluorescence for tyrosine hydroxylase (TH; red) and cytochrome c (green) in horizontal slices of the substantia nigra pars compacta (SNc). A, healthy DA cell with no indication of cytochrome c release (from the hypoxia plus H2O2 group). Note the punctuate cytosolic cytochrome c immunostaining. B, DA cell with cytochrome c release in the cytosol (from the hypoxia group). Note the clustered and fusion pattern of cytochrome c immunoreactivity in the cytosol. Scale bar, 15 μm.
Figure 8. Hypoxia-induced cytochrome c release is inhibited by H2O2.
Histograms (means ±s.d.) of TH-positive neurones (A), neurones releasing cytochrome c (B), and the percentage of TH-positive neurones releasing cytochrome c (double-labelled) neurones (C) in the three experimental groups. *P < 0.05, **P < 0.005; ANOVA.
Two-way ANOVA with group and treatment as main factors demonstrated highly significant effects of group (F= 97.65; P < 0.0001), treatment (F= 121.04; P < 0.0001) and interaction (F= 71.99; P < 0.0001). Post-hoc analysis demonstrated highly significant differences between the control and hypoxic groups in the number of cells releasing cytochrome c (P < 0.001) and in the percentage of TH cells releasing cytochrome c (P < 0.001). Significant differences were also found between the hypoxia and the hypoxia plus H2O2 groups in cytochrome-c-releasing cell number (P < 0.01) and in the percentage of TH cells expressing released cytochrome c (P < 0.001). Significant differences were also found between hypoxia in the H2O2 group and the controls in the percentages of TH cells expressing released cytochrome c (P < 0.01), while no significant difference was present between the same groups in the cell numbers releasing cytochrome c. There was also no significant difference in the number of TH-positive cells.
Discussion
The results of this study indicate that H2O2 reduces the effects of hypoxia on DA neurones of the SNc by counteracting both early and late electrophysiological responses associated with O2 deprivation. In addition, H2O2 reduces events that preclude cell death of the DA neurones in hypoxic conditions, as revealed by cytochrome c release.
When DA neurones are exposed to hypoxic ACSF, they typically undergo a profound membrane hyperpolarization, mainly due to opening of KATP channels (Mercuri et al. 1994a; Guatteo et al. 1998). If H2O2 is added to the hypoxic medium, a rapid recovery of the resting membrane potential is observed and action potential firing is restored. Likewise, the outward current induced by hypoxia is rapidly suppressed by H2O2, although DA neurones are still exposed to the anoxic insult.
The most likely explanation for this effect is that H2O2 reduces the recruitment of KATP conductance by providing an alternative source for O2. We propose this hypothesis on the basis of three experimental observations. (1) Perfusion of H2O2 during exposure to hypoxia resulted in an early transient hyperpolarization (or outward current) prior to full recovery of the resting membrane potential (or holding current; see Fig. 1). This early response is reminiscent of a similar transient hyperpolarization/outward current observed upon reoxygenation after hypoxia, which is due to reactivation of the Na+–K+ electrogenic pump (Mercuri et al. 1994a, b; Guatteo et al. 1998). (2) KATP-channel-dependent hyperpolarization is an early membrane response associated with exposure of DA neurones to a hypoxic and hypoglycaemic medium. However, H2O2 counteracts hypoxia-mediated hyperpolarization only; that due to hypoglycaemia was insensitive to H2O2. This strongly suggests that H2O2 does not act on KATP channels directly, but compensates for their activation secondary to O2 deprivation. (3) Finally, and most importantly, the reduction of hypoxia-mediated outward current by H2O2 was prevented by the block of catalase with 3-AT. This antagonist reacts with the intermediate complex (Compound I) that is formed during catalase-dependent metabolism of H2O2, so that catalase inhibition occurs only in the presence of H2O2 (Walton & Fulton, 1983). The activity of catalase occurs inside the cell and is largely located in peroxisomes (Gaunt & de Duve, 1976; Brannan et al. 1981), where it participates to the clearance of H2O2 by generating H2O and O2 (Halliwell, 1999). Hence, we suggest that H2O2 compensates for the lowered levels of O2 during anoxia through this intracellular metabolic pathway, leading to reduction of KATP channel recruitment.
