Abstract
A rise in glucose concentration depolarizes the β-cell membrane potential leading to electrical activity and insulin release. It is generally believed that closure of KATP channels underlies the depolarizing action of glucose, though work from several laboratories has indicated the existence of an additional anionic mechanism. It has been proposed that glucose activates a volume-regulated anion channel, generating an inward current due to Cl− efflux. This mechanism requires that intracellular [Cl−] is maintained above its electrochemical equilibrium. This hypothesis was tested in rat β-cells by varying [Cl−] in the patch pipette solution using the Cl−-permeable antibiotic amphotericin B to allow Cl− equilibration with the cell interior. Under such conditions, a depolarization and electrical activity could be evoked by 16 mm glucose with pipette solutions containing 80 or 150 mm Cl−. At 40 or 20 mm Cl−, a subthreshold depolarization was usually observed, whilst further reduction to 12 or 6 mm abolished depolarization, in some cases leading to a glucose-induced hyperpolarization. With a pipette solution containing gramicidin, which forms Cl−-impermeable pores, glucose induced a depolarization and electrical activity irrespective of [Cl−] in the pipette solution. Under the latter conditions, glucose-induced electrical activity was prevented by bumetanide, an inhibitor of the Na+–K+–2Cl− co-transporter. This inhibition could be overcome by the use of amphotericin B with a high [Cl−] pipette solution. These findings suggest that the maintenance of high intracellular [Cl−] in the β-cell is an important determinant in glucose-induced depolarization, and support the hypothesis that β-cell stimulation by glucose involves activation of the volume-regulated anion channel and generation of an inward Cl− current.
The secretion of insulin from the pancreatic β-cell in response to a rise in glucose concentration involves a characteristic pattern of electrical activity (see Ashcroft & Rorsman, 1989; Misler et al. 1992; Best & McLaughlin, 2004 for reviews). In the presence of fasting blood glucose levels (3–5 mm), the β-cell membrane potential is normally in the region of −60 mV. A rise in glucose concentration to insulinotropic levels (6–25 mm) causes a gradual depolarization to a threshold potential (−40 to −50 mV) at which voltage-sensitive Ca2+ channels open, generating action potentials or ‘spikes’. This response is important since it results in a rise in cytosolic [Ca2+] ([Ca2+]i) that activates the exocytotic machinery.
The mechanism whereby glucose depolarizes the pancreatic β-cell has been the subject of extensive study. It is generally believed that the depolarization of the β-cell membrane potential by glucose is the result of closure of KATP channels due to increased glucose oxidation and a rise in the intracellular ATP/ADP ratio (Ashcroft & Rorsman, 1989). However, there is considerable evidence for at least one additional ionic mechanism sensitive to changes in glucose concentration. For example, KATP channel activity is sensitive to glucose over the substimulatory range 0–5 mm (Ashcroft et al. 1988; Best, 2000, 2000a), but is virtually unaffected by changes in glucose concentrations over the stimulatory range (5–20 mm; Best, 2000, 2000a). Furthermore, glucose has been shown to induce electrical activity and insulin release even when KATP channel activity is completely inhibited by a maximal concentration of the sulphonylurea tolbutamide (Best et al. 1992; Best, 2002a) or activated by diazoxide (Henquin, 1992).
An increasing amount of evidence from a number of laboratories supports the suggestion that an anionic mechanism could be an important component of the KATP channel-independent glucose-sensing mechanism. For example, glucose has been shown to stimulate 36Cl− efflux from pre-loaded islets with kinetics closely corresponding to those reported for glucose-induced insulin release (Sehlin, 1978; Malaisse et al. 2004). A similar effect of glucose on β-cell Cl− permeability has been demonstrated using fluorimetric measurements (Eberhardson et al. 2000). The conductance underlying these effects is likely to be a volume-regulated anion channel (VRAC; Kinard & Satin, 1995; Best et al. 1996b). Activation of this channel in β-cells by hypotonic cell swelling causes depolarization of the cell membrane potential (due to Cl− efflux) and thus leads to electrical and secretory activity (Best et al. 1996a; Drews et al. 1998). There is evidence that glucose can also activate the VRAC, both at the whole-cell (Best, 1997, 2000) and single channel levels (Best, 1999, 2002b). Consistent with these findings, inhibitors of the VRAC inhibit both glucose-induced electrical activity and insulin release (Best, 1997, 2002a, c; Best et al. 2000, 2004).
The observation that VRAC activation causes β-cell depolarization implies that intracellular [Cl−] ([Cl−]i) must be maintained above its electrochemical equilibrium (i.e. ECl is positive with respect to the resting membrane potential). Estimates of [Cl−]i from the distribution of 36Cl− in both mouse and rat islets suggest that this is indeed the case, with apparent values for ECl of −18 to +2.5 mV being reported (Sehlin, 1978; Malaisse et al. 2004). Fluorimetric measurements of [Cl−]i in β-cells are broadly consistent with these findings (Eberhardson et al. 2000). It is likely that the Na+–K+–2Cl− co-transporter NKCC1 is, at least in part, responsible for Cl− accumulation since there is both functional and molecular evidence for the expression of this co-transporter in β-cells (Lindstrom et al. 1988; Majid et al. 2001).
