Abstract
Sensory information continuously converges on the spinal cord during a variety of motor behaviours. Here, we examined presynaptic control of group Ia afferents in relation to acquisition of a novel motor skill. We tested whether repetition of two motor tasks with different degrees of difficulty, a novel visuo-motor task involving the ankle muscles, and a control task involving simple voluntary ankle movements, would induce changes in the size of the soleus H-reflex. The slope of the H-reflex recruitment curve and the H-max/M-max ratio were depressed after repetition of the visuo-motor skill task and returned to baseline after 10 min. No changes were observed after the control task. To elucidate the mechanisms contributing to the H-reflex depression, we measured the size of the long-latency depression of the soleus H-reflex evoked by peroneal nerve stimulation (D1 inhibition) and the size of the monosynaptic Ia facilitation of the soleus H-reflex evoked by femoral nerve stimulation. The D1 inhibition was increased and the femoral nerve facilitation was decreased following the visuo-motor skill task, suggesting an increase in presynaptic inhibition of Ia afferents. No changes were observed in the disynaptic reciprocal Ia inhibition. Somatosensory evoked potentials (SEPs) evoked by stimulation of the tibial nerve (TN) were also unchanged, suggesting that transmission in ascending pathways was unaltered following the visuo-motor skill task. Together these observations suggest that a selective presynaptic control of Ia afferents contributes to the modulation of sensory inputs during acquisition of a novel visuo-motor skill in healthy humans.
The soleus H-reflex is modulated during a number of different motor tasks (Capaday & Stein, 1986; Katz et al. 1988; Meunier & Pierrot-Deseilligny, 1989; Llewellyn et al. 1990; Nielsen & Kagamihara, 1993b; Faist et al. 1996). It has been demonstrated that much of this modulation is explained by changes in presynaptic inhibition of the synapses of Ia afferents on spinal motorneurones (Hultborn et al. 1987a, b; Meunier & Pierrot-Deseilligny, 1989; Nielsen & Kagamihara, 1993b; Faist et al. 1996). Presumably, gating of sensory inputs to spinal motorneurones is of functional importance in regulating the contribution of the stretch reflex circuitry to the ongoing motor activity (Nielsen & Sinkjær, 2002; Rudomin, 2002). One advantage of regulating this contribution at the presynaptic level is that sensory inputs may be selectively gated, while allowing effective activation of the muscles by central commands (Rudomin, 2002). Furthermore, regulation of sensory information at the presynaptic level may permit conveyance of proprioceptive information to the cortex, which might contribute to adjusting supraspinal motor commands (Llewellyn et al. 1990; Morita et al. 1998).
It is possible that changes in the presynaptic control of sensory inputs may take place during acquisition of a novel motor task in order to modulate the stretch reflex circuitry during the performance of the task. Cross-sectional studies have demonstrated differences in H-reflex size in specific groups of athletes, which may reflect training-induced modifications of the transmission in the monosynaptic Ia pathway through changes in presynaptic inhibition (Rochcongar et al. 1979; Casabona et al. 1990; Nielsen et al. 1993). Animal studies have shown that long-term H-reflex up- and downregulation following operant conditioning is associated with plasticity at multiple sites (Carp & Wolpaw, 1994; Wolpaw et al. 1994; Wolpaw & Tennissen, 2001). Short-term H-reflex changes have been suggested to be related to changes in presynaptic inhibition at the Ia terminal (Wolpaw, 1997).
We have previously demonstrated an increase in the excitability of the leg cortical area in relation to acquisition of a novel visuo-motor skill (Perez et al. 2004). In this study we observed that the size of the H-reflex in one of the muscles involved during the visuo-motor skill training was depressed at the end of the session (unpublished data). We decided to test this observation in more detail and examine presynaptic control of group Ia afferents as a possible mechanism contributing to the H-reflex depression during acquisition of a visuo-motor skill.
We recorded the soleus H-reflex in relation to the acquisition of the same visuo-motor skill task as in the study by Perez et al. (2004). To evaluate presynaptic inhibition of the soleus Ia afferents we measured the size of the femoral nerve monosynaptic Ia facilitation of the soleus H-reflex (Hultborn et al. 1987a, b) and the long-latency (D1) inhibition of the soleus H-reflex induced by peroneal nerve stimulation (Mizuno et al. 1971). Finally, we measured tibial nerve somatosensory evoked potentials to test whether transmission of proprioceptive information to the cortex was affected in relation to acquisition of the visuo-motor skill task.
Methods
General experimental conditions
We studied 19 healthy volunteers (9 women, 10 men) with an average age of 29 ± 7 years (mean ±s.d.). All subjects gave their informed consent to the experimental procedure, which was approved by the local ethics committee. The study was performed in accordance with the Declaration of Helsinki. Ten subjects participated in two sessions separated by at least one week; one involved training of a visuo-motor skill with the ankle muscles, and the other involved repeated voluntary dorsi- and plantarflexion movements without visual feedback of the performed movements (control session). The other nine subjects participated only in the visuo-motor skill training session in which additional measurements were taken.
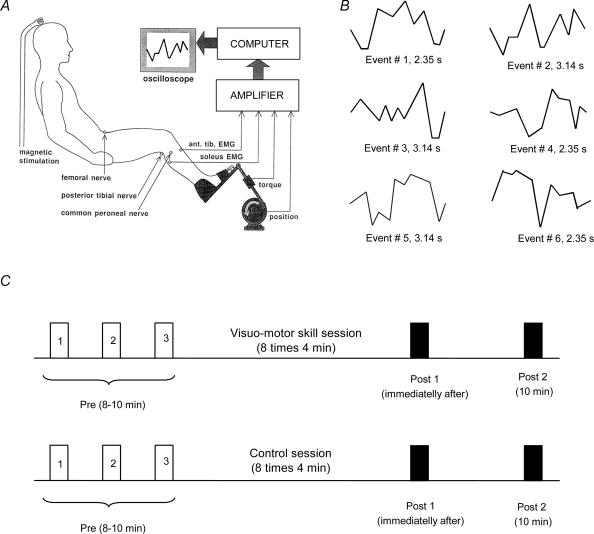
During both training sessions, subjects were seated with the examined leg flexed in the hip (120 deg), knee (160 deg) and ankle (110 deg). The foot was attached to a foot-plate, which was connected to a goniometer and allowed free movements of the ankle joint. The goniometer data were digitally sampled at 2000 Hz with QNX realtime analog to a digital capturing system. There was no resistance to the movements except for the weight of the foot-plate, which was approximately 1 kg. A session consisted of eight sets of 4 min of training, with 2 min of rest in between the sets. Measurements were taken before, immediately after and 10 min after both sessions (Fig. 1C).
Figure 1. General experimental conditions.
