Abstract
Pharmacological modulation of the mitochondrial ATP-sensitive K+ channel (mitoKATP) sensitive to diazoxide and 5-hydroxydecanoate (5-HD) represents an attractive strategy to protect cells against ischaemia/reperfusion- and stroke-related injury. To re-evaluate a functional role for the mitoKATP in brain, we used Percoll-gradient-purified brain nonsynaptosomal mitochondria in a light absorbance assay, in radioisotope measurements of matrix volume, and in measurements of respiration, membrane potential (ΔΨ) and depolarization-induced K+ efflux. The changes in mitochondrial morphology were evaluated by transmission electron microscopy (TEM). Polyclonal antibodies raised against certain fragments of known sulphonylurea receptor subunits, SUR1 and SUR2, and against different epitopes of K+ inward rectifier subunits Kir 6.1 and Kir 6.2 of the ATP-sensitive K+ channel of the plasma membrane (cellKATP), were employed to detect similar subunits in brain mitochondria. A variety of plausible blockers (ATP, 5-hydroxydecanoate, glibenclamide, tetraphenylphosphonium cation) and openers (diazoxide, pinacidil, chromakalim, minoxidil, testosterone) of the putative mitoKATP were applied to show the role of the channel in regulating matrix volume, respiration, and ΔΨ and K+ fluxes across the inner mitochondrial membrane. None of the pharmacological agents applied to brain mitochondria in the various assays pinpointed processes that could be unequivocally associated with mitoKATP activity. In addition, immunoblotting analysis did not provide explicit evidence for the presence of the mitoKATP, similar to the cellKATP, in brain mitochondria. On the other hand, the depolarization-evoked release of K+ suppressed by ATP could be re-activated by carboxyatractyloside, an inhibitor of the adenine nucleotide translocase (ANT). Moreover, bongkrekic acid, another inhibitor of the ANT, inhibited K+ efflux similarly to ATP. These observations implicate the ANT in ATP-sensitive K+ transport in brain mitochondria.
A K+ channel of mitochondrial origin, with properties resembling the properties of the ATP-sensitive K+ channel of the plasma membrane (cellKATP), was first demonstrated in 1991 in patch-clamp studies using fused liver mitoplasts (Inoue et al. 1991). It has been shown that this mitochondrial K+ channel (mitoKATP) is sensitive to openers and blockers of the cellKATP. Later, the mitoKATP was isolated, purified and thoroughly studied in the reconstituted system (Paucek et al. 1992; Garlid et al. 1996; Jaburek et al. 1998; Bajgar et al. 2001; Mironova et al. 2004). From studies with isolated mitochondria, it was suggested that the only function of the mitoKATP is to regulate mitochondrial volume (Kowaltowski et al. 2001). Changes of mitochondrial membrane potential (ΔΨ) and respiration caused by ATP or by pharmacological modulators of the mitoKATP were found to be minimal in heart mitochondria (Kowaltowski et al. 2001). However, in brain mitochondria, substantial changes of mitochondrial respiration associated with opening and closing of the mitoKATP were reported (Bajgar et al. 2001). This could be due to a sevenfold higher amount of mitoKATP in brain mitochondria than in liver or heart mitochondria (Bajgar et al. 2001).
From the studies with reconstituted mitoKATP, it was proposed that this channel consists of two types of subunit: a 55 kDa inwardly rectifying channel subunit ‘mitoKir’, analogous to a Kir 6.X subunit of the cellKATP, and a 63 kDa sulphonylurea receptor subunit ‘mitoSUR’, corresponding to SUR1 or SUR2 of the cellKATP (Grover & Garlid, 2000; Bajgar et al. 2001). Several laboratories have performed immunoblotting analysis by applying polyclonal antibodies raised against peptides of known subunits of cellKATP, and detected Kir 6.1, Kir 6.2 and SUR subunits in isolated mitochondria (Suzuki et al. 1997, 2002; Lacza et al. 2003a, b; Tai et al. 2003). However, these antibodies did not react with proteins in the reconstitutively active purified fraction of the mitoKATP (Grover & Garlid, 2000). Thus, the molecular identity of the mitochondrial subunits of the putative mitoKATP remains unknown.
An implication of the mitoKATP in cardio- and neuroprotection against ischaemia/reperfusion- and stroke-related injuries dramatically raised the interest in its regulation and physiological role (Domoki et al. 1999; Pacher et al. 2002; Shimizu et al. 2002; Liu et al. 2003; O'Rourke, 2004; Sato et al. 2004). The mitoKATP was also suggested to be involved in the preconditioning of cardiomyocytes and neurones (O'Rourke, 2000; Nagy et al. 2004), but recently the role and existence of the mitoKATP have been questioned (Ovide-Bordeaux et al. 2000; Hanley et al. 2002; Das et al. 2003; Kis et al. 2003; Kopustinskiene et al. 2003; Holmuhamedov et al. 2004). Several reports cast doubt on the hypothesis that the mitochondrial effects of pharmacological openers or blockers of the mitoKATP are necessarily related to mitoKATP activity. For example, diazoxide, an opener of the mitoKATP (Garlid et al. 1996), can depolarize mitochondria due to inhibition of succinate dehydrogenase (Grimmsmann & Rustenbeck, 1998; Lim et al. 2002; Hanley et al. 2002). Diazoxide and pinacidil uncouple oxidative phosphorylation in heart mitochondria (Kowaltowski et al. 2001; Kopustinskiene et al. 2002; Holmuhamedov et al. 2004). Glibenclamide, a blocker of the putative mitoKATP, was also found to uncouple rat liver mitochondria (Szewczyk et al. 1997). 5-Hydroxydecanoate (5-HD), a derivative of free fatty acid, and one of the most often used blockers of the putative mitoKATP, is readily converted into 5-HD-CoA, which can be further metabolized in the β-oxidation pathway (Hanley et al. 2002; Lim et al. 2002).
In the present study, we sought to visualize manifestations of the mitoKATP using various experimental approaches to determine to what extent functioning of the mitoKATP contributes to regulation of matrix volume, respiration, ΔΨ, and uncoupler-induced K+ efflux from isolated brain nonsynaptosomal mitochondria. Surprisingly, none of the pharmacological agents applied to brain mitochondria in the various assays pinpointed processes that could be unequivocally associated with the mitoKATP. In addition, immunoblotting analysis did not provide explicit evidence for the presence of the mitoKATP, similar to the cellKATP, in purified brain mitochondria.
Methods
Materials
Diazoxide, glibenclamide, 5-HD, testosterone and 3H2O were purchased from Sigma (St Louis, MO, USA). [14C]Sucrose was purchased from Moravek Biochemicals (Brea, CA, USA). Pinacidil, chromakalim and minoxidil were from Tocris (Ellisville, MO, USA). Tetraphenylphosphonium chloride was obtained from Fluka (Buchs, Switzerland). Carboxyatractyloside (CAT) and bongkrekic acid (BKA) were the generous gift of Professor Martin Klingenberg.
Isolation of brain mitochondria
Male Sprague-Dawley rats, 225–250 g (Harlan, Indianapolis, IN, USA), were killed by decapitation, according to an Institutional Animal Care and Use Committee approved protocol. Nonsynaptosomal mitochondria derived from the brains were isolated in mannitol–sucrose (MS) medium and purified on a discontinuous Percoll gradient (Sims, 1990; Brustovetsky et al. 2002a). Mitochondrial protein was determined by the Bradford method (Bradford, 1976) using BSA as a standard.
Measurements of mitochondrial light absorbance, ΔΨ and respiration
Changes in light absorbance of the mitochondrial suspension were monitored at 525 nm in a 0.3 ml chamber at 37°C, with continuous stirring. ΔΨ was monitored by following the distribution of tetraphenylphosphonium cation (TPP+) between the external medium (initially 1.8 μm TPP+-Cl) and the mitochondrial matrix with a TPP+-sensitive electrode (Kamo et al. 1979) in the KCl-, LiCl- or MS-based incubation medium containing 125 mm KCl, 125 mm LiCl or 215 mm mannitol, plus 70 mm sucrose, respectively. All media contained 2.5 mm MgCl2, 10 mm Hepes, pH 7.4 (adjusted with KOH in KCl-based medium, or with Tris in LiCl- and MS-based media), 0.1% BSA (free from fatty acids) and 1 μm oligomycin, unless stated otherwise. In the experiments with adenine nucleotides, oligomycin was used to block oxidative phosphorylation. A similar approach has been employed by others (Kowaltowski et al. 2001). In addition, the KCl-based medium contained 3 mm KH2PO4, while the LiCl- and MS-based media contained 3 mm NaH2PO4. The incubation media were supplemented with 3 mm pyruvate plus 1 mm malate, or with 3 mm succinate plus 3 mm glutamate. All substrate solutions were K+ free, and pH was adjusted with Tris. A decrease in the external TPP+ concentration corresponded to mitochondrial polarization, while an increase in the TPP+ concentration in the medium corresponded to depolarization. Using a Clark-type electrode in a closed 0.3 ml chamber, oxygen consumption was monitored separately or simultaneously with ΔΨ, under identical conditions. The ΔΨ and respiratory measurements were performed at 37°C in a continuously stirred 0.3 ml chamber. All data traces shown are representative of at least three separate experiments.