In agreement with our results, it was previously reported that H2O2 provides a supplementary source of O2 in slice preparations of the spinal cord (Walton & Fulton, 1983). Notably, catalase has been thought to play a marginal role in H2O2 elimination in the central nervous system, while GPx, which reduces H2O2 into H2O without generating O2, is considered the main factor in brain H2O2 catabolism (Sinet et al. 1980; Choen, 1988; Jain et al. 1991). However, in the SNc, higher activity of catalase has been reported (Hung & Lee, 1998; Avshalumov et al. 2005), thus, H2O2 elimination through catalase in DA neurones may indeed provide an alternative source of O2 in hypoxic conditions to overcome the metabolic stress.
H2O2 protects DA neurones during hypoxia
A reduction of the hyperpolarizing response to O2 deprivation does not necessarily indicate that DA neurones are protected. In fact, membrane hyperpolarization is thought to be a safety mechanism to preserve energy consumption. Therefore, H2O2 may actually aggravate the effects of hypoxia on the DA neurones by restoring their firing activity. We can exclude this hypothesis and affirm a clear protective role exerted by H2O2 during an anoxic insult on the basis of both functional and anatomical results. First of all we found that even after a prolonged exposure to a hypoxic medium, if H2O2 was present in the bathing medium during the hypoxic insult, the large majority of the DA neurones rapidly recovered their firing activity upon reoxygenation. Conversely, in the absence of H2O2, little or no recovery was found, in agreement with previous observations showing an irreversible depolarization induced in DA neurones following prolonged perfusion in an O2-deprived medium (Mercuri et al. 1994a). Secondly, we provided anatomical evidence of reduced release of cytochrome c in DA cells exposed to hypoxia if H2O2 was dissolved in the hypoxic medium. Different lines of evidence indicate that cytochrome c release is a good indicator of the activation of irreversible cell death pathways which are mostly related to apoptosis pathways (Fujimura et al. 2000; Galeffi et al. 2000; Sims & Anderson, 2002). The strategy employed allowed a precise quantification of the cell population under observation thanks to the TH immunolabelling. The use of a double-labelling technique permitted us to limit the observation to TH-positive neurones, thus providing a good match with the physiological data on DA neurones. The efficacy of the technique is further emphasized by the low s.d. values, and by the lack of effects of the different treatments on the total number of TH neurones. The highly significant differences observed between the hypoxia and the hypoxia plus H2O2 groups in the two death index analyses, namely total number of cells releasing cytochrome c and percentage of TH cells also releasing cytochrome c, provide strong support to the physiological evidence of the protective action of H2O2.
Harmful versus protective effects of H2O2
Interestingly, when added to a normoxic ACSF, H2O2 induced an outward current that could be blocked by the sulphonylurea agent tolbutamide, an antagonist of KATP channels. Indeed, H2O2 has been shown to reduce intracellular ATP levels and activate KATP channels in renal epithelial cells (Filipovic & Reeves, 1997), smooth muscle cells (Hattori et al. 2003) and pancreatic beta cells, where it interferes with glucose metabolism by targeting their mitochondria (Maechler et al. 1999), thus reducing ATP intracellular levels (Krippeit-Drews et al. 1999). With regard to the central nervous system, H2O2 has been reported to hyperpolarize hippocampal neurones through activation of a K+ conductance (Seutin et al. 1995). Moreover, in the substantia nigra, voltammetric experiments have demonstrated an H2O2-mediated control of somatodrendritic DA release (Chen et al. 2002) through opening of KATP channels (Avshalumov & Rice, 2003). In DA cells of the SNc in particular, it has recently been shown that H2O2 exerts a tonic modulation of KATP conductance, which may be regulated by its enzymatic degradation (Avshalumov et al. 2005).
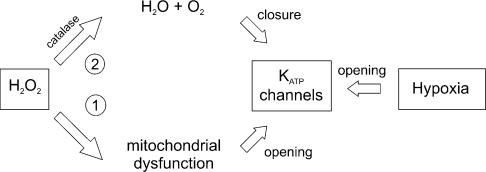
This effect seems to somehow contradict the inhibitory effect of H2O2 on the KATP-dependent hyperpolarization induced by hypoxia. In Fig. 9, we show a theoretical scheme to explain these opposing results, in which we propose a harmful (1) and a protective (2) effect of H2O2 on DA cells. H2O2 impairs mitochondrial function and activates KATP channels (1); however, at the same time, it also undergoes an enzymatic degradation. When this degradation occurs through the catalase pathway (2), it leads to production of H2O and O2. In normoxic conditions the prevailing effect of H2O2 is harmful, causing mitochondrial impairment and membrane hyperpolarization in a manner similar to hypoxia. Conversely, in hypoxic conditions, H2O2 added to the medium is rapidly metabolized into free O2, and counteracts the opening of KATP channels, and eventually protects DA neurones from the hypoxic insult.