A, diagram of the experimental set-up; B, the six randomized figures presented during the visuo-motor skill training session. Event #2 was used to assess motor performance before and after each training session; C, a timeline of the training protocols. Before each session three measurements were taken to establish a baseline (Pre 1, 2 and 3). Also, measurements were taken immediately after training (Post 1, dark bar) and 10 min after the session was stopped (Post 2, dark bar).
Visuo-motor skill training session
For this session we used a purpose-written PC program. The position of the ankle joint was measured by a goniometer and displayed as a cursor on a computer screen located in front of the subject. On the screen six different figures could be presented in random order (Fig. 1A and B). In this way each of the six figures was presented 16 times in each 4 min training session. Each of the figures sketched a series of dorsi- and plantarflexion movements that the subjects were required to perform. The subjects were instructed to make the cursor follow the figures on the screen as accurately as possible by performing voluntary ankle dorsi- and plantarflexion movements. During dorsiflexion the cursor moved to the bottom of the screen, while during plantarflexion the cursor moved to the top of the screen. The cursor moved automatically from the left to the right. To evaluate motor performance, we selected one of the six figures (Fig. 1B) and averaged the ankle joint position for the 16 times that this figure had been presented during each 4 min training session. The obtained averaged trace was divided in 10 equal parts by vertical lines, and the error of performance was measured as the difference between the target (the figure on the computer screen) and the actual position of the ankle joint at each of these 10 points. For each subject a sum error was obtained during the first 4 min of training and compared to the error during the last 4 min of training. The mean and standard error of the mean were calculated for each condition.
Control session (without visual feedback)
Subjects were instructed to voluntarily perform alternating ankle dorsi- and plantarflexion movement. The starting position was neutral (90 deg) and the amplitude of the movements ranged between 25 deg of dorsi- and 30 deg plantarflexion. This amplitude of movement was similar to the movements performed during the visuo-motor skill training session. Subjects were instructed to perform a single dorsi- and plantarflexion movement in 1 s, which corresponded to the average frequency of movement in the visuo-motor skill training session. The speed of movement was approximately 48–64 deg s−1. Motor performance was assessed by instructing the subjects to perform 4 min of the visuo-motor skill task before and after the session (see visuo-motor skill session).
Stimulation and recording
Surface electrodes were used for stimulation and recording electromyographic (EMG) activity. EMG activity was recorded from the soleus (SOL) muscle by bipolar surface electrodes (interelectrode distance, 2 cm). The amplified EMG signals were filtered (band-pass, 25 Hz to 1 kHz), sampled at 2 kHz, and stored on a PC for off-line analysis (CED 1401+ with Signal software, Cambridge Electronic Devices, Cambridge, UK).
SOL H-reflex
The SOL H-reflex was evoked by stimulating the posterior tibial nerve through a monopolar electrode (1 ms rectangular pulse) in the popliteal fossa using a constant-current stimulator (model DS7A, Digitimer, UK). The reflex response was measured as peak-to-peak amplitude of the non-rectified reflex response. SOL H-reflex recruitment curves were assessed by averaging three responses at each stimulus intensity. Stimulus intensities were randomly increased in steps of 0.05 mA, starting below H-reflex threshold and increasing up to supramaximal intensity to measure the maximal motor response (M-max). The sensitivity of the H-reflex to facilitatory or inhibitory conditioning effects has been shown to depend crucially on its size (Crone et al. 1990). During measurements of the effect of a conditioning stimulus, the size of the SOL control reflex was therefore maintained at 20–25% of the M-max. H-reflexes were evoked at an interval of 4 s.
Somatosensory evoked potentials (SEPs)
To record tibial nerve (TN) somatosensory evoked potentials (SEPs), two Ag–AgCl surface electrodes were positioned on the scalp; the active electrode 2 cm behind and the reference 3 cm in front of Cz (Cz′ and F; Morita et al. 1998). Rectangular single electric pulses were delivered to the TN in the popliteal fossa (see SOL H-reflex). The stimulus threshold for SEPs is lower than for H-reflexes. To ensure that the same stimulus intensity was used before and after the visuo-motor skill task, the size of the M-wave was monitored. In our experiments the stimulus intensity was adjusted to evoke an H-reflex in the SOL muscle and an M-wave with a size of approximately 10% of the M-max (mean M-wave size of 9.8% (s.d. ± 2.7%) and 10.06% (s.d. ± 4.1%) of M-max before and after the training, respectively) (Fig. 3B and D). The possibility that the size of the SEP may be contaminated by afferent input from the H-reflex contraction is less likely, since ischaemia in the leg distal to the TN stimulation has no influence on the size of the SEP when subjects are tested at rest (Morita et al. 1998). Stimuli were applied at an interval of 1 s and 100 traces were averaged each time. The first peak of the TN-SEPs was positive and had a latency of approximately 34 ms, which was followed by a negative (42 ms), and a positive (53 ms) component (Fig. 4A). A small negative earlier peak was only observed in two of the subjects, with a latency of approximately 25–27 ms. The peak-to-peak amplitudes of the potentials were measured as N27/P34, P34/N42, and N42/P53. TN-SEPs latencies and amplitudes were analysed before and after the visuo-motor skill task (for specific details see Morita et al. 1998).
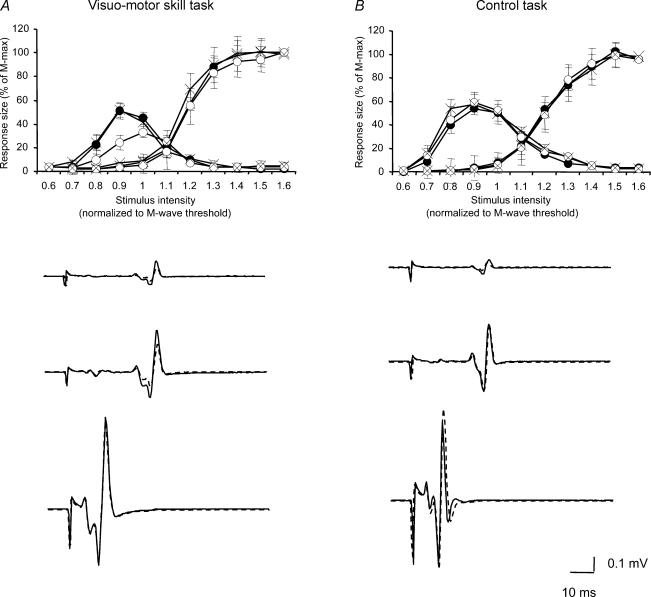
Figure 3. The effect of visuo-motor skill training and control session on the SOL H-reflex excitability recruitment curve.