Matrix volume measurements
Measurements of mitochondrial matrix volume were performed using the radioisotope method (Lim et al. 2002; Brand, 2002). In the control experiments designed to determine matrix volumes at time zero, mitochondria (0.2 mg protein), 3H2O (1 μCi) and [14C]sucrose (0.1 μCi) were added to 0.3 ml of ice-cold KCl-based incubation medium, and matrix volumes were measured immediately. In the experiments with different pretreatments, mitochondria (0.2 mg protein) were incubated with various substances for 5 min at 37°C in 0.3 ml KCl-based incubation medium supplemented with 3H2O (1 μCi) and [14C]sucrose (0.1 μCi). Then, mitochondria were pelleted at 15 800 g for 2 min, and 200 μl of supernatant was transferred into a fresh tube and mixed with 200 μl of distilled water. The pellets were solubilized in 50 μl of 20% Triton X-100, and 350 μl of distilled water was added to equalize pellet and supernatant sample volumes. Both pellet and supernatant samples were transferred into scintillation vials with 3.5 ml of scintillation liquid (EcoLite; MP Biochemicals, Irvine, CA, USA). 3H and 14C were determined using a Tri-Carb 2100TR liquid scintillation analyser (Packard Instrument Co., Meriden, CT, USA). Matrix volume was calculated as previously described (Brand, 2002) and expressed in microlitres per milligram of mitochondrial protein.
Fluorescence of flavoproteins
Fluorescence of total mitochondrial flavoproteins was monitored in a Perkin-Elmer LS 55 luminescence spectrometer equipped with a bio-kinetics accessory using excitation/emission wavelengths 450/515 nm (Richards-Kortum & Sevick-Muraca, 1996). The experiments were performed in a 400 μl cuvette at room temperature (22°C) under continuous stirring.
K+ efflux
K+ efflux from depolarized brain mitochondria was monitored by following the concentration of K+ in the K+-free MS-based medium supplemented with either 3 mm succinate plus 3 mm glutamate, or 3 mm pyruvate plus 1 mm malate, using a miniature K+-selective electrode in a continuously stirred 0.3 ml chamber at 37°C. The miniature K+-selective electrode was constructed in our laboratory using a K+-selective membrane purchased from Fluka (Buchs, Switzerland). K+ efflux was initiated by 1 μm carbonyl cyanide p-(trifluoromethoxy) phenylhydrazone (FCCP).
Transmission electron microscopy (TEM)
Purified brain mitochondria were incubated with or without 50 μm diazoxide or 0.4 nm valinomycin in 125 mm KCl-based medium supplemented with 3 mm succinate plus 3 mm glutamate at 37°C, prior to fixation in 2.5% glutaraldehyde in the same incubation medium at room temperature for 15 min. BSA was omitted from the medium. Mitochondria were then centrifuged at 15 800 g for 5 min, and the supernatant was discarded. The pellet was layered with fresh 2.5% glutaraldehyde solution and left overnight at 4°C. The samples were postfixed in 1% osmium tetroxide for 1 h and dehydrated in a graded series of ethanol before embedding in Polybed 812. Sections were cut on a Reichert Ultracut S microtome, and stained with a methanolic solution of uranyl acetate plus lead citrate. Electron micrographs were obtained using a JEOL-1200 EX electron microscope. To quantitatively evaluate the morphological changes in each experimental protocol, four different TEM micrographs, representing randomly chosen areas in the grid, were selected and analysed in a blinded fashion using the previously described approach (Scorrano et al. 2002). Total mitochondrial population was categorized into three groups according to their morphology: (i) condensed, (ii) intermediate and (iii) swollen. Mitochondria with characteristics bridging morphological groups were assigned to the lower category. Mitochondria were counted and morphological distribution was statistically analysed using one-way ANOVA followed by Bonferroni's post test (GraphPad Prism, 4.0).
Immunoblotting
Brain homogenates and isolated mitochondria pretreated with Protease Inhibitor Cocktail (Sigma) were solubilized by incubation in NuPAGE LDS sample buffer (Invitrogen, Carlsbad, CA, USA) supplemented with a reducing agent at 70°C for 15 min. Bis-Tris Mops gels (4–12%; Invitrogen) were used for electrophoresis (20 μg protein per lane). After electrophoresis, proteins were transferred to Hybond-ECL nitrocellulose membrane (Amersham Biosciences). Blots were incubated with one of the following: primary goat anti-SUR1, anti-SUR2, anti-Kir 6.1 (R14), anti-Kir 6.2 (G16) or anti-Kir 6.2 (N18), or with primary rabbit anti-Kir 6.2 (H55) or anti-Kir 6.1 (H80) antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA,) at 1:500 dilution for 1 h at room temperature in 8% donkey serum, phosphate-buffered saline, pH 7.2, and 0.15% Triton X-100. An incubation of blots with primary antibodies at 4°C overnight produced similar results (not shown). Blots were developed using donkey anti-goat or goat anti-rabbit IgG (1:20000) coupled with horseradish peroxidase (Jackson ImmunoResearch Laboratories, West Grove, PA, USA) and Supersignal West Pico chemiluminescent reagents (Pierce, Rockford, IL, USA). When available, blocking peptides were used to distinguish specific and nonspecific bands, as recommended by the manufacturer. Primary antibodies were incubated with the appropriate blocking peptides (1:5 ratio) for 2 h at room temperature. These mixtures were then incubated with corresponding blots for 1 h at room temperature. Molecular mass markers SeeBlue Plus 2 Standards (5 μl) and MagicMark Western Protein standards (10 μl) (Invitrogen) were used to determine molecular masses of the bands.
Statistics
Statistical analyses of experimental data consisted of one-way ANOVA followed by Bonferroni's post test (GraphPad Prism, 4.0). Data represent means ±s.e.m. of at least three separate experiments.
Results
Immunobloting analysis
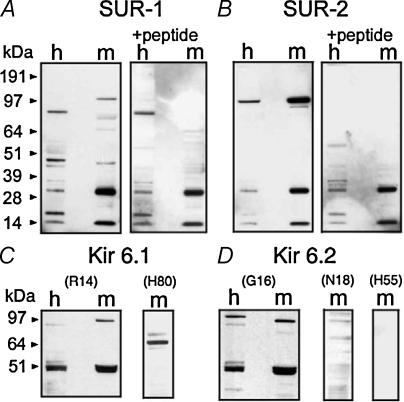
In our experiments, polyclonal anti-SUR1 antibody recognized several immunoreactive bands on the blotting membranes with transferred homogenate and mitochondrial samples (Fig. 1A). Three bands with molecular masses of about 13, 30 and 97 kDa appeared to be enriched in the mitochondrial fraction. A faint 45 kDa band appeared in both homogenate and mitochondrial samples. Only the 45 and 97 kDa bands were eliminated by the blocking peptide. Polyclonal anti-SUR2 antibody detected a significant enrichment of immunoreactive material, with an approximate molecular mass of 97 kDa (Fig. 1B). This band was eliminated by the blocking peptide. The amount of 97 kDa immunoreactive material detected with anti-SUR2 antibody greatly exceeded the amount detected with anti-SUR1 antibody (Fig. 1A and B). In both cases, with anti-SUR1 and anti-SUR2 antibodies, we detected mitochondrially enriched bands of about 13 and 30 kDa, which were insensitive to the blocking peptide. Thus, 13 and 30 kDa bands represented nonspecific binding of antibodies with irrelevant polypeptides. Of note, similar nonspecific bands were found with all antibodies (anti-SUR and anti-Kir 6.X) used in this work and will not be discussed further.
Figure 1. Immunoblotting analysis of brain homogenates (h) and purified mitochondrial fractions (m) with polyclonal antibodies against fragments of SUR1 and SUR2, and different epitopes of Kir 6.1 and Kir 6.2, revealed multiple immunoreactive bands.
Blocking peptides (+peptide) were used to discriminate nonspecific bands. Blocking-peptide-insensitive 13 and 30 kDa bands, seen in A and B with anti-SUR antibodies, were also observed in all immunoblots with anti-Kir 6.X antibodies (not shown).
Polyclonal anti-Kir 6.1 antibody (R14) raised against a peptide corresponding to a near C-terminal region of Kir 6.1 detected two enriched blocking-peptide-sensitive bands in mitochondrial samples with molecular masses of about 51 and 97 kDa (Fig. 1C). However, another anti-Kir 6.1 antibody (H80), raised against a peptide corresponding to the C-terminal of Kir 6.1, did not recognize bands with similar molecular masses (Fig. 1C). Instead, this antibody detected a highly enriched 65 kDa band, and a faint band at approximately 70 kDa. Anti-Kir 6.2 antibody (G16) raised against a peptide corresponding to an internal region of Kir 6.2 recognized two enriched bands in the mitochondrial fraction with molecular masses of about 51 and 97 kDa (Fig. 1D). However, anti-Kir 6.2 antibodies raised against peptides corresponding to the C-terminal fragment (N18) or to N-terminal fragments of Kir 6.2 (H55) did not recognize these bands (Fig. 1D). All these antibodies produced distinct bands with polypeptides recommended by the manufacturer as positive controls (not shown).