Figure 9. Scheme of H2O2 and hypoxia effects on KATP channels in DA neurones of the SNc.
H2O2 produces a harmful effect (1), mainly through dysfunction of the mitochondrial respiratory chain. This leads to opening of KATP channels. During hypoxia, KATP channels are similarly opened. However, in hypoxia, H2O2 provides an alternative source of O2 through conversion into H2O and O2 by catalase (2). This pathway counteracts the metabolic impairment due to O2 deprivation, and eventually protects DA neurones from the hypoxic insult.
In agreement with this hypothetical scheme, we found that when the catalase pathway was blocked by 3-AT, not only was hypoout reduction by H2O2 abolished, but hypoout was increased when the hypoxic insult occurred in the presence of H2O2 (Fig. 4). Indeed, if the catalase pathway is blocked, the protective effects of H2O2 are prevented and the harmful effects of H2O2 exacerbate the metabolic impairment occurring through hypoxia. In addition, we found that the reduction of hypoout by H2O2 was dose dependent within a range of concentrations between 0.1 and 3 mm, while at higher concentrations this effect did not occur (Fig. 3B). Indeed, if high doses of H2O2 are added to the hypoxic medium, the protective effects due to H2O2 degradation by catalase may be masked by the direct harmful effects on mitochondrial function by H2O2.
Concluding remarks
H2O2 is a potentially toxic compound responsible for free-radical-dependent neuronal damage; however, in conditions of reduced O2 supply, it may exert a protective role through its metabolic degradation into O2. At present, direct clinical use of H2O2 during acute stroke may be premature and open to uncontrolled side-effects. However, endogenous H2O2 is indeed produced during anoxia (Russell & Jackson, 1994; Zulueta et al. 1997; Jovanovic et al. 2001; Wilhelm et al. 2003). Therefore, new therapeutical approaches targeting the enzymatic pathways involved in H2O2 degradation may possibly be clinically relevant.
Acknowledgments
We wish to thank Professor M. T. Carri for helpful discussions. This work was supported by FIRB grants RBNE01WY7P_010 and RBNE017555–006 to N.B.M., and Ministero della Salute grant RF03.182 to N.B.
References
- Auerbach JM, Segal M. Peroxide modulation of slow onset potentiation in rat hippocampus. J Neurosci. 1997;17:8695–8701. doi: 10.1523/JNEUROSCI.17-22-08695.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avshalumov MV, Chen BT, Koos T, Tepper JM, Rice ME. Endogenous hydrogen peroxide regulates the excitability of midbrain dopamine neurons via ATP-sensitive potassium channels. J Neurosci. 2005;25:4222–4231. doi: 10.1523/JNEUROSCI.4701-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avshalumov MV, Rice ME. Activation of ATP-sensitive K+ (KATP) channels by H2O2 underlies glutamate-dependent inhibition of striatal dopamine release. Proc Natl Acad Sci U S A. 2003;100:11729–11734. doi: 10.1073/pnas.1834314100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boveris A, Chance B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem J. 1973;134:707–716. doi: 10.1042/bj1340707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brannan TS, Maker HS, Raes IP. Regional distribution of catalase in the adult rat brain. J Neurochem. 1981;36:307–309. doi: 10.1111/j.1471-4159.1981.tb02411.x. [DOI] [PubMed] [Google Scholar]
- Chen BT, Avshalumov MV, Rice ME. H2O2 is a novel, endogenous modulator of synaptic dopamine release. J Neurophysiol. 2001;85:2468–2476. doi: 10.1152/jn.2001.85.6.2468. [DOI] [PubMed] [Google Scholar]
