Abstract
Transition metals block the muscle Cl− channel ClC-1, which belongs to a large family of double-barreled Cl− channels and transporters. In the Torpedo Cl− channel ClC-0, Zn2+ block is closely related to the common gating mechanism that opens and closes both pores of the channel simultaneously, and the mutation C212S, which locks the common gate open, also eliminates the block. In ClC-1, however, previous results suggested that Zn2+ block is independent of gating, and that the cysteine residues involved in Zn2+ binding are in different positions to those that confer Zn2+ sensitivity on ClC-0. In this work, we show that Zn2+ block of ClC-1 is faster at hyperpolarized potentials where the channel is more likely to be in the closed state. Mutation C277S, equivalent to C212S in ClC-0, which locks the common gate in ClC-1 open, virtually eliminates Zn2+ block. A mutation, V321A, which reduces open probability of the common gate, facilitated Zn2+ block. These results demonstrate that Zn2+ block is state dependent, acting on the common gate. The extent of the block, however, is not a simple function of the open probability of the common gate. The Q10 of ∼13 of the time course of Zn2+ block, which is significantly higher than the Q10 of common gating transitions in WT ClC-1, suggests that Zn2+ binds to a very high temperature-dependent low-probability closed substate of the common gate, which has not yet been characterized in this channel.
Earlier electrophysiological studies have shown that Zn2+ inhibits the resting membrane conductance of skeletal muscle, most of which is due to Cl− (Hutter & Warner, 1967; Stanfield, 1970; Bretag et al. 1984). After the cloning of the ClC-1 Cl− channel, which is the major contributor to muscle Cl− conductance, it became possible to investigate the mechanism of Zn2+ block of this conductance using patch clamping and mutagenesis (Kurz et al. 1997, 1999; Rychkov et al. 1997). It has been suggested that cysteine residues at positions 242, 254 and 546 form the transition metal binding site in ClC-1 (Kurz et al. 1999). Zn2+ block was found to be independent of channel gating, and simple obstruction of the pore was suggested as the most likely mechanism (Kurz et al. 1999). Similar studies on a closely related channel from the Torpedo electric organ, ClC-0, suggested a completely different mechanism, showing that Zn2+ facilitates closure of the so-called common gate of the channel. A possible binding site for Zn2+ in ClC-0 also seemed different from that in ClC-1 (Chen, 1998; Lin et al. 1999).
The ClC family of proteins, including both ClC-1 and ClC-0, consists of dimeric, double-pored channels or transporters, with each monomer forming an individual conduction pathway (Dutzler et al. 2002). ClC-0 and ClC-1, which have been studied extensively using electrophysiological techniques, show a complex gating behaviour (Jentsch et al. 2002). Both channels display two types of gating – a faster gating process that opens and closes each protopore independently (the ‘fast’ or ‘single pore’ gates), and a slower gating process that closes both protopores simultaneously (the ‘slow’ or ‘common’ gate) (Middleton et al. 1994, 1996; Ludewig et al. 1996; Saviane et al. 1999).
Gating of both channels depends on permeating anions, and intracellular and extracellular pH (Pusch et al. 1995; Rychkov et al. 1996; Lin et al. 1999; Accardi et al. 2001; Chen & Chen, 2001). Moreover, mutations of equivalent residues in ClC-1 and ClC-0 often have similar effects on gating and permeation. For example, C212S in ClC-0 and C277S in ClC-1 almost completely abolish common gating transitions in corresponding channels (Lin et al. 1999; Accardi et al. 2001).
Despite obvious similarities, common gates in ClC-1 and ClC-0 have opposite voltage dependence, 1000 times different time scales of operation, and, what is more important, they have very different temperature dependence, suggesting vastly different structural changes in the channels during opening and closing transitions. The Q10 of the common gate of ClC-0 is ∼40, one of the highest for known biological processes, while for common gating of ClC-1 it is ∼4 (Pusch et al. 1997; Bennetts et al. 2001). With such differences in common gating between ClC-1 and ClC-0, it would not be surprising if the mechanisms of Zn2+ block were different between these two channels. An indication that they indeed may be different is that Zn2+ block in ClC-1 is irreversible and requires millimolar concentrations, while ClC-0 is blocked reversibly by just 10 µm Zn2+ (Kurz et al. 1997; Rychkov et al. 1997; Chen, 1998).