Light absorbance measurements and radioisotope assay
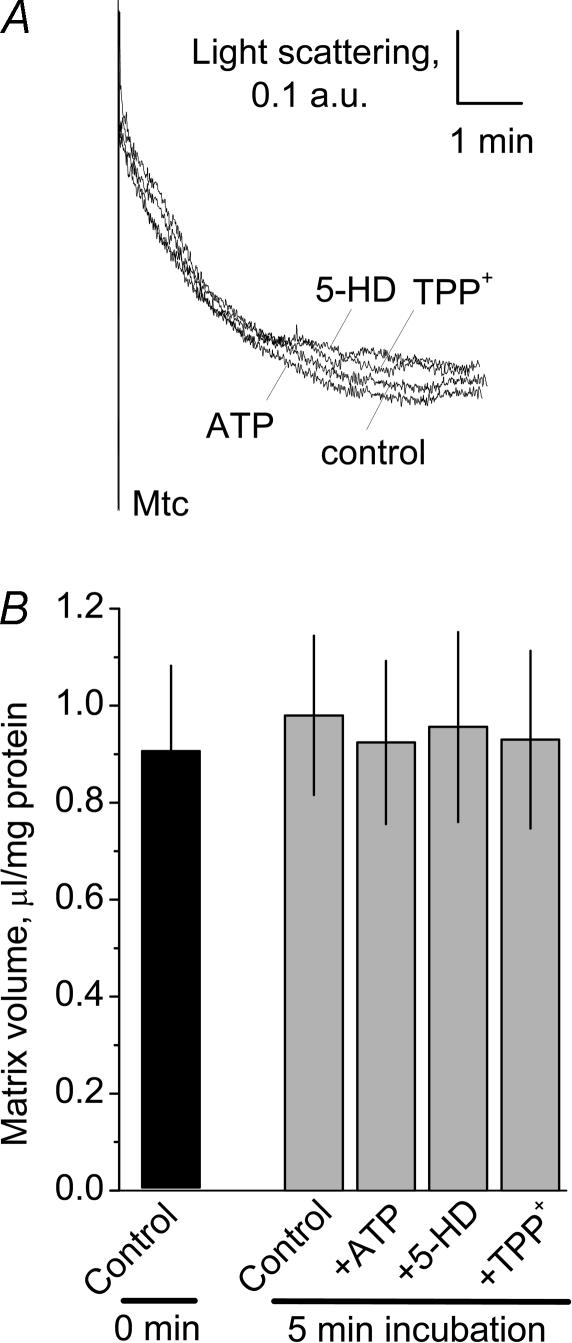
It was suggested that K+ influx through the mitoKATP could be monitored by following light absorbance of mitochondrial suspension (Beavis et al. 1985, 1993; Garlid & Beavis, 1985). Since the mitoKATP in isolated mitochondria incubated without exogenous ATP are supposed to be in an open state, addition of mitochondria into the medium with high K+ and oxidative substrates should cause massive K+ influx, accompanied by a concurrent water influx leading to mitochondrial swelling (Beavis et al. 1993; Kowaltowski et al. 2001). In our experiments, we observed a decrease in light absorbance of mitochondrial suspension after adding brain mitochondria into the KCl-based incubation medium (Fig. 2A). However, in our experiments, we were unable to suppress the decrease with 0.5 mm ATP, either with 0.5 mm 5-HD, or with 4 μm TPP+, which is a recently discovered blocker of the reconstituted putative mitoKATP (Mironova et al. 2004). The initial fall in light absorbance was also observed in K+-free MS-based medium (not shown). Despite the decrease in light absorbance, the radioisotope assay did not reveal statistically significant changes in the matrix volume (Fig. 2B). Thus, the initial decrease in light absorbance of the suspension of brain mitochondria appeared to be independent of matrix volume changes, insensitive to modulators of the mitoKATP, and therefore could not be attributed to the mitoKATP activity.
Figure 2. Blockers of the putative mitochondrial ATP-sensitive K+ channel (mitoKATP) did not affect an initial decrease in light absorbance of suspension of isolated brain mitochondria (A) or mitochondrial matrix volume measured with radioisotopes (B).
Mitochondria were incubated in the KCl-based incubation medium supplemented with 3 mm succinate and 3 mm glutamate. A, representative traces obtained with 0.5 mm ATP, 0.5 mm 5-hydroxydecanoate (5-HD) and 4 μm tetraphenylphosphonium cation (TPP+), and without (control). The blockers were added to the medium prior to adding mitochondria (Mtc). B, summary of the radioisotope measurements of the mitochondrial matrix volume. Matrix volume at time zero was measured on ice as outlined in Methods. In other experiments, mitochondria were incubated for 5 min with or without the blockers of the putative mitoKATP at 37°C prior to determination of the matrix volume. Data are means ±s.e.m., n = 5.
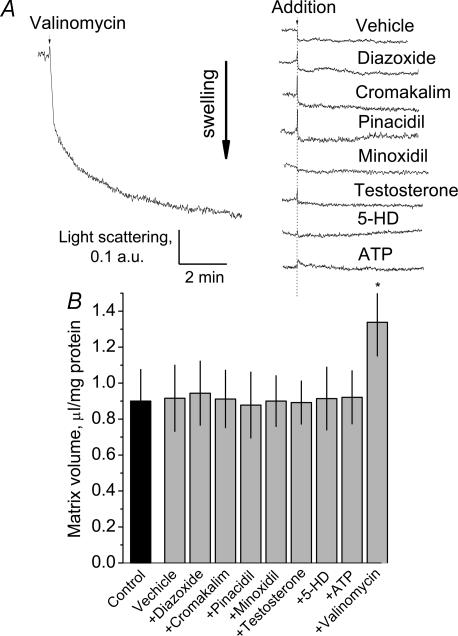
In the subsequent experiments, we addressed to what extent pharmacological openers of the mitoKATP could induce swelling of brain mitochondria. Following a presumed blockade of the putative mitoKATP with 0.5 mm ATP, the openers of the mitoKATP were added to mitochondria to activate the channel and to induce K+ influx into mitochondria leading to mitochondrial swelling. Valinomycin, a K+-selective ionophore, was used to demonstrate mitochondrial swelling due to K+ influx. Valinomycin (0.4 nm) produced both a distinct decrease in light absorbance and an increase in matrix volume measured with isotopes (Fig. 3). However, none of the tested openers (diazoxide, chromakalim, pinacidil, minoxidil and testosterone, each at 50 μm) produced a decrease in light absorbance indicative of mitochondrial swelling or increased matrix volume determined with isotopes. All other tested concentrations of the openers (10, 100 and 250 μm) were also without effect (not shown). In addition, neither ATP (0.5 mm) nor 5-HD (0.5 mm), added to mitochondria incubated in the absence of ATP, induced substantial changes in light absorbance or in matrix volume, as determined with isotopes (Fig. 3). The results obtained in the present study with brain mitochondria mirror the liver and heart mitochondria data obtained by Das et al. (2003). Thus, the light absorbance measurements and radioisotope assay did not produce evidence for the involvement of the putative mitoKATP in the regulation of matrix volume of brain nonsynaptosomal mitochondria.
Figure 3. Openers and blockers of the putative mitoKATP did not affect light absorbance of the suspension of isolated brain mitochondria (A) or mitochondrial matrix volume measured with radioisotopes (B).
Mitochondria were incubated in the KCl-based incubation medium supplemented with 3 mm succinate and 3 mm glutamate. A, representative traces obtained with and without openers and blockers of the mitoKATP, and with valinomycin (0.4 nm). Openers (50 μm each) and blockers (0.5 mm 5-HD, 0.25 mm ATP) of putative mitoKATP were added as indicated to mitochondria 4–5 min after addition of mitochondria into the chamber, when the initial decrease in light absorbance was completed. The openers were added in the presence of 0.5 mm ATP. Higher and lower doses of the openers (10 and 100 μm) did not produce an effect beyond the deflection caused by a vehicle (1.5 μl DMSO, 0.5% final concentration) alone. The presence of the higher concentrations of DMSO (1–1.5% final concentration) in the medium produced a larger effect of the vehicle, but did not improve the effects of diazoxide or other DMSO-dissolved openers. Exclusion of BSA from the medium did not result in changes in the effect of the openers or blockers (not shown). Diazoxide, cromakalim and pinacidil were solubilized in DMSO. Valinomycin, minoxidil and testosterone were solubilized in ethanol. Ethanol as a vehicle (final concentration 0.5%) did not affect light absorbance measurements (not shown). B, summary of the radioisotope measurements of the mitochondrial matrix volume with and without the openers of the mitoKATP, and with valinomycin. Matrix volume in the control was determined in the ice-cold medium. All other matrix volume measurements were performed at 37°C. Vehicle was 0.5% DMSO. *P < 0.05 versus vehicle. Data are means ±s.e.m., n = 3.