- Chen BT, Avshalumov MV, Rice ME. Modulation of somatodendritic dopamine release by endogenous H2O2: susceptibility in substantia nigra but resistance in VTA. J Neurophysiol. 2002;87:1155–1158. doi: 10.1152/jn.00629.2001. [DOI] [PubMed] [Google Scholar]
- Choen G. Oxygen radicals and Parkinson's disease. In: Halliwell B, editor. Oxygen Radicals and Tissue Injury. Kansas: Allen Press; 1988. pp. 130–135. [Google Scholar]
- Cohen G, Farooqui R, Kesler N. Parkinson disease: a new link between monoamine oxidase and mitochondrial electron flow. Proc Natl Acad Sci U S A. 1997;94:4890–4894. doi: 10.1073/pnas.94.10.4890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das DK, Engelman RM, Maulik N. Oxygen free radical signaling in ischemic preconditioning. Ann N Y Acad Sci. 1999;874:49–65. doi: 10.1111/j.1749-6632.1999.tb09224.x. [DOI] [PubMed] [Google Scholar]
- Duranteau J, Chandel NS, Kulisz A, Shao Z, Schumacker PT. Intracellular signaling by reactive oxygen species during hypoxia in cardiomyocytes. J Biol Chem. 1998;273:11619–11624. doi: 10.1074/jbc.273.19.11619. [DOI] [PubMed] [Google Scholar]
- Ebadi M, Srinivasan SK, Baxi MD. Oxidative stress and antioxidant therapy in Parkinson's disease. Prog Neurobiol. 1996;48:1–19. doi: 10.1016/0301-0082(95)00029-1. [DOI] [PubMed] [Google Scholar]
- Farooqui AA, Horrocks LA. Lipid peroxides in the free radical pathophysiology of brain diseases. Cell Mol Neurobiol. 1998;18:599–608. doi: 10.1023/A:1020625717298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipovic DM, Reeves WB. Hydrogen peroxide activates glibenclamide-sensitive K+ channels in LLC-PK1 cells. Am J Physiol Cell Physiol. 1997;272:C737–743. doi: 10.1152/ajpcell.1997.272.2.C737. [DOI] [PubMed] [Google Scholar]
- Fowler JC. Hydrogen peroxide opposes the hypoxic depression of evoked synaptic transmission in rat hippocampal slices. Brain Res. 1997;766:255–258. doi: 10.1016/s0006-8993(97)00699-9. [DOI] [PubMed] [Google Scholar]
- Fridovich I. Superoxide dismutases. An adaptation to a paramagnetic gas. J Biol Chem. 1989;264:7761–7764. [PubMed] [Google Scholar]
- Fujimura M, Morita-Fujimura Y, Noshita N, Sugawara T, Kawase M, Chan PH. The cytosolic antioxidant copper/zinc-superoxide dismutase prevents the early release of mitochondrial cytochrome c in ischemic brain after transient focal cerebral ischemia in mice. J Neurosci. 2000;20:2817–2824. doi: 10.1523/JNEUROSCI.20-08-02817.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galeffi F, Sinnar S, Schwartz-Bloom RD. Diazepam promotes ATP recovery and prevents cytochrome c release in hippocampal slices after in vitro ischemia. J Neurochem. 2000;75:1242–1249. doi: 10.1046/j.1471-4159.2000.0751242.x. [DOI] [PubMed] [Google Scholar]
- Gaunt GL, de Duve C. Subcellular distribution of d-amino acid oxidase and catalase in rat brain. J Neurochem. 1976;26:749–759. doi: 10.1111/j.1471-4159.1976.tb04448.x. [DOI] [PubMed] [Google Scholar]
- Grace AA, Onn SP. Morphology and electrophysiological properties of immunocytochemically identified rat dopamine neurons recorded in vitro. J Neurosci. 1989;9:3463–3481. doi: 10.1523/JNEUROSCI.09-10-03463.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham DG, Tiffany SM, Bell WR, Jr, Gutknecht WF. Autoxidation versus covalent binding of quinones as the mechanism of toxicity of dopamine, 6-hydroxydopamine, and related compounds toward C1300 neuroblastoma cells in vitro. Mol Pharmacol. 1978;14:644–653. [PubMed] [Google Scholar]