This study tests the hypothesis that Zn2+ block of ClC-1 is similar to that of ClC-0, i.e. it depends on common gating. Our results show that two mutations, C277S and C278S, equivalent to those affecting Zn2+ binding in ClC-0, drastically reduce Zn2+ block in ClC-1. The results also suggest that Zn2+ binds to a closed state of ClC-1 when its common gate resides in a very highly temperature-dependent low-probability substate, which has not been envisaged before.
Methods
Cell culture and transfection
Human embryonic kidney (HEK293) cells were grown in Dulbecco's modified Eagle's medium (Invitrogen Australia, Melbourne, Australia), containing 10% (v/v) fetal bovine serum (Trace, Melbourne, Australia), supplemented with l-glutamine (2 mm; Sigma, St Louis, MO, USA), and maintained at 37°C in 5% CO2. Cell cultures were transfected with 700 ng of either wild-type (WT) or mutant pCIneo/hClC-1 cDNA using Lipofectamine Plus reagent (Invitrogen), following the standard protocol described by the manufacturer, in 25 mm culture wells. Cells were cotransfected with 70 ng of green fluorescent protein plasmid cDNA (pEGFP-N1; Clontech, Palo Alto, CA, USA) to allow identification of transfected cells during patch-clamp experiments. Cells were plated for patch clamping at least 3 h after transfection, and electrophysiological measurements were commenced approximately 24 h after transfection.
Electrophysiology
Patch-clamping experiments were performed on transfected HEK293 cells in the whole-cell configuration using a List EPC 7 (List, Darmstadt, Germany) patch-clamp amplifier and associated standard equipment, at room temperature (24 ± 1°C). The standard external solution contained (mm): 140 NaCl, 4 KCl, 2 CaCl2, 1 MgCl2, 5 Hepes, adjusted to pH 7.4 with NaOH. For treatment with Zn2+, ZnSO4 (1–5 mm) was added to normal external solution, and used to perfuse cells. MTSET and MTSEA stock solutions (1 m) were prepared in water and stored frozen. For experiments utilizing these compounds, an aliquot of the stock was diluted in the standard external solution to a final concentration of 1 mm, immediately prior to use. The standard pipette solution contained (mm): 40 CsCl, 10 EGTA-KOH, 10 Hepes-KOH, 75 caesium glutamate, adjusted to pH 7.2 with NaOH. Patch pipettes of 1–3 MΩ were pulled from borosilicate glass. Series resistance did not exceed 5 MΩ, and was 70–85% compensated. Cells with the maximum peak current amplitude between 5 and 15 nA at −140 mV were used for analysis. Cells with smaller and larger currents were discarded due to the errors introduced by background currents and series resistance, respectively. The largest error due to series resistance was less than 10 mV. Currents were filtered at 3 kHz, and collected and analysed using pClamp software (Axon Instruments, Union City, CA, USA). Potentials listed are intracellular potentials relative to outside zero.
For analysis of the temperature dependence of Zn2+ inhibition, the temperature of the bath was varied from room temperature (24 ± 1°C) to 30°C using a Peltier-based device with negative feedback. Bath solution without Zn2+ was replaced with Zn2+-containing external solution heated to the same temperature. Experiments at 30°C were conducted at a −30 mV holding potential only.
Experimental design and data analysis
To investigate voltage dependence of Zn2+ binding on a slow time scale, the effect of Zn2+ on ClC-1 currents was examined at three different membrane potentials of +40, −30 and −60 mV. Cells were held at one of these potentials, and a 12 ms test pulse to −100 mV was applied every 2 s to elicit inward currents. Sufficient time was allowed for the cell to equilibrate after the shift of the membrane potential, and Zn2+ was applied after the amplitude of the peak inward current remained stable at least for 1 min. The magnitude of the instantaneous inward current in response to −100 mV steps was normalized to the magnitude of that current observed before the application of Zn2+, and plotted against time for each holding potential. These data sets were fitted with a single exponential function to approximate the time course of the block.
To analyse the temperature dependence of Zn2+ block, the time course of current inhibition by 1 mm Zn2+ was obtained at −30 mV holding potential, at both room temperature and 30°C, and fitted with a single exponential function. The time constants of the block at the two temperatures were then used to calculate a Q10 value for the process of Zn2+-mediated inhibition of ClC-1.