TEM
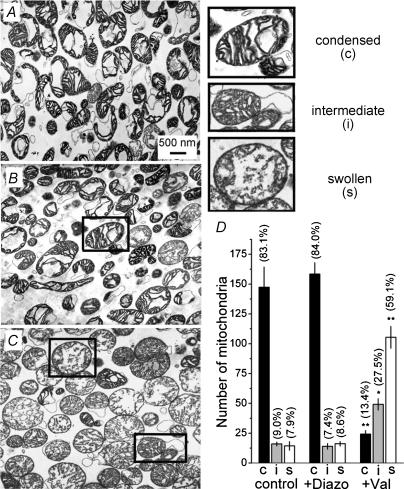
TEM was used to substantiate light absorbance and matrix volume measurements. Most mitochondria in the control sample appeared to be in a condensed state with a dark electron-dense matrix (Fig. 4A). Diazoxide (50 μm) did not cause matrix expansion (Fig. 4B), while valinomycin (0.4 nm) resulted in swelling of the mitochondrial matrix (Fig. 4C). Morphometric analysis did not reveal statistically significant changes in morphological distribution between control and diazoxide-treated mitochondria (Fig. 4D). On the other hand, valinomycin significantly decreased the number of condensed mitochondria and increased the number of swollen mitochondria (Fig. 4D). Thus, TEM confirmed the lack of expansion of mitochondrial matrices after diazoxide treatment, and visualized valinomycin-induced swelling.
Figure 4. Diazoxide failed to induce swelling of brain mitochondria as judged by transmission electron microscopy (TEM) (A and B). A low dose of valinomycin produced a distinct expansion of the mitochondrial matrix (C).
A, mitochondria were incubated in the KCl-based incubation medium supplemented with 3 mm succinate and 3 mm glutamate before fixation. B and C, mitochondria were incubated for 5 min in the same medium additionally containing 50 μm diazoxide (B) or 0.4 nm valinomycin (C). Higher or lower doses of diazoxide (10 and 100 μm) produced similar results (not shown). In all these experiments, BSA was omitted from the medium to avoid possible interference with diazoxide or valinomycin. Frames, representative examples of condensed (c), intermediate (i) and swollen (s) mitochondria. D, morphometric analysis of mitochondria incubated with and without 50 μm diazoxide (+Diazo) or 0.4 nm valinomycin (+Val). The percentages of the mitochondrial populations in the respective groups are given in parentheses. *P < 0.05, **P < 0.01 versus corresponding morphological group in the control without pretreatments. Data are means ±s.e.m., n = 4.
Measurements of respiration and ΔΨ
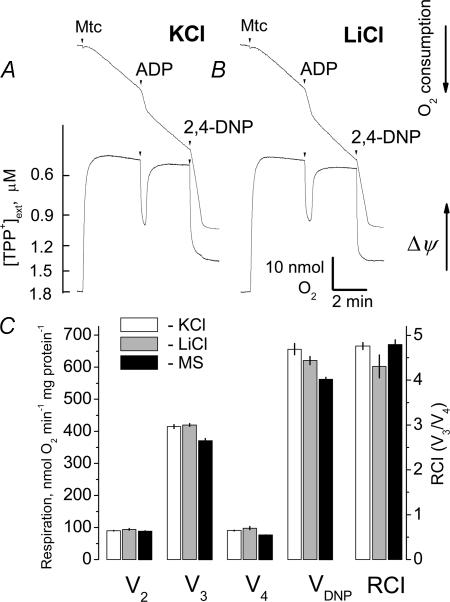
The lack of swelling of brain mitochondria incubated in KCl-based medium could be due to concurrent activation of the K+/H+ exchanger leading to extrusion of excessive K+ from the mitochondrial matrix (Bernardi & Azzone, 1983; Brierley et al. 1984). Simultaneous K+ influx through the putative mitoKATP, and K+ efflux through the K+–H+ exchanger, constitute a continuous K+ cycle that may partially depolarize mitochondria and activate mitochondrial respiration (Kowaltowski et al. 2001; Bajgar et al. 2001). It has been proposed that the mitoKATP is selective for K+ and does not conduct Li+ (Kowaltowski et al. 2001). In K+-free MS-based medium, the mitoKATP should also be inoperative because of the absence of K+. Thus, if K+ cycling involving mitoKATP is supposed to be active in brain mitochondria, omitting K+ from the incubation medium should stop the K+ cycling, polarize mitochondria, and decrease respiratory rate. The respiratory rates of brain mitochondria incubated in KCl-, LiCl- and MS-based media, with or without ADP or 2,4-dinitrophenol (2,4-DNP), were similar (Fig. 5). These experiments were performed in the absence of oligomycin. Accumulation of TPP+ indicative of ΔΨ was also comparable in KCl- and LiCl-based media (Fig. 5A and B). This suggested a lack of K+-dependent processes that could affect respiration and ΔΨ in these experiments. In addition, the lack of any difference in light scattering measurements conducted in K+-containing and K+-free medium argues against the K+–H+ antiporter compensating for any effects of mitoKATP.
Figure 5. Respiration and membrane potential of isolated brain mitochondria in media of various compositions.
A and B, representative traces of respiration (upper traces) and change in membrane potential (ΔΨ; lower traces) obtained with and without 200 μm ADP or 60 μm 2,4-dinitrophenol (2,4-DNP). Mitochondria were incubated in KCl- or LiCl-based media supplemented with 3 mm succinate plus 3 mm glutamate. These experiments were performed in the absence of oligomycin. C, summary plot of the respiratory rates, including measurements in mannitol–sucrose (MS)-based medium. V2 is the rate of respiration prior to addition of ADP, V3 is the respiratory rate in the presence of ADP, V4 is the respiratory rate after all ADP was depleted, VDNP is the respiratory rate in the presence of 2,4-DNP. RCI, respiratory control index, the ratio of the respiratory rate with 200 μm ADP to the respiratory rate after all added ADP has been phosphorylated, without addition of oligomicin. Data are means ±s.e.m., n = 3.
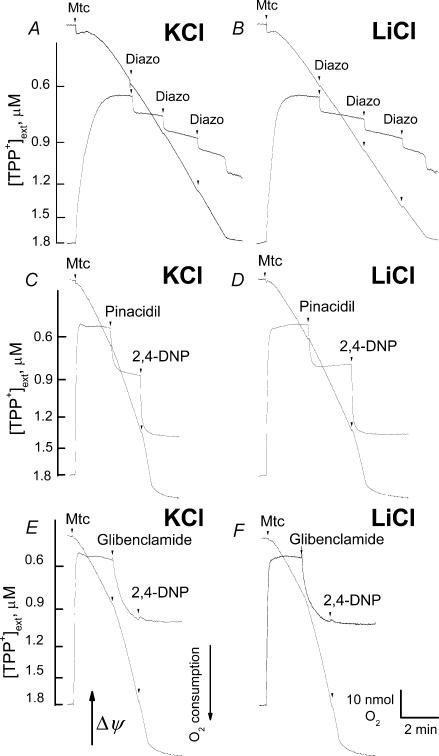
With succinate plus glutamate, consecutive additions of 50 μm diazoxide depolarized mitochondria in both KCl- and LiCl-based media (Fig. 6A and B). In these experiments, succinate was the main substrate, while glutamate was used to prevent oxaloacetate-induced inhibition of SDH (Wojtczak et al. 1969) by eliminating oxaloacetate in the transaminase reaction (Lehninger et al. 1993). Opening of the putative mitoKATP was not involved in these depolarizations since they were observed in both KCl- and LiCl-based media. The diazoxide-induced depolarizations were not accompanied by concomitant activation in respiration (Fig. 6A and B). Diazoxide inhibits succinate dehydrogenase (SDH) (Grimmsmann & Rustenbeck, 1998; Lim et al. 2002; Hanley et al. 2002) and uncouples oxidative phosphorylation acting as a weak protonophore (Kowaltowski et al. 2001; Kopustinskiene et al. 2002; Holmuhamedov et al. 2004). Therefore, depolarization without activation of respiration could be explained by the inhibition of SDH combined with uncoupling. The inhibition of SDH could also account for the suppression of the uncoupled respiration by diazoxide. While basal respiration with succinate plus glutamate remained virtually unaffected, respiration stimulated by 60 μm 2,4-DNP was inhibited by diazoxide: 320 ± 6 nmol O2 min−1 (mg protein)−1 without diazoxide versus 152 ± 7 nmol O2 min−1 (mg protein)−1 with 50 μm diazoxide (mean ±s.e.m., P < 0.001, n = 5). An opener, pinacidil (150 μm), and a blocker, glibenclamide (150 μm), depolarized brain mitochondria, oxidizing 3 mm pyruvate plus 1 mm malate in both KCl- and LiCl-based media (Fig. 6C–F). In this case, however, the depolarizations were accompanied by activation of respiration. Similar results were obtained when mitochondria were fuelled by succinate plus glutamate (not shown). Interestingly, in our experiments, glibenclamide inhibited the uncoupling effect of 2,4-DNP. The mechanism of this inhibition is unclear.