The graphs show the effect of motor skill (A) and control (B) tasks on the SOL H-reflex excitability recruitment curve in 10 subjects. The ordinate shows the size of the SOL H-reflex and M-wave (as a percentage of the M-max). The abscissa shows the intensity of stimulation normalized to motor threshold of the M-wave. Measurements were taken before (•), immediately after (○) and 10 min after (×) each session were stopped. Traces below each graph show the effect of each task on the SOL H-reflex recruitment curve in a single subject (before: dark line and after: dotted line). Bars indicate standard errors (*P < 0.05).
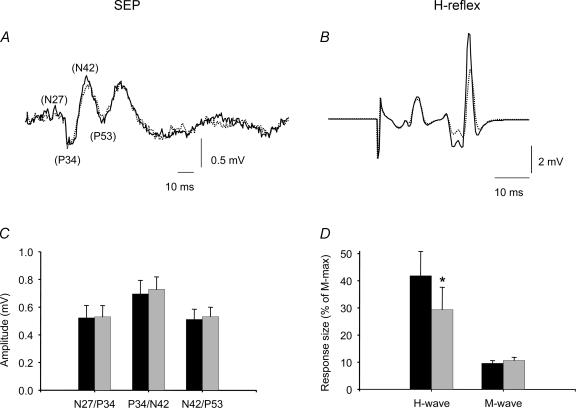
Figure 4. The effect of visuo-motor skill training on SOL H-reflex and TN-SEPs.
A, TN-SEP and B SOL H-reflex before (dark line) and after (dotted line) the visuo-motor skill task in one subject. The bar graphs show the data from all subjects, in C: the ordinate shows the size of the TN-SEPs (mV) and the abscissa shows the N27/P34, P34/N42, and N42/P53 components. In D the ordinate shows the size of the response as a percentage of the M-max and the abscissa shows the response measured (H-wave and M-wave). The black bars indicate that measurements taken before and grey bars after the visuo-motor skill training. Error bars indicate standard errors (*P < 0.05).
Presynaptic inhibition and reciprocal Ia inhibition
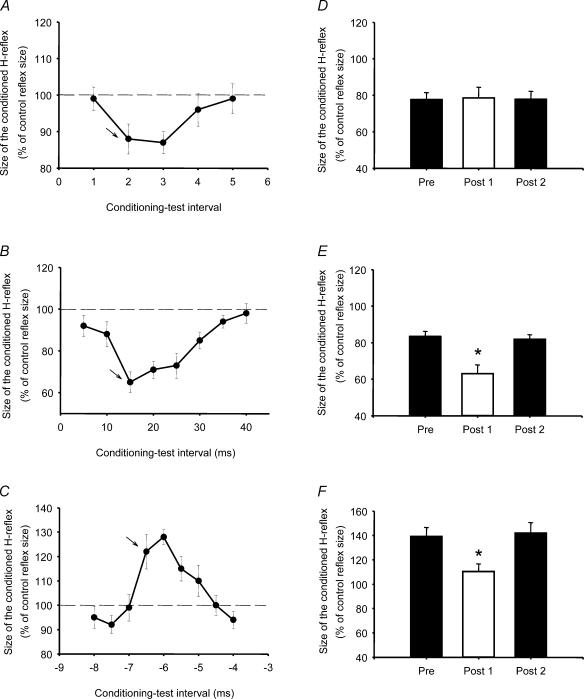
The SOL H-reflex was conditioned by stimulating the common peroneal nerve (CPN) and the femoral nerve (FN). The CPN was stimulated (1 ms rectangular pulse) through bipolar electrodes placed 2 cm apart over the nerve distal to the neck of the fibula. It was checked that the stimulation evoked a motor response in the TA muscle without a motor response in the peroneal muscles. The stimulus strength was expressed in multiples of the motor response threshold (MT) in the TA muscle. CPN stimulation elicits a depression of the SOL H-reflex at conditioning–test intervals of 2–3 ms (Crone et al. 1987), which is in all likelihood mediated by the disynaptic reciprocal Ia inhibitory pathway (Crone et al. 1987). At longer conditioning–test intervals, 15–20 ms, CPN stimulation also elicits a depression of the SOL H-reflex (D1 inhibition, Mizuno et al. 1971), which is in all likelihood caused by presynaptic inhibition of the terminals of Ia afferents on SOL motoneurones.
The FN was stimulated through a monopolar ball electrode placed over the femoral triangle. The indifferent electrode was placed just below the gluteus maximus muscle. The intensity for stimulating the FN was 1.2 × MT in the quadriceps muscle. In each experiment a time course of the effect of the FN facilitation was recorded for every subject (Hultborn et al. 1987a, b). The onset of facilitation was considered to be the earliest interval at which the conditioned (by femoral nerve stimulation) reflex was 10% larger than the control reflex, and measurements were taken at 0.5–1 ms longer than this interval. The size of the facilitation reflects the size of the monosynaptic excitatory postsynaptic potential in the SOL motoneurones evoked by the activation of Ia afferents from the quadriceps muscle, and changes in its size are considered to indicate changes in presynaptic inhibition of the Ia afferents (Hultborn et al. 1987a, b). The quadriceps and SOL muscles are close synergists, and projections of Ia afferents from the quadriceps and SOL onto SOL motoneurones appear to be similarly controlled (Hultborn et al. 1987b). It therefore seems reasonable to use changes in the FN facilitation as an indicator of changes in presynaptic inhibition of SOL Ia afferents. We measured both D1 inhibition and FN facilitation, because both measurements provide independent information about presynaptic inhibition and help us to exclude changes in recruitment gain in the SOL motoneurones (Nielsen & Kagamihara, 1993a). D1 inhibition reflects the level of presynaptic inhibition of SOL Ia afferents evoked by peripheral nerve stimulation, and FN facilitation reflects the level of ongoing presynaptic inhibition of FN Ia afferents (which parallels that of SOL Ia afferents).
Data analysis
Three SOL H-reflex recruitment curves were averaged prior to each session to establish a baseline. Recruitment curves were also measured immediately after and at a 10 min interval after each session. The values of the SOL H-reflex recruitment curve were expressed in relation to the MT of the SOL M-wave by using linear regression analysis. Mean baseline activity for the M-wave was calculated and values one s.d. above the baseline were included in the regression line. M-wave MT was determined by the interaction between the x intercept and the mean baseline using the linear regression formula: Y = a+bx. Measurements were normalized to the M-wave MT intensity and expressed as a percentage of the SOL M-max. During measurements of presynaptic and reciprocal inhibition, 15 control and 15 conditioned reflexes were averaged. Repeated measures ANOVA test was used to determine the effect of each task on the H-max/M-max ratio, the slope of the ascending part of the recruitment curve, H-threshold and M-max with task (skill versus control) and time of measurements (before, immediately after and 10 min after the session) as factors. The effect of each task of the SOL H-reflex recruitment curve was measured by using stimulus intensity and time of measurements as factors. A two-way ANOVA test was used to determine the effect of the visuo-motor skill task (with time of measurement as a factor: pre, post1 and post2) on spinal circuits (with spinal circuits as a factor: D1 inhibition, FN facilitation and reciprocal inhibition). Also, the ANOVA test was used to determine the effect of the visuo-motor skill task (with time of measurements as a factor: pre, post1 and post2) on the amplitude and latency of the TN-SEPs (with SEPs components as a factor). A Bonferroni post hoc test was done on significant comparisons. The paired student t test was used to compare motor performance, baseline level of the short- and long-latency depression of the SOL H-reflex and the monosynaptic femoral nerve facilitation of the SOL H-reflex.