- Guatteo E, Federici M, Siniscalchi A, Knopfel T, Mercuri NB, Bernardi G. Whole cell patch-clamp recordings of rat midbrain dopaminergic neurons isolate a sulphonylurea- and ATP-sensitive component of potassium currents activated by hypoxia. J Neurophysiol. 1998;79:1239–1245. doi: 10.1152/jn.1998.79.3.1239. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Reactive oxygen species and the central nervous system. J Neurochem. 1992;59:1609–1623. doi: 10.1111/j.1471-4159.1992.tb10990.x. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JMC. Antioxidant defence enzyme: the glutathione peroxidase family. In: Halliwell B, Gutteridge JMC, editors. Free Radicals in Biology and Medicine. UK: Oxford Science Publications; 1999. pp. 140pp. 170–172. [Google Scholar]
- Hattori T, Kajikuri J, Katsuya H, Itoh T. Effects of H2O2 on membrane potential of smooth muscle cells in rabbit mesenteric resistance artery. Eur J Pharmacol. 2003;464:101–109. doi: 10.1016/s0014-2999(03)01427-4. [DOI] [PubMed] [Google Scholar]
- Hung HC, Lee EH. MPTP produces differential oxidative stress and antioxidative responses in the nigrostriatal and mesolimbic dopaminergic pathways. Free Radic Biol Med. 1998;24:76–84. doi: 10.1016/s0891-5849(97)00206-2. [DOI] [PubMed] [Google Scholar]
- Jain A, Martensson J, Stole E, Auld PA, Meister A. Glutathione deficiency leads to mitochondrial damage in brain. Proc Natl Acad Sci U S A. 1991;88:1913–1917. doi: 10.1073/pnas.88.5.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic S, Land SC, Olver RE, Wilson SM. Hypoxic activation of an amiloride-sensitive cation conductance in alveolar epithelial cells. Biochem Biophys Res Commun. 2001;286:622–627. doi: 10.1006/bbrc.2001.5432. [DOI] [PubMed] [Google Scholar]
- Klann E, Thiels E. Modulation of protein kinases and protein phosphatases by reactive oxygen species: implications for hippocampal synaptic plasticity. Prog Neuropsychopharmacol Biol Psychiatry. 1999;23:359–376. doi: 10.1016/s0278-5846(99)00002-0. [DOI] [PubMed] [Google Scholar]
- Krippeit-Drews P, Kramer C, Welker S, Lang F, Ammon HP, Drews G. Interference of H2O2 with stimulus-secretion coupling in mouse pancreatic beta-cells. J Physiol. 1999;514:471–481. doi: 10.1111/j.1469-7793.1999.471ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas R, Sugimori M. Electrophysiological properties of in vitro Purkinje cell dendrites in mammalian cerebellar slices. J Physiol. 1980;305:197–213. doi: 10.1113/jphysiol.1980.sp013358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maechler P, Jornot L, Wollheim CB. Hydrogen peroxide alters mitochondrial activation and insulin secretion in pancreatic beta cells. J Biol Chem. 1999;274:27905–27913. doi: 10.1074/jbc.274.39.27905. [DOI] [PubMed] [Google Scholar]
- Marinelli S, Federici M, Giacomini P, Bernardi G, Mercuri NB. Hypoglycemia enhances ionotropic but reduces metabotropic glutamate responses in substantia nigra dopaminergic neurons. J Neurophysiol. 2001;85:1159–1166. doi: 10.1152/jn.2001.85.3.1159. [DOI] [PubMed] [Google Scholar]
- Mercuri NB, Bonci A, Calabresi P, Stefani A, Bernardi G. Properties of the hyperpolarization-activated cation current Ih in rat midbrain dopaminergic neurons. Eur J Neurosci. 1995;7:462–469. doi: 10.1111/j.1460-9568.1995.tb00342.x. [DOI] [PubMed] [Google Scholar]
- Mercuri NB, Bonci A, Calabresi P, Stratta F, Bernardi G. Responses of rat mesencephalic dopaminergic neurons to a prolonged period of oxygen deprivation. Neuroscience. 1994a;63:757–764. doi: 10.1016/0306-4522(94)90520-7. [DOI] [PubMed] [Google Scholar]
- Mercuri NB, Bonci A, Johnson SW, Stratta F, Calabresi P, Bernardi G. Effects of anoxia on rat midbrain dopamine neurons. J Neurophysiol. 1994b;71:1165–1173. doi: 10.1152/jn.1994.71.3.1165. [DOI] [PubMed] [Google Scholar]
- Olanow CW, Tatton WG. Etiology and pathogenesis of Parkinson's disease. Annu Rev Neurosci. 1999;22:123–144. doi: 10.1146/annurev.neuro.22.1.123. [DOI] [PubMed] [Google Scholar]