The above findings suggest that maintenance of a relatively high value for [Cl−]i could be of major importance for the regulation of β-cell electrical activity by glucose. In the present study, this hypothesis has been tested by the use of two distinct approaches for altering [Cl−]i in intact rat β-cells.
Methods
Islet cell preparation
Pancreatic islets were prepared from Sprague-Dawley rats (300–350 g; either sex, killed by stunning and cervical dislocation) by collagenase digestion (Worthington type 4, Cambridge Biosciences, Cambridge, UK). Islets were dispersed into single cells by brief exposure to a Ca2+-free medium consisting of (mm): 130 NaCl, 5 KCl, 2 MgSO4, 4 glucose, 1 EGTA, 1% (w/v) bovine serum albumin and 25 Hepes-NaOH (pH 7.4). Cells were centrifuged at 100 g for 5 min, re-suspended in Hepes-buffered Minimal Essential Medium (MEM; Gibco, Paisley, Scotland) containing 5% (v/v) bovine serum albumin and gentamycin (50 μg ml−1), plated onto 30 mm diameter polystyrene dishes and cultured for 2–10 days in humidified air at 37°C. β-cells were identified by their size (larger than non-β-cells) and granular appearance. The standard incubation medium used for islet cell preparation and incubations consisted of 130 NaCl, 5 KCl, 1 MgSO4, 1 NaH2PO4, 1.2 CaCl2, 25 Hepes-NaOH (pH 7.4) and glucose at the required concentration.
Electrophysiology
Cells were superfused at a rate of approximately 2 ml min−1 with incubation medium. Membrane potential was recorded from single β-cells using the perforated patch technique with a List EPC-7 amplifier (List, Darmstadt, Germany). At specific points during the recording, the amplifier was switched to voltage-clamp mode and inward current (Ii) recorded for a period of 60 s at a holding potential of −65 mV. Under such conditions, VRAC activation is manifest as a characteristically noisy inward current (Best, 1997, 2000). In order to quantify changes in VRAC activity, mean current amplitudes were measured from 50 s segments of recording using pCLAMP6 software (Axon Instruments). Input conductance (Ginput) was measured under similar conditions as an index of whole-cell KATP channel activity (Smith et al. 1990; Best, 2000). Briefly, cells were voltage-clamped at −70 mV and exposed to 200 ms pulses of ±10 mV at 2 s intervals. Single isolated β-cells were used for these experiments in order to avoid contaminating currents from adjacent electrically coupled cells. The basic pipette solution consisted of (mm): 138 KCl, 10 NaCl, 1 MgCl2 and 10 Hepes-NaOH (pH 7.2). Additional solutions were made with lower concentrations of Cl− by substitution with gluconate. In experiments designed to study the effects of varying [Cl−]i, electrical access was achieved by the inclusion of 240 μg ml−1 amphotericin B in the pipette solution. This antibiotic allows electrical access to the cell interior by forming pores permeable to monovalent cations and Cl− (Ermishkin et al. 1977) and is routinely used for perforated patch recording of membrane potential and whole-cell currents (Rae et al. 1991). When normal [Cl−]i was to remain intact, 50 μg ml−1 gramicidin (which forms Cl−-impermeable pores; Myers & Haydon, 1972) was used as perforating agent (Rhee et al. 1994). In both cases, series resistance was < 25 MΩ and whole-cell capacitance within the range 7–13 pF. Following seal formation, a period of approximately 10 min was allowed in order to achieve optimal electrical access and, in the case of amphotericin B, to maximize equilibration of Cl− between the pipette solution and the cell interior. All experiments were carried out at 28–30°C. Where appropriate, data are expressed as mean ± s.e.m., statistical significance being ascribed using Student's paired or unpaired t test.
Results
The first series of experiments investigated the effects of directly manipulating [Cl−]i via the patch pipette solution using amphotericin B. Since this antibiotic forms Cl−-permeable pores, its use provides a convenient method for altering [Cl−]i in an otherwise intact cell.