Results
Motor performance
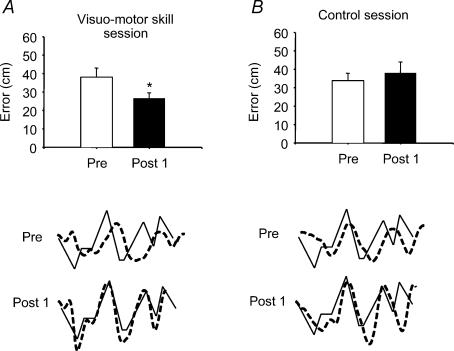
A significant improvement in the performance of the visuo-motor skill was observed in all subjects who trained the task (Fig. 2). The error between the target and the actual movement exerted by the subject was decreased from 37.7 cm to 26.6 cm after 32 min of visuo-motor skill task for the population of subjects (P < 0.01). The mean difference was 11.1 cm with a 95% confidence interval (CI) from 7.1 to 15. This improvement was still observed 10 min after the session. In contrast, when the subjects performed alternating ankle movements without visual feedback for a similar amount of time, no effect on the performance of the visuo-motor skill task was observed (error was 33.6 cm before and 38.0 cm after; P= 0.4).
Figure 2. The effect of visuo-motor skill training and control session on motor performance.
Bar graphs demonstrate the average motor performance before and after visuo-motor skill and control tasks in all subjects. The ordinate shows the mean error value from all subjects (cm). The abscissa shows the time at which measurements were taken (Pre, before training; Post 1, immediately after training). Traces showing the event selected for analysis (continuous line) and the subject performance (broken line) are seen next to the graph bars. Each broken line represents the average of the subject performance for 4 min. The traces shown are from the same subject. Bars indicate standard errors (*P < 0.05).
SOL H-reflex recruitment curve
The visuo-motor skill training induced a significant depression of the SOL H-reflex recruitment curve (ANOVA, F= 3.39, P= 0.02, n = 10; Fig. 3). This depression was observed immediately after the training (P= 0.01), and the values returned to baseline when measurements were taken 10 min after the training was stopped (see discussion) (P= 0.3). The H-max/M-max ratio was significantly depressed from 51% to 31% immediately after the training (ANOVA, F= 7.16, P= 0.01, n = 10), with a return to baseline after 10 min (49%, P= 0.9). This depression was observed in 7 out of 10 subjects. The slope of the ascending part of the SOL H-reflex recruitment curve was also significantly depressed immediately after the training (P < 0.01), but the SOL H-reflex threshold was unchanged (ANOVA, F= 1.07, P= 0.35, n = 10). No changes were observed in the size of the M-max after the visuo-motor skill training (ANOVA, F= 1.01, P= 0.3, n = 10). Pearson correlation analysis showed a weak, but not significant correlation between the strength of depression of the H-max/M-max and performance of the task (r= 0.32, P= 0.4). There were no significant changes in the SOL H-reflex recruitment curve, the H-max/M-max ratio, M-max and the H-reflex threshold following the session where subjects had performed the control task for a similar time as during the visuo-motor skill training session.
TN-SEPs and H-reflex
The effect of changing the intensity of the TN stimulation on the size of SEPs was investigated in three subjects. In all subjects the size of the TN-SEP components was found to increase up to stimulus intensities of approximately 20% of the M-max. At stimulus intensities from 10% to 20% of the M-max, the size of the N27/P34 increased by 8%, P34/N42 increased by 17% and N42/P53 increased by 30%. We consequently decided to use a stimulation intensity of 10% of M-max for the actual experiment, since at this stimulus intensity we could obtain a reasonably sized H-reflex, a small M-wave and SEPs that were of a submaximal size (and hence would be expected to be sensitive to changes in the size of the afferent volley). In nine subjects, TN-SEPs and the SOL H-reflex were measured simultaneously at rest before and after the visuo-motor skill training session. Whereas the H-reflex was significantly depressed from 42% to 29% of M-max following the training session (P < 0.01; Fig. 4B and D), all of the components of the SEPs had the same latency and amplitude before and after the training (Fig. 4A and C). There were also no changes in the size of the small M-wave evoked by the stimulation, indicating that the stimulation was comparable before and after the training (P= 0.6; 9.8% of M-max before and 10.6% of M-max after the training).
Presynaptic inhibition and disynaptic reciprocal Ia inhibition
Figure 5A–C shows time courses of the effect of peroneal (Fig. 5A and B) and FN stimulation (Fig. 5C) on the SOL H-reflex in a single subject. The peroneal nerve stimulation caused a depression of the SOL H-reflex at a conditioning-test interval of 2–3 ms. This inhibition has been shown to be caused by activation of the disynaptic reciprocal Ia inhibitory pathway (Crone et al. 1987). For the actual experiment, a conditioning–test interval of 2 ms (indicted by arrow; Fig. 5A) was used for evaluation of the size of the inhibition before and after the visuo-motor skill training session. The inhibition observed at longer intervals (around 8–20 ms) has been termed D1 (Mizuno et al. 1971), and is in all likelihood caused by presynaptic inhibition of SOL Ia afferents. An interval of 15 ms (indicated by arrow; Fig. 5B) was used to evaluate the size of this inhibition before and after the visuo-motor skill training. The facilitation of the SOL H-reflex induced by femoral nerve stimulation had an onset at a conditioning–test interval between −5.5 and −6.0 ms (negative conditioning–test intervals designate that the control stimulus preceded the conditioning stimulus). In order to ensure that the facilitation was caused solely by transmission in the Ia monosynaptic pathway from the quadriceps to SOL motoneurones, a conditioning–test interval of −5.5 ms (indicated by arrow; Fig. 5C) was used to evaluate the size of the facilitation before and after the visuo-motor skill training (Hultborn et al. 1987a).
Figure 5. The effect of visuo-motor skill training on presynaptic inhibition and disynaptic reciprocal Ia inhibition.