Results
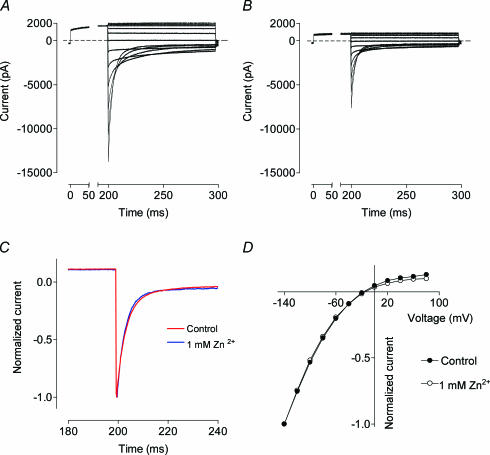
Application of 1 mm Zn2+ in the bath solution almost completely inhibited the WT hClC-1, as reported previously (Kurz et al. 1997). The block developed slowly over several minutes and was virtually irreversible at normal pH. Currents recorded at the time when approximately half of the channels were blocked showed the same basic characteristics as the control recordings (Fig. 1). On the time scale in the range of hundreds of milliseconds, there was no change in the current kinetics at either negative or positive potentials (Fig. 1A–C), nor there was any change in the shape of the current–voltage relationships (Fig. 1D). In addition, there was no shift in voltage dependence of the apparent open probability (Po) of the ClC-1 in the presence of Zn2+ (not shown). Voltage-dependent changes in the current kinetics and current rectification in the presence of an ion blocker are usually taken as evidence of that blocker binding in the channel pore (Hille, 2001). In the absence of such changes, the results given above indicate that the fast voltage-dependent block of an open pore cannot account for the mechanism of ClC-1 inhibition by Zn2+, which is consistent with the conclusions made previously by others (Kurz et al. 1997).
Figure 1. Effects of Zn2+ on wild-type (WT) human muscle Cl− channel (hClC-1) gating and rectification.
Examples of ClC-1 current traces in the absence (A) and presence (B) of 1 mm Zn2+. Currents were recorded in response to the following voltage protocol: holding potential was −30 mV; 200 ms prepulse to +40 mV was followed by 100 ms pulses to membrane potentials ranging from −140 to +80 mV in 20 mV increments. C, normalized superimposed currents in response to a −140 mV step in the absence and presence of 1 mm Zn2+. D, current–voltage (I–V) plots of the peak current in the absence and presence of 1 mm Zn2+. Currents were normalized to peak instantaneous current at −140 mV.
As Zn2+-mediated attenuation of the ClC-1 current happened on a very slow time scale, voltage dependence of the block might not have been obvious in recordings shown in Fig. 1. Therefore we further investigated voltage dependence of the block caused by Zn2+, this time on a longer time scale by holding cells at three different holding potentials: −60, −30 and +40 mV (see Methods). The block of the WT hClC-1 by Zn2+ occurred much more quickly if the cell was held at more hyperpolarized potentials. The times to half-maximal inhibition (τ50) for the WT hClC-1 were 148 s at −60 mV and 355 s at −30 mV (Fig. 2). The time course of Zn2+ block was not a simple single exponential, but rather a sigmoidal function, which was obvious at a holding potential of −60 mV; however, for simplicity this was ignored while determining τ50 (Fig. 2). Fitting single exponential functions to the data obtained at −60 and −30 mV suggested that at both potentials the current would plateau at a value of 7% of the initial current, which is comparable in size with the leakage in these cells (Fig. 2). At +40 mV, the block was too slow to achieve the same level of inhibition within feasible duration of a patch-clamping experiment on these cells (15–20 min). Therefore, to obtain τ50 of WT hClC-1 inhibition at +40 mV, it was assumed, as Zn2+ binding was irreversible, that the extent of the block would be the same at all potentials, with only the time course of the block changing. When the value of plateau was fixed to 7% of the initial current, the single exponential fit to the data points obtained at +40 mV gave τ50 of 1081 s.
Figure 2. Voltage dependence of the time course of the WT hClC-1 block by Zn2+.
The currents were obtained by stepping the membrane potential to −120 mV for 12 ms from a holding potential of either +40, −30 or −60 mV, as indicated. The points represent the means (±s.e.m., indicated by dotted lines, n = 3–7 cells) of the peak current amplitude at −120 mV in the presence of 1 mm Zn2+ normalized to the current obtained before application of Zn2+. Sudden steps visible in the data are due to a variable number of cells used in the averaging procedure at different time points, because of the difficulty in maintaining seals over the entire time period. Continuous lines represent single exponential fits to the data.