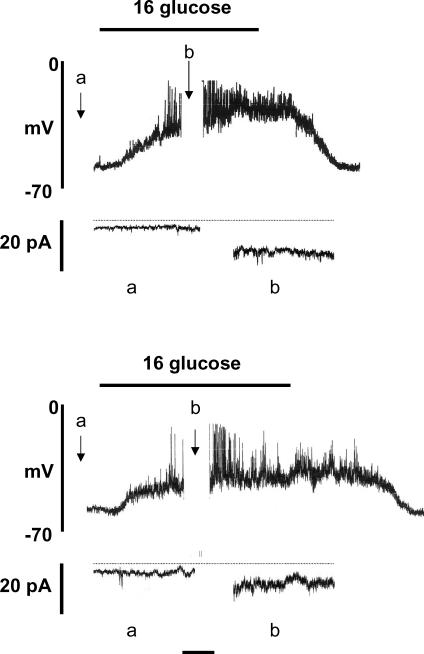
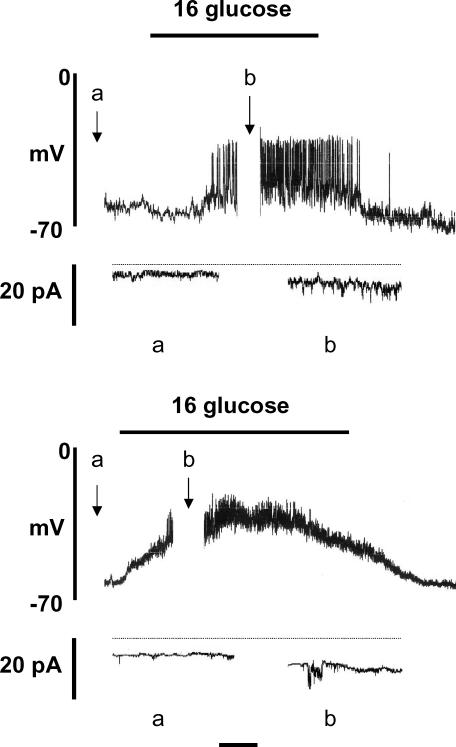
Figure 1 shows the effects of raising the glucose concentration from 4 to 16 mm on membrane potential and whole-cell current. Using amphotericin B and a pipette solution chloride concentration ([Cl−]p) of 150 mm Cl−, the resting membrane potential in the presence of 4 mm glucose was −64.3 ± 2.0 mV (n = 7; Fig. 1, upper panel). The corresponding whole-cell inward current (Ii) at a holding potential of −65 mV was −1.96 ± 0.68 pA (n = 7). In all cells studied, raising the glucose concentration to 16 mm depolarized the cell membrane potential, resulting in the generation of action potentials. Under such conditions, a marked, significant (P < 0.01) inward shift in holding current was apparent (−11.94 ± 1.58 pA, n = 7) together with increased current noise, representing activation of the volume-regulated anion channel (VRAC; Best et al. 1996a; Best, 1997, 2000). A return to 4 mm glucose was followed by repolarization of the membrane potential and cessation of electrical activity.
Figure 1. Amphotericin-perforated patch recordings from rat pancreatic β-cells.
The pipette solution contained 150 mm (upper) and 80 mm (lower) Cl−. Whole-cell current was recorded during the periods marked a and b. Horizontal time calibration bar: 1 min (membrane potential) or 15 s (current recordings). Horizontal dotted line, zero current. The recordings shown are representative of 7 similar experiments in each case.
Virtually identical findings were obtained when [Cl−]p was reduced to 80 mm, 16 mm glucose depolarizing 7/7 cells and generating electrical activity in 6/7 (Fig. 1, lower panel). However, further reduction of [Cl−]p resulted in a progressive impairment in the β-cell response to glucose stimulation. Thus, with 40 or 20 mm[Cl−]p, glucose-induced electrical activity was recorded in only 2/7 and 1/7 cells, respectively, a subthreshold depolarization being evoked in the majority (11/14) of cells (Fig. 2, upper panel). When [Cl−]p was reduced to 12 mm, 16 mm glucose had no effect on membrane potential in 7/7 cells. With a further reduction of [Cl−]p to 6 mm, glucose stimulation was found to cause a modest hyperpolarization in 4/7 cells (Fig. 2, lower panel).
Figure 2. Amphotericin-perforated patch recordings from rat pancreatic β-cells.
The pipette solution contained 20 mm (upper) and 6 mm (lower) Cl−. Whole-cell current was recorded during the periods marked a and b. Horizontal time calibration bar: 1 min (membrane potential) or 15 s (current recordings). Horizontal dotted line, zero current. The recordings shown are representative of 7 experiments in each case.
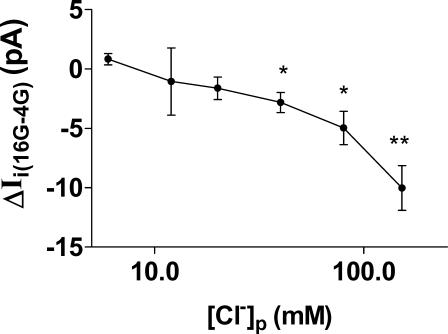
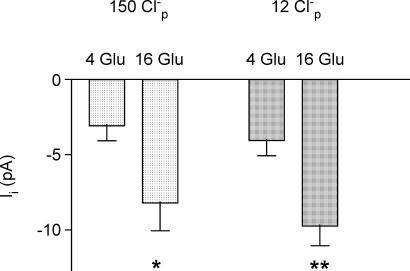
At a concentration of 4 mm glucose, neither the resting membrane potential nor the mean amplitude of Ii at a holding potential of −65 mV depended on [Cl−]p. For example, the values for resting membrane potential in the presence of 4 mm glucose with a [Cl−]p of 40 and 6 mm were −63.0 ± 0.9 and −59.4 ± 0.9 mV, respectively, with corresponding values for Ii of −2.46 ± 0.55 and −3.18 ± 0.40 pA (n = 7 in all cases). This suggests that [Cl−]i and VRAC activity do not make a major contribution to β-cell membrane potential at substimulatory glucose concentrations. However, as shown in Fig. 3, the inward current induced by 16 mm glucose (ΔIi(16G-4G)) showed a clear dependence on [Cl−]p. Indeed, a significant increase in current amplitude could only be evoked by 16 mm glucose when [Cl−]p was 40 mm or more. For a Cl−-selective current, reversal under the conditions used would be predicted with a value for [Cl−]i of approximately 11 mm. This compares with the observed value for current reversal with a [Cl−]p of approximately 7.5 mm. This small discrepancy could be explained in part by incomplete equilibration of Cl− between the pipette solution and the cell interior and by the significant permeability of the VRAC to ionic species other than [Cl−] (Best et al. 2001).