The effect of repeating a visuo-motor skill task on A, the short-latency depression of the SOL H-reflex (2–3 ms conditioning-test interval); B, the long-latency depression of the SOL H-reflex (D1 inhibition, 15–20 ms conditioning–test interval); and C, FN facilitation of the SOL H-reflex (−4 to −8 ms conditioning–test interval, the negative value indicates that the control pulse preceded the conditioning pulse). The bar graphs show the data from all subjects (D, reciprocal Ia inhibition; E, D1 inhibition; and F, FN facilitation). The ordinate shows the size of the conditioned SOL H-reflex (as a percentage of the control reflex size), and the abscissa shows the time at which measurements were taken (Pre, before training; Post 1, immediately after and Post 2, 10 min after the session). The traces are from three different subjects and the ordinate shows the size of the conditioned SOL H-reflex (as a percentage of the control reflex size) and the abscissa shows the conditioning-test interval. At each condition 15 control and 15 conditioned H-reflexes were averaged. Bars indicate standard errors (*P < 0.05).
Figure 5D–F shows the average size of the disynaptic reciprocal Ia inhibition (Fig. 5D), D1 inhibition (Fig. 5E) and FN facilitation (Fig. 5F), before (left columns), immediately after (middle columns) and 10 min after (right columns) the visuo-motor skill training in all subjects. In 4 out of the 10 subjects who participated in both training sessions, it was not possible to evoke a facilitation of the SOL H-reflex by FN stimulation, and/or stimulation of the peroneal nerve failed to produce any inhibition of the SOL H-reflex. This experiment was therefore only performed in six subjects. A two-way ANOVA test showed a significant interaction between the time of measurement and D1 inhibition, FN facilitation and reciprocal inhibition following the visuo-motor skill session (ANOVA, F= 2.96, P= 0.02). Post hoc testing revealed an increase of D1 inhibition (P < 0.001, n = 6, Fig. 5E) and a decrease of the FN facilitation (P= 0.01, n = 6, Fig. 5F) immediately after the visuo-motor skill session. In contrast, no changes were observed in the disynaptic reciprocal Ia inhibition (P= 0.8, n = 6; Fig. 5D). At the measurement 10 min after the end of the training session, all three parameters had returned to the baseline level. The average size of the D1 inhibition was 17% (i.e. the size of the conditioned reflex expressed, as a percentage of the control reflex was 83%. The size of the inhibition ranged between 10% and 24%, s.e.m.±2.2%), it increased to 37% (i.e. the size of the conditioned reflex expressed, as a percentage of the control reflex was 63%. The size of the inhibition ranged between 19% and 53%, s.e.m.±4.6%) immediately after the training and returned to baseline after 10 min. The average size of the FN facilitation from all subjects before the motor skill session was 139% (range: 123% to 172%, s.e.m.±7.3%, size of the conditioned reflex expressed as a percentage of the control reflex), it decreased to 110% (range: 87% to 127%, s.e.m.±6.4%, size of the conditioned reflex expressed as a percentage of the control reflex) immediately after training, and returned to baseline values at 10 min. Pearson correlation analysis showed a not significant correlation between both the strength of the femoral nerve facilitation and the D1 inhibition and the performance of the task, respectively (r= 0.41, P= 0.4 and r= 0.47, P= 0.3).
Discussion
The present experiments have shown that the SOL H-reflex is depressed following training of a novel visuo-motor skill involving the ankle muscles, while no changes were observed following repetition of alternating dorsi- and plantarflexion movements where no visual feedback about their motor performance was provided. The H-reflex depression was accompanied by increased D1 inhibition and decreased FN facilitation of the SOL H-reflex, whereas disynaptic reciprocal Ia inhibition was unchanged. There were also no changes in the size of TN-SEPs following the visuo-motor skill training. Together, these observations suggest that selective presynaptic control of Ia afferents may contribute to modulate sensory inputs during acquisition of novel visuo-motor skills.
Acquisition of a novel visuo-motor skill, but not simple repetition of movement decreases the size of the SOL H-reflex
A depression of the SOL H-reflex in relation to repetition of a motor task has been reported in several previous studies (deVries et al. 1981; Bulbulian & Darabos, 1986; Trimble & Koceja, 2001; Schneider & Capaday, 2003; Motl & Dishman, 2003). In some of these studies, the depression of the H-reflex has been related to improvements in motor performance and thus may represent a purposeful adaptation of transmission in the central component of the stretch reflex circuit (Voigt et al. 1998; Trimble & Koceja, 2001; Schneider & Capaday, 2003). However, in other studies the H-reflex depression has been suggested to be caused by non-specific mechanisms induced by exercise and unrelated to any changes in motor performance (deVries et al. 1981; Bulbulian & Darabos, 1986; Motl & Dishman, 2003). In addition, H-reflex depression has also been observed following sustained and fatiguing contractions, and it may thus reflect the inhibitory effect of increased group III/IV afferent discharge following development of muscle fatigue (Walton et al. 2002; Kato et al. 2003). It was therefore important in our study to make sure that the H-reflex depression was related to the visuo-motor skill training and not just to a non-specific response to exercise or fatigue-related changes. This is why our subjects also performed voluntary dorsi- and plantarflexion movements without visual feedback and any requirement of improving the performance. No changes in the H-reflex were observed in relation to alternating ankle movements, when no learning was taking place (Fig. 3B). This finding is in agreement with the results of Hess et al. (2003), who found that the size of the H-reflex was modulated only when individuals repeated a motor task that required a high degree of attention, such as stepping over an obstacle, but changes were not present during normal walking, which requires less attention. Therefore, the depression of the H-reflex following the visuo-motor skill training is not likely to be caused by a non-specific exercise-induced mechanism. An influence of muscle fatigue in the present study is also unlikely, since all movements were dynamic and submaximal and since motor performance was improved following the visuo-motor skill training (Fig. 2).
Contribution of presynaptic inhibition to the depression of the SOL H-reflex
The visuo-motor skill training investigated here was originally designed with the aim of inducing changes in the disynaptic reciprocal Ia inhibition. However, as shown in Fig. 5D we observed no such changes. Rather, the SOL H-reflex depression appeared to be caused by increased presynaptic inhibition of the Ia afferents, since we observed a significant increase in the long-latency depression of the H-reflex (D1 inhibition) and a decrease of the FN-induced facilitation of the SOL H-reflex. These two measures provide independent evidence of changes in presynaptic inhibition of SOL Ia afferents (Hultborn et al. 1987a, b), and taken together strongly suggest that presynaptic inhibition of the SOL Ia afferents is increased following the visuo-motor skill task.
There is good evidence from other studies that changes in presynaptic inhibition of the synapses between sensory afferents and motoneurones is fundamental in the adaptation of the reflex circuitry during motor learning. Habituation of the monosynaptic gill-withdrawal reflex in Aplysia has been shown to be caused by a depression of synaptic transmission between the sensory afferents and motoneurones through changes in presynaptic inhibition (Kandel et al. 2000). In rats and monkeys, a change in motoneurone firing threshold seems to be the main mechanism associated with long-term downregulation of the H-reflex during classical operant conditioning (Carp & Wolpaw, 1994; Wolpaw et al. 1994; Wolpaw & Tennissen, 2001), whereas short-term downregulation of the H-reflex is likely to be related to changes in presynaptic inhibition (Wolpaw, 1997), which agrees with the results observed in our study.