Faster block at more negative potentials could be due to an increased electrostatic attraction of Zn2+ to a binding site within the electric field. On the other hand, Po of the WT ClC-1 changes from about 0.4 at −60 mV to 0.95 at +40 mV (Rychkov et al. 1996), and thus there is a significant change in the ratio between the number of open and closed channels in this voltage range. Therefore, voltage dependence of Zn2+ block could also be a result of voltage dependence of ClC-1 gating if Zn2+ binding was state dependent.
To analyse further the possible dependence of Zn2+ block on the gating processes of ClC-1, we studied the effects of Zn2+ on various ClC-1 mutants that we have shown previously to have altered gating. Each of these mutations affected only one type of gating, leaving the other one unchanged. The three mutations investigated were: C277S, which increases minimum Po of the common gate to ∼0.75 effectively locking it in the open state (Accardi et al. 2001; Duffield et al. 2003); C278S, which increases minimum Po of the single pore gate from ∼0.05 to ∼0.4 (Duffield et al. 2003); and V321S, which shifts Po of the common gate to more positive potentials compared with the WT, reducing the common gate Po at any given potential (Duffield et al. 2003). Application of 1 mm Zn2+ to the bath caused no block of either C277S or C278S currents. At a higher concentration of 5 mm, a maximal current reduction for both mutants was between 15 and 20% even after 10 min of incubation (Fig. 3A). In contrast, the V321S mutant showed a much faster time course for Zn2+ block, with a τ50 of about 12 s at −30 mV, compared with 355 s in WT (Fig. 3B). Also, in contrast to WT, Zn2+ block of V321S was independent of voltage between −30 and +40 mV (Fig. 3B).
Figure 3. Effects of point mutations altering hClC-1 gating on Zn2+ block.
The time course of Zn2+ block of C277S and C278S (A) and V321S (B) mutants (n = 5–6 cells). For comparison, data from WT channels are presented. The voltage protocol used is the same as that used in Fig. 2. Holding potentials are indicated in parentheses. Zn2+ concentration was 5 mm for cysteine mutants, and 1 mm for V321S and WT. Continuous lines represent single exponential fits to the data.
Zn2+ inhibits ClC-0 by facilitating common gating (Chen, 1998). The results above also suggest that common gating of ClC-1 may be an important determinant of the mechanism of Zn2+ block. However, in ClC-1, Zn2+ block is too slow to be a simple function of the Po of either the single pore gate or the common gate. This raises the possibility that Zn2+ interacts with another state of ClC-1, similar to that of the inactivated state of ClC-0, which is normally overlooked in ClC-1 due to its slow kinetics, low probability, and the same voltage dependence as the other two gating processes. A startling feature of the ClC-0 common gate is its temperature dependence with a Q10 of ∼40 (Pusch et al. 1997). The Q10 of Zn2+ block in ClC-0 is approximately the same as the Q10 of common gating, which supports those authors' conclusion that in ClC-0 Zn2+ acts on the common gate (Chen, 1998). In ClC-1, the Q10 of common gating at pH 7.4 is ∼4 (Bennetts et al. 2001). To determine whether the state on which Zn2+ acts is different from the known ClC-1 common gating, we have investigated the temperature dependence of Zn2+ block. Results show that ClC-1 inhibition occurs much faster at higher temperatures (Fig. 4). At 24°C, the time to half-maximum inhibition of the current was 355 ± 1 s, whilst at 30°C it was 46 ± 1 s. This corresponds to a Q10 value for the Zn2+ block of ∼13.
Figure 4. Temperature dependence of the time course of WT hClC-1 block by 1 mm Zn2+.
The voltage protocol used is the same as that used in Fig. 2. Holding potential was −30 mV. Temperature is shown in parentheses. Continuous lines represent single exponential fits to the data.
Zn2+-binding sites often contain a number of cysteine residues (Karlin & Zhu, 1997; Auld, 2001). Methanethiosulphonate reagents are able to react with, and crosslink, cysteine residues within a peptide (Akabas et al. 1992). To investigate further the possible role of cysteine residues, and their potential movements during channel gating, the reagents MTSET and MTSEA were applied to the WT ClC-1 channel. Neither of these reagents was observed to cause any attenuation, or affect channel kinetics, even following more than 10 min of perfusion (data not shown).