Figure 3. Relationship between chloride concentration of pipette solution ([Cl−]p) (plotted on log scale) and mean amplitude of inward current induced by 16 mm glucose (ΔIi(16G-4G)) during amphotericin-perforated patch recordings.
The values were calculated from mean current amplitudes during stimulation with 16 mm glucose minus the corresponding unstimulated mean current amplitudes in the presence of 4 mm glucose taken from 7 experiments of the type shown in Figs 1 and 2. Asterisks denote significant difference between whole-cell currents evoked by 16 and 4 mm glucose, ascribed by paired t test (*P < 0.01; **P < 0.001).
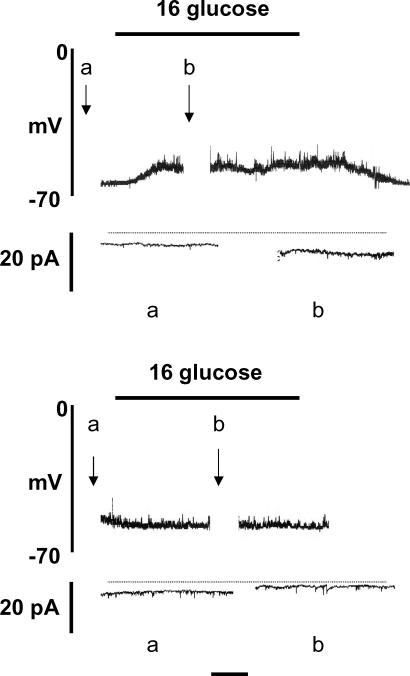
The net cellular K+ conductance should be small and remain constant under the conditions used. However, in order to ascertain whether β-cell KATP channel activity was affected by altered [Cl−]i, input conductance (Ginput) was measured under conditions similar to those described above (Fig. 4). With [Cl−]p of 150 mm and in the presence of 4 mm glucose, Ginput was 0.59 ± 0.06 nS (n = 6). Raising the glucose concentration to 16 mm resulted in a modest though significant (P < 0.05) increase in this value to 0.75 ± 0.07 nS, consistent with a previous report from this laboratory and possibly reflecting VRAC activation (Best, 2000). When [Cl−]p was reduced to 6 mm, Ginput in the presence of 4 mm glucose was 0.58 ± 0.06 nS (n = 6), a value virtually identical to that obtained with 150 mm [Cl−]p. Furthermore, under the latter conditions, a rise in glucose to 16 mm again significantly (P < 0.05) increased Ginput to 0.96 ± 0.15 nS. Thus, neither KATP channel activity nor the glucose-induced increase in Ginput appear to be affected by reduced [Cl−]i.
Figure 4. Amphotericin-perforated patch recordings from rat pancreatic β-cells.
The pipette solution contained 150 mm (upper) and 6 mm (lower) Cl−. Horizontal time calibration bar: 1 min (membrane potential) or 4 s (Ginput). The recordings shown are representative of 6 similar experiments.
Experiments were next carried out to investigate whether the dependence on [Cl−]i of glucose-induced depolarization was apparent when gramicidin was substituted for amphotericin B in the pipette solution as a pore-forming agent. In contrast to amphotericin B, gramicidin forms Cl−-impermeable pores and can therefore be used for perforated patch recording without disrupting [Cl−]i. As shown in Fig. 5, under these conditions 16 mm glucose depolarized the cells and induced electrical activity irrespective of [Cl−]p. Correspondingly, glucose stimulation increased the mean amplitude of the inward VRAC current to a similar extent with 150 or 12 mm [Cl−]p (Fig. 6).
Figure 5. Gramicidin-perforated patch recordings from rat pancreatic β-cells.
The pipette solution contained 150 mm (upper) and 12 mm (lower) Cl−. Whole-cell current was recorded during the periods marked a and b. Horizontal time calibration bar: 1 min (membrane potential) or 15 s (current recordings). Horizontal dotted line, zero current. The recordings shown are representative of 6–7 similar experiments.
Figure 6. Mean inward current amplitudes (Ii) in β-cells voltage-clamped at −65 mV under gramicidin-perforated patch conditions in the presence of 4 or 16 mm glucose.
The pipette solution contained 150 mm (n = 6) or 12 mm (n = 7) Cl−. *P < 0.02; **P < 0.005 compared with 4 mm glucose controls.