Figure 6. Diazoxide, pinacidil and glibenclamide induce K+-independent depolarization of brain mitochondria.
Pinacidil and glibenclamide activated respiration in a K+-independent manner concurrently with depolarization. Mitochondria were incubated either in KCl-based (A, C and E) or LiCl-based (B, D and F) medium supplemented either with 3 mm succinate and 3 mm glutamate (A and B) or with 3 mm pyruvate plus 1 mm malate (C–F). BSA was omitted in all experiments. ΔΨ and respiration were monitored simultaneously. A and B, three consecutive additions of diazoxide, 50 μm each, were made as indicated. Each addition of diazoxide introduced 0.5% DMSO into the medium as a vehicle. 0.5–1.5% DMSO alone affected neither mitochondrial respiration nor ΔΨ (not shown). C and D, 150 μm pinacidil was added as indicated. E and F, 150 μm glibenclamide was added as indicated. 60 μm 2,4-DNP was added at the end of the experiments to induce maximal depolarization and activation of respiration (C–F).
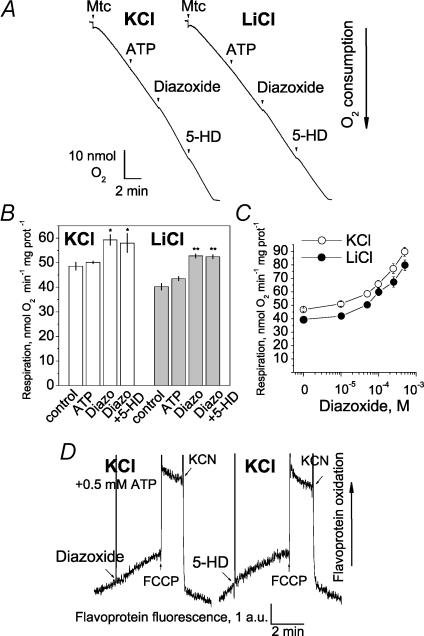
Earlier, an inhibition of respiration by ATP followed by reactivation with diazoxide and consecutive suppression with 5-HD or glyburide was demonstrated, and considered to be evidence for the presence of the mitoKATP in heart and brain mitochondria (Kowaltowski et al. 2001; Bajgar et al. 2001). In our hands, the respiration of brain mitochondria in KCl- or LiCl-based media supplemented with pyruvate and malate was not affected by 0.5 mm ATP, while 50 μm diazoxide slightly activated respiration (Fig. 7A and B). These experiments were performed without addition of TPP+ into the media to avoid possible interaction of TPP+ with the putative mitoKATP (Mironova et al. 2004). 5-HD (0.5 mm) was added after diazoxide without effect. Figure 7C shows the dose–response curves for the effect of diazoxide on respiration in KCl- and in LiCl-based media. The effect of diazoxide on respiration of brain mitochondria was K+ independent, and therefore could not be attributed to the putative mitoKATP.
Figure 7. Effects of ATP, diazoxide and 5-HD on the respiration and flavoprotein redox status of isolated brain mitochondria in KCl- and LiCl-based medium.
Mitochondria were incubated either in KCl-based medium or in LiCl-based medium (A and B). In both cases, the medium was supplemented with 3 mm pyruvate plus 1 mm malate. BSA was excluded from the media. 0.5 mm ATP, 50 μm diazoxide and 0.5 mm 5-HD were added to mitochondria as indicated. An addition of diazoxide introduced 0.5% DMSO into the medium as a vehicle. 0.5–1.5% DMSO alone did not affect mitochondrial respiration. The higher final concentrations of the vehicle (1–1.5% DMSO) did not improve the effect of diazoxide (not shown). B, summary plot of respiratory rates in KCl- and LiCl-based media. *P < 0.05, **P < 0.01 versus respiratory rate without additions (V2). Data are means ±s.e.m., n = 4. C, diazoxide dose–response curves for the respiration in KCl- and LiCl-media. Substrates: 3 mm pyruvate plus 1 mm malate. Data are means ±s.e.m., n = 4. D, fluorescence of mitochondrial flavoproteins was monitored in KCl-based medium. Similar results were obtained in LiCl- and MS-based media (not shown). 50 μm diazoxide, 0.5 mm 5-HD, 1 μm FCCP and 2 mm KCN were added as indicated.
Flavoprotein redox status
In our experiments, both diazoxide and 5-HD failed to change redox status of mitochondrial flavoproteins (FP) (Fig. 7D). The slight increase in FP oxidation induced by diazoxide might reflect mild uncoupling. The tiny increase in FP oxidation was also observed in LiCl-based medium (not shown). On the other hand, FCCP produced distinct oxidation of FP, while KCN resulted in reduction of FP (Fig. 7D). Thus, FP redox status appeared to be independent from the putative changes in the mitoKATP activity.
Recently, it has been reported that testosterone applied to cardiac myocytes or to cardiac mitoplasts produces 5-HD-sensitive opening of mitoKATP in a KCl-dependent manner leading to oxidation of FP (Er et al. 2004). In our experiments with isolated brain mitochondria, we found no influence of testosterone (10–50 μm) on the light absorbance of mitochondrial suspension, ΔΨ and redox status of FP (not shown).
K+ efflux measurements
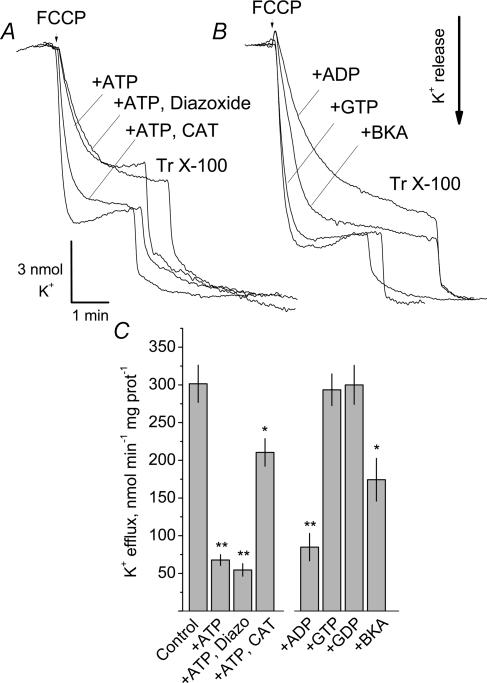
Mitochondria incubated in K+-free medium respond to depolarization by releasing intramitochondrial K+ (Jung & Brierley, 1981; Baranova et al. 2000). Sensitivity of K+ release to ATP and to pharmacological modulators of mitoKATP activity could directly implicate this channel in K+ transport in brain mitochondria. In our experiments, brain mitochondria incubated in K+-free MS-based medium retained K+ during the course of the experiment. FCCP-induced depolarization caused a massive release of mitochondrial K+ into the medium (Fig. 8). The release of K+ was partially inhibited by 0.15 mm ATP added to mitochondria prior to FCCP. ADP had a similar effect. A higher concentration of ATP or ADP (0.5 mm) did not provide a stronger inhibition (not shown). GTP and GDP (0.15 mm) were without effect (Fig. 8B and C). Diazoxide (50 μm) added after ATP did not reverse ATP-induced suppression of K+ efflux. Neither a lower nor a higher dose of diazoxide (10 or 100 μm) affected K+ release (not shown). Thus, while the release of K+ from depolarized brain mitochondria could be partially inhibited by ATP, diazoxide proved to be ineffective. However, the release of K+ from ATP-treated mitochondria could be re-activated by CAT (10 μm), an inhibitor of adenine nucleotide translocase (ANT), applied either prior to (not shown) or 2 min after addition of ATP to mitochondria (Fig. 8A). In addition, BKA, another inhibitor of the ANT, partially blocked K+ release (Fig. 8B and C). These observations implicate the ANT in the ATP-sensitive FCCP-induced K+ efflux.
Figure 8. Uncoupler-induced release of K+ from isolated brain mitochondria incubated in K+-free MS-based medium.
ATP, ADP and bongkrekic acid (BKA) partially inhibited K+ efflux. Diazoxide did not reactivate the ATP-suppressed release of K+, while carboxyatractyloside (CAT) reactivated the ATP-suppressed K+ efflux. GTP and GDP did not influence K+ efflux. Mitochondria were incubated in the MS-based medium supplemented with 3 mm succinate and 3 mm glutamate. 0.15 mm ATP and 50 μm diazoxide (in the presence of 0.5% DMSO as a vehicle) were added into the chamber prior to mitochondria. Higher and lower doses of diazoxide (10 and 100 μm) produced similar results. The higher final concentrations of the vehicle (1–1.5% DMSO) did not improve the effect of diazoxide (not shown). A, 10 μm CAT was added to mitochondria 2 min after addition of ATP. B, 0.15 mm ADP, 0.15 mm GTP, 0.15 mm GDP or 20 μm BKA was added prior to mitochondria in the absence of ATP. A and B, the medium was supplemented with 1 μm oligomycin. In both cases, FCCP (1 μm) was added to depolarize mitochondria and to trigger K+ efflux. Similar results were obtained with 60 μm 2,4-DNP (not shown). At the end of the experiment, 0.1% Triton X-100 (Tr X-100) was added to release a residual K+. C, summary of the results of the experiments. *P < 0.05, **P < 0.01 versus control. Data are means ±s.e.m., n = 4.