Discussion
The main conclusion of this work is that despite a 100-fold difference in apparent affinity, Zn2+ block of ClC-1 seems mechanistically similar to that of ClC-0 (Chen, 1998). Mutations of three cysteine residues – C212S, C213G and C480S – have been found to affect Zn2+ block in ClC-0, with the C212S mutant being the least susceptible to inhibition by Zn2+ (Chen, 1998). Mutations of corresponding cysteine residues in ClC-1 studied here (C277S and C278S) and previously (C546A; Kurz et al. 1999) also significantly reduced Zn2+ block. As with C212S in ClC-0, mutation C277S caused the greatest reduction, allowing a maximum inhibition by 5 mm Zn2+ of just ∼15%.
It has been shown that Zn2+ blocks ClC-0 by facilitating closure of the common gate, and that the block and relaxation of the common gate are occurring on the same time scale (Chen, 1998). Common gating of ClC-1 operates on a time scale that is 1000 times faster than Zn2+ block; therefore, the time constants of current relaxations are not affected by Zn2+ in ClC-1. Faster block at more hyperpolarized potentials does, however, suggest that Zn2+ binds to a closed channel. At this stage, faster block at more negative potentials solely due to increased electrostatic attraction to a binding site in the pore can be ruled out, as the temperature dependence of the block is greatly exceeds the temperature dependence of ion diffusion across an energy barrier (DeCoursey & Cherny, 1998). Virtual elimination of Zn2+ block in the C277S mutant, which has little common gating, and stronger and faster block in the V321S mutant which has more common gating, add to the argument that Zn2+ binds to the channel with its common gate closed. This conclusion is supported by our previous results on Cd2+ block of the R304E mutant of rat ClC-1, where the affinity for Cd2+ is reduced several fold compared with the WT channel (Rychkov et al. 1997). Re-examination of the published data on R304E (Rychkov et al. 1997) shows that the minimum Po of the common gate is increased to 0.49 in R304E compared with 0.25 in WT (M. D. Duffield, G. Y. Rychkov, A. H. Bretag & M. L. Roberts, unpublished). Elimination of 80% of Zn2+ block in C278S found here cannot be attributed to its effects on the common gate, as this mutation only affects the single pore gate (Duffield et al. 2003). Whether the effect of the C278S mutation on Zn2+ block is due to its effect on single pore gating or due to its effects on the Zn2+ binding site is hard to verify, as this is the only mutation known to affect the pore gate without affecting the common gate at the same time.
All cysteine and histidine residues in ClC-1 that are conserved in ClC-0 have been mutated, and the mutant channels have been investigated for Zn2+ block either in this work or previously (Kurz et al. 1999). Mutations of Cys-277, Cys-278 and Cys-546 have the strongest effects. It is likely that at least one or two of these residues are participating in coordination of the Zn2+ ion. With the crystal structure of a homologous ClC bacterial channel available (Dutzler et al. 2002), it is possible to look for the potential Zn2+-binding sites in ClC-1. It is clear from the ClC-1 modelling that adjacent Cys-277 and Cys-278 cannot both coordinate the same Zn2+ ion since their side chains are pointing in different directions, while Cys-546 is too far from either of these cysteines to be a part of the same binding site (Fig. 5). The crystal structure also reveals that Arg-304 and Val-321 are positioned far from all cysteine residues and far from each other (Fig. 5). This supports the notion that changes in Zn2+ block in these mutants are not due to their local effects but are due to their effect on gating.
Figure 5. Homology model of ClC-1.
A, top view from extracellular side; B, side view from the dimer interface. Locations of residues affecting Zn2+ block (C277, C278, C546, V321 and R304) and the residue implicated in fast gating mechanism (E232; Dutzler et al. 2003) are shown.
The differences observed in the time course of Zn2+ inhibition cannot be explained completely by the differences in the Po of the common gate. The Po of the V321S mutant common gate at +40 mV is close to 70%, which is approximately equivalent to the Po of the WT ClC-1 channel common gate at −60 mV (Duffield et al. 2003). Nevertheless, the rate of Zn2+ inhibition of the V321S channel at +40 mV is still significantly faster than that seen in the WT channel at −60 mV. Similarly, the rates of Zn2+ block seen in V321S are very similar at both holding potentials used, −30 and +40 mV, even though there is a difference of about 40% in the Po of the V321S common gate between these two potentials (Duffield et al. 2003).