It is not possible from our data to make any certain conclusions regarding the exact mechanism of the increased presynaptic inhibition following the visuo-motor skill training, but some speculations may be made. It seems unlikely that changes in sensory feedback to the spinal interneurones, which convey the inhibition, should be involved. It was assured that the displacement of the ankle joint was similar during both the visuo-motor skill and the control tasks; therefore it is likely that the sensory feedback from cutaneous and joint afferents was comparable. It is a possibility that the visuo-motor skill training session resulted in selective changes in γ motor activity and thereby increased spindle afferent discharge. Experiments in cats have shown an increase in γ motor activity during performance of a novel motor task (Prochazka, 1989). Activity in flexor muscle Ia afferents is a major source for presynaptic inhibition of SOL Ia afferents (Baldissera et al. 1981), and could explain the decreased H-reflex size observed in our study. However, experiments in humans have shown that during the process of learning a new visuo-motor task, the firing rate of muscle spindles is transiently reduced (Jones et al. 2001). Another possibility is that more co-contraction between ankle antagonistic muscles occurred during the visuo-motor skill training session compared to the control task. Evidence has shown that during co-contraction of ankle muscles, the level of presynaptic inhibition at the terminal of Ia afferents is increased (Nielsen & Kagamihara, 1993b). However, if anything, a decrease of co-contraction between the antagonist muscles is observed following the visuo-motor skill training task (unpublished observations).
There is more evidence to suggest that a change in the descending drive to the interneurones conveying the inhibition could be involved. Firstly, in our previous study (Perez et al. 2004) the same visuo-motor skill training induced an increased excitability of corticospinal neurones projecting to the ankle muscles. The changes in corticospinal activity, which are probably associated with these excitability changes, would probably result in changes in presynaptic inhibition through the projections from the corticospinal tract to the pathway which mediates presynaptic inhibition (Lundberg & Voorhoeve, 1962; Iles, 1996; Meunier & Pierrot-Deseilligny, 1998). Although studies in cats and humans have shown that a single TMS pulse depresses transmission in the pathways mediating presynaptic inhibition of Ia afferents (Lundberg & Vyklicky, 1963; Rudomin et al. 1983; Valls-Sole et al. 1994; Iles, 1996; Meunier & Pierrot-Deseilligny, 1998), the precise manner in which descending pathways interact with the presynaptic inhibitory pathway during motor learning still remains unknown (Rudomin & Schmidt, 1999). Secondly, the corticospinal tract has been found to be involved in downregulation of the H-reflex during operant conditioning in animal experiments (Chen & Wolpaw, 1997, 2002).
Selective gating of group Ia afferents during visuo-motor skill acquisition
The selective decrease of the SOL H-reflex without any changes in the amplitude of the TN-SEPs (Fig. 4), suggests that the increase of presynaptic inhibition is selective for the terminals of Ia afferents on the spinal motoneurones. In line with this, Morita et al. (1998) observed that the SOL H-reflex was depressed during co-contraction of antagonistic muscles relative to its size during plantarflexion, whereas the TN-SEP had the same size during the two tasks. There is good evidence from cat experiments to suggest that primary afferent synapses on ascending neurones and motoneurones are inhibited by different populations of interneurones (Jankowska & Padel, 1984). Indeed, the network responsible for controlling presynaptic inhibition of primary afferents seems in general to be organized to ensure selective control of afferent input to specific populations of both motoneurones and ascending neurones (Rudomin, 1999, 2002).
Significance of increased presynaptic inhibition for acquisition and performance of visuo-motor skills
It seems unreasonable from our data to suggest that the increased presynaptic inhibition and the depression of the SOL H-reflex were important for the motor performance. Our subjects were able to complete the visuo-motor task with similar accuracy immediately and 10 min after training, although 10 min after training the SOL H-reflex had returned to the baseline level. There was also only a weak and non-significant correlation between changes in the SOL H-reflex size and the improved performance. We would, rather, like to suggest that the present findings are related to the process of learning the motor task. Acquisition of visuo-motor skills, such as the one investigated in our study, requires that visual input and proprioceptive information are integrated centrally to optimize the central motor programme for the movement (Johansson, 2002; Buneo et al. 2002). At the same time, increased attention to the task is necessary. Previous studies have demonstrated that the excitability of the motor cortex is strongly modulated by the level of attention (Stefan et al. 2004; Rosenkranz & Rothwell, 2004). Therefore, the increased motor cortical excitability observed in our and in previous studies in relation to motor skill acquisition (Karni et al. 1995; Pascual-Leone et al. 1995; Classen et al. 1998; Perez et al. 2004), probably reflect adaptations in the motor cortex in relation to this optimization and increased attention. The purpose of gating sensory input to spinal motoneurones (i.e. depression of the H-reflex) without a concomitant gating of ascending sensory input to the cortex (i.e. lack of depression of SEPs) during skill acquisition is unclear, but it seems reasonable to suggest that optimization of the task requires that visual and sensory feedback are closely integrated at a cortical level. During our visuo-motor skill task, subjects performed continuous adjustments in muscle activity to follow the trajectory. Sensory feedback contributes to the timing and strength of muscle activity during alternating movements (Meunier & Pierrot-Deseilligny, 1989). In our study it may be speculated that unexpected sensory feedback, by overshooting the target, may contribute to the muscle activity and perturb the movement trajectory. During learning, gating of such peripheral influence may facilitate the direct cortical control of muscle activity. Our findings may thus reflect that descending systems maintain a tight control of spinal circuits during acquisition of novel visuo-motor tasks, whereas a less tight control (i.e. no depression of the H-reflex) might be expected once full optimization of the task has been achieved. However, further studies are necessary to confirm this hypothesis.
Acknowledgments
This study was supported by The Danish Society of Multiple Sclerosis, The Danish Health Research Council, The Danish Ministry of Culture and the Elsass Foundation.