Discussion
The results presented in this paper cast doubt on a functional role of the mitoKATP in CNS mitochondria, although we can not completely rule out the existence of mitoKATP in brain. We presume that if mitoKATP exists, it has a different molecular identity than cellKATP. The lack of knowledge at the molecular and genetic levels represents a substantial impediment in the study of the channel. While the molecular structure of mitoKATP remains unknown, the evidence for its presence and role in mitochondria is almost utterly pharmacological, based on the apparent selectivity of the putative openers and blockers of the mitoKATP. However, in our study, the pharmacological modulators of the putative mitoKATP failed to affect functions of brain mitochondria in a manner that could be clearly associated with the activity of the putative mitoKATP. In addition, immunoblotting analysis did not provide explicit evidence for the presence of Kir 6.1, Kir 6.2, SUR1 and SUR2 in brain mitochondria. On the other hand, CAT-induced reactivation of ATP-suppressed K+ efflux and BKA inhibition of K+ release suggest involvement of the ANT in the K+ transport in brain mitochondria.
Previously, in mitochondria of PC 12 cells, anti-SUR1 antibody recognized a 155 kDa protein, while anti-SUR2 antibody did not detect a band (Tai et al. 2003). In contrast, in mitochondria from mouse, rat and piglet brain, the anti-SUR2 antibodies recognized mitochondrially enriched polypeptides with molecular masses around 30 and 130 kDa (Lacza et al. 2003a). The anti-SUR2 antibodies also recognized a 25 kDa polypeptide in mouse brain and liver mitochondria, and in rat heart mitochondria (Lacza et al. 2003b). With anti-SUR1 and anti-SUR2 antibodies, we found a blocking-peptide-sensitive 97 kDa band enriched in mitochondrial samples. This band did not correspond to the expected molecular masses of SUR1, SUR2 or mitoSUR and, therefore, it could not be attributed to any of these proteins. In contrast to other groups (Shimizu et al. 2002; Lacza et al. 2003a), our experiments with anti-SUR2 antibody, did not reveal the 130 kDa band. In addition, the 30 kDa band detected previously with anti-SUR2 antibody in brain mitochondrial samples (Lacza et al. 2003a) appeared to be insensitive to the blocking peptide and thus appeared to be nonspecific. Therefore, it could not be attributed to mitoSUR.
Earlier, the 50 kDa polypeptides tentatively corresponding to Kir 6.1 or Kir 6.2 were found in heart mitochondria (Lacza et al. 2003b). A 50 kDa ‘mitochondrial Kir 6.1’ but not ‘Kir 6.2’ was detected in PC 12 cells (Tai et al. 2003). Also, a 50 kDa polypeptide putatively representing Kir 6.1 was found in brain, liver, and skeletal muscle mitochondria (Suzuki et al. 1997; Shimizu et al. 2002; Lacza et al. 2003a). In contrast, Thomzig et al. (2001) did not detect Kir 6.1 in brain mitochondria. In our experiments with purified brain nonsynaptosomal mitochondria, we detected 50 kDa bands enriched in mitochondrial samples with both anti-Kir 6.1 and anti-Kir 6.2 antibodies. These might represent spliced variants of Kir 6.1 or Kir 6.2 subunits of the cellKATP. However, in contrast to heart mitochondria (Lacza et al. 2003b), in brain mitochondria we did not detect 50 kDa bands with anti Kir 6.X antibodies raised against other epitopes of Kir 6.2 or Kir 6.1. Thus, the 50 kDa bands might not represent either Kir 6.1 or Kir 6.2, but might instead belong to another protein with cross-reactivity to certain anti-Kir 6.X antibodies. In line with this, Grover & Garlid (2000) reported that antibodies against Kir 6.1 reacted with an unknown mitochondrial protein, but did not react with any protein in the reconstitutively active purified fraction of the mitoKATP.
Based on studies with isolated mitochondria, it has been suggested that the main function of a putative mitoKATP is to regulate mitochondrial matrix volume (Kowaltowski et al. 2001). Opening of the putative mitoKATP with diazoxide caused an increase in matrix volume evaluated with light absorbance assay (Kowaltowski et al. 2001; Bajgar et al. 2001). However, in the present study, using three different assays (light absorbance measurements, radioisotope measurements and TEM), we did not find evidence for the changes of matrix volume following treatment of brain mitochondria with blockers or openers of the putative mitoKATP. The recent radioisotope measurements of matrix volume in liver and heart mitochondria also challenged the role of mitoKATP in the regulation of matrix volume (Das et al. 2003). The possible cause of the differences between our results and the previously reported data (Bajgar et al. 2001) is not clear. The use of different types of mitochondrial preparations could be one of the possible explanations. In our work, we used Percoll-gradient-purified nonsynaptosomal mitochondria, while others used brain mitochondria isolated with the use of digitonin without a further density gradient purification step (Bajgar et al. 2001). Although the conflicting data might be caused by irreversible inactivation of the putative mitoKATP during purification over Percoll gradient, isolated Percoll-gradient-purified brain mitochondria were used in other studies of mitoKATP without loss of activity (Shimizu et al. 2002; Lacza et al. 2003a). It is also possible that concurrent activation of the K+–H+ exchanger compensated K+ influx through a mitoKATP, preventing detectable matrix volume changes in our experiments.
It has been reported that diazoxide causes slight activation of mitochondrial respiration, presumably due to acceleration of futile K+ cycling across the inner mitochondrial membrane (IMM) (Kowaltowski et al. 2001; Bajgar et al. 2001; Garlid & Paucek, 2003). In heart mitochondria, opening the mitoKATP resulted in a negligible depolarization (1–2 mV) that was practically impossible to detect (Kowaltowski et al. 2001). In isolated brain mitochondria, the amount of the mitoKATP was estimated to be sevenfold higher than in liver or heart mitochondria (Bajgar et al. 2001). Correspondingly, in brain mitochondria, the blockade of a putative mitoKATP with ATP was accompanied by a stronger inhibition of respiration followed by greater re-activation of respiration induced by diazoxide (Bajgar et al. 2001). In contrast to the previous studies with isolated brain mitochondria (Bajgar et al. 2001), we did not observe an inhibition of respiration with exogenous ATP. Diazoxide produced subtle K+-independent activation of respiration without reversal by 5-HD. With succinate, diazoxide noticeably depolarized brain mitochondria. However, this effect of diazoxide was observed in KCl-based as well as in KCl-free media. Therefore, the diazoxide-induced depolarization could not be attributed to opening the putative mitoKATP. The effects of diazoxide in our experiments could be better explained by protonophoric properties of diazoxide (Kopustinskiene et al. 2002; Holmuhamedov et al. 2004) and by inhibition of SDH (Schafer et al. 1971), rather than by opening the putative mitoKATP. An increased electron flow in the respiratory chain due to activation of respiration could cause oxidation of mitochondrial FP, including FADH2 and FMN (Nicholls & Ferguson, 2002). In neurones, diazoxide increased FP fluorescence that was interpreted as an indication of opening the mitoKATP, leading to activation of electron flow in the respiratory chain due to membrane depolarization (Liu et al. 1998). 5-HD, added with diazoxide, blocked a putative mitoKATP and attenuated electron flow preventing oxidation of FP (Liu et al. 1998). In contrast, in our experiments with isolated brain mitochondria, neither diazoxide nor 5-HD affected redox status of FP. Our observations are consistent with results from two other groups that reported a failure of diazoxide to induce detectable changes in FP fluorescence in cardiomyocytes (Lawrence et al. 2001; Hanley et al. 2002). Recently, testosterone has been shown to be an effective opener of the mitoKATP in cardiomyocytes and cardiac mitoplasts (Er et al. 2004). However, in our experiments with brain mitochondria, testosterone did not influence mitochondrial volume, ΔΨ or redox status of FP. Thus, the effect of testosterone could be tissue specific. An electrophysiological study of mitochondrial multiprotein complex including SDH, α-subunit of ATP synthase, phosphate carrier, mitochondrial ATP-binding cassette protein 1 and ANT, reconstituted into proteoliposomes or a planar lipid bilayer, revealed a channel sensitive to blockers and openers of a putative mitoKATP (Ardehali et al. 2004). It is unknown whether all five proteins of the multiprotein complex that resembles the putative mitoKATP are required for channel activity (Ardehali et al. 2004). It is also unclear which of them is a pore-forming component of the putative channel. The presence of the ANT in the multiprotein complex invigorates the hypothesis that the ANT might be responsible for the mitochondrial K+ uniport sensitive to adenine nucleotides (Panov et al. 1980; Halestrap, 1989). It has been previously shown that 2,4-DNP-induced release of K+ from rat liver mitochondria is inhibited by ATP and ADP (Baranova et al. 2000). Atractyloside, a specific inhibitor of the ANT, completely eliminated the inhibitory effect of adenine nucleotides. In accord with this observation, in our experiments, CAT, another specific inhibitor of the ANT, reversed ATP–induced inhibition of FCCP-evoked K+ release from brain mitochondria. Initially, the adenine nucleotide binding site of the mitoKATP was proposed to face the mitochondrial matrix (Inoue et al. 1991). Later, the adenine nucleotide binding site of the putative mitoKATP was found to face the cytosol (Yarov-Yarovoy et al. 1997). If this is the case, the atractyloside- and CAT-sensitive effects of adenine nucleotides could be attributed to the ANT rather than to the putative mitoKATP. In line with this, BKA, an inhibitor of the ANT, suppressed K+ efflux induced by depolarization mimicking the effect of adenine nucleotides. Since the ANT-linked channel is regulated by adenine nucleotides (Halestrap, 1989; Brustovetsky et al. 2002b), it could be nominally identified as a mitoKATP. The other constituents of the multiprotein complex described by Ardehali et al. (2004) might cooperate with the ANT, providing regulatory mechanisms of a putative mitoKATP. However, additional studies are required to substantiate this hypothesis.