- Pellmar TC. Peroxide alters neuronal excitability in the CA1 region of guinea-pig hippocampus in vitro. Neuroscience. 1987;23:447–456. doi: 10.1016/0306-4522(87)90068-6. [DOI] [PubMed] [Google Scholar]
- Roeper J, Hainsworth AH, Ashcroft FM. Tolbutamide reverses membrane hyperpolarisation induced by activation of D2 receptors and GABAB receptors in isolated substantia nigra neurones. Pflugers Arch. 1990;416:473–475. doi: 10.1007/BF00370758. [DOI] [PubMed] [Google Scholar]
- Russell WJ, Jackson RM. Hydrogen peroxide release by mitochondria from normal and hypoxic lungs. Am J Med Sci. 1994;308:239–243. doi: 10.1097/00000441-199430840-00005. [DOI] [PubMed] [Google Scholar]
- Seutin V, Scuvee-Moreau J, Massotte L, Dresse A. Hydrogen peroxide hyperpolarizes rat CA1 pyramidal neurons by inducing an increase in potassium conductance. Brain Res. 1995;683:275–278. doi: 10.1016/0006-8993(95)00436-t. [DOI] [PubMed] [Google Scholar]
- Sims NR, Anderson MF. Mitochondrial contributions to tissue damage in stroke. Neurochem Int. 2002;40:511–526. doi: 10.1016/s0197-0186(01)00122-x. [DOI] [PubMed] [Google Scholar]
- Sinet PM, Heikkila RE, Cohen G. Hydrogen peroxide production by rat brain in vivo. J Neurochem. 1980;34:1421–1428. doi: 10.1111/j.1471-4159.1980.tb11222.x. [DOI] [PubMed] [Google Scholar]
- Topper JN, Cai J, Falb D, Gimbrone MA., Jr Identification of vascular endothelial genes differentially responsive to fluid mechanical stimuli: cyclooxygenase-2, manganese superoxide dismutase, and endothelial cell nitric oxide synthase are selectively up-regulated by steady laminar shear stress. Proc Natl Acad Sci U S A. 1996;93:10417–10422. doi: 10.1073/pnas.93.19.10417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traystman RJ, Kirsch JR, Koehler RC. Oxygen radical mechanisms of brain injury following ischemia and reperfusion. J Appl Physiol. 1991;71:1185–1195. doi: 10.1152/jappl.1991.71.4.1185. [DOI] [PubMed] [Google Scholar]
- Walton K, Fulton B. Hydrogen peroxide as a source of molecular oxygen for in vitro mammalian CNS preparations. Brain Res. 1983;278:387–393. doi: 10.1016/0006-8993(83)90280-9. [DOI] [PubMed] [Google Scholar]
- Wilhelm J, Vankova M, Maxova H, Siskova A. Hydrogen peroxide production by alveolar macrophages is increased and its concentration is elevated in the breath of rats exposed to hypoxia: relationship to lung lipid peroxidation. Physiol Res. 2003;52:327–332. [PubMed] [Google Scholar]
- Wung BS, Cheng JJ, Chao YJ, Hsieh HJ, Wang DL. Modulation of Ras/Raf/extracellular signal-regulated kinase pathway by reactive oxygen species is involved in cyclic strain-induced early growth response-1 gene expression in endothelial cells. Circ Res. 1999;84:804–812. doi: 10.1161/01.res.84.7.804. [DOI] [PubMed] [Google Scholar]
- Yermolaieva O, Brot N, Weissbach H, Heinemann SH, Hoshi T. Reactive oxygen species and nitric oxide mediate plasticity of neuronal calcium signaling. Proc Natl Acad Sci U S A. 2000;97:448–453. doi: 10.1073/pnas.97.1.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HY, McPherson BC, Liu H, Baman TS, Rock P, Yao Z. H2O2 opens mitochondrial K (ATP) channels and inhibits GABA receptors via protein kinase C-epsilon in cardiomyocytes. Am J Physiol Heart Circ Physiol. 2002;282:H1395–1403. doi: 10.1152/ajpheart.00683.2001. [DOI] [PubMed] [Google Scholar]
- Zulueta JJ, Sawhney R, Yu FS, Cote CC, Hassoun PM. Intracellular generation of reactive oxygen species in endothelial cells exposed to anoxia-reoxygenation. Am J Physiol Lung Cell Mol Physiol. 1997;272:L897–902. doi: 10.1152/ajplung.1997.272.5.L897. [DOI] [PubMed] [Google Scholar]