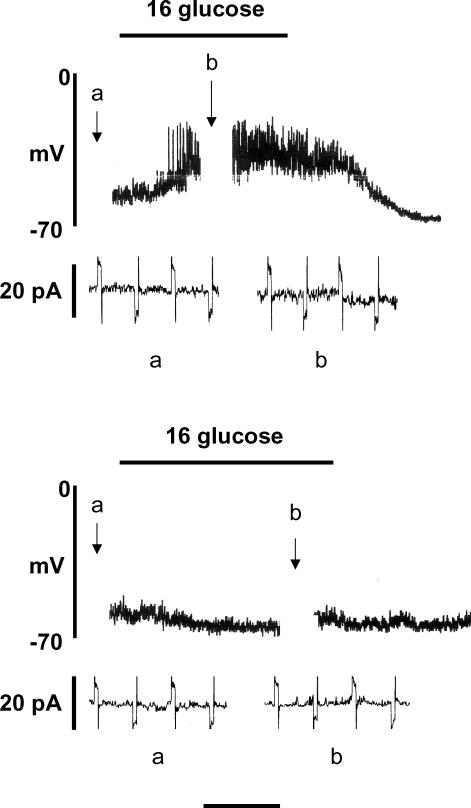
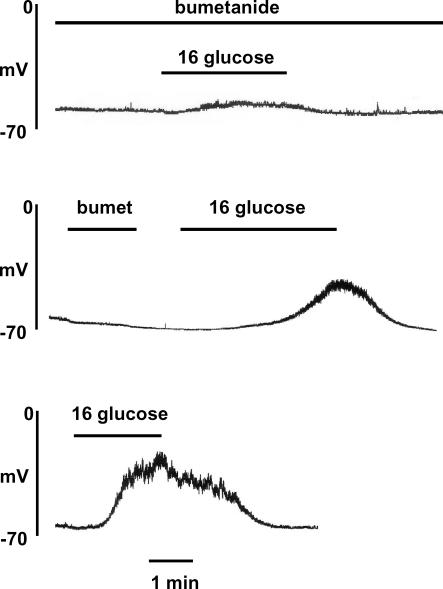
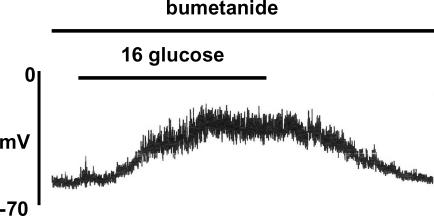
As noted earlier, ECl is maintained at positive values in the β-cell, probably due to activity of the Na+–K+–2Cl− co-transporter NKCC1. The next series of experiments investigated the effects of pharmacological inhibition of this co-transporter by the loop diuretic bumetanide. Unless otherwise stated, gramicidin was used in the pipette solution in these experiments in order to maintain normal [Cl−]i. The resting membrane potential (at 4 mm glucose) of cells incubated in the presence of 10 μm bumetanide (−61.3 ± 1.64 mV, n = 8) was not significantly different from that in cells in the absence of the diuretic (−57.3 ± 1.6 mV, n = 8), consistent with the earlier suggestion that [Cl−]i does not make a major contribution to β-cell membrane potential at substimulatory concentrations of glucose. However, as shown in Fig. 7 (upper panel), pre-treatment of the cells with 10 μm bumetanide markedly suppressed the subsequent response to 16 mm glucose. A delayed and diminished response to high glucose persisted for several minutes following withdrawal of the diuretic (Fig. 7, middle panel), but a normal response could generally be restored by a prolonged period of drug ‘washout’ (Fig. 7, lower panel). When similar experiments were carried out using amphotericin B (with 150 mm [Cl−]p) instead of gramicidin, bumetanide pre-treatment failed to prevent glucose-induced electrical activity (Fig. 8). This indicates that the imposition of a high [Cl−]i via the pipette solution could effectively counteract the reduction of [Cl−]i resulting from NKCC1 inhibition by the diuretic.
Figure 7.
Inhibition of glucose-induced electrical activity by pre-treatment with 10 μm bumetanide (upper and middle recordings) and 30 min following exposure to bumetanide (lower recording). The pipette solution contained gramicidin and 150 mm Cl−. The traces are representative of at least 5 similar recordings in each case.
Figure 8. Lack of inhibition of glucose-induced electrical activity by 10 μm bumetanide under amphotericin-perforated patch conditions with a pipette solution containing 150 mm Cl−.
The trace is representative of 5 similar recordings.
Discussion
The purpose of the present study was to test the hypothesis that glucose-induced depolarization and electrical activity in the pancreatic β-cell are dependent on maintenance of a positive value for ECl with respect to the resting membrane potential. As noted earlier, the available estimates for [Cl−]i in the β-cell indicate that intracellular Cl− is accumulated above its electrochemical equilibrium, at least in part through the action of NKCC1. Two distinct experimental approaches have therefore been used to alter [Cl−]i whilst maintaining cellular integrity.
The first approach took advantage of the Cl− permeability of amphotericin B used for perforated patch recording, allowing exchange of Cl− between the pipette solution and the cell interior. As noted earlier, a limitation of this technique is that, even after a period of equilibration, the precise value of [Cl−]i (and hence ECl) cannot be certain, since these will depend on the relative rates of Cl− exchange via the amphotericin B pores in the cell patch and via the various Cl− exchange mechanisms in the plasma membrane. However, it was clear from these studies that both electrical activity and magnitude of the VRAC current evoked by 16 mm glucose were markedly dependent on [Cl−]i. Thus, 16 mm glucose routinely caused depolarization and electrical activity only when [Cl−]p was 150 or 80 mm, broadly corresponding to estimates of [Cl−]i in intact cells. In contrast, the use of lower [Cl−]p progressively impaired depolarization and reduced the amplitude of Ii induced by 16 mm glucose. When gramicidin, which forms Cl−-impermeable pores, was used as perforating agent instead of amphotericin B, responses to 16 mm glucose were unaffected by altered [Cl−]p.