Acknowledgments
This work was supported by NIH/NINDS RO1 NS050131 to N.B.
References
- Ardehali H, Chen Z, Ko Y, Mejia-Alvarez R, Marban E. Multiprotein complex containing succinate dehydrogenase confers mitochondrial ATP-sensitive K+ channel activity. Proc Natl Acad Sci U S A. 2004;101:11880–11885. doi: 10.1073/pnas.0401703101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajgar R, Seetharaman S, Kowaltowski AJ, Garlid KD, Paucek P. Identification and properties of a novel intracellular (mitochondrial) ATP-sensitive potassium channel in brain. J Biol Chem. 2001;276:33369–33374. doi: 10.1074/jbc.M103320200. [DOI] [PubMed] [Google Scholar]
- Baranova OV, Skarga YY, Negoda AE, Mironova GD. Inhibition of 2,4-dinitrophenol-induced potassium efflux by adenine nucleotides in mitochondria. Biochemistry (Mosc) 2000;65:218–222. [PubMed] [Google Scholar]
- Beavis AD, Brannan RD, Garlid KD. Swelling and contraction of the mitochondrial matrix. I. A structural interpretation of the relationship between light scattering and matrix. J Biol Chem. 1985;260:13424–13433. [PubMed] [Google Scholar]
- Beavis AD, Lu Y, Garlid KD. On the regulation of K+ uniport in intact mitochondria by adenine nucleotides and nucleotide analogs. J Biol Chem. 1993;268:997–1004. [PubMed] [Google Scholar]
- Bernardi P, Azzone GF. Electroneutral H+–K+ exchange in liver mitochondria. Regulation by membrane potential. Biochim Biophys Acta. 1983;724:212–223. doi: 10.1016/0005-2728(83)90140-8. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brand MD. Measurement of mitochondrial protonmotive force. In: Brown GC, Cooper CE, editors. Bioenergetics. A Practical Approach. Oxford: IRL Press; 2002. pp. 39–62. [Google Scholar]
- Brierley GP, Jurkowitz MS, Farooqui T, Jung DW. K+/H+ antiport in heart mitochondria. J Biol Chem. 1984;259:14672–14678. [PubMed] [Google Scholar]
- Brustovetsky N, Brustovetsky T, Jemmerson R, Dubinsky JM. Calcium-induced cytochrome c release from CNS mitochondria is associated with the permeability transition and rupture of the outer membrane. J Neurochem. 2002a;80:207–218. doi: 10.1046/j.0022-3042.2001.00671.x. [DOI] [PubMed] [Google Scholar]
- Brustovetsky N, Tropschug M, Heimpel S, Heidkamper D, Klingenberg M. A large Ca2+-dependent channel formed by recombinant ADP/ATP carrier from Neurospora crassa resembles the mitochondrial permeability transition pore. Biochemistry. 2002b;41:11804–11811. doi: 10.1021/bi0200110. [DOI] [PubMed] [Google Scholar]
- Das M, Parker JE, Halestrap AP. Matrix volume measurements challenge the existence of diazoxide/glibencamide-sensitive KATP channels in rat mitochondria. J Physiol. 2003;547:893–902. doi: 10.1113/jphysiol.2002.035006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domoki F, Perciaccante JV, Veltkamp R, Bari F, Busija DW. Mitochondrial potassium channel opener diazoxide preserves neuronal–vascular function after cerebral ischemia in newborn pigs. Stroke. 1999;30:2713–2718. doi: 10.1161/01.str.30.12.2713. [DOI] [PubMed] [Google Scholar]
- Garlid KD, Beavis AD. Swelling and contraction of the mitochondrial matrix. II. Quantitative application of the light scattering technique to solute transport across the inner membrane. J Biol Chem. 1985;260:13434–13441. [PubMed] [Google Scholar]
- Garlid KD, Paucek P. Mitochondrial potassium transport: the K+ cycle. Biochim Biophys Acta. 2003;1606:23–41. doi: 10.1016/s0005-2728(03)00108-7. [DOI] [PubMed] [Google Scholar]
- Garlid KD, Paucek P, Yarov-Yarovoy V, Sun X, Schindler PA. The mitochondrial KATP channel as a receptor for potassium channel openers. J Biol Chem. 1996;271:8796–8799. doi: 10.1074/jbc.271.15.8796. [DOI] [PubMed] [Google Scholar]
- Grimmsmann T, Rustenbeck I. Direct effects of diazoxide on mitochondria in pancreatic B-cells and on isolated liver mitochondria. Br J Pharmacol. 1998;123:781–788. doi: 10.1038/sj.bjp.0701663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover GJ, Garlid KD. ATP-sensitive potassium channels: a review of their cardioprotective pharmacology. J Mol Cell Cardiol. 2000;32:677–695. doi: 10.1006/jmcc.2000.1111. [DOI] [PubMed] [Google Scholar]
- Halestrap AP. The regulation of the matrix Volume of mammalian mitochondria in vivo and in vitro and its role in the control of mitochondrial metabolism. Biochim Biophys Acta. 1989;973:355–382. doi: 10.1016/s0005-2728(89)80378-0. [DOI] [PubMed] [Google Scholar]
- Hanley PJ, Mickel M, Loffler M, Brandt U, Daut J. KATP channel-independent targets of diazoxide and 5-hydroxydecanoate in the heart. J Physiol. 2002;542:735–741. doi: 10.1113/jphysiol.2002.023960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmuhamedov EL, Jahangir A, Oberlin A, Komarov A, Colombini M, Terzic A. Potassium channel openers are uncoupling protonophores: implication in cardioprotection. FEBS Lett. 2004;568:167–170. doi: 10.1016/j.febslet.2004.05.031. [DOI] [PubMed] [Google Scholar]
- Inoue I, Nagase H, Kishi K, Higuti T. ATP-sensitive K+ channel in the mitochondrial inner membrane. Nature. 1991;352:244–247. doi: 10.1038/352244a0. [DOI] [PubMed] [Google Scholar]
- Jaburek M, Yarov-Yarovoy V, Paucek P, Garlid KD. State-dependent inhibition of the mitochondrial KATP channel by glyburide and 5-hydroxydecanoate. J Biol Chem. 1998;273:13578–13582. [PubMed] [Google Scholar]
- Jung DW, Brierley GP. On the relationship between the uncoupler-induced efflux of K+ from heart mitochondria and the oxidation–reduction state of pyridine nucleotides. J Biol Chem. 1981;256:10490–10496. [PubMed] [Google Scholar]
- Kamo N, Muratsugu M, Hongoh R, Kobatake Y. Membrane potential of mitochondria measured with an electrode sensitive to tetraphenyl phosphonium and relationship between proton electrochemical potential and phosphorylation potential in steady state. J Membr Biol. 1979;49:105–121. doi: 10.1007/BF01868720. [DOI] [PubMed] [Google Scholar]
- Kis B, Rajapakse NC, Snipes JA, Nagy K, Horiguchi T, Busija DW. Diazoxide induces delayed pre-conditioning in cultured rat cortical neurons. J Neurochem. 2003;87:969–980. doi: 10.1046/j.1471-4159.2003.02072.x. [DOI] [PubMed] [Google Scholar]
- Kopustinskiene DM, Jovaisiene J, Liobikas J, Toleikis A. Diazoxide and pinacidil uncouple pyruvate-malate-induced mitochondrial respiration. J Bioenerg Biomembr. 2002;34:49–53. doi: 10.1023/a:1013870704002. [DOI] [PubMed] [Google Scholar]
- Kopustinskiene DM, Toleikis A, Saris NE. Adenine nucleotide translocase mediates the KATP-channel-opener-induced proton and potassium flux to the mitochondrial matrix. J Bioenerg Biomembr. 2003;35:141–148. doi: 10.1023/a:1023746103401. [DOI] [PubMed] [Google Scholar]
- Kowaltowski AJ, Seetharaman S, Paucek P, Garlid KD. Bioenergetic consequences of opening the ATP-sensitive K+ channel of heart mitochondria. Am J Physiol Heart Circ Physiol. 2001;280:H649–657. doi: 10.1152/ajpheart.2001.280.2.H649. [DOI] [PubMed] [Google Scholar]