These observations are likely to reflect the fact that Zn2+ binds to a closed substate of the common gate that has very low probability in the WT channel, therefore having little effect on its kinetics. As Zn2+ binding is irreversible, the low probability of that substate has little effect on the extent of the block. With sufficient Zn2+ present in the bath for long enough, all channels eventually will go through this substate and will bind Zn2+. It is quite possible that Zn2+ itself induces the substate of the common gate of the WT channel that binds Zn2+, which would be similar to the facilitation of common gating by Zn2+ in ClC-0. Apparently, in the V321S mutant, the probability of the channel residing in a substate that binds Zn2+ is much higher than in the WT. The suggestion that Zn2+ binds to a previously unknown substate of ClC-1 is supported by a very high temperature dependence of Zn2+ block. The Q10 value of ∼13 for Zn2+ inhibition indicates that it involves structural rearrangement in the channel that is far more complex than those caused by either the ClC-1 single pore gating or common gating processes, which have Q10 values of ∼3 and ∼4, respectively (Bennetts et al. 2001). The temperature dependence of ClC-1 block by Zn2+ is more akin to the temperature dependence of ClC-0 common gating in the presence of Zn2+ (Chen, 1998) than to the temperature dependence of ClC-1 gating.
In conclusion, we have presented results that strongly suggest that there is a highly temperature-dependent gating transition in ClC-1 that leads to a channel substate that binds Zn2+ ions. While it is now clear that the mechanism of ClC-1 block by Zn2+ is similar to that of ClC-0, the exact the nature of the gating transition that forms the binding site for Zn2+ in both channels requires further investigation.
Acknowledgments
We would like to thank Dr Chris Bagley of Hanson Institute in Adelaide for helpful discussions and for homology modelling of ClC-1, and Professor T. J. Jentsch of the Center for Molecular Neurobiology in Hamburg (Germany) for providing the human ClC-1 clone. This work was supported by the Australian Research Council.
References
- Accardi A, Ferrera L, Pusch M. Drastic reduction of the slow gate of human muscle chloride channel (ClC-1) by mutation C277S. J Physiol. 2001;543:745–752. doi: 10.1111/j.1469-7793.2001.00745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akabas MH, Stauffer DA, Xu M, Karlin A. Acetylcholine receptor channel structure probed in cysteine-substitution mutants. Science. 1992;258:307–310. doi: 10.1126/science.1384130. [DOI] [PubMed] [Google Scholar]
- Auld DS. Zinc coordination sphere in biochemical zinc sites. Biometals. 2001;14:273–313. doi: 10.1023/a:1012976615056. [DOI] [PubMed] [Google Scholar]
- Bennetts B, Roberts ML, Bretag AH, Rychkov GY. Temperature dependence of human muscle ClC-1 chloride channel. J Physiol. 2001;535:83–93. doi: 10.1111/j.1469-7793.2001.t01-1-00083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretag AH, Fietz MJ, Bennet RRJ. The effects of zinc and other transition metal ions on rat skeletal muscle. Proc Aust Physiol Pharmacol Soc. 1984;15:146P. [Google Scholar]
- Chen TY. Extracellular zinc ion inhibits ClC-0 chloride channels by facilitating slow gating. J Gen Physiol. 1998;112:715–726. doi: 10.1085/jgp.112.6.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MF, Chen TY. Different fast-gate regulation by external Cl− and H+ of the muscle-type ClC chloride channels. J Gen Physiol. 2001;118:23–32. doi: 10.1085/jgp.118.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCoursey TE, Cherny VV. Temperature dependence of voltage-gated H+ currents in human neutrophils, rat alveolar epithelial cells, and mammalian phagocytes. J Gen Physiol. 1998;112:503–522. doi: 10.1085/jgp.112.4.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffield M, Rychkov G, Bretag A, Roberts M. Involvement of helices at the dimer interface in ClC-1 common gating. J Gen Physiol. 2003;121:149–161. doi: 10.1085/jgp.20028741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutzler R, Campbell EB, Cadene M, Chait BT, MacKinnon R. X-ray structure of a ClC chloride channel at 3.0 Å reveals the molecular basis of anion selectivity. Nature. 2002;415:287–294. doi: 10.1038/415287a. [DOI] [PubMed] [Google Scholar]