References
- Baldissera F, Hultborn H, Illert M. Integration in spinal neuronal systems. In: Brooks VB, editor. Handbook of Physiology, Section 1, The Nervous System, Vol. 2, Motor Control. Bethesda: American Physiological Society; 1981. pp. 509–595. [Google Scholar]
- Bulbulian R, Darabos BL. Motor neuron excitability: the Hoffmann reflex following exercise of low and high intensity. Med Sci Sports Exerc. 1986;18:697–702. [PubMed] [Google Scholar]
- Buneo CA, Jarvis MR, Batista AP, Andersen RA. Direct visuomotor transformations for reaching. Nature. 2002;416:632–636. doi: 10.1038/416632a. [DOI] [PubMed] [Google Scholar]
- Capaday C, Stein RB. Amplitude modulation of the soleus H-reflex in the human during walking and standing. J Neurosci. 1986;6:1308–1313. doi: 10.1523/JNEUROSCI.06-05-01308.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carp JS, Wolpaw JR. Motoneuron plasticity underlying operantly conditioned decrease in primate H-reflex. J Neurophysiol. 1994;72:431–442. doi: 10.1152/jn.1994.72.1.431. [DOI] [PubMed] [Google Scholar]
- Casabona A, Polizzi C, Perciavalle V. Differences in H-reflex between athletes trained for explosive contractions and non-trained subjects. Eur J Appl Physiol Occup Physiol. 1990;61:26–32. doi: 10.1007/BF00236689. [DOI] [PubMed] [Google Scholar]
- Chen XY, Wolpaw JR. Dorsal column but not lateral column transection prevents down-conditioning of H reflex in rats. J Neurophysiol. 1997;78:1730–1734. doi: 10.1152/jn.1997.78.3.1730. [DOI] [PubMed] [Google Scholar]
- Chen XY, Wolpaw JR. Probable corticospinal tract control of spinal cord plasticity in the rat. J Neurophysiol. 2002;87:645–652. doi: 10.1152/jn.00391.2001. [DOI] [PubMed] [Google Scholar]
- Classen J, Liepert J, Wise SP, Hallett M, Cohen LG. Rapid plasticity of human cortical movement representation induced by practice. J Neurophysiol. 1998;79:1117–1123. doi: 10.1152/jn.1998.79.2.1117. [DOI] [PubMed] [Google Scholar]
- Crone C, Hultborn H, Jespersen B, Nielsen J. Reciprocal Ia inhibition between ankle flexors and extensors in man. J Physiol. 1987;389:163–185. doi: 10.1113/jphysiol.1987.sp016652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone C, Hultborn H, Mazieres L, Morin C, Nielsen J, Pierrot-Deseilligny E. Sensitivity of monosynaptic test reflexes to facilitation and inhibition as a function of the test reflex size: a study in man and the cat. Exp Brain Res. 1990;81:35–45. doi: 10.1007/BF00230098. [DOI] [PubMed] [Google Scholar]
- deVries HA, Wiswell RA, Bulbulian R, Moritani T. Tranquilizer effect of exercise. Acute effects of moderate aerobic exercise on spinal reflex activation level. Am J Phys Med. 1981;60:57–66. [PubMed] [Google Scholar]
- Faist M, Dietz V, Pierrot-Deseilligny E. Modulation, probably presynaptic in origin, of monosynaptic Ia excitation during human gait. Exp Brain Res. 1996;109:441–449. doi: 10.1007/BF00229628. [DOI] [PubMed] [Google Scholar]
- Hess F, Van Hedel HJ, Dietz V. Obstacle avoidance during human walking: H-reflex modulation during motor learning. Exp Brain Res. 2003;151:82–89. doi: 10.1007/s00221-003-1415-7. [DOI] [PubMed] [Google Scholar]
- Hultborn H, Meunier S, Morin C, Pierrot-Deseilligny E. Assessing changes in presynaptic inhibition of Ia fibres: a study in man and the cat. J Physiol. 1987a;389:729–756. doi: 10.1113/jphysiol.1987.sp016680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H, Meunier S, Pierrot-Deseilligny E, Shindo M. Changes in presynaptic inhibition of Ia fibres at the onset of voluntary contraction in man. J Physiol. 1987b;389:757–772. doi: 10.1113/jphysiol.1987.sp016681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iles JF. Evidence for cutaneous and corticospinal modulation of presynaptic inhibition of Ia afferents from the human lower limb. J Physiol. 1996;491:197–207. doi: 10.1113/jphysiol.1996.sp021207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Padel Y. On the origin of presynaptic depolarization of group I muscle afferents in Clarke's column in the cat. Brain Res. 1984;295:195–201. doi: 10.1016/0006-8993(84)90967-3. [DOI] [PubMed] [Google Scholar]
- Johansson RS. Dynamic use of tactile afferent signals in control of dexterous manipulation. Adv Exp Med Biol. 2002;508:397–410. doi: 10.1007/978-1-4615-0713-0_45. [DOI] [PubMed] [Google Scholar]
- Jones KE, Wessberg J, Vallbo A. Proprioceptive feedback is reduced during adaptation to a visuomotor transformation: preliminary findings. Neuroreport. 2001;12:4029–4033. doi: 10.1097/00001756-200112210-00035. [DOI] [PubMed] [Google Scholar]
- Kandel ER, Schwartz JH, Jessel TM. Principles of neural science. 4. USA: The Mcgraw-Hill Companies, Inc; 2000. pp. 1247–1279. Chapter 63. [Google Scholar]
- Karni A, Meyer G, Jezzard P, Adams MM, Turner R, Ungerleider LG. Functional MRI evidence for adult motor cortex plasticity during visuo-motor skill learning. Nature. 1995;377:155–158. doi: 10.1038/377155a0. [DOI] [PubMed] [Google Scholar]
- Kato T, Takeda Y, Tsuji T, Kasai T. Further insights into post-exercise effects on H-reflexes and motor evoked potentials of the flexor carpi radialis muscles. Motor Control. 2003;7:82–99. doi: 10.1123/mcj.7.1.82. [DOI] [PubMed] [Google Scholar]
- Katz R, Meunier S, Pierrot-Deseilligny E. Changes in presynaptic inhibition of Ia fibres in man while standing. Brain. 1988;111:417–437. doi: 10.1093/brain/111.2.417. [DOI] [PubMed] [Google Scholar]
- Llewellyn M, Yang JF, Prochazka A. Human H-reflexes are smaller in difficult beam walking than in normal treadmill walking. Exp Brain Res. 1990;83:22–28. doi: 10.1007/BF00232189. [DOI] [PubMed] [Google Scholar]
- Lundberg A, Voorhoeve P. Effects from the pyramidal tract on spinal reflex arcs. Acta Physiol Scand. 1962;56:201–219. doi: 10.1111/j.1748-1716.1962.tb02498.x. [DOI] [PubMed] [Google Scholar]
- Lundberg A, Vyklicky L. Inhibitory interaction between spinal reflexes to primary afferents. Experientia. 1963;19:247–248. doi: 10.1007/BF02151360. [DOI] [PubMed] [Google Scholar]