- Lacza Z, Snipes JA, Kis B, Szabo C, Grover G, Busija DW. Investigation of the subunit composition and the pharmacology of the mitochondrial ATP-dependent K+ channel in the brain. Brain Res. 2003a;994:27–36. doi: 10.1016/j.brainres.2003.09.046. [DOI] [PubMed] [Google Scholar]
- Lacza Z, Snipes JA, Miller AW, Szabo C, Grover G, Busija DW. Heart mitochondria contain functional ATP-dependent K+ channels. J Mol Cell Cardiol. 2003b;35:1339–1347. doi: 10.1016/s0022-2828(03)00249-9. [DOI] [PubMed] [Google Scholar]
- Lawrence CL, Billups B, Rodrigo GC, Standen NB. The KATP channel opener diazoxide protects cardiac myocytes during metabolic inhibition without causing mitochondrial depolarization or flavoprotein oxidation. Br J Pharmacol. 2001;134:535–542. doi: 10.1038/sj.bjp.0704289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehninger AL, Nelson DL, Cox MM. Principles of Biochemistry. 2. New York: Worth Publishers; 1993. [Google Scholar]
- Lim KHH, Javadov SA, Das M, Clarke SJ, Suleiman MS, Halestrap AP. The effects of ischaemic preconditioning, diazoxide and 5-hydroxydecanoate on rat heart mitochondrial volume and respiration. J Physiol. 2002;545:961–974. doi: 10.1113/jphysiol.2002.031484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Sato T, O'Rourke B, Marban E. Mitochondrial ATP-dependent potassium channels: novel effectors of cardioprotection? Circulation. 1998;97:2463–2469. doi: 10.1161/01.cir.97.24.2463. [DOI] [PubMed] [Google Scholar]
- Liu D, Slevin JR, Lu C, Chan SL, Hansson M, Elmer E, Mattson MP. Involvement of mitochondrial K+ release and cellular efflux in ischemic and apoptotic neuronal death. J Neurochem. 2003;86:966–979. doi: 10.1046/j.1471-4159.2003.01913.x. [DOI] [PubMed] [Google Scholar]
- Er F, Michels G, Gassanov N, Rivero F, Hoppe UC. Testosterone induces cytoprotection by activating ATP-sensitive K+ channels in the cardiac mitochondrial inner membrane. Circulation. 2004;110:3100–3107. doi: 10.1161/01.CIR.0000146900.84943.E0. [DOI] [PubMed] [Google Scholar]
- Mironova GD, Negoda AE, Marinov BS, Paucek P, Costa AD, Grigoriev SM, Skarga YY, Garlid KD. Functional distinctions between the mitochondrial ATP-dependent K+ channel (mitoKATP) and its inward rectifier subunit (mitoKIR) J Biol Chem. 2004;279:32562–32568. doi: 10.1074/jbc.M401115200. [DOI] [PubMed] [Google Scholar]
- Nagy K, Kis B, Rajapakse NC, Bari F, Busija DW. Diazoxide preconditioning protects against neuronal cell death by attenuation of oxidative stress upon glutamate stimulation. J Neurosci Res. 2004;76:697–704. doi: 10.1002/jnr.20120. [DOI] [PubMed] [Google Scholar]
- Nicholls DG, Ferguson SJ. Bioenergetics 3. London: Academic Press; 2002. [Google Scholar]
- O'Rourke B. Myocardial KATP channels in preconditioning. Circ Res. 2000;87:845–855. doi: 10.1161/01.res.87.10.845. [DOI] [PubMed] [Google Scholar]
- O'Rourke B. Evidence for mitochondrial K+ channels and their role in cardioprotection. Circ Res. 2004;94:420–432. doi: 10.1161/01.RES.0000117583.66950.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovide-Bordeaux S, Ventura-Clapier R, Veksler V. Do modulators of the mitochondrial KATP channel change the function of mitochondria in situ? J Biol Chem. 2000;275:37291–37295. doi: 10.1074/jbc.M005772200. [DOI] [PubMed] [Google Scholar]
- Pacher P, Thomas AP, Hajnoczky G. Ca2+ marks: miniature calcium signals in single mitochondria driven by ryanodine receptors. Proc Natl Acad Sci U S A. 2002;99:2380–2385. doi: 10.1073/pnas.032423699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panov A, Filippova S, Lyakhovich V. Adenine nucleotide translocase as a site of regulation by ADP of the rat liver mitochondria permeability to H+ and K+ ions. Arch Biochem Biophys. 1980;199:420–426. doi: 10.1016/0003-9861(80)90298-2. [DOI] [PubMed] [Google Scholar]
- Paucek P, Mironova G, Mahdi F, Beavis AD, Woldegiorgis G, Garlid KD. Reconstitution and partial purification of the glibenclamide-sensitive, ATP-dependent K+ channel from rat liver and beef heart mitochondria. J Biol Chem. 1992;267:26062–26069. [PubMed] [Google Scholar]
- Richards-Kortum R, Sevick-Muraca E. Quantitative optical spectroscopy for tissue diagnosis. Annu Rev Phys Chem. 1996;47:555–606. doi: 10.1146/annurev.physchem.47.1.555. [DOI] [PubMed] [Google Scholar]
- Sato T, Li Y, Saito T, Nakaya H. Minoxidil opens mitochondrial K (ATP) channels and confers cardioprotection. Br J Pharmacol. 2004;141:360–366. doi: 10.1038/sj.bjp.0705613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer G, Portenhauser R, Trolp R. Inhibition of mitochondrial metabolism by the diabetogenic thiadiazine diazoxide. I. Action on succinate dehydrogenase and TCA-cycle oxidations. Biochem Pharmacol. 1971;20:1271–1280. doi: 10.1016/0006-2952(71)90358-3. [DOI] [PubMed] [Google Scholar]
- Scorrano L, Ashiya M, Buttle K, Weiler S, Oakes SA, Mannella CA, Korsmeyer SJ. A distinct pathway remodels mitochondrial cristae and mobilizes cytochrome c during apoptosis. Dev Cell. 2002;2:55–67. doi: 10.1016/s1534-5807(01)00116-2. [DOI] [PubMed] [Google Scholar]
- Shimizu K, Lacza Z, Rajapakse N, Horiguchi T, Snipes J, Busija DW. MitoKATP opener, diazoxide, reduces neuronal damage after middle cerebral artery occlusion in the rat. Am J Physiol Heart Circ Physiol. 2002;283:H1005–1011. doi: 10.1152/ajpheart.00054.2002. [DOI] [PubMed] [Google Scholar]
- Sims NR. Rapid isolation of metabolically active mitochondria from rat brain and subregions using Percoll density gradient centrifugation. J Neurochem. 1990;55:698–707. doi: 10.1111/j.1471-4159.1990.tb04189.x. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Kotake K, Fujikura K, Inagaki N, Suzuki T, Gonoi T, Seino S, Takata K. Kir6.1: a possible subunit of ATP-sensitive K+ channels in mitochondria. Biochem Biophys Res Commun. 1997;241:693–697. doi: 10.1006/bbrc.1997.7891. [DOI] [PubMed] [Google Scholar]
- Szewczyk A, Czyz A, Nalecz MJ. ATP-regulated potassium channel blocker, glibenclamide, uncouples mitochondria. Pol J Pharmacol. 1997;49:49–52. [PubMed] [Google Scholar]
- Tai KK, McCrossan ZA, Abbott GW. Activation of mitochondrial ATP-sensitive potassium channels increases cell viability against rotenone-induced cell death. J Neurochem. 2003;84:1193–1200. doi: 10.1046/j.1471-4159.2003.01625.x. [DOI] [PubMed] [Google Scholar]
- Thomzig A, Wenzel M, Karschin C, Eaton MJ, Skatchkov SN, Karschin A, Veh RW. Kir6.1 is the principal pore-forming subunit of astrocyte but not neuronal plasma membrane KATP channels. Mol Cell Neurosci. 2001;18:671–690. doi: 10.1006/mcne.2001.1048. [DOI] [PubMed] [Google Scholar]
- Wojtczak L, Wojtczak AB, Ernster L. The inhibition of succinate dehydrogenase by oxalacetate. Biochim Biophys Acta. 1969;191:10–21. doi: 10.1016/0005-2744(69)90310-6. [DOI] [PubMed] [Google Scholar]
- Yarov-Yarovoy V, Paucek P, Jaburek M, Garlid KD. The nucleotide regulatory sites on the mitochondrial KATP channel face the cytosol. Biochim Biophys Acta. 1997;1321:128–136. doi: 10.1016/s0005-2728(97)00051-0. [DOI] [PubMed] [Google Scholar]