- Dutzler R, Campbell EB, MacKinnon R. Gating the selectivity filter in ClC chloride channels. Science. 2003;300:108–112. doi: 10.1126/science.1082708. [DOI] [PubMed] [Google Scholar]
- Hille B. Ion Channels of Excitable Membranes. Sunderland: Sinauer Associates; 2001. [Google Scholar]
- Hutter OF, Warner AE. Action of some foreign cations and anions on the chloride permeability of frog muscle. J Physiol. 1967;189:445–460. doi: 10.1113/jphysiol.1967.sp008178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch TJ, Stein V, Weinreich F, Zdebik AA. Molecular structure and physiological function of chloride channels. Physiol Rev. 2002;82:503–508. doi: 10.1152/physrev.00029.2001. [DOI] [PubMed] [Google Scholar]
- Karlin S, Zhu ZY. Classification of mononuclear zinc metal sites in protein structures. Proc Natl Acad Sci U S A. 1997;94:14231–14236. doi: 10.1073/pnas.94.26.14231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurz LL, Klink H, Jakob I, Kuchenbecker M, Benz S, Lehmann-Horn F, Rüdel R. Identification of three cysteines as targets for the Zn2+ blockade of the human skeletal muscle chloride channel. J Biol Chem. 1999;274:11687–11692. doi: 10.1074/jbc.274.17.11687. [DOI] [PubMed] [Google Scholar]
- Kurz L, Wagner S, George ALJ, Rüdel R. Probing the major skeletal muscle chloride channel with Zn2+ and other sulfhydryl-reactive compounds. Pflugers Arch. 1997;433:357–363. doi: 10.1007/s004240050288. [DOI] [PubMed] [Google Scholar]
- Lin YW, Lin CW, Chen TY. Elimination of the slow gating of ClC-0 chloride channel by a point mutation. J Gen Physiol. 1999;114:1–12. doi: 10.1085/jgp.114.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludewig U, Pusch M, Jentsch TJ. Two physically distinct pores in the dimeric ClC-0 chloride channel. Nature. 1996;383:340–343. doi: 10.1038/383340a0. [DOI] [PubMed] [Google Scholar]
- Middleton RE, Pheasant DJ, Miller C. Purification, reconstitution, and subunit composition of a voltage-gated chloride channel from Torpedo electroplax. Biochemistry. 1994;33:13189–13198. doi: 10.1021/bi00249a005. [DOI] [PubMed] [Google Scholar]
- Middleton RE, Pheasant DJ, Miller C. Homodimeric architecture of a ClC-type chloride ion channel. Nature. 1996;383:337–340. doi: 10.1038/383337a0. [DOI] [PubMed] [Google Scholar]
- Pusch M, Ludewig U, Jentsch TJ. Temperature dependence of fast and slow gating relaxations of ClC-0 chloride channels. J Gen Physiol. 1997;109:105–116. doi: 10.1085/jgp.109.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusch M, Ludewig U, Rehfeldt A, Jentsch TJ. Gating of the voltage-dependent chloride channel CIC-0 by the permeant anion. Nature. 1995;373:527–531. doi: 10.1038/373527a0. [DOI] [PubMed] [Google Scholar]
- Rychkov GY, Astill DS, Bennetts B, Hughes BP, Bretag AH, Roberts ML. pH-dependent interactions of Cd2+ and a carboxylate blocker with the rat C1C-1 chloride channel and its R304E mutant in the Sf-9 insect cell line. J Physiol. 1997;501:355–362. doi: 10.1111/j.1469-7793.1997.355bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rychkov GY, Pusch M, Astill DS, Roberts ML, Jentsch TJ, Bretag AH. Concentration and pH dependence of skeletal muscle chloride channel ClC-1. J Physiol. 1996;497:423–435. doi: 10.1113/jphysiol.1996.sp021778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saviane C, Conti F, Pusch M. The muscle chloride channel ClC-1 has a double-barreled appearance that is differentially affected in dominant and recessive myotonia. J Gen Physiol. 1999;113:457–468. doi: 10.1085/jgp.113.3.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanfield PR. The differential effects of tetraethylammonium and zinc ions on the resting conductance of frog skeletal muscle. J Physiol. 1970;209:231–256. doi: 10.1113/jphysiol.1970.sp009164. [DOI] [PMC free article] [PubMed] [Google Scholar]