Comparable findings have been reported in other cell types. For example, studies in neocortical neurones have shown that changes in membrane potential and excitability mediated via chloride channels are influenced by altering [Cl−]i, using the conventional whole-cell recording configuration (Nakanishi & Kukita, 2000). A similar dependency on [Cl−]i has been demonstrated for membrane potential and the polarity of Cl− currents with the perforated patch technique in neurones and smooth muscle cells using the Cl−-permeable antibiotics nystatin and amphotericin (Abe et al. 1994; Rhee et al. 1994; Kyrozis & Reichling, 1995). In each of these studies, artefactual changes in [Cl−]i could be eliminated by the use of gramicidin as perforating agent.
The present study is consistent with the hypothesis that depolarization of the pancreatic β-cell by glucose involves activation of the VRAC and the generation of an inward anion current (Best et al. 1997; Best & McLaughlin, 2004). However, these findings could also have significant implications regarding previously published perforated patch studies with the glucagon-secreting α-cell. The mechanism by which glucose inhibits glucagon release is at present unclear. Indeed, reports that glucose hyperpolarizes (Barg et al. 2000) and depolarizes (Gromada et al. 2004) the α-cell have originated from the same laboratory. The pipette solution used in these studies was reported to contain amphotericin B with 22 mm Cl−, conditions that, in the present study, generated a modest depolarization in β-cells. It is conceivable the imposition of an abnormal [Cl−]i could profoundly influence the α-cell response to glucose. In this respect, it should be borne in mind that, in contrast to the pancreatic β-cell, α-cells express the outward K+–Cl− co-transporter KCC which would be expected to maintain ECl at a negative value (i.e. low [Cl−]i; Davies et al. 2004). In these circumstances, VRAC activation by glucose would be expected to generate an outward (hyperpolarizing) current (see Best & McLaughlin, 2004 for further discussion). Taken together, the above factors suggest that future perforated patch-clamp studies of islet cells should include the use of gramicidin in order to avoid possible pitfalls resulting from the unwitting alteration of [Cl−]i.
The second experimental approach to investigate the role of [Cl−]i in β-cell function involved the use of the loop diuretic bumetanide to inhibit NKCC1 and thereby reduce [Cl−]i. Bumetanide has been previously shown to hyperpolarize arterial smooth muscle (Davis, 1992) and skeletal muscle (van Mil et al. 1997), indicating an important role for [Cl−]i in regulating membrane potential in these tissues. In the present study, the resting membrane potential in the presence of 4 mm glucose was not significantly affected by bumetanide, consistent with the idea that [Cl−]i does not make a major contribution to β-cell membrane potential at low concentrations of glucose. However, treatment with the diuretic caused a marked impairment of depolarization in response to a stimulatory concentration of glucose and effectively blocked electrical activity. The finding that this inhibitory effect could be reversed by raising [Cl−]i strongly suggests that the primary action of bumetanide on the β-cell was inhibition of NKCC1 resulting in a reduction in [Cl−]i and a consequent impairment in glucose-induced VRAC current. This conclusion is consistent with the earlier suggestion of Sandstrom (1990), who demonstrated an inhibition of insulin release by bumetanide, and could explain the diabetogenic action of loop diuretics (Furman, 1981).
In conclusion, the results of the present study support the hypothesis that VRAC activation, generating an inward anion current, is an important step in depolarization of the β-cell membrane potential and the induction of electrical activity by glucose. The primary physiological function of the KATP channel, which is regulated by glucose within a substimulatory concentration range, could be to hyperpolarize and lower the electrical resistance of the β-cell during hypoglycaemia, thereby preventing insulin release.
Acknowledgments
This work was supported in part by the Wellcome Trust. I should like to thank Dr Peter Brown for helpful discussion.