- Meunier S, Pierrot-Deseilligny E. Gating of the afferent volley of the monosynaptic stretch reflex during movement in man. J Physiol. 1989;419:753–763. doi: 10.1113/jphysiol.1989.sp017896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier S, Pierrot-Deseilligny E. Cortical control of presynaptic inhibition of Ia afferents in humans. Exp Brain Res. 1998;119:415–426. doi: 10.1007/s002210050357. [DOI] [PubMed] [Google Scholar]
- Mizuno Y, Tanaka R, Yanagisawa N. Reciprocal group I inhibition on triceps surae motoneurons in man. J Neurophysiol. 1971;34:1010–1017. doi: 10.1152/jn.1971.34.6.1010. [DOI] [PubMed] [Google Scholar]
- Morita H, Petersen N, Nielsen J. Gating of somatosensory evoked potentials during voluntary movement of the lower limb in man. Exp Brain Res. 1998;120:143–152. doi: 10.1007/s002210050388. [DOI] [PubMed] [Google Scholar]
- Motl RW, Dishman RK. Acute leg-cycling exercise attenuates the H-reflex recorded in soleus but not flexor carpi radialis. Muscle Nerve. 2003;28:609–614. doi: 10.1002/mus.10479. [DOI] [PubMed] [Google Scholar]
- Nielsen J, Crone C, Hultborn H. H-reflexes are smaller in dancers from The Royal Danish Ballet than in well-trained athletes. Eur J Appl Physiol Occup Physiol. 1993;66:116–121. doi: 10.1007/BF01427051. [DOI] [PubMed] [Google Scholar]
- Nielsen J, Kagamihara Y. Differential projection of the sural nerve to early and late recruited human tibialis anterior motor units: change of recruitment gain. Acta Physiol Scand. 1993a;147:385–401. doi: 10.1111/j.1748-1716.1993.tb09515.x. [DOI] [PubMed] [Google Scholar]
- Nielsen J, Kagamihara Y. The regulation of presynaptic inhibition during co-contraction of antagonistic muscles in man. J Physiol. 1993b;464:575–593. doi: 10.1113/jphysiol.1993.sp019652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen JB, Sinkjær T. Reflex excitation of muscles during human walking. Adv Exp Med Biol. 2002;508:369–375. [PubMed] [Google Scholar]
- Pascual-Leone A, Nguyet D, Cohen LG, Brasil-Neto JP, Cammarota A, Hallett M. Modulation of muscle responses evoked by transcranial magnetic stimulation during the acquisition of new fine visuo-motor skills. J Neurophysiol. 1995;74:1037–1045. doi: 10.1152/jn.1995.74.3.1037. [DOI] [PubMed] [Google Scholar]
- Perez MA, Lungholt BK, Nyborg K, Nielsen JB. Motor skill training induces changes in the excitability of the leg cortical area in healthy humans. Exp Brain Res. 2004;159:197–205. doi: 10.1007/s00221-004-1947-5. [DOI] [PubMed] [Google Scholar]
- Prochazka A. Sensorimotor gain control: a basic strategy of motor systems? Prog Neurobiol. 1989;33:281–307. doi: 10.1016/0301-0082(89)90004-x. [DOI] [PubMed] [Google Scholar]
- Rochcongar P, Dassonville J, Le Bars R. Modification of the Hoffmann reflex in function of athletic training. Eur J Appl Physiol Occup Physiol. 1979;40:165–170. doi: 10.1007/BF00426939. [DOI] [PubMed] [Google Scholar]
- Rosenkranz K, Rothwell JC. The effect of sensory input and attention on the sensorimotor organization of the hand area of the human motor cortex. J Physiol. 2004;561:307–320. doi: 10.1113/jphysiol.2004.069328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudomin P. Selectivity of presynaptic inhibition: a mechanism for independent control of information flow through individual collaterals of single muscle spindle afferents. Prog Brain Res. 1999;123:109–117. doi: 10.1016/s0079-6123(08)62848-4. [DOI] [PubMed] [Google Scholar]
- Rudomin P. Selectivity of the central control of sensory information in the mammalian spinal cord. Adv Exp Med Biol. 2002;508:157–170. doi: 10.1007/978-1-4615-0713-0_19. [DOI] [PubMed] [Google Scholar]
- Rudomin P, Jimenez I, Solodkin M, Duenas S. Sites of action of segmental and descending control of transmission on pathways mediating PAD of Ia- and Ib-afferent fibers in cat spinal cord. J Neurophysiol. 1983;50:743–769. doi: 10.1152/jn.1983.50.4.743. [DOI] [PubMed] [Google Scholar]
- Rudomin P, Schmidt RF. Presynaptic inhibition in the vertebrate spinal cord revisited. Exp Brain Res. 1999;129:1–37. doi: 10.1007/s002210050933. [DOI] [PubMed] [Google Scholar]
- Schneider C, Capaday C. Progressive adaptation of the soleus H-reflex with daily training at walking backward. J Neurophysiol. 2003;89:648–656. doi: 10.1152/jn.00403.2002. [DOI] [PubMed] [Google Scholar]
- Stefan K, Wycislo M, Classen J. Modulation of associative human motor cortical plasticity by attention. J Neurophysiol. 2004;92:66–72. doi: 10.1152/jn.00383.2003. [DOI] [PubMed] [Google Scholar]
- Trimble MH, Koceja DM. Effect of a reduced base of support in standing and balance training on the soleus H-reflex. Int J Neurosci. 2001;106:1–20. doi: 10.3109/00207450109149734. [DOI] [PubMed] [Google Scholar]
- Valls-Sole J, Alvarez R, Tolosa ES. Vibration-induced presynaptic inhibition of the soleus H reflex is temporarily reduced by cortical magnetic stimulation in human subjects. Neurosci Lett. 1994;170:149–152. doi: 10.1016/0304-3940(94)90261-5. [DOI] [PubMed] [Google Scholar]
- Voigt M, Chelli F, Frigo C. Changes in the excitability of soleus muscle short latency stretch reflexes during human hopping after 4 weeks of hopping training. Eur J Appl Physiol Occup Physiol. 1998;78:522–532. doi: 10.1007/s004210050455. [DOI] [PubMed] [Google Scholar]
- Walton DM, Kuchinad RA, Ivanova TD, Garland SJ. Reflex inhibition during muscle fatigue in endurance-trained and sedentary individuals. Eur J Appl Physiol. 2002;87:462–468. doi: 10.1007/s00421-002-0670-9. [DOI] [PubMed] [Google Scholar]
- Wolpaw JR. The complex structure of a simple memory. Trends Neurosci. 1997;20:588–594. doi: 10.1016/s0166-2236(97)01133-8. [DOI] [PubMed] [Google Scholar]
- Wolpaw JR, Maniccia DM, Elia T. Operant conditioning of primate H-reflex: phases of development. Neurosci Lett. 1994;170:203–207. doi: 10.1016/0304-3940(94)90319-0. [DOI] [PubMed] [Google Scholar]
- Wolpaw JR, Tennissen AM. Activity-dependent spinal cord plasticity in health and disease. Annu Rev Neurosci. 2001;24:807–843. doi: 10.1146/annurev.neuro.24.1.807. [DOI] [PubMed] [Google Scholar]