References
- Abe Y, Furukawa K, Itoyama Y, Akaike N. Glycine response in acutely dissociated ventromedial hypothalamic neuron of the rat: new approach with gramicidin perforated patch-clamp technique. J Neurophysiol. 1994;72:1530–1537. doi: 10.1152/jn.1994.72.4.1530. [DOI] [PubMed] [Google Scholar]
- Ashcroft FM, Ashcroft SJH, Harrison DE. Properties of single potassium channels modulated by glucose in rat pancreatic β-cells. J Physiol. 1988;400:501–527. doi: 10.1113/jphysiol.1988.sp017134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft FM, Rorsman P. Electrophysiology of the pancreatic β -cell. Prog Biophys Molec Biol. 1989;54:87–143. doi: 10.1016/0079-6107(89)90013-8. [DOI] [PubMed] [Google Scholar]
- Barg S, Galvanovskis J, Gopel SO, Rorsman P, Eliasson L. Tight coupling between electrical activity and exocytosis in mouse glucagon-secreting α-cells. Diabetes. 2000;49:1500–1510. doi: 10.2337/diabetes.49.9.1500. [DOI] [PubMed] [Google Scholar]
- Best L. Glucose and a-ketoisocaproate induce transient inward currents in rat pancreatic beta cells. Diabetologia. 1997;40:1–6. doi: 10.1007/s001250050635. [DOI] [PubMed] [Google Scholar]
- Best L. Cell-attached recordings of the volume-sensitive anion channel in rat pancreatic B-cells. Biochim Biophys Acta. 1999;1419:248–256. doi: 10.1016/s0005-2736(99)00071-1. [DOI] [PubMed] [Google Scholar]
- Best L. Glucose-sensitive conductances in rat pancreatic beta-cells: contribution to electrical activity. Biochim Biophys Acta. 2000;1468:311–319. doi: 10.1016/s0005-2736(00)00272-8. [DOI] [PubMed] [Google Scholar]
- Best L. Evidence that glucose-induced electrical activity in rat pancreatic β-cells does not require KATP channel inhibition. J Membr Biol. 2002a;185:193–200. doi: 10.1007/s00232-001-0114-1. [DOI] [PubMed] [Google Scholar]
- Best L. Study of a glucose-activated anion-selective channel in rat pancreatic beta-cells. Pflugers Arch. 2002b;445:97–104. doi: 10.1007/s00424-002-0893-y. [DOI] [PubMed] [Google Scholar]
- Best L. Inhibition of glucose-induced electrical activity by 4-hydroxytamoxifen in rat pancreatic β-cells. Cellular Signalling. 2002c;14:69–73. doi: 10.1016/s0898-6568(01)00223-6. [DOI] [PubMed] [Google Scholar]
- Best L, Brown PD, Sheader EA, Yates AP. Selective inhibition of glucose-stimulated beta-cell activity by an anion channel inhibitor. J Membr Biol. 2000;177:169–175. doi: 10.1007/s002320001110. [DOI] [PubMed] [Google Scholar]
- Best L, Brown PD, Tomlinson S. Anion fluxes, volume regulation and electrical activity in the mammalian pancreatic β-cell. Exp Physiol. 1997;82:957–966. doi: 10.1113/expphysiol.1997.sp004081. [DOI] [PubMed] [Google Scholar]
- Best L, McLaughlin J. Nutrients as regulators of endocrine and neuroendocrine secretion. In: Winderickx JG, Taylor PM, editors. Nutrient-Induced Responses in Eucaryotic Cells. Vol. 7. Berlin: Springer-Verlag; 2004. pp. 79–111. Topics in Current Genetics. [Google Scholar]
- Best L, Miley HE, Yates AP. Activation of an anion conductance and B-cell depolarization during hypotonically-induced insulin release. Exp Physiol. 1996a;81:927–933. doi: 10.1113/expphysiol.1996.sp003993. [DOI] [PubMed] [Google Scholar]
- Best L, Sheader EA, Brown PD. A volume-activated anion conductance in insulin-secreting cells. Pflugers Arch. 1996b;431:363–370. doi: 10.1007/BF02207273. [DOI] [PubMed] [Google Scholar]
- Best L, Speake T, Brown PD. Characterization of the volume-sensitive anion channel in rat pancreatic beta-cells. Exp Physiol. 2001;86:145–150. doi: 10.1113/eph8602118. [DOI] [PubMed] [Google Scholar]
- Best L, Yates AP, Decher N, Steinmeyer K, Nilius B. Inhibition of glucose-induced electrical activity in rat pancreatic β-cells by DCPIB, a selective inhibitor of volume-sensitive anion currents. Eur J Pharmacol. 2004;489:13–19. doi: 10.1016/j.ejphar.2004.02.030. [DOI] [PubMed] [Google Scholar]
- Best L, Yates AP, Tomlinson S. Stimulation of insulin secretion by glucose in the absence of diminished potassium (86Rb+) permeability. Biochem Pharmacol. 1992;43:2483–2485. doi: 10.1016/0006-2952(92)90330-l. [DOI] [PubMed] [Google Scholar]
- Davis JP. The effects of Na+–K+–Cl−– co-transport and Cl−–HCO3− exchange blockade on the membrane potential and intracellular chloride levels of rat arterial smooth muscle, in vitro. Exp Physiol. 1992;77:857–862. doi: 10.1113/expphysiol.1992.sp003652. [DOI] [PubMed] [Google Scholar]
- Davies SL, Roussa E, Le Rouzic P, Thevenod F, Alper SL, Best L, Brown PD. Expression of K/Cl cotransporters in the α-cells of rat endocrine pancreas. Biochim Biophys Acta. 2004;1667:7–14. doi: 10.1016/j.bbamem.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Drews G, Zempel G, Krippeit-Drews P, Britsch S, Busch GL, Kaba NK, Lang F. Ion channels involved in insulin release are activated by osmotic swelling of pancreatic B-cells. Biochim Biophys Acta. 1998;1370:8–16. doi: 10.1016/s0005-2736(97)00240-x. [DOI] [PubMed] [Google Scholar]
- Eberhardson M, Patterson S, Grappengiesser E. Microfluorometric analysis of Cl− permeability and its relation to oscillatory Ca2+ signalling in glucose-stimulated pancreatic β-cells. Cellular Signalling. 2000;12:781–786. doi: 10.1016/s0898-6568(00)00122-4. [DOI] [PubMed] [Google Scholar]
- Ermishkin LN, Kasumov KM, Potseluyev VM. Properties of amphotericin B channels in a lipid bilayer. Biochim Biophys Acta. 1977;470:357–367. doi: 10.1016/0005-2736(77)90127-4. [DOI] [PubMed] [Google Scholar]
- Furman BL. Impairment of glucose tolerance produced by diuretics and other drugs. Pharmacol Ther. 1981;12:613–649. doi: 10.1016/0163-7258(81)90102-9. [DOI] [PubMed] [Google Scholar]
- Gromada J, Ma X, Hoy M, Bokvist K, Salehi A, Berggren P-O, Rorsman P. ATP-sensitive K+ channel-dependent regulation of glucagon release and electrical activity by glucose in wild-type and SUR1−/− mouse α-cells. Diabetes. 2004;53(Suppl. 3):S181–S189. doi: 10.2337/diabetes.53.suppl_3.s181. [DOI] [PubMed] [Google Scholar]
- Henquin JC. ATP-sensitive K+ channels may not be the sole regulators of glucose-induced electrical activity in pancreatic B-cells. Endocrinology. 1992;131:127–131. doi: 10.1210/endo.131.1.1611991. [DOI] [PubMed] [Google Scholar]
- Kinard TA, Satin LS. An ATP-sensitive Cl− channel current that is activated by cell swelling, cAMP and glyburide in insulin-secreting cells. Diabetes. 1995;44:1461–1466. doi: 10.2337/diab.44.12.1461. [DOI] [PubMed] [Google Scholar]
- Kyrozis A, Reichling DB. Perforated-patch recording with gramicidin avoids artifactual changes in intracellular chloride concentration. J Neurosci Meth. 1995;57:27–35. doi: 10.1016/0165-0270(94)00116-x. [DOI] [PubMed] [Google Scholar]
- Lindstrom P, Norlund L, Sandstrom P-E, Sehlin J. Evidence for co-transport of sodium, potassium and chloride in mouse pancreatic islets. J Physiol. 1988;400:223–236. doi: 10.1113/jphysiol.1988.sp017118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majid A, Speake T, Best L, Brown PD. Expression of the Na-K-2Cl cotransporter in α and β cells isolated from the rat pancreas. Pflugers Arch. 2001;442:570–576. doi: 10.1007/s004240100566. [DOI] [PubMed] [Google Scholar]
- Malaisse WJ, Zhang Y, Louchami K, Jijakli H. Stimulation by d-glucose of 36Cl− efflux from prelabeled rat pancreatic islets. Endocrine. 2004;25:23–25. doi: 10.1385/ENDO:25:1:23. [DOI] [PubMed] [Google Scholar]
- Misler S, Barnett DW, Gillis KD, Pressel DM. Electrophysiology of stimulus-secretion coupling in human β-cells. Diabetes. 1992;41:1221–1228. doi: 10.2337/diab.41.10.1221. [DOI] [PubMed] [Google Scholar]
- Myers VB, Haydon DA. Ion transfer across lipid membranes in the presence of gramicidin A. II. The ion selectivity. Biochim Biophys Acta. 1972;274:313–322. doi: 10.1016/0005-2736(72)90179-4. [DOI] [PubMed] [Google Scholar]
- Nakanishi K, Kukita F. Intracellular [Cl−] modulated synchronous electrical activity in rat neocortical neurones in culture by way of GABAergic inputs. Brain Res. 2000;863:192–204. doi: 10.1016/s0006-8993(00)02152-1. [DOI] [PubMed] [Google Scholar]
- Rae J, Cooper K, Gates P, Watsky M. Low access resistance perforated patch recordings using amphotericin B. J Neurosci Meth. 1991;37:15–26. doi: 10.1016/0165-0270(91)90017-t. [DOI] [PubMed] [Google Scholar]
- Rhee JS, Ebihara S, Akaike N. Gramicidin perforated patch-clamp technique reveals glycine-gated outward chloride current in dissociated nucleus solitarii neurons of the rat. J Neurophysiol. 1994;72:1103–1108. doi: 10.1152/jn.1994.72.3.1103. [DOI] [PubMed] [Google Scholar]
- Sandstrom P-E. Bumetanide reduces insulin release by a direct effect on the pancreatic β-cell. Eur J Pharmacol. 1990;187:377–383. doi: 10.1016/0014-2999(90)90365-d. [DOI] [PubMed] [Google Scholar]
- Sehlin J. Interrelationship between chloride fluxes in pancreatic islets and insulin release. Am J Physiol. 1978;235:E501–E508. doi: 10.1152/ajpendo.1978.235.5.E501. [DOI] [PubMed] [Google Scholar]
- Smith PA, Ashcroft FM, Rorsman P. Simultaneous recordings of glucose-induced electrical activity and ATP-regulated K+-currents in isolated mouse pancreatic beta-cells. FEBS Lett. 1990;261:187–190. doi: 10.1016/0014-5793(90)80667-8. [DOI] [PubMed] [Google Scholar]
- van Mil HG, Geukes Foppen RJ, Siegenbeek van Heukelom J. The influence of bumetanide on the membrane potential of mouse skeletal muscle cells in isotonic and hypertonic media. Br J Pharmacol. 1997;120:39–44. doi: 10.1038/sj.bjp.0700887. [DOI] [PMC free article] [PubMed] [Google Scholar]